Abstract
Animal models are crucial to the study of the neurobiological bases of psychiatric disorders, but schizophrenia is a particularly challenging disorder to model given the complexity and heavily verbal nature of its symptoms. This article describes a developmental surgical rodent model of schizophrenia, the neonatal ventral hippocampal lesion (NVHL) model. This widely used model produces reliable behavioral abnormalities that are comparable to those observed in patients, as well as anatomical and neurophysiological disruptions in forebrain areas that are also implicated in schizophrenia. A brief background of the development and validity of the NVHL model is discussed here, along with detailed procedures for producing the model in rats. Critical issues particular to neonatal surgery are discussed, and representative histological and behavioral results are presented.
Keywords: schizophrenia, developmental, hippocampus, prefrontal cortex, rodent model
INTRODUCTION
Animal models are critical for advancing knowledge regarding the neurobiological substrates of neuropsychiatric disease states, and for assessing the efficacy of potential therapeutic agents. Schizophrenia is a disorder for which it is challenging but also particularly useful to develop animal models, given the severity of the disorder, the relative lack of understanding of its etiology, and the shortcomings of currently available treatments (van Os and Kapur, 2009). While animal models of schizophrenia are necessarily incomplete, as symptoms such as disordered language cannot be reproduced in rats, there are aspects of behavior and cognition that can be successfully modeled. These include hypersensitivity to dopaminergic agents, social withdrawal, and cognitive impairments (Lipska and Weinberger, 2000; Tseng et al., 2009). However, this degree of face validity is not sufficient for a useful model of schizophrenia; other known etiological and neurobiological constructs must also be replicated to the extent possible. For example, schizophrenia is now widely considered to be a neurodevelopmental disorder, encompassing a perinatal neurological insult that does not manifest behaviorally until late adolescence or early adulthood (Lewis and Levitt, 2002), and thus a successful animal model should include a neurodevelopmental component.
The neonatal ventral hippocampal lesion (NVHL) model, originally developed by Barbara Lipska and colleagues (Lipska et al., 1993), fulfills many of the requirements for a useful and clinically translational model of schizophrenia. This surgical model involves the infusion of an excitotoxin into the hippocampus during the first postnatal week, a time point roughly comparable to the third trimester of human development (Tseng et al., 2009). Resulting behavioral abnormalities are widespread and include, hyperlocomotion, hypersensitivity to stimulants, disrupted sensorimotor gating, social withdrawal, impaired working memory, impaired behavioral flexibility, and addictive behavior (Brady et al., 2008; Brady et al., 2010; Chambers et al., 1996; Chambers and Self, 2002; Lipska et al., 2002; Lipska et al., 1993; Lipska et al., 1995; Palacorolla et al., 2013; Placek et al., 2013; Sams-Dodd et al., 1997). Importantly, most of these abnormalities do not emerge until post-adolescence, and some can be reversed by antipsychotic treatment (Lipska and Weinberger, 1994; Sams-Dodd et al., 1997). In addition, the NVHL manipulation produces disruptions in many brain areas and neural circuits that have been implicated in schizophrenia. The site of the lesion itself is the ventral hippocampus, which may be disrupted or disorganized in schizophrenia (Weinberger, 1999). However, many if not all of the behavioral abnormalities appear to be mediated in downstream target areas, particularly the prefrontal cortex which has been heavily implicated in schizophrenia (Brady et al., 2010; O'Donnell, 2012; Placek et al., 2013). Disruption of the development of such efferent hippocampal pathways, rather than damage or cell loss in the hippocampus per se, appears to drive the abnormalities seen in the NVHL model (O'Donnell, 2012).
This article describes procedures for producing the NVHL manipulation in postnatal day 7 rats. Protocols are provided for pre-surgical preparation of animals, and for the basic surgical procedure. Instructions for constructing a modified stereotaxic frame, and recipes for the required solutions, are also provided. All procedures involving live animals should be reviewed for approval by the user's Institutional Animal Care and Use Committee (IACUC). This article follows up and expands on a previously published detailed protocol describing the NVHL manipulation (Chambers and Lipska, 2011).
BASIC PROTOCOL 1
PRE-SURGICAL PREPARATION OF ANIMALS
The NVHL surgery is performed on approximately postnatal day (PD) 7. However, attention must be paid to dams and pups during the first postnatal week. This protocol describes the care of pregnant females and offspring prior to surgery. Most of the published literature utilizing the NVHL manipulation has used Sprague-Dawley rats, but other strains may be used (e.g., (Gruber et al., 2010).
