Abstract
Background
Cyclic AMP-dependent protein kinase (PKA) signaling is a key target for the action of alcohol and may therefore play a role in the pathophysiology of alcohol withdrawal seizures (AWSs). Here, we investigated the role of PKA activity with respect to increased seizure susceptibility in rats that were subjected to alcohol withdrawal.
Methods
Adult male Sprague-Dawley rats received three daily doses of ethanol (or vehicle) for four consecutive days. Rats were then tested for susceptibility to acoustically evoked AWSs 3, 24, and 48 hours after the last alcohol dose. In separate experiments, the inferior colliculus (IC) was collected at these same time points from rats subjected to alcohol withdrawal and control rats following alcohol withdrawal. PKA activity, catalytic Cα (PKACα) protein, regulatory RIIα (PKARIIα) protein, and RIIβ (PKARIIβ) protein were measured in the IC. Lastly, in situ pharmacological studies were performed to evaluate whether inhibiting PKA activity in the IC suppressed AWSs.
Results
In the ethanol-treated group, AWSs were observed at the 24-hour time point, but not at the 3-hour or 48-hour time points. In the IC, PKA activity was significantly higher both 3 hours (i.e., before AWS susceptibility) and 24 hours after the last alcohol dose (when AWS susceptibility peaked) than in control rats. Consistent with these findings, protein levels of the PKACα subunit were significantly increased in the IC both 3 hours and 24 hours after the last alcohol dose. Lastly, in situ inhibition of PKA activity within the IC suppressed AWSs.
Conclusions
The increase in PKA activity and PKACα protein expression in the IC preceded the occurrence of AWSs, and inhibiting PKA activity within the IC suppressed acoustically evoked AWSs. Together, these findings suggest that altered PKA activity plays a key role in the pathogenesis of AWSs.
Keywords: alcohol intoxication, audiogenic seizures, phosphorylation, PKA
INTRODUCTION
Chronic alcohol intoxication followed by the acute cessation of alcohol consumption typically induces a withdrawal syndrome that is characterized by increased susceptibility to acoustically evoked generalized tonic-clonic seizures (for review, see N’Gouemo and Rogawski, 2006). The mechanisms that underlie the neuronal hyperexcitability that drives these acoustically evoked alcohol withdrawal–induced seizures (AWSs) are poorly understood; however, increased susceptibility to AWSs may involve specific cellular events such as cAMP-dependent protein kinase (PKA) signaling. Interestingly, the PKA signaling pathway is a major target for ethanol and has been implicated in the regulation of alcohol intake (Pandey et al., 2005). Moreover, alcohol exposure increases neuronal PKA activity in the brain (Balino et al., 2014), while chronic alcohol intoxication followed by acute withdrawal does not affect expression of the PKA catalytic Cα (PKACα) subunit in either the cortex or amygdala (Pandey et al., 2001; Pandey et al., 2003).
The inferior colliculus (IC) plays a central role in the initiation of acoustically evoked AWSs (Frye et al., 1983; Faingold and Riaz, 1995; McCown and Breese, 1990; Chakravarty and Faingold, 1998). However, the precise intracellular signaling pathways that underlie the generation of AWSs have not been identified (Evans et al., 2000; N'Gouemo and Morad 2003; N'Gouemo et al., 1996). Susceptibility to acoustically evoked AWSs begins after the 7th hour of alcohol withdrawal, peaks in the 24th hour, and dissipates by the 48th hour (Faingold 2008). Thus, identifying the cellular and/or molecular changes that occur within the first seven hours of alcohol withdrawal may reveal factors that are critical to the development of acoustically evoked AWSs. PKA is an important regulator of synaptic activity and may play a role in cellular and molecular events that lead to seizure generation following alcohol withdrawal. Interestingly, infusing cAMP derivatives directly into the IC of healthy naive rats increases their susceptibility to develop acoustically evoked seizures (Ludvig and Moshe, 1987). These findings suggest that increased PKA activity in the IC may contribute to enhance seizure susceptibility, including AWSs. Here, we evaluated the extent to which: i) alcohol withdrawal alters PKA activity and expression in the IC at 3, 24, and 48 hours (when the susceptibility to AWS is absent, maximal, and dissipated, respectively), and ii) acoustically evoked AWSs are suppressed by inhibiting PKA activity within the IC.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (250-300 g) were obtained from Taconic (Germantown, MD) and housed in a temperature- and humidity-controlled room under a 12-hour/12-hour light/dark cycle with food and water available ad libitum. All efforts were made to minimize the number of animals used in these experiments. All experimental procedures were approved by the Georgetown University Animal Care and Use Committee and were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.) et al., 2011).
Cannula guide implantation
Animals were anesthetized with an intraperitoneal injection of ketamine (85 mg/kg) and xylazine (3 mg/kg) and placed in a Kopf stereotaxic frame. Guide cannulas (21-gauge, Plastics One, Roanoke, VA) were implanted bilaterally over the IC (-9.15 mm posterior to Bregma, ±1.5 mm lateral to Bregma), and the injection cannula was subsequently advanced vertically to a final position of 4.5 mm below the surface of the brain (Paxinos and Watson, 2005). A stylet was placed in each guide cannula to prevent blockage when not in use.
