Abstract
This study sought to investigate the effects of environmentally relevant gestational followed by continued chronic exposure to the herbicide, atrazine, on motor function, cognition, and neurochemical indices of nigrostriatal dopamine (DA) activity in male rats. Dams were treated with 100 µg/kg atrazine, 10 mg/kg atrazine, or vehicle on gestational day 1 through postnatal day 21. Upon weaning, male offspring continued daily vehicle or atrazine gavage treatments for an additional six months. Subjects were tested in a series of behavioral assays, and 24 h after the last treatment, tissue samples from the striatum were analyzed for DA and 3,4-dihydroxyphenylacetic acid (DOPAC). At 10 mg/kg, this herbicide was found to produce modest disruptions in motor functioning, and at both dose levels it significantly lowered striatal DA and DOPAC concentrations. These results suggest exposures to atrazine have the potential to disrupt nigrostriatal DA neurons and behaviors associated with motor functioning.
Keywords: atrazine, dopamine, striatum, locomotor activity, motor function, behavior
Introduction
Atrazine is an herbicide used extensively to control broadleaf and grassy weeds on crops such as corn, sorghum, and sugarcane (US EPA, 2012). With approximately 73–78 million pounds being applied per year, atrazine is the second most widely used pesticide in the United States (Grube et al., 2011). Currently, the adverse effects of this herbicide on human health are not fully understood. This is alarming since atrazine is quite prevalent in the environment. Not only has atrazine been detected more often than any other herbicide in U.S drinking water (Barbash et al., 2001), there have been several reports of community water supply samples exceeding the EPA’s maximum contaminant level (MCL) of 3 µg/L (Kolpin et al., 1997; US EPA, 2003). Further, human exposure has been confirmed through reports of atrazine metabolites in urine (Curwin et al., 2007). With such risk for human exposure, it is important that the health effects of atrazine be thoroughly investigated.
Atrazine is an endocrine disrupter that alters ovarian and testicular hormone secretion and adversely effects both female and male reproductive systems (Abarikwu et al., 2010; Cooper et al., 2007; Hayes et al., 2011; Stanko et al., 2010; Wetzel et al., 1994). Despite the fact that the hormones shown to be affected by atrazine also play a major role in the development of the central nervous system (Schantz and Widholm, 2001), less research has examined the neurobehavioral consequences of atrazine exposure. A growing body of research suggests that atrazine is a dopamine (DA) neuronal toxicant. Studies in rodents revealed atrazine exposure produces dose-dependent impairment of DA neurons in the substantia nigra pars compacta (Coban and Filipov, 2007). Atrazine also decreases striatal DA content (Bardallus et al., 2011; Coban and Filipov, 2007; Filipov et al., 2007; Li et al., 2014a, 2014b; Rodriguez et al., 2013; Sun et al., 2014), an effect believed to be due to increases in cytosolic DA, which is prone to oxidative breakdown (Hossain and Filipov, 2008).
Atrazine has also been reported to alter behaviors associated with the nigrostriatal (NS) DA system. The NSDA pathway plays a central role in motor control (Haber, 2014). In rodents atrazine has been shown to alter locomotor activity (Bardullas et al., 2011; Belloni et al., 2011; Peruzovic et al., 1995; Rodriguez et al., 2013; Ugazio et al., 1991), delay righting reflexes (Belloni et al., 2011), and impair rotarod performance (Bardullas et al., 2011). These tasks have all been shown to measure behaviors that are controlled, in part, by NSDA neurons (Adeosun et al., 2012; Galli et al., 2014; Kravitz et al., 2010; Liu et al., 2014; Verhave et al., 2009; Yin et al., 2009). The walking beam assay assesses the ability of rats to traverse a narrow elevated beam and has proven to be very useful in the assessment of motor coordination (Goldsten, 2003). This task is disrupted after lesions to the striatum (Jimenez-Martin et al., 2015; Urakawa et al., 2007), and it is sensitive to detecting motor impairments after exposure to other endocrine disrupters (Abou-Donia et al., 2001; Arcadia et al., 1998), but has not yet been utilized to assess the effects of atrazine exposure.
While atrazine has been shown to disrupt NSDA neurons, it is possible that the effects of atrazine on brain function are more widespread, affecting the mesolimbic and mesocortical DA pathways. Together, these pathways are involved in aspects of higher cognitive functions, such as reward-based learning (Haber, 2014). Only a few studies have examined the effects of atrazine exposure on cognitive function, and these studies yielded mixed results. Bardullas et al. (2011) found that rats exposed to a moderate dose of atrazine (10 mg/kg) for 11 and 12 months displayed slightly more errors than control rats in a spontaneous alternation task and a non-delayed random foraging parading. In another study, Lin et al. (2013) revealed that doses of atrazine ≥ 25 mg/kg for 10 days impaired the performance of mice in a novel object recognition task. In contrast, other studies revealed atrazine exposure to improve performance in avoidance learning tasks (Belloni et al., 2011; Peruzovic et al., 1995).
Although studies have examined the neurobehavioral effects of atrazine in rats when administered chronically (Bardullas et al., 2011) and at environmentally-relevant exposure levels during gestation (Belloni et al., 2007; 2011), no studies have examined the neurobehavioral effects of atrazine exposure beginning during gestation and continuing through adulthood. Arguably, this mode of exposure closely mimics that of individuals living in small farming communities (Kolpin et al., 1997; U.S. EPA, 2001). Therefore, studying the effects of this exposure paradigm may provide relevant information to ascertain the health risks associated with atrazine exposure in humans. Additionally, no studies have examined the effects of atrazine on complex operant learning or its possible anxiogenic effects. Thus, the primary aim of this study was to utilize a novel paradigm of exposure (gestational plus chronic up to 7 months old) to assess the effects of oral exposure to environmentally-relevant atrazine levels on locomotor activity, motor coordination, learning and memory, anxiety, and neurochemical estimates of nigrostriatal DA neurons in male and female Sprague-Dawley rats. As a secondary aim, this study also sought to use four novel behavioral measures in order to help elucidate the effects of atrazine exposure on neurobehavioral development.
Methods
Animals and dosing
Female Sprague Dawley rats (10 weeks old) were mated and upon detection of vaginal plugs were housed individually. Twelve pregnant females were randomly assigned to one of three treatment groups on gestation day 1 (GD1): corn oil (vehicle control), 100 µg/kg atrazine (ATZ low), or 10 mg/kg atrazine (ATZ high), for a total of 4 dams per treatment group. The 100 µg/kg dose was selected because it is relevant to the EPA’s current maximum contaminant level (MCL; 3µg/l), and this dose has been shown to induce neuronal damage in the hypothalamus, dentate gyrus, and striatum (Giusi et al., 2006). The 10 mg/kg dose was examined because it has been shown to alter locomotor activity, impair motor coordination, disrupt learning, and decrease DA levels in the striatum when administered chronically to rats beginning at 1 month of age (Bardallus et al., 2011). Atrazine (Lot 1912-24-9; BOC Sciences, Shirley, NY) was dissolved in corn oil and administered to the dams daily at a volume of 2 ml/kg through oral gavage on GD 1 through postnatal day (PND) 21. Oral gavage was chosen because it mimics the most common mode of human exposure (drinking water), and it allows for the delivery of exact amounts of atrazine.
At weaning (PND 21) litters were cross-fostered and culled to 10 pups (5 males and 5 females), and pups continued daily administration of corn oil vehicle, 100 µg/kg atrazine, or 10 mg/kg atrazine through oral gavage for an additional six months. Thirty male pups were randomly selected from 8 dams (2–3 dams per treatment group) for behavioral testing, with a total of three treatment groups for each sex with 10 rats in each group, although the sample size used for each assay varied slightly. Females were not used due to the impact that estrus cycling has been shown to have on several of the behavioral measured gathered in this study (Craft and Leitl, 2008; Sayin et al., 2014; Takeo and Sakuma, 1995; Vinogradova, 1999; Warren and Juraska,1997). Offspring were housed in same sex pairs for the duration of the study with ad libitum access to food and water in the home cage, except during caloric restriction experiments. Behavioral testing occurred between 1 and 7 months of age (Figure 1). Daily dosing always occurred after behavioral testing between 1900–2200 hr for locomotor activity and light dark box assessments, and between 1300–1700 hr for all other assays.
