Abstract
Hypothyroidism is a very common disorder worldwide, for which the usual treatment is monotherapy with levothyroxine (L-T4). However, a number of patients treated with L-T4 continue to report symptoms of hypothyroidism despite seemingly normal levels of thyroid stimulating hormone (TSH), free-T3 (FT3), and free-T4 (FT4) measured by immunoassay. This review summarizes the limitations of the immunoassays commonly used to measure thyroid hormones and emphasizes the advantages of the role of liquid chromatography-tandem mass spectrometry (LC-MSMS). Immunoassays for free thyroid hormone are affected by alterations in serum binding proteins that occur in many physiologic and disease states. Multiple studies show falsely normal values for T3, FT3, and FT4 by immunoassay that are below the reference interval when measured by (ultrafiltration) LC-MSMS, a reference method. We suggest evaluation of thyroid hormone levels by ultrafiltration LC-MSMS for patients who continue to experience hypothyroid symptoms on LT-4. This may help identify the approximately 20% subset of patients who would benefit from addition of T3 to their treatment regimen (combination therapy).
Keywords: total thyroid hormone, free thyroid, immunoassay, LC-MSMS
Introduction
Hypothyroidism is a common disorder. Overt hypothyroidism occurs in approximately 5% of the population in developed countries, while subclinical hypothyroidism (defined by elevated thyroid stimulating hormone [TSH] with normal immunoassay free thyroxine [FT4]) occurs in 4 – 10% of people (1, 2). Measurement of those samples for FT4 and free 3,5,3′-triiodothyronine (FT3) by ultrafiltration LC-MS/MS demonstrates that 2/3 of individuals classified as subclinical by immunoassay have FT4 or FT3’s below the 2.5TH percentiles and are therefore now classified as clinically hypothyroid (3). Hypothyroidism is more commonly found with increasing age and female gender, with a female to male ratio of approximately 9:1 (4). The signs and symptoms of hypothyroidism are dependent on the degree of thyroid hormone deficiency, the acuity of disease development, and the duration of disease. The consequences of untreated or wrongly treated hypothyroidism can be significant, ranging from nonspecific symptoms to life-threatening manifestations. Even the syndrome “subclinical hypothyroidism” may have clinical consequences, and has been linked to infertility, pregnancy complications, psychiatric illness, neuromuscular symptoms, cardiac dysfunction, and mortality (5, 6). Thus, accurate and timely diagnosis of hypothyroidism is essential. Extra-thyroidal diseases are also important conditions requiring accurate measurement of thyroid hormones. For example, euthyroid sick syndrome occurs in critically ill patients and is characterized by low FT3, normal to low TSH, frequently elevated reverse T3, and low FT4 if of prolonged duration; an accurate FT3 is especially important to the diagnosis (7). Other important conditions for measurement of free thyroid hormones include those associated with drugs that affect thyroid function, peripheral metabolism of thyroid hormones such as amiodarone (8), or binding of thyroid hormones to plasma proteins.
The two major thyroid hormones produced by the thyroid gland are 3,5,3′-triiodothyronine (T3) and thyroxine (T4). The thyroid gland exclusively synthesizes T4, and produces approximately 20% of T3. The majority of T3 is generated by 5′-deiodination catalyzed by type I and type II T4–5′-deiodinases in peripheral tissue. Specifically, approximately 65% of peripheral-derived T3 is produced by type II deiodinase, a microsomal enzyme with activity in the brain, muscle, pituitary, and placenta; the remaining 35% is derived from type I deiodinase activity, a plasma membrane enzyme located in the thyroid, liver, and kidney (9). A type III deiodinase inactivates T3 and T4 to produce reverse T3 and diiodothyronine (10). The majority of circulating thyroid hormones are bound to serum proteins, allowing for their transport and extension of half-life; however, carrier protein binding makes thyroid hormones unavailable to tissues and thus protein bound T3 and T4 are considered inactive. Thyroxine-binding globulin (TBG) is a high affinity, but low concentration, binding protein that binds approximately 80% of T3 and 75% of T4 (11). The remainder of circulating T3 and T4 are bound to the low-affinity proteins albumin and transthyretin. Changes in binding protein concentrations occur in a number of conditions, and can significantly impact the total thyroid hormone concentrations. Only approximately 0.04% of total T3 and 0.02% of T4 are available in circulation as free hormones, and are considered the biologically active forms because the free hormone is accessible to peripheral tissue. According to the free hormone hypothesis, only the free thyroid hormone is available for cellular uptake, while the bound form is not immediately available (12). Recently it has been shown that T4 has a very poor affinity for the thyroid hormone nuclear receptor while T3 is avidly bound to the latter and responsible for the clinical effects, indicating that T4 is the prehormone and needs to be converted to the active hormone T3 (13, 14).