Ethanol administration
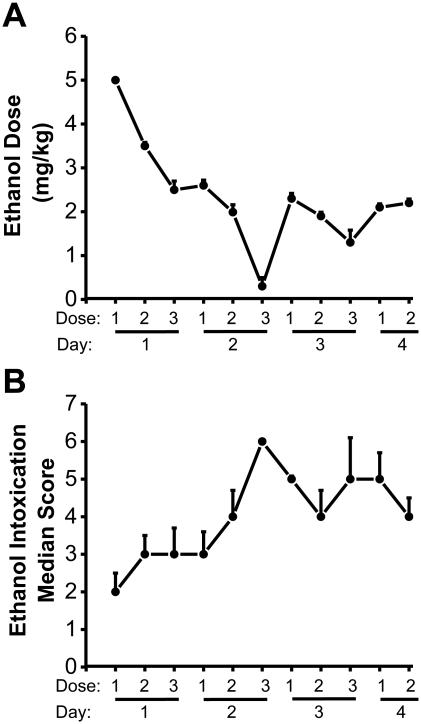
Ethanol was prepared from a 95% stock solution and administered by oral gavage as a 30% (v/v) solution in ISOMIL Infant Formula Concentrate (the ISOMIL formula was first diluted 1:1 with water). Ethanol was administered three times per day (at 8-hour intervals) for four days (see Fig. 1), and the level of intoxication was evaluated using a standard behavior-rating scale (Faingold, 2008). The first dose of ethanol was 5 g/kg body weight, and subsequent doses were adjusted empirically for each animal in order to achieve a moderate (i.e., an accentuated staggering gait and considerable elevation of the pelvis) but not severe (i.e., lethargy without pelvic or abdominal elevation) degree of intoxication (Faingold, 2008); on average, the total amount of ethanol administered was 2-12 g/kg/day. On the fourth day, ethanol treatment was terminated after the second dose. The behavioral signs of ethanol intoxication (with the corresponding subsequent doses of ethanol given in parentheses) were determined based on a well-described intoxication scale (Faingold, 2008; Majchrowicz, 1975) as follows: 0, neutrality or absence of signs of intoxication or withdrawal (5 g/kg); 1, sedation (4 g/kg); 2, ataxia 1 characterized by the lowest degree of gait impairment (4 g/kg); 3, ataxia 2 corresponding to an intermediate degree of gait impairment (3 g/kg); 4, ataxia 3 characterized by a marked level of intoxication or recovery of the righting reflex (2 g/kg); 5, loss of the righting reflex (1 g/kg); and 6, coma or absence of movement, closed eyes, and/or the absence of the eye–blink reflex (0 g/kg). The behavioral signs of ethanol withdrawal include hyperactivity, tremors, tail spasticity, spontaneous seizures (myoclonus and forelimb clonic seizures), and acoustically evoked startle responses and seizures (N’Gouemo et al., 2006; Faingold, 2008). Control (sham-treated) animals were treated under similar conditions but received the ISOMIL formula alone (without ethanol).
Figure 1.
Ethanol doses administered and ethanol intoxication scores
Data are shown as the mean±SEM dosages of ethanol administered (A) and the median±MAD ethanol intoxication scores measured (B) at 8-hour intervals over four days (n=24 rats).
Blood ethanol concentration
In a separate set of experiments, blood ethanol concentration (BEC) was measured in animals from the control and the ethanol-treated groups 3, 24, and 48 hours after the last ethanol dose. The rats were anesthetized with Nembutal (50 mg/kg; i.p.), and blood was extracted by cardiac puncture using a 21-gauge needle. The concentration of ethanol in the serum was measured using a GM7 or AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA) calibrated against a 300 mg/dL ethanol standard.
Seizure testing
To determine the animals’ susceptibility to develop acoustically evoked AWSs, ten animals were selected at random from each treatment group (i.e., ethanol-treated rats and control rats) and tested for seizures 2-3 hours (the 3-hour group), 23-24 hours (the 24-hour group), and 47-48 hours (the 48-hour group) after the last dose of ethanol or control treatment. The acoustic stimulus consisted of 100-110-decibel sound pressure level pure tones (Med Associates, St. Albans, VT) or mixed sounds (delivered via an electrical bell) which were presented until a seizure was elicited (or for a maximum of 60 sec if no seizure activity was observed). Convulsive seizure behavior was classified based on the scale for acoustically evoked seizures (Jobe et al., 1992) as follows: 0, no seizures in response to acoustic stimuli; 1, one episode of wild running seizures (WRSs) and/or jumping; 2, two episodes of WRSs; 3, one episode of WRSs and/or jumping followed by bouncing generalized tonic-clonic seizures (clonus) involving forelimbs and hindlimbs; 4, two episodes of WRSs followed by clonus; 5, one episode of WRSs and clonus followed by tonic forelimb extension (FLE) and clonus of the hindlimbs; 6, two episodes of WRSs followed by clonus, tonic forelimb extension (FLE) and partial tonic extension of the hindlimbs; 7, one episode of WRSs followed by clonus, tonic FLE and partial tonic hindlimb extension (HLE); 8, two episodes of WRSs followed by clonus, tonic FLE and full tonic HLE; 9, one episode of WRSs followed by clonus, tonic FLE and full tonic HLE. Wild running corresponds to the pre-convulsive phase while clonus and tonic FLE represent the convulsive phases of acoustically evoked seizures. Animals that did not exhibit a seizure during the first trial were tested again two hours later. The animals used in the molecular studies were not subjected to acoustically evoked seizure testing, as evoked seizures can induce a long-lasting increase in extracellular GABA levels (Ueda and Tsuru, 1995), and such GABA release together with and various degree of AWS severity and/or duration may have altered PKA activity.
Intra-IC microinjection
For intra-IC microinjection, the stylets were removed from the implanted cannula guide tubes, and vehicle (phosphate-buffered saline, pH 7.4) was infused through an injection cannula (26 gauge, Plastics One) at a rate of 0.25 µl/min for 2 min based on the report that a 0.5 µl infusion volume results in site-specific diffusion (Millan et al., 1986). Animals were then tested for susceptibility to acoustically evoked seizure 22-26 hours after ethanol withdrawal; only animals that exhibited seizures were used for subsequent pharmacological studies. Rp-cAMPS and KT5720 were reconstituted in phosphate buffered saline (PBS, pH 7.4), and in PBS containing 10% dimethyl sulfoxide, respectively. These drugs were selected on the basis of the selective inhibition of intracellular cAMP-PKA activity (Van Haastert et al., 1984; Vianna et al., 1999). Animals were closely monitored following the administration of Rp-cAMPS (5.0 µg/hemisphere) or KT5720 (2.5 µg/hemisphere). Animals were then tested for seizure susceptibility 0.5, 1, 2, and 4 hours after infusion. In another set of experiments, animals that exhibited AWSs after microinjection of the vehicle were tested for seizure susceptibility at 0.5, 1, 2 and 4 hour as a control. At the end of the experiment, the animals received bilateral infusions of Fast Green (0.5 µl/hemisphere, Electron Microscopy Science, Hatfield, PA) at the microinjection sites. The animals were then deeply anesthetized with Nembutal (50 mg/kg i.p.), and coronal sections of the IC were obtained in order to visually verify the sites of microinjection. For a given rat, AWS data were included in the analysis only if the sites of microinjections were confirmed within the IC central nucleus.