Figure 1.
Timeline of experimental procedures
All rats were housed in polycarbonate cages with corn cob bedding in a light (12/12 hr light/dark cycle, lights on 0700 to 1900 hr) and temperature (20±2°C) controlled vivarium. Rats were maintained according to the general principles of animal husbandry outlined by the Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies, 2011), and the experimental protocol was approved by the Institutional Animal Care and Use Committee of Western Michigan University.
Locomotor activity
Locomotor activity assessments occurred at 1 month of age (control n = 8, ATZ low n = 10, ATZ high n = 10) and again at 6 months of age (n = 8) in eight custom-designed Plexiglas chambers housed within a Versamax® animal activity monitoring system equipped with infrared sensors. Each rat was placed individually in a chamber for 1 hr, and several measures of ambulatory activity were obtained, including horizontal activity, vertical activity, stereotypy, and time spent in center area. Locomotor activity sessions took place during the dark cycle, beginning at 1900 hr, and the lights were turned off in the testing room.
Walking beam
In order to assess the effects of atrazine on motor coordination, rats (n = 8 per treatment group) were tested on their ability to traverse a balance beam. Performance was assessed on postnatal (PND) 37–46 and again at 22 weeks of age. The procedure consisted of three phases: shaping, training and testing and was conducted between 0900 and 1600 hr. The test apparatus and procedures were adapted from those described by Carter et al. (2001). The test apparatus consisted of a metal rod 122 cm in length elevated 76 cm above the floor, and A 20 W light was located at the start of the beam and utilized as an avoidance stimulus. At the opposite end of the beam was a goal box. Three beams of various widths (2.5, 1.5, and 1 cm) were used to assess motor coordination.
During Phase 1 (shaping) rats were gradually trained to traverse a 2.5 cm training beam over a series of 4 trials. For the first trial, the rat was placed 30 cm away from the goal box and for each successive trial, the rat was placed further and further away from the goal box until it could successively traverse the entire length of the beam (Phase 1 was not conducted at 22 weeks of age). During Phase 2 (training), rats were required to cross the entire length of the 2.5 cm training beam for two consecutive days, and each daily session consisted of three consecutive trials. Phase 3 (testing) began immediately following the last day of training and took place within one day. During testing, rats were given two trials on three different beam widths (2.5, 1.5, and 1.0 cm, respectively). Data were collected on duration of time to traverse the beam, number of falls, and number of and foot slips. During testing, if the rat did not traverse the beam within 120 s, this was considered a fail.
Spatial discrimination reversal
Rats were placed on food restriction one week prior to training in the spatial discrimination reversal task. Feeding was adjusted daily in order to maintain the animals on a slight schedule of caloric restriction that still allowed for developmentally-appropriate gains in body weight. This feeding regimen was continued for the entire duration of the learning task. Rats were assessed in the spatial discrimination reversal learning task from approximately 2 – 5 months of age (n = 8). Testing occurred in eight standard operant chambers equipped with three levers and MED-PC software version 4 (MED Associates Inc., St. Albans, VT).
Beginning at two months of age, rats were trained to press the center-lever using an autoshaping procedure. Once subjects obtained 100 reinforcers a session for two consecutive sessions, they were trained to press a lever associated with a particular spatial location under a fixed ratio (FR) 20 schedule of reinforcement. Pressing the right lever was reinforced in half of the rats from each treatment group, and pressing the left lever was reinforced in the other half. The house light was illuminated during each session. Each trial began with a 3 s inter-trial interval (ITI), and the extension of all three levers into the chamber. A session ended once the rat obtained 50 reinforcers under a FR 20 schedule of reinforcement or 30 min had elapsed. Spatial discrimination 1 was mastered when a rat achieved 95% correct responding for 5 consecutive sessions, and if its accuracy for the first 10 trials of each of session was greater than or equal to 80%. If a rat failed to meet these criteria, it remained under the FR 20 schedule of reinforcement on the original lever until the mastery criteria were met.
After the spatial discrimination on the original lever was mastered, the rats were required to learn a new spatial discrimination. Under this procedure, the rats that were initially reinforced for pressing the left lever were subsequently reinforced for pressing the right lever under a FR 20 schedule of reinforcement, and vice versa. Responding on incorrect levers had no programmed consequences, and the mastery criteria for this phase were the same as the first spatial discrimination. Data were collected on percent correct responding for each trial, percent correct responding for each session, and the number of presses until the first FR 20 was met. At the end of the spatial discrimination assay (5 months of age), all rats were placed back on free access to food.
Morris Water Maze
Performance in a Morris Water Maze was assessed beginning at 26 weeks of age (n = 8). The assay consisted of two phases: training and testing. The apparatus consisted of a circular pool 183 cm in diameter and 38 cm deep. The escape platform was a clear acrylic circle, 11.5 cm in diameter. The room was filled with several cues to aid in spatial navigation. During all training and testing sessions, the experimenter sat in the same spot and served as another visual cue. At the beginning of each session, the pool was filled with water that was 26 °C, and this temperature was monitored and maintained throughout the entire session.
During the first day of training, the platform was placed in the center of the pool and elevated 1 cm above the water. For the first trial, the rat was placed on the escape platform with its nose facing North (N) in order to habituate the rat to the platform and familiarize the animal with its location. For the remaining three trials, the start location varied for each trial and went in the following order: N, South (S), and East (E). For the next four days of training, the escape platform was submerged 1 cm below the water in the center of the pool. The rats were given four trials a day from each of the start locations N, S, E, West (W), with the order of start locations varying each day. For each trial, the rats were given a maximum of 60 s to reach the escape platform. If the rat did not reach the escape platform within 60 s, it was guided to the platform by the experimenter. Once the rat reached the escape platform, it was allowed to remain on the platform for 15 sec in order to orient to its surroundings. Data were collected on the latency to reach the escape platform for each trial, and an average latency score was calculated for each day.
Testing occurred on day six, immediately following the five days of training. During testing, the escape platform was removed from the pool, and a novel start position (NW) was created. Each rat was given a single 60 s trial from this start location. Data were collected on the number of times the rat crossed the area of the pool where the escape platform was previously located and the duration of time the rat spent in the center quadrant of the pool.
Light-dark box
The light-dark box was conducted at 7 months of age at the beginning of the dark-cycle. Rats were tested in four acrylic chambers equipped with infrared sensors. Each chamber (40.3 × 40.3 × 40.3 cm) was divided into two equal compartments, a light compartment and a dark compartment, by an acrylic wall. The light compartment did not have a ceiling and was comprised of all white walls with a 53 watt light bulb located directly above. The dark compartment was comprised of all black walls and a black ceiling. The acrylic divider contained a small (10.16 × 10.16 cm) opening that allowed for movement between the two sides. Each rat was individually placed in the chamber for 5 min. Rats were initially placed in the central opening between the compartments with the nose facing the light-side of the compartment. Data were collected on the duration of time spent on each side, number of transitions between sides, and horizontal activity counts.
Neurochemical analysis
The day following the light-dark box assessment and 24 hr after the last atrazine or vehicle treatment, rats were euthanized by rapid decapitation and brains were immediately frozen on dry ice for tissue analysis (control n = 10, ATZ low n = 9, ATZ high n = 9). A cryostat set at −10°C was used to prepare frozen coronal sections (500 µm), and bilateral tissue punches of the striatum were placed into cold tissue buffer (15% methanol/ 0.05 M sodium phosphate/ 0.03 citrate pH 2.5) and centrifuged for 5 sec at 4 °C (Beckman Coulter Microfuge, Palo Alto, CA). Samples were then sonicated with three consecutive 1 s bursts (Heat Systems Ultrasonics, Plainview, NY), and protein was pelleted by centrifugation at 12,000 rpm for 1 min.
High performance liquid chromatography coupled with electrochemical detection (HPLC-ED) using a Waters 515 pump (Waters Corporation, Milford, MA) and an ESA Coulochem 5100A electrochemical detector with an oxidation potential of +0.4 V was used to determine the content of DA and DOPAC in supernatants. DA and DOPAC content of samples was quantified by comparing the peak heights of each sample to that of standards. Tissue pellets were re-suspended in 1 N NaOH and assayed for protein using the bicinchoninic acid (BCA) protein assay (Walker, 1994). In order to correct for differences in sample size, DA and DOPAC content was normalized to the amount of protein in each sample and expressed as a concentration in ng per mg protein.