Obtain pregnant female rats, either through breeding or ordering of timed pregnant females. If timed pregnant females are obtained, schedule delivery to the laboratory between embryonic day (ED) 15-17. Gestation is approximately 22 days.
Single house pregnant rats and ensure access to food and water. As the end of the gestational period nears, monitor dams at least twice a day for signs of labor and appearance of pups. Note the date of birth of the offspring, which is designated as PD 0.
- On about PD 4-5, sex and weigh the pups.Use care in removing and replacing pups from the home cage, as the dam will be protective and potentially aggressive.
- To determine the sex of pups, examine the anogenital region. In males, there is a larger distance between the genitals and the anal opening, and the genital organ is typically (but not always) more prominent.Pups where the sex is ambiguous or unable to be determined should not be used.
- If only one sex will be used for the study, pups of the other sex may be left in the cage until surgery, or culled if the litter size is unusually high.Typical litter size is 10-14 pups. If litters are unusually small or large, pups may not reach the target weight (15-20 g) during the appropriate time window for the NVHL procedure (PD 6-8). Pups in standard-size litters of 10-14 pups can reasonably be expected to gain 2-3 g per day in the first week.
If both sexes will be used for the study, but the litter size is high, the smallest pups in the litter should be culled.
Cull any pups that are markedly smaller (3-4 g) than the rest of the litter and/or are visibly unhealthy, weak, or show motor impairments or other abnormalities.
Culled pups may be euthanized by rapid decapitation or by prolonged hypothermia. Seek IACUC approval and veterinary input for the chosen method of euthanasia.
BASIC PROTOCOL 2
The NVHL Surgical Procedure
The NVHL manipulation is produced by infusing 30 nl/hemisphere of ibotenic acid (10 ug/ul) bilaterally into the hippocampus of early postnatal pups. This protocol describes the steps of this procedure, including anesthesia, infusion of the toxin, post-surgical (short-term) recovery period, and long-term recovery period. The surgical procedure should take place on PD 7 ± 1 day, i.e., between PD 6 to 8.
Materials
Artificial CSF (see Reagents and Solutions)
Ibotenic acid (see Reagents and Solutions)
Stereotaxic frame(s), modified (see Support Protocol 1)
Injection cannula assembly –26 gauge needle with sharp bevel (e.g., Hamilton 775802) with depth marker (see Support Protocol 1)
Polyethylene PE-20 tubing, i.d. 0.38-0.4 mm, o.d. 1.09-1.1 mm
1 ml syringes with 23-25 ga needles
10 μl infusion syringe(s) (e.g., Hamilton 80300)
Syringe infusion pump(s) capable of delivering 15 nl/min
Rat dams and litters
Crushed ice and bucket
Self-adhesive label tape
Scalpel blades
Clip applier and clips (e.g., Autoclip system), or wound closure glue
Clip remover (if clips are used)
Toothed iris tissue forceps (1 × 2 teeth, straight 4 inch)
Ear punch, or tattooing equipment
Alcohol, or instrument heat sterilizer (e.g., Germinator 500)
Rubber pipet bulbs
Warming pads (e.g., Deltaphase 8 × 8 pads)
- Weigh all pups.Rats need to be between 15-20 g on the day of surgery to ensure both adequate overall health, and consistency with standard stereotaxic coordinates. Pups that do not meet this weight requirement within the time window specified (PD 6-8) should not be used.
- Place cages (with dams) in or near the surgical location. Pups should remain in the home cage with the dams as much as possible, except for the induction of anesthesia, the surgery, and the immediate post-surgical period.Use care in removing and replacing pups from the home cage, as the dam will be protective and potentially aggressive.
Before beginning the first surgery, fill the PE-20 tubing and injection cannula assembly with aCSF (for sham surgeries) and/or ibotenic acid (for lesion surgeries).
- When preparing for lesion surgeries, first flush and fill the entire tubing/cannula assembly with aCSF using a 1 ml syringe with a 23-25 ga needle, then attach a 10 μl infusion syringe and backfill the cannula and tubing with a small amount (3-5 μl) of 10 μg/μl ibotenic acid solution. You should see the toxin enter the tubing above the injection cannula. Do not overfill as the ibotenic solution is sensitive to light.Be sure to place an air bubble in between the aCSF and the ibotenic acid, and monitor it so that you can refill the tubing/cannula with toxin as necessary.