Preparation of brain homogenates
Three, 24, and 48 hours after the last ethanol dose, the animals were deeply anesthetized with pentobarbital (100 mg/kg; i.p.), and the colliculi were dissected immediately and stored at −80°C until use. Tissue homogenates (10% w/v) from each animal were lysed in cold buffer (4°C) containing 50 mM Tris-HCl (pH 7.4), 8.5% (w/v) sucrose, 2 mM EDTA, 10mM β-mercaptoethanol, 0.2% Triton 100-X and phosphatase inhibitor (Thermo Scientific, Rockford, IL) for PKA activity studies. For Western blot studies, tissues were lysed in 50 mM Tris-HCl (pH 7.4), 300 mM NaCl, 1% octylphenoxypolyethoxyethanol (Sigma-Aldrich, St Louis, MO), 10% of glycerol, 1 mM ethylenediaminetetracetic acid and 1 mM Na3VO4 (Thermo Scientific). The samples were placed on ice for ten minutes and homogenized further using a CV18 ultrasonic processor (Sonics, Newtown, CT). The homogenates were cleared by centrifugation (14,000×g at 4°C for 30 min) using a Z326K centrifuge (Hermle Labnet, Edison, NJ); the supernatants were collected, transferred to sterile microtubes, and stored at −80°C until use. Protein concentration was determined using the Pierce BCA Protein Assay kit (Thermo Scientific) and an Epoch spectrophotometer (Biotek, Winooski, VT).
PKA activity assay
PKA activity was measured using the non-radioactive PepTag assay (Promega, Madison, WI), which uses a brightly fluorescent peptide substrate that is highly specific to PKA. Phosphorylation changes the substrate’s net charge from +1 to −1; thus, non-phosphorylated peptides migrate toward the anode, whereas phosphorylated peptides migrate toward the cathode. To start the reaction, an aliquot of the PKA sample was incubated for 30 min at room temperature in PepTag PKA reaction buffer containing 0.4 µg/µl Kemptide PepTag A1 (L-R-R-A-S-L-G;Promega). The reaction was terminated by heating at 95°C for 10 min. The samples were separated in a 0.8% agarose gel at 100 V for 15 min. The gel was then scanned and imaged using an Odyssey Fc Imager (LI-COR Biosciences, Lincoln, NE).
Western blot analysis
The PKA holoenzyme contains two catalytic (C) subunits and a regulatory (R) subunit dimer (for review, see Spauling 1993). In this study, only the catalytic Cα isoform was evaluated, as the expression of both Cα and Cβ is co-localized in the hindbrain, with the Cα subunit giving a relatively stronger signal (Cadd and McKnight, 1989). For each sample, 60 µg of total protein was separated by electrophoresis in a 7.5% sodium dodecyl sulfate-polyacrylamide gel and electro-transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences) for one hour, then probed overnight at 4°C with a primary rabbit antibody against the PKACα subunit (1:1000; Cell Signaling Technology, Inc., Danvers, MA), the PKARIIα (1:1000; BD Transduction Laboratories, San Jose, CA) or the PKARIIβ (1:1000; BD Transduction Laboratories); the membranes were also incubated with anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH) antibody (1:2500; Abcam, Cambridge, MA) as a loading control. The membranes were then washed with tris-buffered saline containing 1% Tween-20 and blocked with blocking buffer. The membranes were probed with goat anti-mouse IRDye800 (1:10,000; LI-COR Biosciences) and goat anti-rabbit IRDye680 (1:10,000; LI-COR Biosciences) for one hour at room temperature, then scanned using an Odyssey Fc Imager (LI-COR Biosciences).
Data analysis
Data were analyzed using OriginPro 2016 software (OriginLab, Northampton, MA). Differences in BEC between groups and various time points (i.e., 3-, 24-, and 48-hours after the last alcohol dose) were analyzed using two-way ANOVA with Bonferroni correction for post hoc comparisons. Differences in body weights were also analyzed using two-way ANOVA with Bonferroni correction. For each animal in a given group, the ethanol dosages (mg/kg body weight) and ethanol intoxication levels were recorded at each time point. Similarly, for each animal in a given group, the prevalence of WRSs, clonus, and tonic FLE were recorded and changes between groups were analyzed using the Fisher’s exact test. The time interval from the start of the acoustic stimulus to the appearance of the first episode of seizure was recorded as the seizure latency. Seizure duration corresponded to the time between the onset and end of seizure episode. The seizure severity score was determined for each animal. The seizure severity scores between the control group and 3-h, 24-h and 48-h groups were analyzed using the Kruskal-Wallis test with Student-Newman-Keuls correction for post hoc comparisons. To quantify PKA activity, the gels were imaged and quantified using the Odyssey Image Studio v5.2 software (LI-COR Biosciences) and data are expressed relative to the mean control value. The protein levels of the PKACα, PKARIIα, and PKARIIβ subunits were measured for the corresponding band using densitometry (Odyssey Image Studio v5.2 software, LI-COR Biosciences). The integrated intensity of each band was normalized to the intensity of GAPDH. The mean of the control values (i.e., from vehicle-treated animals) was calculated, and the normalized intensity of each control and ethanol-treated sample was calculated relative to this mean control value. To analyze differences in PKA activity and protein levels between groups, a two-way ANOVA was performed followed by a Bonferroni correction. For in situ pharmacological studies, following administration of vehicle, KT5720 or Rp-cAMPS, animals that did not display an AWS within the 60-sec acoustic stimulation period were considered to lack seizure activity. For each treatment, the prevalence of WRSs, clonus and tonic FLE seizures were recorded, and data between controls and KT5720 or Rp-cAMPS group were analyzed using the Chi-square (χ2) test . The seizure severity scores between groups were analyzed using the Kruskal-Wallis test with Student-Newman-Keuls correction. Data regarding BEC, body weight, ethanol dose, seizure latency, and seizure duration are presented as the mean±SEM. The prevalence of each AWS component (i.e., WR, clonus, or tonic FLE) is presented as an absolute percentage (%). The seizure severity scores and degree of alcohol intoxication are presented as the median±median absolute deviation (MAD). PKA activity and the PKACα, PKARIIα, and PKARIIβ subunit protein data are presented as percent±S.E.M. relative to the mean control value. Before to perform an ANOVA, data were first subjected to a normality test and test for homogeneity of variance. Differences between groups were considered significant at P<0.05.