The laboratory assays for assessment of thyroid function include thyroid stimulating hormone (TSH), total triiodothyronine (TT3), total thyroxine (TT4), FT3, and FT4. The measurement of FT3 and FT4 are the most clinically relevant for the evaluation of thyroid disorders, with total thyroid hormones being affected by variations in binding protein concentrations. It is essential that free thyroid hormone measurement accurately assess hormone concentration even in the presence of significant variation in the concentration of binding proteins that occur in a variety of physiological and disease states. This is clearly achievable using equilibrium dialysis or ultrafiltration to effect the separation and then measure the free thyroid concentrations in the dialysate or ultrafiltrate.
Increasing evidence by our group and others indicates inaccurate values are being generated for thyroid hormones by the commonly used immunoassay platforms. SJS directed the chemistry laboratory at Children’s National Medical Center from 1988–2008. Shortly after arriving he met with Dr Wellington Hung, the head of endocrinology and a thyroid expert. Dr Hung emphasized that FT4 tests run in the laboratory by immunoassay often did not agree with TSH, especially true when the TSH’s were elevated. Repeating the immunoassay FT4’s and FT3’s gave results that were very similar; ie. immunoassays gave very precisely the wrong result. To establish why this happened, we sent these samples to Nichols laboratory for measurement of FT4 by equilibrium dialysis followed by immunoassay. Almost all these results were found to agree with the high TSH’s thereby demonstrating conclusively that a distinct problem existed with currently used immunoassay platforms for the measurement of FT4 and FT3. Work by our team over the past 2 decades has verified that immunoassays for FT4, FT3, and TT3 frequently overestimate values for all 3 tests especially at low concentrations (3, 14–18). Furthermore, these papers have shown a poor correlation of immunoassay values for FT4, FT3, and TT3 with log TSH or TSH as well as with the patient’s clinical condition. The Food and Drug Administration (FDA) is partially responsible for this as they have approved immunoassay platforms for the measurement of all 3 analytes and did not check whether the results generated by these assays agreed with TSH or the patient’s clinical condition (19). An important distinction between the immunoassay and LC-MSMS methods is that the latter remove the thyroid binding proteins prior to measurement of FT4 and FT3. This review discusses the assays available for total and free thyroid hormone measurement. Specific limitations of commonly used assays are discussed, and issues relevant to treatment are highlighted.
Limitations of current free thyroid hormone immunoassays
The majority of laboratories use one-step direct analog immunoassay for determination of free thyroid hormone. A complete review of assay methodology for total and free thyroid hormone measurement has been published elsewhere (14). A number of disease and physiological states have been shown to impact free thyroid hormone measurement. Furthermore, free thyroid measurements show poor agreement between various assays.
Numerous protein binding variations can affect free thyroid hormone measurement (14). Briefly, pregnancy is a well-established cause of increased TBG and lowered albumin levels, resulting in inaccurate free thyroid hormone by immunoassay (20). Genetic variations in binding proteins occur such as congenital TBG deficiency or excess, or mutations in transthyretin and albumin that confound free hormone measurement by immunoassay (21). Many commonly used medications disrupt thyroid hormone binding to serum proteins; dilution used by immunoassay methods can open more free thyroid hormone binding spots on proteins by reducing the competing drugs with potential alteration of free hormone measurement (14). For example, heparin administration in both fractionated and unfractionated forms causes displacement of T3 and T4 from binding proteins causing apparent elevations in measured FT3 and FT4 values, possibly due to heparin activation of lipoprotein lipase (22). Other common medications that displace T3 and T4 from serum binding proteins with implications on thyroid testing include furosemide, salicylate, and anti-epileptics (23, 24). Patients with certain medical conditions such as end-stage renal disease, major cardiac surgery, and critical illness have free thyroid hormones levels that are inaccurate by analog immunoassay methods (25, 26). The dependence of FT4 and FT3 on thyroid hormone binding proteins thus makes most of these immunoassays unsuitable for a variety of populations. Indeed, several studies have shown the dependence of FT4 measurement on serum binding protein conditions (27–29).
A number of studies have shown inconsistencies between the results of various free thyroid hormone immunoassays. An investigation by Giovannini et al. (30) evaluated 54 control samples representing a range of values for FT3 and FT4 measurement by 1000 laboratories. Substantial differences, of over 20%, were found for both FT3 and FT4 immunoassay methods. Furthermore, low concentrations of FT3 and FT4 showed higher imprecision compared to normal range samples (30). A College of American Pathologist study of fresh frozen serum sent to 3900 clinical laboratories reported significant analytic bias for nine of eleven free T3 and 11 of 13 for free T4 immunoassays (31). A number of other studies report significant differences in free thyroid hormone results according to the immunoassay method performed (32–36). The differences in free thyroid hormone values by the various immunoassay methods may reflect differential assay susceptibility to the various alterations in serum binding proteins. Regardless, the often times substantial differences in free thyroid hormone values according to the method performed have significant clinical implications.