Statistical analyses
Statistical analyses were conducted using the SAS system for Windows, release 9.3. In order to control for litter effects, body weight, DA content, and performance in behavioral assessments were analyzed using a linear mixed model with dam as a random effect nested in treatment groups. Planned comparisons using a t-test were performed following each ANOVA. A one-way ANOVA was performed to compare differences in average dam weights throughout gestation between experimental groups. Means and standard errors (SE) were calculated as simple statistics and presented graphically using Graphpad Prism 6.
Results
General appearance and health
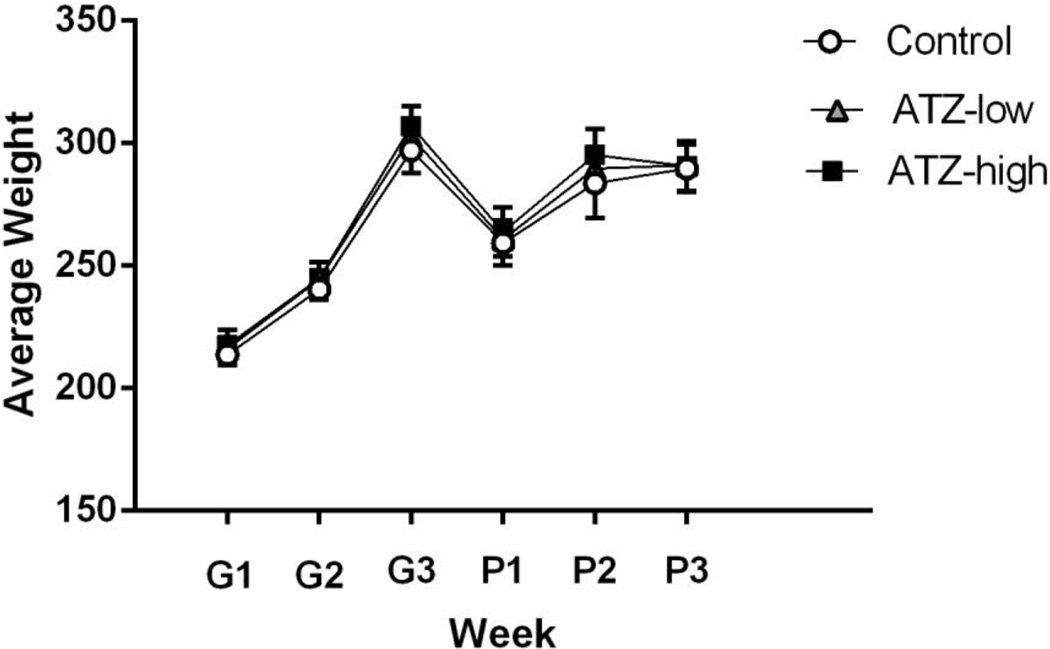
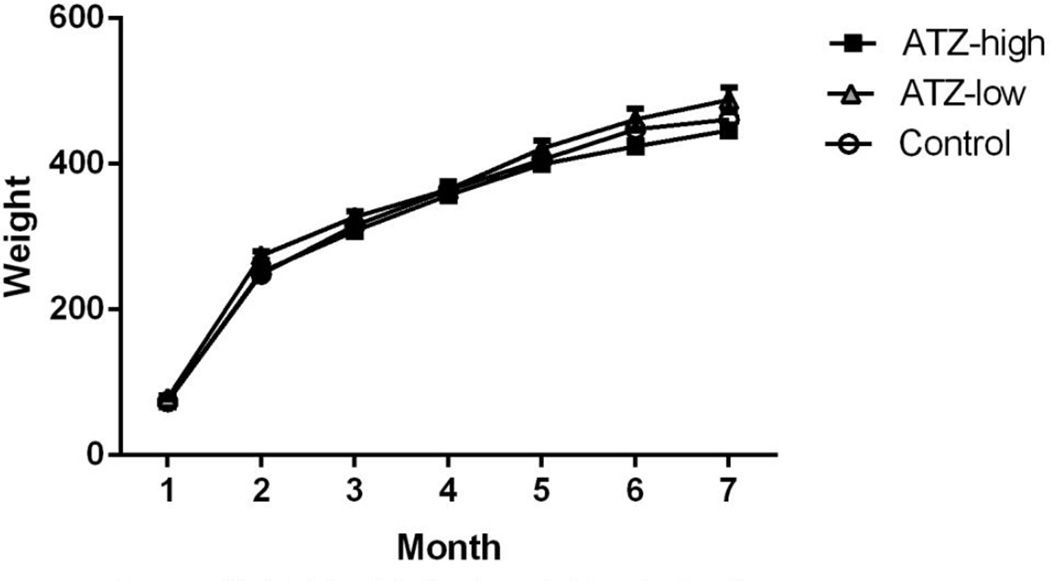
There were no differences in general appearance or body weights between atrazine treated dams and controls during the gestational period (Appendix A). There were also no differences in general appearance or body weights between atrazine treated offspring and controls (Appendix B). Further, during the course of the study, there were no mortalities within any of the treatment groups.
Locomotor activity
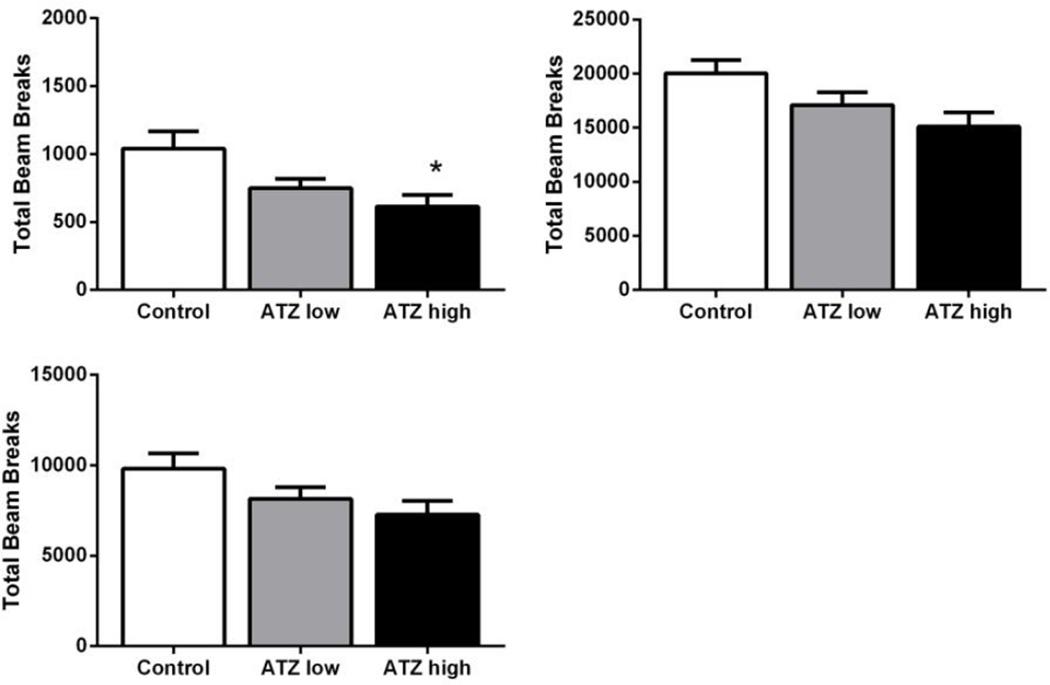
There were no differences in locomotor activity at one month of age. At six months of age, rats receiving the high dose of ATZ displayed significantly less horizontal activity (t = −3.29, p < 0.05) in comparison to controls, with a similar trend observed for vertical activity (p = 0.06) and stereotypy (p = 0.09; Figure 2).
Figure 2. Locomotor Activity.