Results
Blood ethanol concentration and susceptibility to acoustically evoked alcohol withdrawal seizures
We first measured BEC in control-treated rats and in rats that were intoxicated for four days and then subjected to ethanol withdrawal. We found that following ethanol withdrawal, the BEC levels were significantly higher in the ethanol-treated group compared to controls rats measured at the same time points (F7,56=167.03, P=0.0001). Three hours after the last ethanol dose, BEC levels were significantly higher in the ethanol treated group (0.25±0.019 g/dL, n=8) than in the control-treated group (0.005±0.001 g/dL, n=8, t(3)=18.38, P=0.001); similar results were obtained when the 3-hour time point after the last ethanol dose was compared to the 24-hour (0.010±0.001 g/dL, n=8, t(3)=18.05, P=0.001) and 48-hour (0.011±0.001 g/dL, n=8, t(3)=17.93, P=0.001) time points.
Next, in a separate set of experiments, we measured acoustically evoked seizures, which consist of WRSs that evolved into clonus and tonic FLE; no tonic HLE was observed. Twenty-four hours after the last ethanol dose, 80% (8 out of 10), 60% (6 out of 10), and 60% (6 out 10) of the rats tested developed WRSs (P=0.001), clonus (P=0.011), and FLE (P=0.011), respectively. The seizure latency (in rats that exhibited seizures), seizure duration, and the median seizure severity score were 10.63±0.68 sec (n=8), 36.75±7.35 sec (n=8), and 5.0±2.16 (n=10), respectively. No seizures were observed 3 or 48 hours after ethanol withdrawal, nor were any seizures observed in the control (non-ethanol-treated) group. The seizure severity was significantly (P<0.05) higher 24 hours following ethanol withdrawal compared to the 3-hour and 48-hour time points, and the control group (Kruskal-Wallis(3)=28.98, P=0.001).
Ethanol intoxication–related behaviors, amount of ethanol administered to maintain intoxication, and changes in body weight
Fig. 1 summarizes the amount of ethanol administered (Fig. 1A) and the median scores of ethanol intoxication–related behaviors (Fig. 1B). The amount of ethanol administered decreased steadily over the four days and stabilized at approximately 2 g/kg (from an initial 5 g/kg; Fig. 1A). The median score of ethanol intoxication–related behavior increased steadily to the level of ataxia (Fig. 1B). Before ethanol treatment, the body weight of rats were 274.75±5.86 g (n=8) for the control group and 284.13±8.96 g (n=8), 272.50±3.13 (n=8), and 280.00±4.14 (n=8) for the 3-hour, 24-hour and 48-hour post-withdrawal groups, respectively. No significant difference in body weight was measured between the control and ethanol intoxication/withdrawal groups prior to the start of ethanol administration regimen. However, the rats subjected to ethanol intoxication/withdrawal had significantly altered body weight compared to control-treated animals (F7,56=11.59, P=0.0001). Specifically, although the ethanol-treated rats did not differ significantly from control rats 3 hours after the last ethanol dose (264.37±11.21, n=8, P=0.069), body weights were significantly lower in the ethanol-treated 24 (245.63±6.36 g, n=8, t(3)=3.98, P=0.001) and 48 (258.63±6.60, n=8, t(3)=3.03, P=0.02) hours after withdrawal compared to control-treated rats (300.00±5.71 g, n=8).
Alcohol withdrawal increases PKA activity in the IC
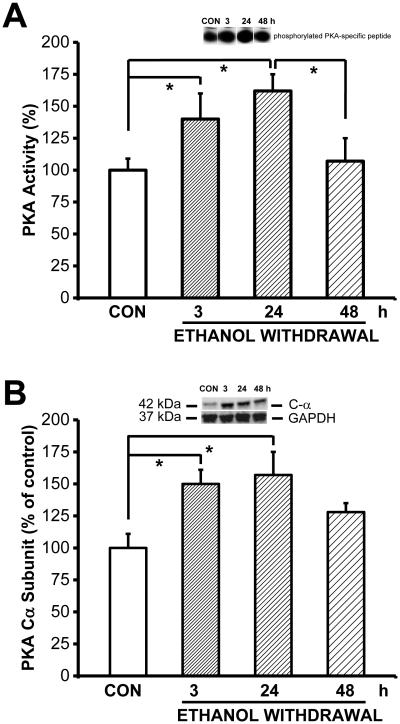
Next, to determine whether alcohol withdrawal affects PKA activity, we measured PKA activity in the IC of control-treated rats and in ethanol-treated rats at various time points following ethanol withdrawal (Fig. 2A) Quantification revealed that PKA activity was altered in the IC of rats subjected to ethanol withdrawal (F7,48=4.42, P=0.0001). Compared to control-treated rats, PKA activity in the IC was significantly higher 3 hours (i.e., before the onset of seizure susceptibility, t(3)=2.32, P=0.024) and 24 hours (i.e., when seizure susceptibility was maximum, t=2.88, P=0.006) after the last ethanol dose compared to control-treat rats. PKA activity was also significantly higher in the IC of ethanol-treated rats 24 hours after withdrawal compared to 48 hours after withdrawal (t=2.24, P=0.029). By 48 hours (when rats are no longer more susceptible to develop acoustically evoked seizures), PKA activity had returned to control levels.
Figure 2.
Ethanol withdrawal upregulates PKA activity and PKACα subunit expression.
Ethanol withdrawal transiently increases PKA activity and PKACα subunit levels in the IC. A. PKA activity was measured in the IC of control-treated rats (CON) and ethanol-treated rats 3, 24, and 48 hours after the last ethanol dose was administered (n=7 rats per group). The inset above the graph shows an example gel containing the phosphorylated PKA-specific peptide. PKA activity was significantly higher 3 and 24 hours after the last ethanol dose, then returned to control levels by the 48-hour time point. B. Ethanol cessation significantly increased the protein levels of the catalytic PKACα subunit in the IC at the 3-hour (n=8) and 24-hour (n=8) time points compared to the control group (n=7). By the 48-hour time point (n=7), PKA C-α levels decreased, but had not returned to control levels. The inset above the graph shows an example western blot at the indicated time points; GAPDH was included as a loading control. *P<0.05 (ANOVA followed by the Bonferroni correction).