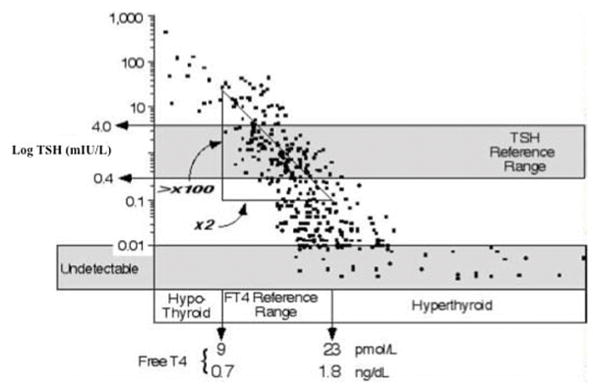
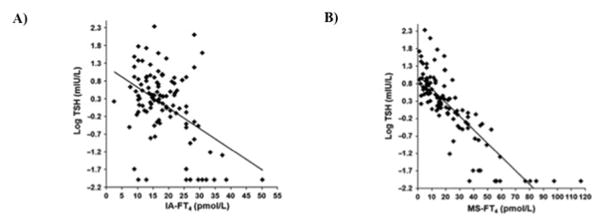
Another indicator of the suboptimal performance of free thyroid hormone immunoassays is the poor correlation of their results on the inverse log-linear relationship between TSH and FT4. The levels of thyroid hormone are tightly controlled by negative regulation of pituitary secretion of TSH such that increased thyroid hormone results in decreased production of TSH, while low thyroid hormone levels induce TSH secretion. The inverse log-linear association between TSH and FT4 has been well documented (14). This relationship is shown graphically in Figure 1 (37). An evaluation of three commercially available immunoassays found relatively poor correlation coefficients between log-transformed TSH and FT4 ranging from 0.72 – 0.76 in a euthyroid population (38). Worse correlation coefficients (0.08 – 0.58) are reported when the population includes patients with thyroid disease (16–18, 39). A representative image of the log TSH and FT4 performed by immunoassay is shown in Figure 2A. In contrast, substantially better correlation coefficients (0.77 – 0.90) are reported for the log-transformed TSH and FT4 measured by ultrafiltration LC-MSMS (Figure 2B) (16, 18, 39).
Figure 1.
Relationship between the log TSH and FT4. Adapted from Spencer et al. (37) with permission.
Figure 2.
Relationship of FT4 with the log TSH. A) TSH and FT4 performed on the Siemens Immulite 2500 analyzer show a poor correlation. B) Improved correlation of the inverse log-linear relationship between TSH and FT4 when FT4 is measured by ultrafiltration followed by LC-MSMS. Adapted from (18) with permission from the American Association for Clinical Chemistry.
Emerging role of LC-MSMS
LC-MSMS identifies analytes by column retention time in combination with the mass-to-charge ratio (m/z) of parent and fragmentation product ions. Quantitation is accomplished by comparing the ratio of measured analyte to a labeled internal standard (isotope dilution tandem mass spectrometry). LC-MSMS assays are highly specific, sensitive, precise, and can detect hormones found in low concentrations. Isotope dilution LC-MSMS has been successfully applied to the measurement of a number of hormones including gonadal and adrenal steroids, thyroid hormones, and 25-hydroxyvitamin D (40–43). Methodology to measure FT3 and FT4 simultaneously by isotope dilution tandem mass spectrometry has also been described (44).
Several studies have compared immunoassays on a variety of platforms to ultrafiltration and equilibrium dialysis LC-MSMS. Reasonable correlation of immunoassay to LC-MSMS is often noted in the euthyroid range; however, the assays frequently disagree at the low and high values for concentrations of thyroid hormone, which are the most critical because these values represent the points that clinical decisions are made. Jonklaas et al. (15) reported lower TT3 values by ultrafiltration LC-MSMS compared to a chemiluminescent immunoassay platform in patients with TSH over 4.5 mIU/L. A different investigation with 63 outpatients and 37 inpatients noted discrepant values at the high and low values of the reference interval, especially for TT3, FT3, and FT4, with low values more frequently shown by LC-MSMS; furthermore, patients with high concentrations for TSH often had thyroid hormones below the reference interval for LC-MSMS that were misclassified as normal by immunoassay (45). A study of 40 patients classified as subclinical hypothyroidism by elevated TSH with normal FT4 levels by conventional immunoassay found that 65% of such patients actually had FT3 or FT4 levels below the reference range by ultrafiltration LC-MSMS (3). Laboratory assays must have acceptable performance throughout the entire analytical range. The assay performance is especially important at the decision thresholds, specifically high and low concentrations of free hormones. Inaccurate values at the high and low range of free hormone can have significant clinical implications by delaying appropriate diagnosis and treatment of thyroid disorders.