Vertical (top left), horizontal (top right) and stereotypy counts (bottom left) at 6 months of age. Means and SE presented were calculated as simple statistics. Analyses were performed using a mixed model ANOVA with t tests for planned comparisons. * indicates different from control with p < 0.05
Walking beam
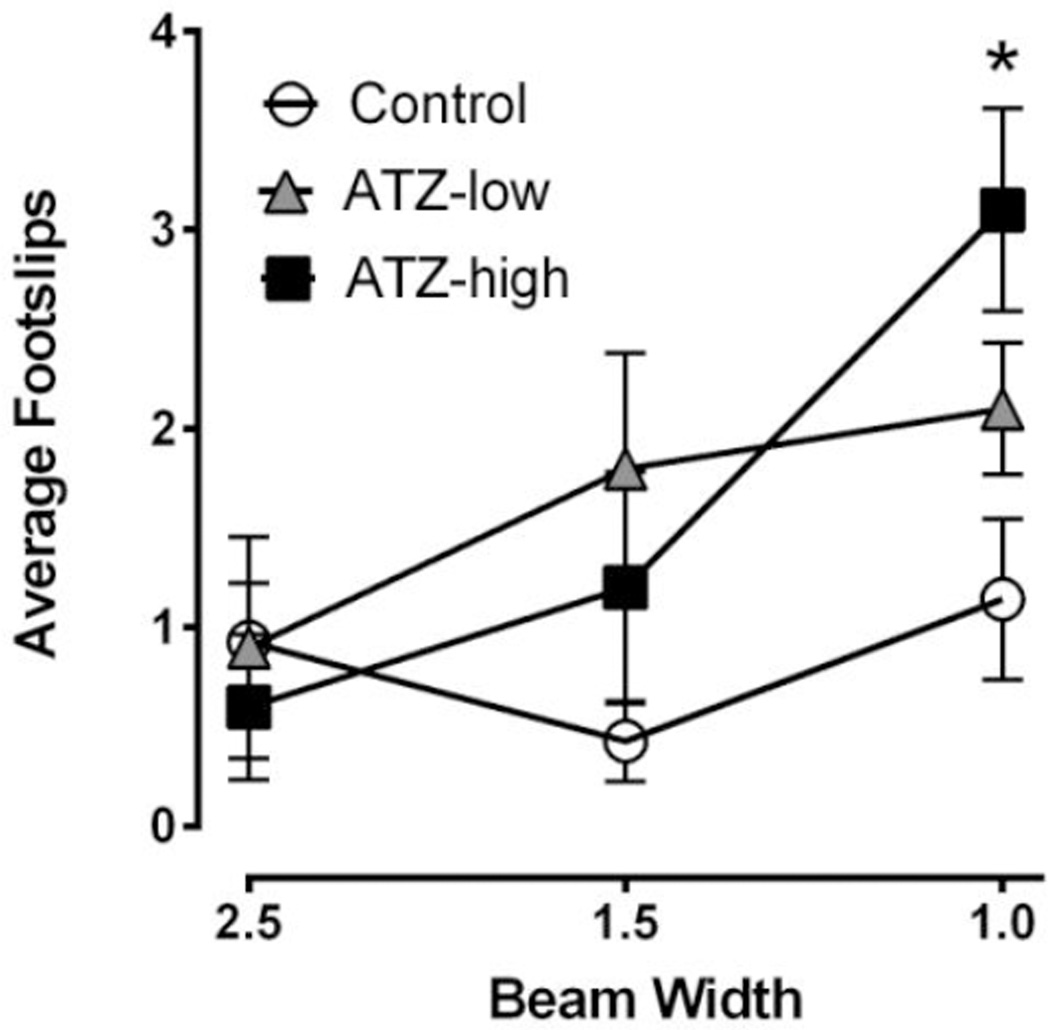
No significant differences were seen in walking beam performance after developmental exposure to atrazine. At 22 weeks of age, no significant differences were seen in walking beam performance during training sessions. For test sessions, rats receiving high dose ATZ engaged in significantly more foot slips than controls (t = 3.59, p < 0.05; Figure 3).
Figure 3. Walking beam.
Average number of footslips at 22 weeks of age. Means and SE presented were calculated as simple statistics. Analyses were performed using mixed model ANOVAs with t tests for planned comparisons. * Indicates ATZ high was significantly different from control with p < 0.05. Data only included for rats that successively traversed each beam.
Learning and memory assays
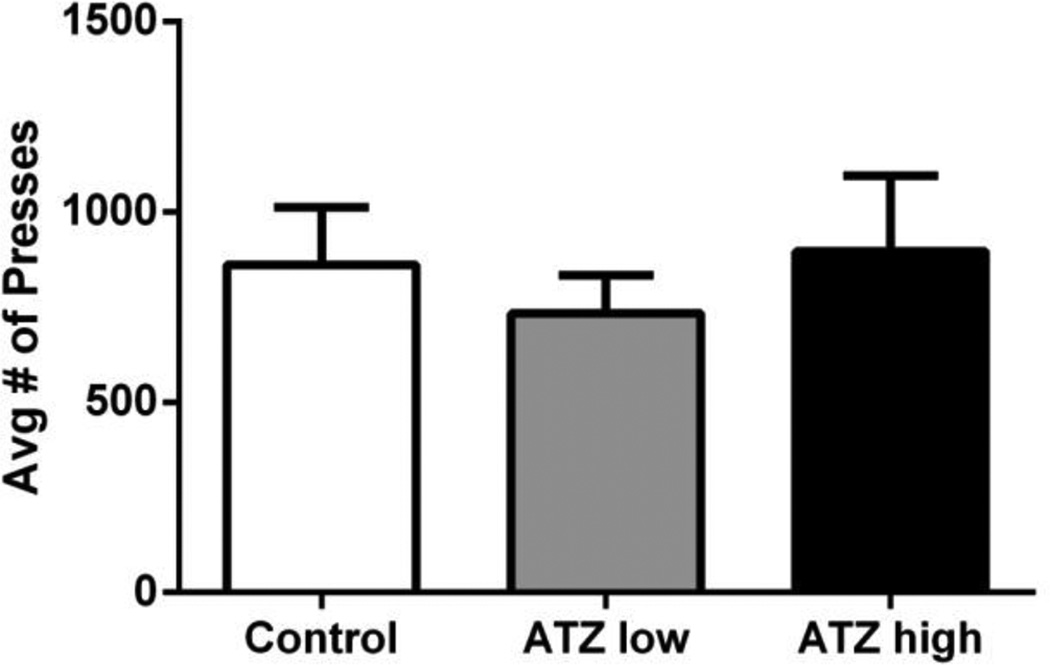
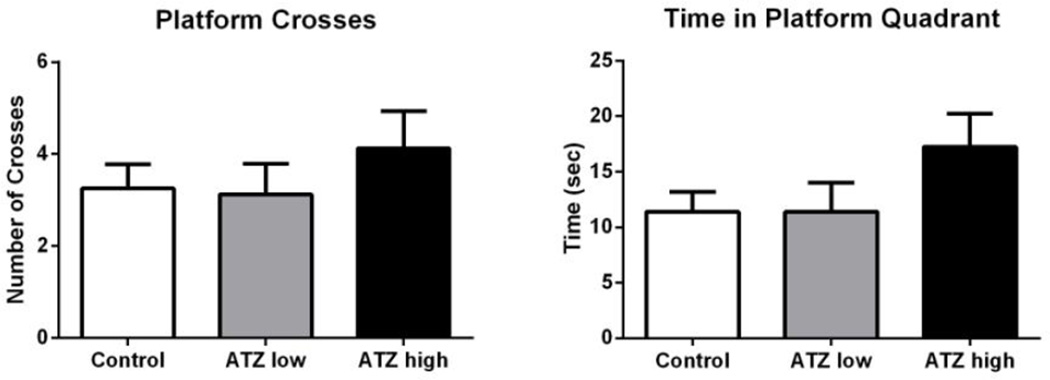
Results revealed no significant differences between treatment groups in performance on the spatial discrimination reversal task (Figure 4) or in the Morris Water Maze (Figure 5).
Figure 4.
The average number of lever presses until the first reinforcer was achieved during the reversal of the spatial discrimination task. Means and SE presented were calculated as simple statistics. Analysis was performed using a mixed model ANOVA with individual t tests for multiple comparisons
Figure 5.