Altered PKA subunit expression in the IC following ethanol withdrawal
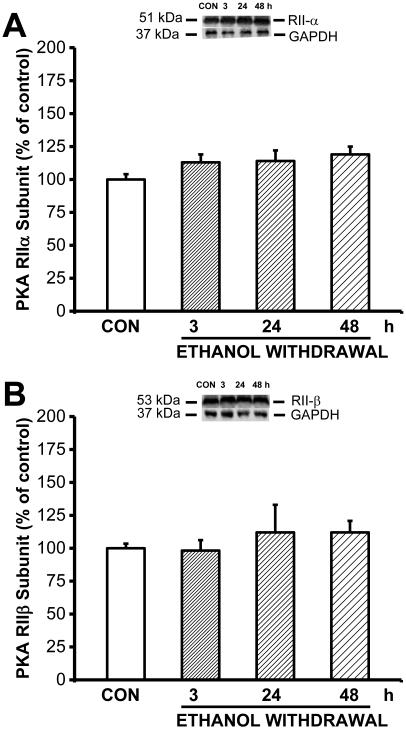
To determine whether the increased PKA activity was due to increased expression of PKA, we next measured the levels of the catalytic PKACα subunit and the regulatory PKARIIα and PKARIIβ subunits using western blot analysis (Fig. 2B). Our analysis revealed that PKACα expression was altered in the IC of rats subjected to ethanol withdrawal (F3,26=3.52, P=0.034). The level of PKACα protein in the IC was significantly (F3,26=3.52, P=0.034) higher both 3 (t(3)= 2.52, P=0.015) and 24 (t(3)=2.95, P=0.005) hours after the last ethanol dose compared to the control-treated group. Similar to PKA activity measured in the IC, the level of PKACα protein returned to control values by 48 hours. In contrast, the expression level of PKARIIα and PKARIIβ subunits were not altered during ethanol withdrawal compared to the control group (Fig. 3).
Figure 3.
Ethanol withdrawal has no effect on expression of the regulatory PKARIIα and PKARIIβ subunits in the IC.
Expression of the regulatory PKARIIα (A) and PKARIIβ (B) subunits was measured in the IC of ethanol-intoxicated and control rats (n=8 for each group) at the indicated times following ethanol withdrawal. The insets above the graphs show example western blots at the indicated time points; GAPDH was included as a loading control. Ethanol withdrawal had no significant effect on the protein levels of the PKARIIα or PKARIIβ subunits.
Focal delivery of PKA inhibitors in the IC suppresses acoustically evoked alcohol withdrawal seizures
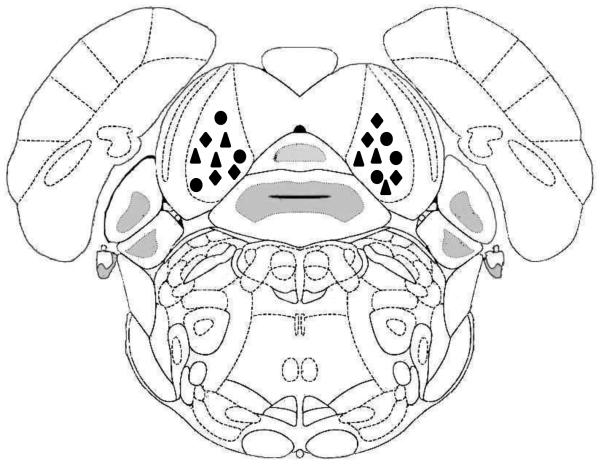
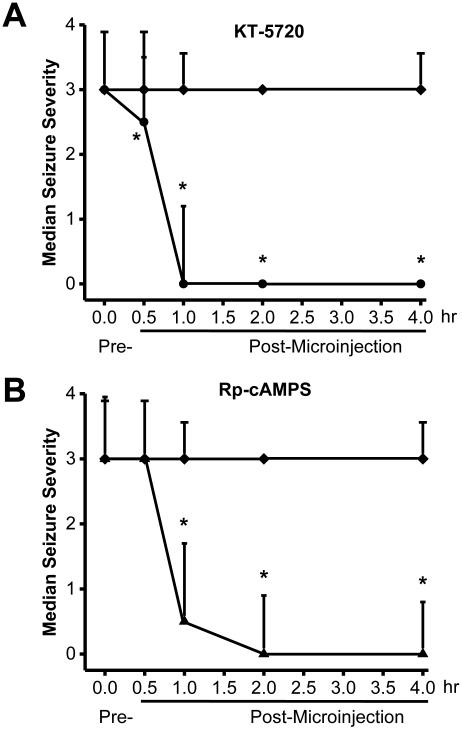
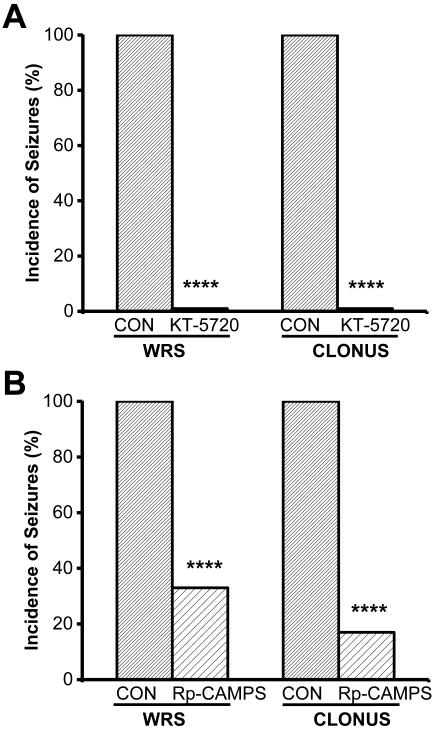
Next, we placed microinjection cannulas into the central nuclei of the IC (Fig. 4) and examined whether administering PKA inhibitors has anticonvulsant effects (Fig. 5). Fig. 5A and 5B show the effects of bilateral intra-IC microinjection of KT-5720 (2.5 μg/hemisphere, n=6) or Rp-cAMPS (5 μg/hemisphere, n=6), respectively, on seizure severity at various times following microinjection. Within 30 min of injection, KT-5720 (P<0.05) but not Rp-cAMPS significantly affected seizure severity (Kruskal-Wallis(2)=9.25, P=0.010). Within 1 hour of microinjection, however, both KT-5720 (P<0.05) and Rp-cAMPS (P<0.05) significantly decreased seizure severity (Kruskal-Wallis(2)=8.30, P=0.017), and complete suppression of seizures was reached within 2 hours (Kruskal-Wallis(2)=9.16, P=0.010) and lasted up to 4 hours, at least (Kruskal-Wallis(2)=13.65, P=0.001). A quantitative analysis of seizure 2 hours after microinjection revealed that KT-5720 (Fig. 6A) completely eliminated the occurrence of WRSs and clonus seizures (χ2(1)=196.02, P=0.0001), and Rp-cAMPS (Fig. 6B) reduced the incidence of WRSs and clonus seizures by 67% (χ2(1)=97.77, df=1, P=0.0001) and 83% (χ2(1)=138.48, P=0.0001), respectively. As a control, bilateral microinjection of vehicle into the IC of ethanol-treated rats (n=6) had no effect on the incidence or severity of acoustically evoked AWSs; a median seizure severity score of 3 was measured 0.5, 1, 2, and lasted at least up to four hours (Fig.5, filled diamonds). The sites of microinjections were located bilaterally in the IC central nucleus in 6 out of 7-8 tested rats/group.