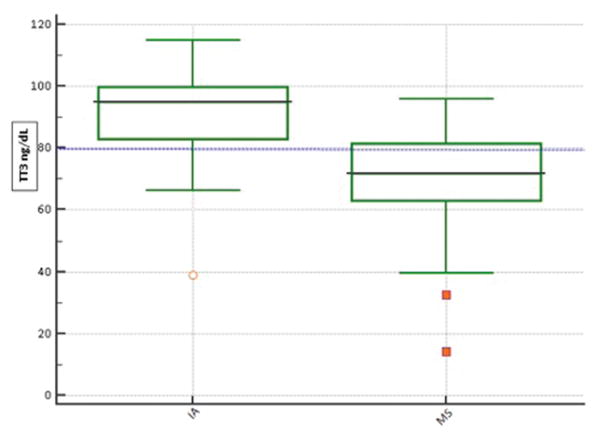
Another indicator of the superiority of LC-MSMS is the improved correlation coefficient for free thyroid hormone and the log-transformed TSH compared to immunoassay. An evaluation of 38 pediatric samples, 200 euthyroid adults, and 128 pregnant women reported an improved log-TSH correlation with free thyroid hormones when measured by LC-MSMS compared to immunoassay (16). Other studies have also reported improved correlation coefficients, ranging from 0.86 – 0.90 (15, 18). The inverse relationship between TSH and T3 is also superior when evaluated by LC-MSMS compared to immunoassay (Figure 3) (15, 18, 46). A recent investigation found significantly lower TT3 values when measured by iostope dilution LC-MSMS compared to immunoassay in patients with high TSH values, confirming the superior correlation with TSH (19).
Figure 3.
TT3 by immunoassay and LC-MSMS at TSH’s above the 95th percentile (3.70 mIU/L). Immunoassay TT3 and TSH was measured on the Roche Cobas 6000. Low values of TT3 are considered <80 ng/dL. More patients were classified as having low TT3 when measured by LC-MSMS, indicating a superior correlation with TSH compared to immunoassay.
Clinical implications
The normal thyroid gland produces both T3 and T4; however, only levothyroxine (L-T4) is the usual replacement treatment used for patients with hypothyroidism in the United States. L-T4 is one of the most commonly given drugs worldwide, and overall has an excellent efficacy and safety record. The majority of patients respond well to L-T4 monotherapy, and patients administered L-T4 can have adequate levels of T3 formed by monodeiodination of T4 (47). However, a subset of patients treated with L-T4 monotherapy continue to report symptoms suggestive of hypothyroidism (48, 49); such patients may account for as many as 20% of hypothyroid patients. These patients usually have within range immunoassay values for TSH, total and free T3, and FT4, giving the suggestion of appropriate L-T4 monotherapy.
The numerous studies reviewed in the above sections demonstrate that the immunoassays used by most laboratories are inaccurate for thyroid hormones, particularly at the low end of the reference interval. Specifically, many patients are classified as having normal levels of thyroid hormones by the commonly used immunoassay methods that are actually below the reference interval by the reference method LC-MSMS. This overestimation by immunoassay can be significant; for example, a study of euthyroid inpatients placed on approximately 6–8 medications found 48% of T3 values were below the 2.5th percentile by LC-MSMS compared to only 11% by immunoassay (45). We hypothesize that many patients who still experience hypothyroid symptoms despite treatment with L-T4 may actually have low levels of T3 that are incorrectly classified by the common immunoassay methods. We recommend patients who continue to experience symptoms of hypothyroidism despite treatment with L-T4 be evaluated for T3, FT3, and FT4 measurement by LC-MSMS. This may identify patients who would benefit by addition of T3 to their treatment regimen. Thyroid hormone measurement by LC-MSMS is available through most large commercial laboratories.
The following case reports are of complicated patients seen at the NIH that highlight the ability of ultrafiltration LC-MSMS to correctly classify their thyroid status. The first case highlights a patient with a protein binding abnormality and the inability of immunoassays to correctly classify these patients (50). The second and third cases demonstrate patients who clinically benefited from measurement of thyroid hormones by ultrafiltration LC-MSMS.
Case report one
The patient is a 30 year-old male with Acquired Immunodeficiency Syndrome and Kaposi’s sarcoma. The patient was clinically euthyroid with a TSH within the reference interval (Table 1). However, free and total thyroid hormones measured by immunoassay were decreased. Laboratory medicine was consulted to provide an explanation for the decreased free and total thyroid hormones in the presence of a normal TSH. A TBG level was ordered by the senior clinical chemistry staff. TBG was decreased at 4.3 ug/mL, indicating a protein binding abnormality as the likely cause of the discrepant thyroid hormone values. Thyroid hormone measurement was repeated on the same sample by LC-MSMS, which showed FT3 and FT4 values within the reference interval, which correlates with the patient’s clinical symptoms. This case example highlights the utility of LC-MSMS to accurately classify thyroid hormone status in the presence of severe protein binding abnormalities. Also important is the inability of thyroid data generated by routine immunoassay platforms to correctly classify these patients.
Table 1.