The total duration of time spent in platform quadrant (left) and the number of platform crosses (right) during MWM test sessions (with escape platform removed). Means and SE presented were calculated as simple statistics. Analysis was performed using a mixed model ANOVA with individual t tests for multiple comparisons
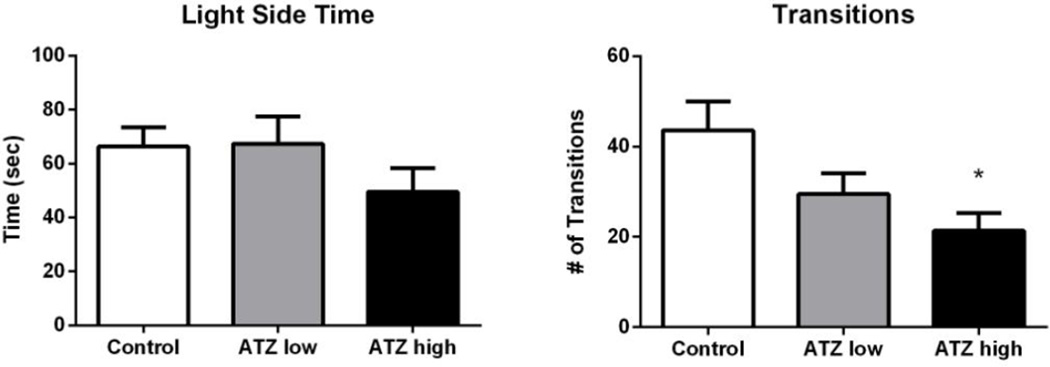
Light-dark box
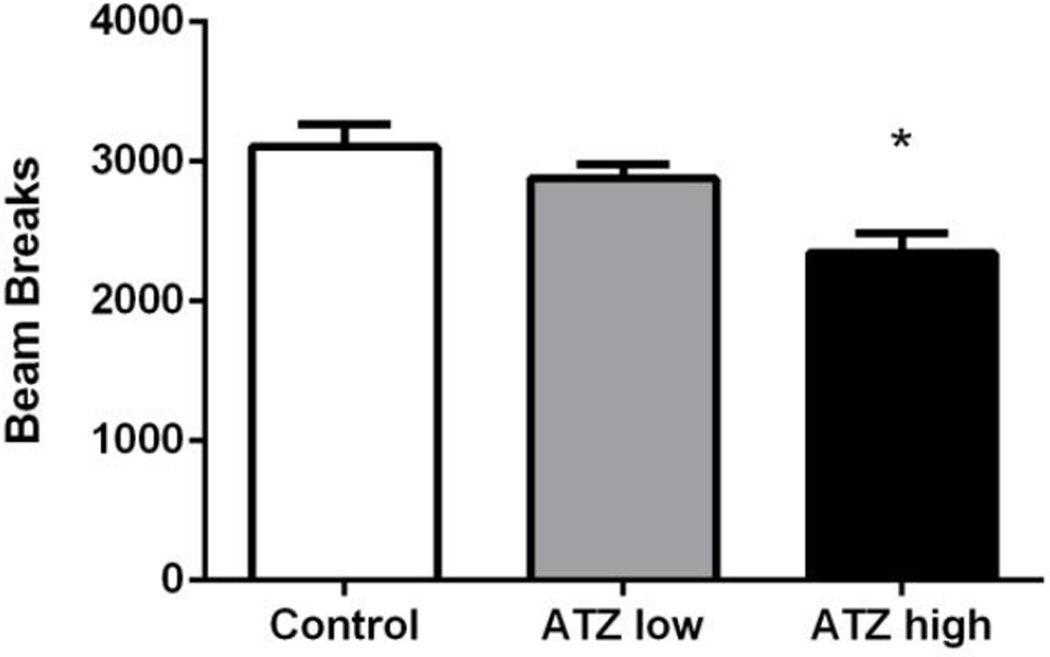
At 7 months of age, rats receiving the high dose of ATZ made significantly fewer transitions into the light side of the chamber in comparison to controls (t = −3.30, p < 0.05; Figure 6). Further, when total horizontal activity (light and dark side) was analyzed, rats in the high dose ATZ group displayed significantly decreased activity in comparison to controls (t = −4.16, p < 0.05; Figure 7)
Figure 6.
The average duration of time spent in the light side of the chamber (left), and the average number of transitions between light and dark compartments (right). * indicates different from control group with p < 0.05. Means and SE were calculated as simple statistics. Analyses were performed using a mixed model ANOVA with t tests for planned comparisons.
Figure 7.
Total horizontal activity at 7 months of age.* indicates significantly different from control with p < 0.05. Means and SE were calculated as simple statistics. Analysis was performed using a mixed model ANOVA with t tests for planned comparisons.
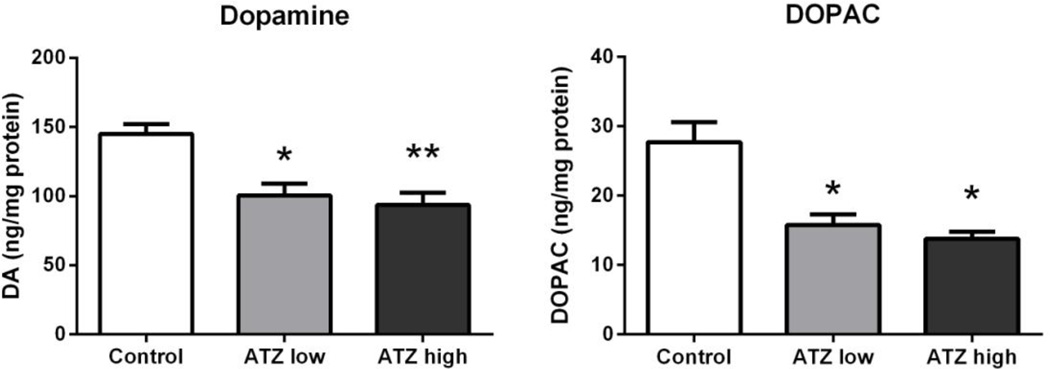
Neurochemical analyses
At 7 months of age, both low and high doses of ATZ produced a similar decrease in striatal DA concentrations (Figure 8). There was a significant main effect of treatment on striatal DA (F2, 4 = 13.47, p < 0.05) and DOPAC (F2, 4 = 10.89, p < 0.05). Planned comparisons indicated rats receiving either low dose (t = −4.09, p < 0.05) or high dose of ATZ (t = −4.75 p < 0.01) had decreased striatal DA in comparison to controls. Further, rats in the low dose ATZ group (t = −3.53 p < 0.05) and high dose ATZ group (t = −4.30, p < 0.05) had decreased DOPAC in comparison to controls (Figure 8).
Figure 8.
Striatal dopamine (DA; left) and DOPAC (right) at 7 months of age. Means and SE presented were calculated as simple statistics. Analyses were performed using a mixed model ANOVA with t tests for planned comparisons. * indicates significantly different from control group with p < 0.05. ** indicates significantly different from control with p < 0.01.
Discussion
This study utilized a variety of behavioral assays to evaluate the consequences of gestational exposure followed by six additional months of daily atrazine treatment in male rats. This herbicide was found to produce disruptions in motor functioning, and it significantly lowers striatal DA and DOPAC concentrations. Atrazine was also revealed to produce a potential anxiogenic effect but did not impair performance in the learning and memory assessments. These results suggest that exposures to atrazine target NSDA neurons and produce disruptions to behaviors associated with motor functioning.
Atrazine impacted locomotor activity and motor coordination. Although no impairments were observed after gestational and early postnatal exposure, an additional 5 months of atrazine exposure decreased vertical activity in male offspring, with significant decreases occurring in rats treated with the high dose (10 mg/kg). Consistent with these findings, atrazine has been shown to alter locomotor activity in previous studies (Bardullas et al., 2011; Belloni et al., 2011; Lin et al., 2013; Peruzovic et al., 1995; Rodriguez et al., 2013; Ugazio et al., 1991). Further, high dose atrazine exposure impaired walking beam performance in male offspring after gestational followed by 22 weeks of daily oral feedings. These findings are consistent with previous studies. Bardullas et al. (2011) reported 10 months of exposure to 10 mg/kg atrazine produced impairments in a rotarod task in male Sprague Dawley rats, and Belloni et al. (2007) reported delayed righting reflexes in male and female mice pups treated with 100 µg/kg atrazine from GD14-PND21.