Figure 4.
Schematic depiction of the intra-IC microinjections sites confirmed by cannula placement, based on the atlas of Paxinos and Watson (2005). The sites where vehicle (filled diamonds), KT-5720 (2.5 μg/hemisphere, filled circles) and Rp-cAMPS (5 μg/hemisphere, filled triangles) were microinjected are shown.
Figure 5.
Intra-IC injection of PKA inhibitors reduces the severity of acoustically evoked AWSs. A. Vehicle (filled diamonds) or KT-5720 (filled circles) was microinjected bilaterally into the IC (2.5 μg/hemisphere), and seizure severity was measured at the indicated times points (n=6). B. Vehicle (filled diamonds) or Rp-cAMPS (filled triangles) was microinjected bilaterally into the IC (5 μg/hemisphere), and seizure severity was measured at the indicated time points (n=6). *P<0.05 (Kruskal-Wallis and Student-Newman-keuls correction).
Figure 6.
Intra-IC injection of PKA signaling inhibitors decreases the prevalence of acoustically evoked AWSs. The percentage of post-drug was corrected to the percentage of vehicle (control, CON). A. KT-5720 (or CON) was microinjected bilaterally into the IC (2.5 μg/hemisphere) of rats following ethanol withdrawal. Two hours after microinjection, the prevalence of WRSs and clonus seizures was measured. B. Rp-cAMPS (or CON) was microinjected bilaterally into the IC (5 μg/hemisphere) of rats following ethanol withdrawal. Two hours after microinjection, the incidence of wild running seizures (WRS) and clonus seizures was measured. ****P<0.0001 (χ2 test; n=6 per group).
Discussion
Here, we report that PKA activity in the IC increases significantly 3 and 24 hours after the last alcohol dose time points that correspond to before the onset of seizure susceptibility, and maximal seizure susceptibility, respectively. This increase in PKA activity in the IC was reflected by an increase in the protein levels of the catalytic PKACα subunit at these same time points. In contrast, ethanol withdrawal—and by extension, increased susceptibility to alcohol withdrawal–induced seizures—did not affect the levels of the regulatory PKARIIα and PKARIIβ subunits in the IC. Thus, the increase in PKA activity in the IC is likely due—at least in large part—to upregulation of the catalytic PKACα subunit. Interestingly, these changes in PKA activity and expression occurred prior to the increase in the animal’s susceptibility to develop AWSs. These findings suggest that upregulation of the catalytic PKACα in the IC is not the result of seizure activity; rather this upregulation may predispose the animals to develop AWSs. Consistent with our molecular findings, inhibiting PKA activity in the IC by microinjecting either a PKA inhibitor (KT-5720) or cAMP antagonist (Rp-cAMPS) markedly suppressed both the severity and incidence of AWSs. Together, these findings indicate that increased PKA activation in the IC plays a role in the generation of AWSs.
The PKA holoenzyme is comprised of a dimer of regulatory (PKAR) subunits with a catalytic (PKAC) subunit bound to each PKAR subunit. When cAMP binds to the PKAR subunits, PKA dissociates, releasing the active PKAC subunits, which phosphorylate specific protein substrates in the cytoplasm and nucleus. PKA activity is reduced by the binding of PKAR subunits to free PKAC subunits (for review, see Spauling 1993). Thus, the increased expression of the catalytic PKACα subunit together with the lack of change in the regulatory PKARIIα and PKARIIβ subunit expression suggests increased PKA activity ultimately enhances susceptibility to develop AWSs. This notion is supported by our finding that AWSs were suppressed by the infusion of PKA inhibitors into the IC, which suggests that increased PKA activity in the IC is necessary for the generation of AWSs. In our study, BEC was still significantly elevated 3 hours after the last alcohol dose (i.e., before the onset of acoustically evoked AWSs), but was significantly reduced 24 hours after alcohol withdrawal (i.e., when the prevalence of acoustically evoked AWSs peaks). Interestingly, however, PKA activity was increased a both time points suggesting that PKA activity was elevated continuously starting 3 hours after the last alcohol dose. Alternatively, PKA activity was transiently elevated at both 3 and 24 hours after alcohol cessation.
The increased PKA activity in the IC can contribute to AWS susceptibility via several downstream mechanisms, including CREB (cAMP response element–binding protein), which binds to genes that contain a cAMP response element (CRE). Interestingly, CREB is a major nuclear target for the dissociated PKAC subunit and a likely target for increased PKA activity. Decreased CRE binding was reported in the cortex of rats subjected to withdrawal from chronic alcohol exposure (Pandey et al., 1999); however, whether this decreased CRE binding also occurs in the IC following alcohol withdrawal is currently unknown. Alternatively, PKA may increase neuronal excitability by regulating the activity of several receptors and/or ion channels that play important roles in the pathogenesis of AWSs. In our study, we found increased PKA activity in the IC following an alcohol intoxication/withdrawal regimen associated with increased seizure susceptibility. This upregulation of PKA activity can phosphorylate GABAA receptors, therefore reducing the strength of GABA-mediated inhibition, ultimately promoting seizure activity (Moss et al., 1999). Interestingly, we previously reported a loss of GABA-mediated inhibition in the IC of rats exhibiting increased susceptibility to AWSs (N’Gouemo et al., 1996).