Induce anesthesia via hypothermia (Danneman and Mandrell, 1997). Place the pup on top of the ice, or in a slight hollow in the ice, and put a lid on the container (e.g., ice bucket). Ensure that the mouth and nose remain uncovered to allow for unrestricted air flow.
- Check on the animal after 10-12 minutes. If there is movement of the limbs, hypothermia is not complete. Replace the animal on the ice for 1-2 minutes at a time until the distal limbs are no longer pink, and no spontaneous limb movement is observed. This level of hypothermic anesthesia should last for approximately 15 minutes.Take care not to leave the pup on the ice for too long. Appearance of a gray or white color throughout the body indicates over-exposure and can lead to death.
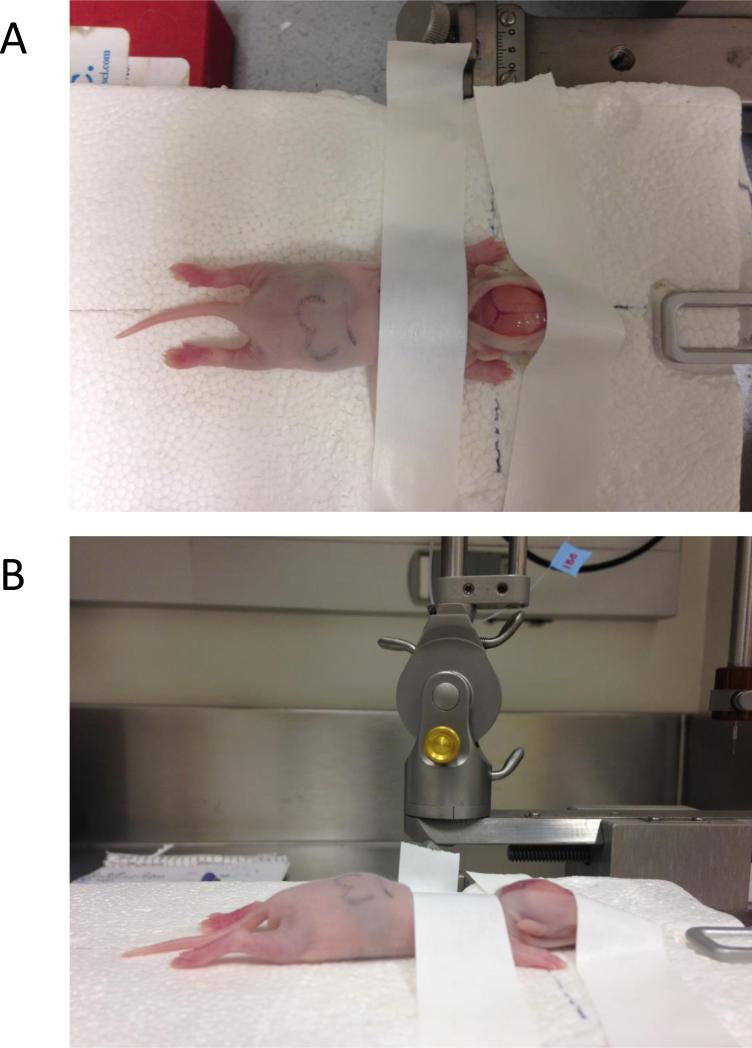
Center the animal on the stereotaxic platform. Spread out the limbs and gently flatten the animal as much as possible. Affix the pup to the platform using a piece of adhesive tape placed across the shoulders, just behind the head (Fig. 1).
- Use a scalpel blade to make a longitudinal cut down the center of the scalp.Because the pup is hypothermic, there should be little to no bleeding.
- Use a second piece of tape to secure the front of the head to the platform. The back of this piece of tape should overlap with the incision so that the tape helps to holds the incision open (Fig. 1).Observe the animal carefully to make sure that the surface of the skull is horizontally level. Gently manipulate the head to ensure a level skull before taping.
- Locate bregma (the intersection of skull plates) on the skull surface, using the cannula. Note the anterior-posterior (AP) and medial-lateral (ML) coordinates.Note that the skull is very soft and thin. Take care not to pierce the skull at this step.