Laboratory values by immunoassay and LC-MSMS on the same sample for a 30 year-old euthyroid male with AIDS-related Kaposi’s sarcoma.
| Value | Reference range | |
|---|---|---|
|
| ||
| Immunoassay results | ||
| FT4 | 0.66 ng/dL | 0.8 – 1.5 ng/dL |
| FT3 | 1.41 pg/mL | 1.8 – 4.2 pg/mL |
| TT3 | 42 ng/dL | 90 – 215 ng/dL |
| TT4 | 3.1 ug/dL | 4.5 – 12.5 ug/dL |
| TSH | 3.52 uIU/mL | 0.36 – 4.0 uIU/mL |
| TBG | 4.3 ug/mL | 13 – 39 ug/mL |
|
| ||
| LC-MSMS results | ||
| FT4 | 1.8 ng/dL | 0.8 – 2.0 ng/dL |
| FT3 | 1.7 pg/mL | 1.5 – 6.0 pg/mL |
| TT3 | 25.2 ng/dL | 80 – 187 ng/dL |
| TT4 | 2.4 ug/dL | 4.9 – 10.5 ug/dL |
| rT3 | 10.7 ng/dL | 9 – 21 ng/dL |
AIDS = Acquired immunodeficiency syndrome
T3 ng/dLx 0.0154 = T3 nmole/L
T4 ug/dL x 12.87=T4 nmole/L
Case report two
A 68 year-old-woman with a past medical history of hypothyroidism reports feeling lethargic despite treatment with L-T4. Her laboratory studies are shown in Table 2. Immunoassay results show thyroid function tests within normal limits. However, LC-MSMS showed TT3 and FT3 values below the reference interval. T3 was added to the patient’s regimen, with improvement of symptoms and normalized LC-MSMS measurements. The patient’s cholesterol also decreased from 220 mg/dL to 160 mg/dL after addition of T3. Of note, the patient was found to be heterozygous for the D2 Thr92Ala polymorphism.
Table 2.
Laboratory values by immunoassay and LC-MSMS on the same sample for a 68 year-old woman with persistent symptoms of hypothyroidism while on L-T4 therapy.
| Value | Reference range | |
|---|---|---|
|
| ||
| Immunoassay results | ||
| FT4 | 1.2 ng/dL | 0.8 – 1.5 ng/dL |
| FT3 | 2.5 pg/mL | 1.8 – 4.2 pg/mL |
| TT3 | 99 ng/mL | 90 – 215 ng/dL |
| T4 | 8.0 ug/dL | 4.5 – 12.5 ug/dL |
|
| ||
| LC-MSMS results | ||
| FT4 | 1.2 ng/dL | 0.8 – 2.0 ng/dL |
| FT3 | 1.5 pg/mL | 1.5 – 6.0 pg/mL |
| TT3 | 64 ng/dL | 80 – 187 ng/dL |
| TT4 | 7.2 ug/dL | 4.9 – 10.5 ug/dL |
| rT3 | 8.7 ng/dL | 9 – 21 ng/dL |
T3 ng/dLx 0.0154 = T3 nmole/L
T4 ug/dL x 12.87=T4 nmole/L
Case report three
A 70 year-old woman with a history of Hashimoto’s thyroiditis is taking LT-4, but reports feeling sluggish and unwell. Immunoassay results for TSH, TT3, TT4, FT3, and FT4 were within normal limits, and thus the patient’s primary care physician was reluctant to change the LT-4 regimen. Ultrafiltration LC-MSMS performed at our institution revealed decreased TT3 of 74 ng/dL (reference interval 80 – 187 ng/dL) and low-normal FT3 of 1.8 pg/mL (1.5 – 6.0 pg/mL). T3 was added to the patient’s regimen, after which the FT3 increased to 3.3 pg/mL and the patient’s symptoms resolved.
There are several potential mechanisms to account for why certain patients, such as cases two and three reported above, continue to experience symptoms of hypothyroidism while on L-T4 and have normal levels of TSH. First, treatment with L-T4 monotherapy may not result in sufficient peripheral conversion to T3 to provide adequate amounts of T3 at the tissue level. Patients with certain deiodinase polymorphisms have been shown to have reduced T3 levels (51, 52). For example, the Thr92Ala polymorphism of D2 occurs at a frequency of 0.35, and is associated with reduced D2 activity and a decreased rate of T3 release from thyroid releasing hormone stimulation (53, 54). Second, the normalization of TSH that occurs during L-T4 therapy is likely due to the known effect of T4 as an important regulator of TSH release; however, TSH is not an indicator of the tissue bioavailability of active T3 (48). LC-MSMS is a potential tool for the laboratory evaluation of patients who may have issues converting L-T4 to bioavailable T3.
In the United States, L-T3 and L-T4 combination therapy remains a controversial issue. The guidelines of the American Thyroid Association suggest L-T4 monotherapy for the treatment of hypothyroidism, while the European Thyroid Association states LT-3/LT-4 combination therapy may be used in selected patients (49, 55). Regardless, LT-3 and LT-4 combination is still considered an experimental treatment modality in the United States; however, this is not the case in many countries such as South Africa and parts of Canada, where combination therapy is routinely employed in around 20% of patients. The use of LC-MSMS may provide objective laboratory evidence to assist in the identification of the subset of patients who may benefit from combination therapy. Both the American and the European Thyroid Associations provide a set of research goals for optimizing therapy for patients with hypothyroidism. We recommend that future clinical trials evaluating LT-3 treatment incorporate LC-MSMS as a tool for identification of patients with issues converting L-T4 to T3 who may benefit the most by combination therapy.