The neuronal network in which acoustically evoked AWSs are initiated is located primarily in the brainstem (Faingold et al., 1998). Moreover, altered PKA activity and expression may be specific to the brainstem, as alcohol withdrawal does not change expression of the PKACα subunit in other brain regions, including the amygdala, cortex, and cerebellum (Pandey et al., 2003; Pandey et al., 2001; Yang et al., 1998). Alcohol withdrawal does not change the expression of the PKARIIα and PKARIIβ subunits in the IC (our study) or cortex (Pandey et al., 2001). The cAMP-PKA signaling pathway has also been implicated in the pathogenesis of other seizure types that initiate in the IC. For example, infusing cAMP derivatives into the IC of healthy rats triggers acoustically evoked seizures that are similar to AWSs (Ludvig and Moshe, 1987). Moreover, infusing cAMP derivatives into the IC of genetically epilepsy-prone rats triggers acoustically evoked seizures that evolve into status epilepticus (Ludvig and Moshe, 1989). Together, these findings suggest that increased levels of cAMP in the IC can promote epileptogenesis and/or exacerbate pre-existing epilepsy. Here, we found that PKA activity in the IC was increased significantly 24 hours after alcohol withdrawal, the same time point at which the animals’ susceptibility to AWSs peaked, supporting the hypothesis that PKA plays a prominent role in seizure-related neuronal hyperexcitability. Thus, altered PKA activity may represent a general mechanism underlying the pathophysiology of seizures that initiate in the IC.
Previous studies found that the PKA signaling pathway is also altered in other models of seizures and epilepsy. For example, administering cAMP agonists to rats increases neuronal excitability in both the neocortex and hippocampus (Boulton et al., 1993; Higashima et al., 2002). Increased PKA activity was also found in the hippocampus and cortex of Krushinski-Molodkina rats, which are genetically prone to acoustically evoked seizures (Yechikhov et al., 2001). However, repetition of acoustically evoked seizures (i.e., audiogenic kindling) in Krushinski-Molodkina rats increases PKA activity in the neocortex, but not in the hippocampus (Yechikhov et al., 2001). Furthermore, increased PKA activity was reported in the cortex—but not in the hippocampus—following pilocarpine-induced status epilepticus (Bracey et al., 2009). Finally, cAMP levels increase in the brain during seizure activity (Ferrendelli et al., 1980); this increase in cAMP occurs after the onset of EEG abnormalities, suggesting that the change in cAMP levels may be a consequence—rather than a cause—of seizure activity. Evidence also suggests that upregulation of PKA may predispose the animals to developing seizures. Interestingly, intra-amygdala injection of cAMP triggers kindling epileptogenesis, whereas infusing PKA inhibitors suppresses picrotoxin-induced generalized seizures (Yokoyama et al., 1989; Vazquez-Lopez et al., 2005). In the genetically epilepsy-prone rat, activation or inhibition of adenylate cyclase in the amygdala increases or suppresses seizure severity, respectively (Tupal and Faingold, 2010a,b). Together, these findings support the notion that PKA activity plays a causative role in epileptogenesis.
One possible limitation of this study is the use of pentobarbital for anesthesia immediately prior to removal of the IC for analysis, as pentobarbital exposure can affect PKA activity (Galisteo et al., 1999). However, pentobarbital-induced sleep is not antagonized by PKA inhibitors, ruling out pentobarbital-induced modulation of PKA activity (Lai et al., 2007). Therefore, such a lack of PKA modulation by pentobarbital may also occur in the IC following pentobarbital-induced anesthesia.
In summary, we identified a molecular signaling mechanism that is upregulated in rats prior to increased susceptibility to alcohol withdrawal–induced seizures. These results suggest that increased PKA activity and increased expression of the catalytic PKACα subunit in the IC may represent important factors in the etiology of AWSs and may serve as a possible therapeutic target for treating generalized seizures that initiate in the IC.
Acknowledgments
This publication was made possible by the National Institutes of Health Public Health Service grant R01AA020073 (to PN), and its contents are the responsibility of the authors and do not necessarily represent the official views of NIH. All experimental procedures used in this study were performed in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals and were approved by the Georgetown University Animal Care and Use Committee. The authors have no conflict of interest to disclose.
References
- Balino P, Ledesma JC, Aragon CM. In vivo study ethanol-activated brain protein kinase A: manipulations of Ca2+ distribution and flux. Alcohol Clin Exp Res. 2014;38:629–640. doi: 10.1111/acer.12289. [DOI] [PubMed] [Google Scholar]
- Boulton CL, McCrohan CR, O’Shaughnessy CT. Cyclic AMP analogues increase excitability and enhance epileptiform activity in rat neocortex in vitro. Eur J Pharmacol. 1993;236:131–136. doi: 10.1016/0014-2999(93)90235-a. [DOI] [PubMed] [Google Scholar]
- Bracey JM, Kurz JE, Low B, Churn SB. Prolonged seizure activity leads to increased Protein Kinase A activation in the rat pilocarpine model of status epilepticus. Brain Res. 2009;1283:167–176. doi: 10.1016/j.brainres.2009.05.066. [DOI] [PubMed] [Google Scholar]
- Cadd G, McKnight GS. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989;3:71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Chakravarty DN, Faingold CL. Comparison of neuronal response patterns in the external and central nuclei of inferior colliculus during ethanol administration and ethanol withdrawal. Brain Res. 1998;783:102–108. doi: 10.1016/s0006-8993(97)01193-1. [DOI] [PubMed] [Google Scholar]
- Evans MS, Li Y, Faingold CL. Inferior colliculus intracellular response abnormalities in vitro associated with susceptibility to ethanol withdrawal seizures. Alcohol Clin Exp Res. 2000;24:1180–1186. [PubMed] [Google Scholar]
- Faingold CL. The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Curr Protocol Neurosci. 2008 doi: 10.1002/0471142301.ns0928s44. Chapter 9:Unit9.28. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Riaz A. Ethanol withdrawal induces increased firing in the inferior colliculus neurons associated with audiogenic seizure susceptibility. Exp Neurol. 1995;132:91–98. doi: 10.1016/0014-4886(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Faingold CL, N’Gouemo P, Riaz A. Ethanol and neurotransmitter interaction- from molecular to integrative effects. Prog Neurobiol. 1998;55:509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Ferrendelli JA, Blank AC, Gross RA. Relationships between seizure activity and cyclic nucleotide levels in brain. Brain Res. 1980;200:93–103. doi: 10.1016/0006-8993(80)91097-5. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra or medial septum. J Pharmacol Exp Ther. 1983;227:663–670. [PMC free article] [PubMed] [Google Scholar]