In correspondence to the observed alterations to behavior, atrazine also decreased DA and DOPAC concentrations in axon terminals of NSDA neurons located in the striatum. Neurochemical assays revealed decreased striatal DA and DOPAC in rats exposed to 100 µg/kg atrazine and 10 mg/kg atrazine. These findings are consistent with those of Bardallus et al. (2011) who reported decreased striatal DA in adult male Sprague-Dawley rats treated with 10 mg/kg atrazine for 12 months (Bardallus et al., 2011). These findings are also consistent with previous studies that found acute exposures to 100 mg/kg atrazine decreased striatal DA and DOPAC in adult male Sprague-Dawley rats (Rodriguez et al., 2013) and male C57BL/6 mice (Coban and Filipov, 2007). Additionally, these findings are supported by that of Filipov et al. (2007) who demonstrated exposure of rat striatal tissue slices to 50–500 µM atrazine for 4 hr produced a dose-dependent decrease in DA levels (highest effect concentration 100 µM) and a dose-dependent increase in DA turnover (highest effect concentration 50 µM). These effects are suggested to be due to the ability of atrazine to decrease DA uptake into striatal synaptic vesicles, thus increasing cytosolic DA, which is subject to generation of toxic oxidative metabolites (Hossain and Filipov, 2008). Further, atrazine has also been shown to decrease tyrosine hydroxylase positive cells in the substantia nigra pars compacta (Coban and Filipov, 2007; Rodriguez et al., 2013), suggesting that decreases in striatal dopamine could also be due to cell death in the substantia nigra.
Neurochemical analyses of neurotransmitter and metabolite levels in the striatum reflect the integrity (DA) and metabolic activity (DOPAC) of axon terminals of NSDA neurons (Lundbald et al., 2012; O’Malley, 2010). In the present study, chronic atrazine exposure at both low and high doses produced concurrent decreases in striatal DA and DOPAC, but no change in the DOPAC/DA ratio (data not shown), results consistent with loss of NSDA axon terminals (Burke and O’Malley, 2013; Lundbald et al., 2012; Nordstorm et al., 2015). Decreases in striatal DA concentrations after 7 months of toxicant exposure correspond with the impairments in walking beam performance and decreases in locomotor activity observed in the high dose ATZ group. Since the NSDA pathway plays a central role in motor control (Haber, 2014), it is plausible that these disruptions to motor activity could be due to loss of axon terminals of NSDA neurons. Although DA and DOPAC concentrations were measured 1-1.5 months after the behavior assessments, it is likely that atrazine-induced loss of NSDA axon terminals was present at 5.5 and 6 months of age. Rats exposed to low dose ATZ had decreased striatal DA and DOPAC yet only a trend for decreased locomotor activity and impaired motor coordination. It is possible that the extent of axonopathy was smaller at the time of behavioral testing in these animals. Given the small sample size used in these groups, it is likely a larger numbers of animals would yield a significant effect of low dose ATZ on behavioral measures of motor control.
Atrazine did not affect performance in the spatial discrimination reversal task conducted at 2–5 months, nor did it affect performance in MWM conducted at 6.5 months of age. The spatial discrimination reversal task measures discriminative learning and behavioral flexibility (Reed et al., 2006). The dorsomedial striatum (Castane et al., 2010) and orbitofrontal cortex (Boulougouris et al., 2007; McAlonan and Brown, 2003) have been shown to be critical for flexible responding during the reversal component. The MWM measures hippocampal-mediated spatial learning and reference memory (Vorhees and Williams, 2006). Similar to the findings of this study, Lin et al. (2013) revealed ≥ 25 mg/kg but not 5 mg/kg atrazine impaired the performance of adult male C57BL/6 mice in a novel object recognition task (Lin et al., 2013). The novel object recognition task assesses recognition memory, a brain function also supported by the hippocampus (Broadbent et al., 2014). Taken together, these findings suggest that ATZ may only affect hippocampal-dependent memory when given at excessively high doses.
In a previous study, Bardullas et al. (2011) demonstrated adult rats exposed to 10 mg/kg atrazine for 12 months displayed slightly more errors in session 3 of a spontaneous alternation task while having no effect in a delayed alternation task. The spontaneous alternation task is designed to measure the natural tendency or rats to shift between alternative spatial responses when exploring a new environment (Bardullas et al., 2011). The delayed alternation task also measures the ability of rats to alternate between spatial responses, except now reinforcement delivery is made contingent on alternating responses (Rodriguez et al., 2001). Therefore, in the delayed alternation task, the rat relies on memory of the previous spatial location in order to obtain a reinforcer, whereas the spontaneous alternation task does not require this. Although both tasks have been suggested to involve aspects of the prefrontal cortex, hippocampus, and striatum (Bardullas et al., 2011; Dudchenko et al., 2000; Lapante et al., 2012; Yang et al., 2014), a recent study suggests that the delayed alternation task may not involve the striatum. Hallock et al. (2013) revealed that inactivation of the hippocampus but not the striatum impaired performance in the delayed alternation task. The authors suggest that the delayed alternation task and other tasks where responding is driven by spatial cues primarily involve the hippocampus, whereas tasks that require unconscious procedural memory are maintained by the striatum (Hallock et al., 2013). Therefore, findings from Bardullas et al. (2011) may suggest that 10 mg/kg atrazine affects striatal-dependent but not hippocampal-dependent brain functions. In agreement, the current study also did not find atrazine to impact hippocampal-dependent brain functions. However, in contrast, the current study did not find atrazine to impact striatal and oribotofrontal cortex-dependent reversal learning at 2–5 months of age either. This suggests that impairment to striatal-dependent cognitive function does not become apparent until after longer durations of exposure as seen in Bardullas et al. (2011).
Other studies have demonstrated atrazine exposure to improve avoidance learning (Belloni et al., 2011; Peruzovic et al., 1995). These improvements in avoidance learning could be due to the ability of atrazine to produce anxiogenic effects, thus resulting in decreased latency to engage in the avoidance response. Indeed the results of the current study revealed gestational exposure followed by daily oral treatment with 10 mg/kg atrazine to alter the behavior of rats in the light-dark box test, suggesting a possible anxiogenic effect at 7 months of age. Although atrazine had no effects on the duration of time spent on the light side of the light-dark box, 10 mg/kg atrazine significantly decreased the number of transitions rats made into the light side of the compartment in comparison to controls. The light-dark box is based on the tendency of rodents to avoid brightly illuminated environments while at the same time explore novel environments. Decreased duration of time and transitions into the light compartment of the chamber are generally viewed as an index of anxiety (Hascouet and Bourin, 2003). Thus, these findings suggest that exposure to moderate doses of atrazine produce anxiogenic effects in male Sprague-Dawley rats.
These findings are in agreement with a previous report that atrazine increased the duration of time zebrafish spent on the dark portion of an aquarium (Steinberg et al., 1995) and are supported by reports that atrazine enhances secretion of the stress hormone corticosterone in rodents. Specifically, high doses of atrazine enhance secretion of adrenocorticotropic hormone and corticosterone in adult mice (≥150 mg/kg; Pruett et al., 2003) and rats (≥ 50 mg/kg Fraites et al., 2009; Laws et al. 2009). Corticosterone mediates many components of the stress response in rodents, and has been shown to mediate anxiety-like behavior in various test paradigms (Pego et al., 2010). Thus, it is plausible that elevated corticosterone could have played a role in the anxiety-like behavior observed in the rats exposed to high dose ATZ during the light-dark box.
It should be noted that results presented here also reveal that high dose (10 mg/kg) atrazine significantly decreases the total amount of horizontal activity (when light side and dark side activity were combined). Therefore, atrazine-induced decreases in the number of transitions rodents made into the light compartment may not be indicative of anxogenic activity, but may simply be related to the effects of atrazine on overall activity resulting in fewer transitions. Future research should assess the effects of atrazine on sensitivity to aversive stimuli in additional behavioral paradigms.