There are a number of important limitations to the routine incorporation of thyroid hormone measurement by LC-MSMS. First, the technique requires use of specialized and very expensive equipment. Second, trained and experienced personnel are required. While thyroid hormone measurement by LC-MSMS is available at most major commercial reference laboratories and many teaching hospitals, the cost is higher than measurement by immunoassays performed on automated platforms. Despite this higher cost it does not come close to the cost of misdiagnosing a patient requiring small doses of T3. Finally, LC-MSMS does have a longer turnaround time even if performed in house, and cannot provide rapid results in emergency settings.
Conclusions
Measurement of T3, FT3, and FT4 by the immunoassay methods commonly used by the majority of laboratories is problematic, especially for low levels of thyroid hormones. A significant number of patients are misclassified as having normal levels of thyroid hormones when they actually have levels below the reference range by LC-MSMS. There are approximately 19 million patients with hypothyroidism in the United States alone. The misclassification of patients by a commonly used laboratory strategy thus has the potential to adversely impact a substantial number of patients. Laboratory assays are expected to be accurate over the entire analytical range, especially at the high or low end of the range where clinical decision points are often made. Assays for TT3, FT3, and FT4 should correlate with both log TSH and the patient’s clinical condition, which is not achieved on any of the commercially available FDA approved immunoassay platforms. LC-MSMS provides better correlation with these parameters over the entire analytical range. We suggest evaluation of patients employing LC-MSMS measurement of thyroid hormones when they continue to report symptoms of hypothyroidism despite therapy with LT-4. This approach may aid in identification of patients who may benefit from combination treatment with LT-3 and LT-4.
Acknowledgments
The authors are supported by the National Institutes of Health Intramural Research Award.
Biography

Dr. Steven Soldin currently oversees the mass spectrometry section of the clinical chemistry service and research programs at the NIH Cliical Center, Bethesda, MD, USA. He is also involved in the routine laboratory work and directs the postdoctoral training of PhD and MD clinical chemistry fellows. He has served as President of several National organizations including the National Academy of Clinical Biochemistry, the American Board of Clinical Chemistry and the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. His research interests focus on the role of specificity in improving patient diagnosis and treatment. Current foci include steroid, and thyroid hormone profiles; his group are also assessing the role of T3 and choline during pregnancy regarding fetal maturation. He has to date published 269 papers in peer reviewed journals.
Footnotes
Declaration of interests: The authors have no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 2.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 3.Gounden V, Jonklaas J, Soldin SJ. A pilot study: subclinical hypothyroidism and free thyroid hormone measurement by immunoassay and mass spectrometry. Clin Chim Acta. 2014;430:121–124. doi: 10.1016/j.cca.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 5.Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc. 2009;84:65–71. doi: 10.4065/84.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee CM, Brent GA, Kovesdy CP, Soldin OP, Nguyen D, Budoff MJ, Brunelli SM, Kalantar-Zadeh K. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrol Dial Transplant. 2015;30:724–737. doi: 10.1093/ndt/gfu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 8.Iervasi G, Clerico A, Bonini R, Manfredi C, Berti S, Ravani M, Palmieri C, Carpi A, Biagini A, Chopra IJ. Acute effects of amiodarone administration on thyroid function in patients with cardiac arrhythmia. J Clin Endocrinol Metab. 1997;82:275–280. doi: 10.1210/jcem.82.1.3675. [DOI] [PubMed] [Google Scholar]
- 9.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohrle J. The selenoenzyme family of deiodinase isozymes controls local thyroid hormone availability. Rev Endocr Metab Disord. 2000;1:49–58. doi: 10.1023/a:1010012419869. [DOI] [PubMed] [Google Scholar]
- 11.Schussler GC. The thyroxine-binding proteins. Thyroid. 2000;10:141–149. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]
- 12.Ekins R. The free hormone hypothesis and measurement of free hormones. Clin Chem. 1992;38:1289–1293. [PubMed] [Google Scholar]