- Galisteo M, Marc N, Fautrel A, Guillouzo A, Corcos L, Lagadic-Gossmann D. Involvement of cyclic nucleotide- and calcium-regulated pathways in phenobarbital-induced cytochrome P-450 3A expression in mouse primary hepatocytes. J Pharmacol Exp Ther. 1999;290:12701277. [PubMed] [Google Scholar]
- Higashima M, Ohno K, Koshino Y. Cyclic AMP-mediated modulation of epileptiform afterdischarge generation in rat hippocampal slices. Brain Res. 2002;949:157–161. doi: 10.1016/s0006-8993(02)02976-1. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Mishra PK, Dailey JW. Genetically epilepsy-prone rats—Actions of antiepileptic drugs and monoaminergic neurotransmitters. In: Faingold CL, Fromm GH, editors. Drugs for Control of Epilepsy— Actions on Neuronal Networks Involved in Seizure Disorders. CRC Press; New York: 1992. pp. 253–276. [Google Scholar]
- Lai C-C, Kuo T-I, Lin H-H. The role of protein kinase A in acute ethanol-induced neurobehavioral actions in rats. Anesth Analg. 2007;105:89–96. doi: 10.1213/01.ane.0000263030.13249.36. [DOI] [PubMed] [Google Scholar]
- Ludvig N, Moshe SL. Cyclic AMP derivatives injected into the inferior colliculus induce audiogenic seizure-like phenomena in normal rats. Brain Res. 1987;437:193–196. doi: 10.1016/0006-8993(87)91545-9. [DOI] [PubMed] [Google Scholar]
- Ludvig N, Moshe SL. Different behavioral and electrographic effects of acoustic stimulation and dibutyryl cyclic AMP injection into the inferior colliculus in normal and in genetically epilepsy-prone rats. Epilepsy Res. 1989;3:185–190. doi: 10.1016/0920-1211(89)90022-3. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence on alcohol and the associated metabolic and behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Millan MH, Meldrum BS, Faingold CL. Induction of audiogenic seizure susceptibility by focal infusion of excitant amino acid or bicuculline into the inferior colliculus of normal rats. Exp Neurol. 1986;91:634–639. doi: 10.1016/0014-4886(86)90059-2. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science. 1992;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.), Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the care and use of laboratory animal. 8th National Academies Press; Washington DC: 2011. [Google Scholar]
- N’Gouemo P, Caspary DM, Faingold CL. Decreased GABA effectiveness in the inferior colliculus neurons during ethanol withdrawal in rat susceptible to audiogenic seizures. Brain Res. 1996;724:200–204. doi: 10.1016/0006-8993(96)00304-6. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Morad M. Ethanol withdrawal seizure susceptibility is associated with upregulation of L- and P-type Ca2+ channels currents in rat inferior colliculus neurons. Neuropharmacol. 2003;45:429–437. doi: 10.1016/s0028-3908(03)00191-6. [DOI] [PubMed] [Google Scholar]
- N'Gouemo P, Rogawski MA. Alcohol withdrawal seizures. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier Academic Press; Amsterdam: 2006. pp. 161–177. [Google Scholar]
- N’Gouemo P, Yasuda RP, Morad M. Ethanol withdrawal is accompanied by downregulation of calcium channel alpha 1B subunit in rat inferior colliculus neurons. Brain Res. 2006;1108:216–220. doi: 10.1016/j.brainres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Mittal N. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat cortex. J Pharmacol Exp Ther. 2001;296:857–868. [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Chartoff EH, Carlezon WA, Jr, Zou J, Zhang H, Kreibich AS, Blendy JA, Crews FT. CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcohol Clin Exp Res. 2005;29:176–184. doi: 10.1097/01.alc.0000153550.31168.1d. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang D, Mittal Nayyar D. Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. J Pharmacol Exp Ther. 1999;288:866–878. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coodinates. fifth Academic Press; San Diego: 2005. [Google Scholar]
- Spaulding SW. The ways in which hormones change cyclic adenosine 3',5'-monophosphate-dependent protein kinase subunits, and how such changes affect cell behavior. Endocr Rev. 1993;14:632–50. doi: 10.1210/edrv-14-5-632. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold CL. Precipitous induction of audiogenic kindling by activation of adenylyl cyclase in the amygdala. Epilepsia. 2010a;51:354–361. doi: 10.1111/j.1528-1167.2009.02263.x. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold C. Inhibition of adenylyl cyclase in amygdala blocks the effect of audiogenic seizure kindling in genetically epilepsy-prone rats. Neuropharmacology. 2010b;59:107–111. doi: 10.1016/j.neuropharm.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Tsuru N. Simultaneous monitoring of the seizure related change in extracellular glutamate and gamma-aminobutyric acid concentration in bilateral hippocampi following development of amygdaloid kindling. Epilepsy Res. 1995;20:213–219. doi: 10.1016/0920-1211(94)00081-7. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJ, Van Driel R, Jastorff B, Baraniak J, Stec WJ, De Wit RJ. Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem. 1984;259:10020–10024. [PubMed] [Google Scholar]
- Vianna MR, Izquierdo LA, Barros DM, Medina JH, Izquierdo I. Intrahippocampal infusion of an inhibitor of protein kinase A separates short- from long-term memory. Behav Pharmacol. 1999;10:223–227. doi: 10.1097/00008877-199903000-00011. [DOI] [PubMed] [Google Scholar]
- Vazquez-Lopez A, Sierra-Paredes G, Sierra-Marcuno G. Role of cAMP-dependent protein kinase on acute picrotoxin-induced seizures. Neurochem Res. 2005;30:613–618. doi: 10.1007/s11064-005-2748-3. [DOI] [PubMed] [Google Scholar]
- Yang X, Horn K, Baraban JM, Wan GS. Chronic ethanol administration decreases phosphorylation of cyclic AMP-response element binding protein in granule cells of rat cerebellum. J Neurochem. 1998;70:224–232. doi: 10.1046/j.1471-4159.1998.70010224.x. [DOI] [PubMed] [Google Scholar]
- Yechikhov S, Morenkov E, Chulanova T, Godukhin O, Shchipakina T. Involvement of cAMP- and Ca(2+)/calmodulin-dependent neuronal protein phosphorylation in mechanisms underlying genetic predisposition to audiogenic seizures in rats. Epilepsy Res. 2001;46:15–25. doi: 10.1016/s0920-1211(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Mori N, Kumashiro H. Chemical kindling induced by cAMP and transfer to electrical kindling. Brain Res. 1989;492:158–162. doi: 10.1016/0006-8993(89)90898-6. [DOI] [PubMed] [Google Scholar]