In summary, these findings provide evidence that gestational and continued chronic exposure to atrazine significantly decreases locomotor activity, impairs motor coordination, and alters DA and DOPAC concentrations in male Sprague-Dawley rats. These results suggest that exposures to atrazine may have the potential to significantly impact brain regions and behaviors specific to motor functioning. Thus, in order to advance informed environmental policy and decision making and corresponding public health protection, further investigation of the potential impact of atrazine exposure on neurological development in humans may be necessary.
Highlights.
Male rats received gestational and chronic exposure to ATZ (10 mg/kg and 100 µg/kg).
ATZ altered locomotor activity and impaired motor coordination.
ATZ lowered striatal DA and DOPAC concentrations.
ATZ produced a potential anxiogenic effect.
ATZ did not impair performance in learning and memory assessments.
Abbreviations
- ATZ
atrazine
- DOPAC
3,4 dihydroxyphenylacetic acid
- DA
dopamine
- NS
nigrostriatal
- GD
gestational day
- PND
postnatal day
- MCL
maximum contaminant level
- FR
fixed ratio
- ITI
inter-trial interval
- HPLC-ED
high performance liquid chromatography coupled with electrochemical detection
Appendix A
Average weight of the dams during gestational (G) weeks 1–3 and postnatal (P) weeks 1–3 for each experimental group.
Appendix B
Monthly body weights of offspring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer L. Walters, Email: Jennifer.l.walters@wmich.edu.
Theresa A. Lansdell, Email: lansdel1@msu.edu.
Keith J. Lookingland, Email: lookingl@msu.edu.
Lisa E. Baker, Email: lisa.baker@wmich.edu.
References
- Abarikwu SO, Adesiyan AC, Oyeloja TO, Oyeyemi MO, Farombi EO. Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch. Environ. Contam. Toxicol. 2010;58:874–882. doi: 10.1007/s00244-009-9371-2. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MG, Goldstein LB, Jones KH, Abdel-Rahman AA, Damodaran TV, Dechkovskaia AM, Bullman SL, Amir BE, Khan WA. Locomotor and sensorimotor performance deficit in rats following exposure to pyridostigmine bromide, DEET, and permethrin, alone and in combination. Toxicological Sciences. 2001;60:305–314. doi: 10.1093/toxsci/60.2.305. [DOI] [PubMed] [Google Scholar]
- Adeosun SO, Hou X, Jiao Y, Zheng B, Henry S, Hill R, Wang JM. Allopregnanolone reinstates tyrosine hydroxylase immunoreactive neurons and motor performance in an MPTP-lesioned mouse model of Parkinson’s disease. PLOS One. 2012;7:e50040. doi: 10.1371/journal.pone.0050040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcadia FA, Costa C, Imperatore C, Marchese A, Rapisarda A, Salemi M, Trimarchi GR, Costa G. Oral toxicity of bis (2-Ethlyhexyl) phthalate during pregnancy and suckling in the long-evans rat. Food and Chemical Toxicology. 1998;36:963–970. doi: 10.1016/s0278-6915(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Barbash JE, Thelin GP, Kolpin DW, Gilliam RJ. Major herbicides in ground water: Results from the National Water-Quality Assessment. J. Environ. Qual. 2001;30:831–845. doi: 10.2134/jeq2001.303831x. [DOI] [PubMed] [Google Scholar]
- Bardullas U, Giordano M, Rodriguez M. Chronic atrazine exposure causes disruption of the spontaneous locomotor activity and alters the striatal dopaminergic system of the male Sprague-Dawley rat. Neurotoxicology and Teratology. 2011;33:263–272. doi: 10.1016/j.ntt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Belloni V, Alleva E, Dessi-Fulgheri F, Zaccaroni M, Santucci D. Effects of low doses of atrazine on the neurobehavioural development of mice. Ethology and Ecology & Evolution. 2007;19:309–322. [Google Scholar]
- Belloni V, Dessi-Fulgheri F, Zaccaroni M, Consigilo ED, Angelis GD, Testai E, Santucci D. Early exposure to low dose of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology. 2011;279:19–26. doi: 10.1016/j.tox.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav. Brain Res. 2007;179(2):219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and rodent hippocampus. Learning and Memory. 2014;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, O’Malley K. Axon degeneration in Parkinson’s disease. Experimental Neurology. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, Morton J, Dunnett SB. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001:8–10. doi: 10.1002/0471142301.ns0812s15. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DEH, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behavioural Brain Research. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban A, Filipov NM. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. Journal of Neurochemistry. 2007;100:1177–1187. doi: 10.1111/j.1471-4159.2006.04294.x. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Laws SC, Parikshit DC, Narotsky MG, Goldman JM, Tyrey LE, Stoker TE. Atrazine and reproductive function: Mode and mechanism of action studies. Birth Defects Research. 2007;80:98–112. doi: 10.1002/bdrb.20110. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicological Sciences. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, Alavanja M. Urinary pesticide concentrations among children, mothers, and fathers living in farm and non-farm household in Iowa. Ann. Occup. Hyg. 2007;51:53–65. doi: 10.1093/annhyg/mel062. [DOI] [PubMed] [Google Scholar]
- Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of Delta9-tetrahydrocannabinol in male and female rats. Eur J Pharmacol. 2008;578(1):37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. Journal of Neuroscience. 2000;20(8):2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipov NM, Stewart MA, Carr RL, Sistrunk SC. Dopaminergic toxicity of the herbicide atrazine in rat striatal slices. Toxicology. 2007;232:68–78. doi: 10.1016/j.tox.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraites MJP, Cooper RL, Buckalew A, Jayaraman S, Mills L, Laws SC. Characterization of the hypothalamic-pituitary-adrenal axis response to atrazine and metabolites in the female rat. Toxicological Sciences. 2009;112:88–99. doi: 10.1093/toxsci/kfp194. [DOI] [PubMed] [Google Scholar]
- Galli S, Lopes DM, Ammari R, Kopra J, Millar SE, Gibb A, Salinas PC. Deficient Wnt signaling triggers striatal synaptic degeneration and impaired motor behavior in adult mice. Nature Communications. 2014;5:4992. doi: 10.1038/ncomms5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusi G, Facciolo RM, Alleva E, Belloni V, Fulgheri FF, Santuccit D. The endocrine disruptor atrazine accounts for a dimorphic somatostatinergic neuronal expression pattern in mice. Toxicological Sciences. 2006;89:257–264. doi: 10.1093/toxsci/kfj012. [DOI] [PubMed] [Google Scholar]
- Goldstein Model of recovery of locomotor ability after sensorimotor cortex injury in rats. Institute for Laboratory Animal Research (ILAR) Journal. 2003;44:125–129. doi: 10.1093/ilar.44.2.125. [DOI] [PubMed] [Google Scholar]
- Grube Donaldson, Kiley, La Wu. Pesticides industry and sales usage: 2006 and 2007 market estimates. 2011 Retrieved from United States Environmental Protection Agency http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf.