- 13.Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin Endocrinol (Oxf) 2014;81:633–641. doi: 10.1111/cen.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Deventer HE, Soldin SJ. The expanding role of tandem mass spectrometry in optimizing diagnosis and treatment of thyroid disease. Adv Clin Chem. 2013;61:127–152. doi: 10.1016/b978-0-12-407680-8.00005-1. [DOI] [PubMed] [Google Scholar]
- 15.Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA. 2008;299:769–777. doi: 10.1001/jama.299.7.769. [DOI] [PubMed] [Google Scholar]
- 16.Jonklaas J, Kahric-Janicic N, Soldin OP, Soldin SJ. Correlations of free thyroid hormones measured by tandem mass spectrometry and immunoassay with thyroid-stimulating hormone across 4 patient populations. Clin Chem. 2009;55:1380–1388. doi: 10.1373/clinchem.2008.118752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soldin SJ, Cheng LL, Lam LY, Werner A, Le AD, Soldin OP. Comparison of FT4 with log TSH on the Abbott Architect ci8200: Pediatric reference intervals for free thyroxine and thyroid-stimulating hormone. Clin Chim Acta. 2010;411:250–252. doi: 10.1016/j.cca.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem. 2011;57:122–127. doi: 10.1373/clinchem.2010.154088. [DOI] [PubMed] [Google Scholar]
- 19.Masika LS, Zhao Z, Soldin SJ. Is measurement of TT3 by immunoassay reliable at low concentrations? A comparison of the Roche Cobas 6000 vs. LC-MSMS. Clin Biochem. 2016 doi: 10.1016/j.clinbiochem.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soldin OP, Tractenberg RE, Soldin SJ. Differences between measurements of T4 and T3 in pregnant and nonpregnant women using isotope dilution tandem mass spectrometry and immunoassays: are there clinical implications? Clin Chim Acta. 2004;347:61–69. doi: 10.1016/j.cccn.2004.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockigt JR. Free thyroid hormone measurement. A critical appraisal. Endocrinol Metab Clin North Am. 2001;30:265–289. doi: 10.1016/s0889-8529(05)70187-0. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson HP, Archbold GP, Johnston P, Young IS, Sheridan B. Misleading serum free thyroxine results during low molecular weight heparin treatment. Clin Chem. 1998;44:1002–1007. [PubMed] [Google Scholar]
- 23.Lim CF, Bai Y, Topliss DJ, Barlow JW, Stockigt JR. Drug and fatty acid effects on serum thyroid hormone binding. J Clin Endocrinol Metab. 1988;67:682–688. doi: 10.1210/jcem-67-4-682. [DOI] [PubMed] [Google Scholar]
- 24.Surks MI, DeFesi CR. Normal serum free thyroid hormone concentrations in patients treated with phenytoin or carbamazepine. A paradox resolved. JAMA. 1996;275:1495–1498. [PubMed] [Google Scholar]
- 25.Csako G, Zweig MH, Benson C, Ruddel M. On the albumin-dependence of measurements of free thyroxin. II. Patients with non-thyroidal illness. Clin Chem. 1987;33:87–92. [PubMed] [Google Scholar]
- 26.Iitaka M, Kawasaki S, Sakurai S, Hara Y, Kuriyama R, Yamanaka K, Kitahama S, Miura S, Kawakami Y, Katayama S. Serum substances that interfere with thyroid hormone assays in patients with chronic renal failure. Clin Endocrinol (Oxf) 1998;48:739–746. doi: 10.1046/j.1365-2265.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 27.Nelson JC, Wang R, Asher DT, Wilcox RB. The nature of analogue-based free thyroxine estimates. Thyroid. 2004;14:1030–1036. doi: 10.1089/thy.2004.14.1030. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JC, Weiss RM, Wilcox RB. Underestimates of serum free thyroxine (T4) concentrations by free T4 immunoassays. J Clin Endocrinol Metab. 1994;79:76–79. doi: 10.1210/jcem.79.1.8027258. [DOI] [PubMed] [Google Scholar]
- 29.Nelson JC, Yoo EW, Wilcox RB. Accuracy issues in free thyroxine testing methods. Semin Perinatol. 2008;32:403–406. doi: 10.1053/j.semperi.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Giovannini S, Zucchelli GC, Iervasi G, Iervasi A, Chiesa MR, Mercuri A, Renieri A, Prontera C, Conte R, Clerico A. Multicentre comparison of free thyroid hormones immunoassays: the Immunocheck study. Clin Chem Lab Med. 2011;49:1669–1676. doi: 10.1515/CCLM.2011.647. [DOI] [PubMed] [Google Scholar]
- 31.Steele BW, Wang E, Klee GG, Thienpont LM, Soldin SJ, Sokoll LJ, Winter WE, Fuhrman SA, Elin RJ. Analytic bias of thyroid function tests: analysis of a College of American Pathologists fresh frozen serum pool by 3900 clinical laboratories. Arch Pathol Lab Med. 2005;129:310–317. doi: 10.5858/2005-129-310-ABOTFT. [DOI] [PubMed] [Google Scholar]
- 32.d’Herbomez M, Forzy G, Gasser F, Massart C, Beaudonnet A, Sapin R. Clinical evaluation of nine free thyroxine assays: persistent problems in particular populations. Clin Chem Lab Med. 2003;41:942–947. doi: 10.1515/CCLM.2003.143. [DOI] [PubMed] [Google Scholar]