- Hallock HL, Arreola AC, Shaw CL, Griffin AL. Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiol Learn Mem. 2013;100:108–116. doi: 10.1016/j.nlm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Hascoet M, Bourin M. A new approach to the light/dark procedure in mice. Pharmacology Biochem. Behav. 1998;60:645–653. doi: 10.1016/s0091-3057(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Anderson LL, Beasley VR, de Solla SR, Iguschi T, Ingraham H, Kestemont P, Kniewald J, Willingham E. Demasculinization and feminization of male gonads by atrazine: Consistent effect across vertebrate classes. Journal of Steroid Biochemistry and Molecular Biology. 2011;127:64–73. doi: 10.1016/j.jsbmb.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248–257. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Filipov NM. Alteration of dopamine uptake into rat striatal vesicles and synaptosomes caused by an in vitro exposure to atrazine and some of its metabolites. Toxicology. 2008;248:52–58. doi: 10.1016/j.tox.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Martin J, Blanco-Lezcano L, Gonzalez-Fraguela ME, Diaz-Hung ML, Serrano-Sanchez T, Almenares JL, Francis-Turner L. Effect of neurotoxic lesion of pedunculopontine nucleus in nigral and striatal redox balance and motor performance in rats. Neuroscience. 2015;289:300–314. doi: 10.1016/j.neuroscience.2014.12.056. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Sneck-Fahrer D, Hallberg GR, Libra RD. Temporal trends of selected agricultural chemicals in Iowa’s groundwater, 1982-1995: Are things getting better? J. Environ. Qual. 1997;26:1007–1017. [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante F, Zhang ZW, Huppe-Gourgues F, Dufresne MM, Vaucher E, Sullivan RM. Cholinergic depletion in nucleus accumbens impairs mesocortical dopamine activation and cognitive function in rats. Neuropharmacology. 2012;63(6):1075–1084. doi: 10.1016/j.neuropharm.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Laws SC, Hotchkiss M, Ferrell J, Jayaraman S, Mills L, Modic W, Tinfo N, Fraites M, Stoker T, Cooper R. Chlorotriazine herbicides and metabolites activate an ACTH-dependent release of corticosterone in male Wistar rats. Toxicological Sciences. 2009;112:78–87. doi: 10.1093/toxsci/kfp190. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun Y, Yang J, Wu Y, Yu J, Li B. Age-dependent dopaminergic dysfunction following fetal exposure to atrazine in SD rats. Environ. Toxicol. Pharmacol. 2014a;37(3):1275–1282. doi: 10.1016/j.etap.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun Y, Yang J, Wu Y, Yu J, Li B. The long-term effects of the herbicide atrazine on the dopaminergic system following exposure during pubertal development. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014b;763:23–29. doi: 10.1016/j.mrgentox.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Lin Z, Dodd CA, Filipov NM. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicology and Teratology. 2013;39:26–35. doi: 10.1016/j.ntt.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Liu HF, Lu S, Ho PW, Tse HM, Pang SY, Kung MH, Ho SL. LRRK2 R1441G mice are more liable to dopamine depletion and locomotor inactivity. Ann. Clin. Transl. Neurol. 2014;1(3):199–208. doi: 10.1002/acn3.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Decressac M, Mattsson B, Bjorklund A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(9):3213–3219. doi: 10.1073/pnas.1200575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 2003;146(1-2):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: The National Academies Press; 2011. [Google Scholar]
- Nordstrom U, Beauvais G, Ghosh A, Pulikkaparambil Sasidharan BC, Lundblad M, Fuchs J, Brundin P. Progressive nigrostriatal terminal dysfunction and degeneration in the engrailed1 heterozygous mouse model of Parkinson's disease. Neurobiol Dis. 2015;73:70–82. doi: 10.1016/j.nbd.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley KL. The role of axonopathy in Parkinson's disease. Exp Neurobiol. 2010;19(3):115–119. doi: 10.5607/en.2010.19.3.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pego JM, Sousa JC, Almeida OFX, Sousa N. Animal models of anxiety and anxiolytic drug action. In: Stein M, Steckler T, editors. Behavioral Neurobiology of Anxiety and its Treatment. Berlin: Springer; 2010. pp. 122–144. [Google Scholar]
- Peruzovic M, Kniewald J, Capkun V, Milkovic K. Effect of atrazine ingested prior to mating on rat females and their offspring. Acta Physiologica Hungarica. 1995;83:79–89. [PubMed] [Google Scholar]
- Pruett SB, Fan R, Fan R, Zheng Q, Herbert P. Modeling and predicting immunological effects of chemical characterization of a quantitative biomarker for immunological changes caused by atrazine and ethanol. Toxicological Sciences. 2003;75:343–354. doi: 10.1093/toxsci/kfg200. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Schwab C. Patterns of immunotoxicity associated with chronic as compared with acute exposure to chemical or physical stressors and their relevance with regard to the role of stress and with regard to immunotoxicity testing. Toxicol Sci. 2009;109(2):265–275. doi: 10.1093/toxsci/kfp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: effects on a spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27(5):721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez VM, Carrizales L, Jimenez-Capdeville ME, Dufour L, Giordano M. The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Research Bulletin. 2001;55:301–308. doi: 10.1016/s0361-9230(01)00477-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Limon-Pacheco JH, Mendoza-Trejo MS, Gonzalez-Gallardo A, Hernandez-Plata I, Giordano M. Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. NeuroToxicology. 2013;34:82–94. doi: 10.1016/j.neuro.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Sayin A, Derinoz O, Yuksel N, Sahin S, Bolay H. The effects of the estrus cycle and citalopram on anxiety-like behaviors and c-fos expression in rats. Pharmacol Biochem Behav. 2014;124:180–187. doi: 10.1016/j.pbb.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ. Health Perspect. 2001;109:1197–206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanko JP, Enoch RR, Ranyer JL, Davis CC, Wolf DC, Malarkey DE, Fenton SE. Effects of prenatal exposure to a low dose atrazine metabolite mixture on pubertal timing and prostate development of male Long-Evans rats. Reproductive Toxicology. 2010;30:540–549. doi: 10.1016/j.reprotox.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg CEW, Lorenz R, Spieser OH. Effects of atrazine on swimming behavior of zebrafish, Brachydanio Rerio. Wat. Res. 1995;29:981–985. [Google Scholar]
- Sun Y, Li YS, Yang JW, Yu J, Wu YP, Li BX. Exposure to atrazine during gestation and lactation periods: toxicity effects on dopaminergic neurons in offspring by downregulation of Nurr1 and VMAT2. Int. J. Mol. Sci. 2014;15(2):2811–2825. doi: 10.3390/ijms15022811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo T, Sakuma Y. Diametrically opposite effects of estrogen on the excitability of female rat medial and lateral preoptic neurons with axons to the midbrain locomotor region. Neurosci Res. 1995;22(1):73–80. doi: 10.1016/0168-0102(95)00885-w. [DOI] [PubMed] [Google Scholar]
- Ugazio G, Bosio A, Burdino E, Ghigo L, Nebbia C. Lethanlity, hexobarbital nacrocis and behavior in rats exposed to atrazine, bentazon, or molinate. Research Communications in Chemical Pathology and Pharmacology. 1991;74:349–361. [PubMed] [Google Scholar]
- United States Environmental Protection Agency. Drinking water exposure assessment for atrazine and various chloro-triazine and hydroxy-triazine degradates (NTIS Publication No. PB2001-103163) Washington, DC: National Technical Information Service; 2001. [Google Scholar]
- United States Environmental Protection Agency. Interim registration eligibility decision (IRED), Docket Number 0062. 2003 Retrieved from http://www.epa.gov/pesticides/reregistration/atrazine/atrazineadd.pdf.
- United States Environmental Protection Agency. Atrazine updates. 2012 Retrieved from http://www.epa.gov/opp00001/reregistration/atrazine/atrazine_update.htm.
- Urakawa S, Hida H, Masuda T, Misumi D, Kima TS, Nishnow H. Environmental enrichment brings a beneficial effect on beam walking and enhances the migration of doublecortin-positive cells following striatal lesions in rats. Neuroscience. 2007;144:920–933. doi: 10.1016/j.neuroscience.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Verhave PS, Vanwersch RA, van Helden HP, Smit AB, Philippens IH. Two new test methods to quantify motor deficits in a marmoset model for Parkinson's disease. Behav. Brain Res. 2009;200(1):214–219. doi: 10.1016/j.bbr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Vinogradova EP. [The effect of different stages of the sex cycle on rat behavior in a plus maze] Zh Vyssh Nerv Deiat Im I P Pavlova. 1999;49(6):1039–1045. [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994;32:5–8. doi: 10.1385/0-89603-268-X:5. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111(2):259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wetzel LT, Luempert LG, Breckenridge CB, Tisdel MO, Stevens JT, Thakur AK, Extrom PJ, Eldridge JC. Chrnoic effects of atrazine on estrus and mammary tumor formation in female Sprague-Dawley and Fischer 344 rats. Journal of Toxicology and Environmental Health. 1994;43:169–182. doi: 10.1080/15287399409531913. [DOI] [PubMed] [Google Scholar]
- Yang ST, Shi Y, Wang Q, Peng JY, Li BM. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol Brain. 2014;7:61. doi: 10.1186/s13041-014-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MRF, Clouse E, Holloway R, Davis MI, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]