- 33.Monneret D, Guergour D, Vergnaud S, Laporte F, Faure P, Gauchez AS. Evaluation of LOCI technology-based thyroid blood tests on the Dimension Vista analyzer. Clin Biochem. 2013;46:1290–1297. doi: 10.1016/j.clinbiochem.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Roberts RF, La’ulu SL, Roberts WL. Performance characteristics of seven automated thyroxine and T-uptake methods. Clin Chim Acta. 2007;377:248–255. doi: 10.1016/j.cca.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Sapin R, d’Herbomez M. Free thyroxine measured by equilibrium dialysis and nine immunoassays in sera with various serum thyroxine-binding capacities. Clin Chem. 2003;49:1531–1535. doi: 10.1373/49.9.1531. [DOI] [PubMed] [Google Scholar]
- 36.Zucchelli GC, Pilo A, Chiesa MR, Masini S. Systematic differences between commercial immunoassays for free thyroxine and free triiodothyronine in an external quality assessment program. Clin Chem. 1994;40:1956–1961. [PubMed] [Google Scholar]
- 37.Spencer CA, LoPresti JS, Patel A, Guttler RB, Eigen A, Shen D, Gray D, Nicoloff JT. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab. 1990;70:453–460. doi: 10.1210/jcem-70-2-453. [DOI] [PubMed] [Google Scholar]
- 38.Serdar MA, Ozgurtas T, Ispir E, Kenar L, Senes M, Yucel D, Bilgi C, Kurt I. Comparison of relationships between FT4 and log TSH in Access DXI 800 Unicel, Modular E170 and ADVIA Centaur XP Analyzer. Clin Chem Lab Med. 2012;50:1849–1852. doi: 10.1515/cclm-2012-0193. [DOI] [PubMed] [Google Scholar]
- 39.Jonklaas J, Soldin SJ. Tandem mass spectrometry as a novel tool for elucidating pituitary-thyroid relationships. Thyroid. 2008;18:1303–1311. doi: 10.1089/thy.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albrecht L, Styne D. Laboratory testing of gonadal steroids in children. Pediatr Endocrinol Rev. 2007;5(Suppl 1):599–607. [PubMed] [Google Scholar]
- 41.Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta. 2006;372:76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 42.Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Bunker AM, Fitzgerald RL, Meikle AW. Performance characteristics of a novel tandem mass spectrometry assay for serum testosterone. Clin Chem. 2006;52:120–128. doi: 10.1373/clinchem.2005.052167. [DOI] [PubMed] [Google Scholar]
- 43.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125:914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 44.Gu J, Soldin OP, Soldin SJ. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin Biochem. 2007;40:1386–1391. doi: 10.1016/j.clinbiochem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Jonklaas J, Sathasivam A, Wang H, Gu J, Burman KD, Soldin SJ. Total and free thyroxine and triiodothyronine: measurement discrepancies, particularly in inpatients. Clin Biochem. 2014;47:1272–1278. doi: 10.1016/j.clinbiochem.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soukhova N, Soldin OP, Soldin SJ. Isotope dilution tandem mass spectrometric method for T4/T3. Clin Chim Acta. 2004;343:185–190. doi: 10.1016/j.cccn.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R. Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. Am J Physiol. 1990;258:E715–726. doi: 10.1152/ajpendo.1990.258.4.E715. [DOI] [PubMed] [Google Scholar]
- 48.Biondi B, Wartofsky L. Combination treatment with T4 and T3: toward personalized replacement therapy in hypothyroidism? J Clin Endocrinol Metab. 2012;97:2256–2271. doi: 10.1210/jc.2011-3399. [DOI] [PubMed] [Google Scholar]
- 49.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J. 2012;1:55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gounden V, Celi FS, Soldin SJ. Academy of Clinical Laboratory Physicians and Scientists. San Francisco: 2014. Case studies illustrating the clinical utility of liquid chromatography-tandem mass spectrometry for the assessment of free thyroid hormone status. [Google Scholar]
- 51.de Jong FJ, Peeters RP, den Heijer T, van der Deure WM, Hofman A, Uitterlinden AG, Visser TJ, Breteler MM. The association of polymorphisms in the type 1 and 2 deiodinase genes with circulating thyroid hormone parameters and atrophy of the medial temporal lobe. J Clin Endocrinol Metab. 2007;92:636–640. doi: 10.1210/jc.2006-1331. [DOI] [PubMed] [Google Scholar]
- 52.Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, Visser TJ. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 53.Butler PW, Smith SM, Linderman JD, Brychta RJ, Alberobello AT, Dubaz OM, Luzon JA, Skarulis MC, Cochran CS, Wesley RA, et al. The Thr92Ala 5′ type 2 deiodinase gene polymorphism is associated with a delayed triiodothyronine secretion in response to the thyrotropin-releasing hormone-stimulation test: a pharmacogenomic study. Thyroid. 2010;20:1407–1412. doi: 10.1089/thy.2010.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mentuccia D, Proietti-Pannunzi L, Tanner K, Bacci V, Pollin TI, Poehlman ET, Shuldiner AR, Celi FS. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor. Diabetes. 2002;51:880–883. doi: 10.2337/diabetes.51.3.880. [DOI] [PubMed] [Google Scholar]
- 55.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]