Calculate the target coordinates for the toxin infusions. The targets (bilateral ventral hippocampus) are located at 3.0 mm posterior and ± 3.5 mm lateral to bregma.
- Move the stereotaxic arm posteriorly and laterally to place the cannula above the target on one side.In a given set of surgeries, always begin with the same hemisphere, for consistency and to minimize the possibility of error (e.g., accidentally infusing twice on the same side).
- Hold the head steady with the thumb and forefinger, and lower the cannula to the skull surface. Use the sharp tip of the cannula to pierce the skull using a quick, short twisting clockwise motion of the stereotaxic manipulator, moving ventrally.Continue to hold the head steady throughout Steps 13-15.
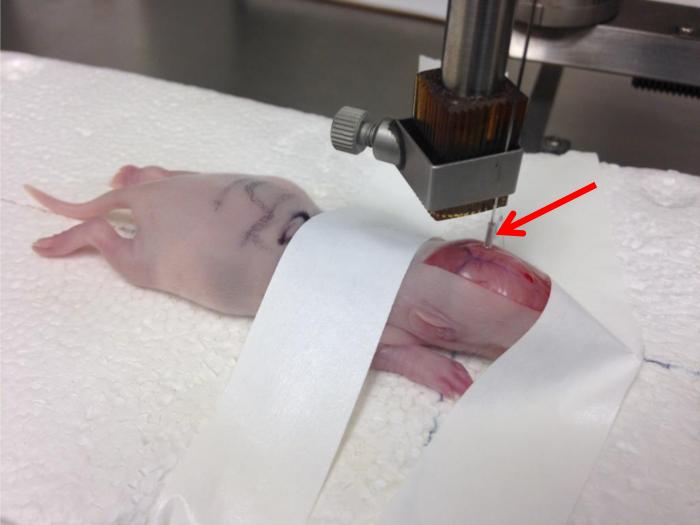
Once the skull is pierced, slowly continue to lower the cannula so that the bottom edge of the tubing depth marker (see Support Protocol 1) is touching the skull (Fig. 2). The skull will compress as the cannula is being lowered.
Once the tubing is touching the skull, slowly raise the cannula back up until the skull rounds again and the skull sutures fill with blood (Fig. 2).
- Turn on the pump and infuse 30 nl of the appropriate solution (either toxin, to produce a lesion, or aCSF, to produce a sham) at 15 nl/min (0.015 μl/min). The infusion should take 2 min.Be sure to set the infusion pump to the correct setting for the syringe size. For example, the display may indicate 0.15 μl/min, but this equals 0.015 μl/min when using a 10 μl syringe.
Once the infusion is complete, leave the cannula in place for 2-3 min to allow for diffusion of the toxin (or aCSF) into the tissue.
Hold the head steady once again with the thumb and forefinger, and slowly raise the cannula.
Move the stereotaxic arm laterally to the target on the opposite side, and repeat Steps 13-18.
- Once the bilateral infusions are complete and the injection cannula has been removed, mark the animal as a lesion or sham using an ear punch, or a tattooing system.The specific marking system may vary, but establish a systematic procedure that is followed with all animals. For example, lesion animals may be marked with a punch in the left ear and sham animals with a punch in the right ear. Or, lesion animals may be tattooed on the left forelimb, and sham animals tattooed on the right forelimb. Toe clipping is discouraged as a marking system.
- Close the scalp wound with clips or wound closure glue.If clips are used, use the toothed forceps to hold the sides of the wound together and lift the skin up well away from the skull before clipping.
- Carefully remove the tape to remove the pup from the platform.As long as the animal is still anesthetized, the tape may be removed prior to Steps 20 and/or 21 if needed to facilitate marking the animal and closing the wound.
Hold the pup gently just behind the ears, letting the body dangle, so that the airway is open.
Use a rubber pipet bulb placed over the nose and mouth to gently resuscitate the pup. Watch the abdomen to ensure that air is entering.
Once breaths are observed, place the pup on a warming pad. Watch for color to return and respiration to normalize, typically within about 10 min.
- Sterilize surgical instruments (scalpel blade, forceps, clip applier, ear punch) in alcohol or a heat-based sterilizer in between animals.Bleeding should be minimal or nonexistent due to the hypothermia. Clean off any blood before placing instruments in alcohol or heat.
- Return the pup to the dam when color, respiration, and activity levels are back to normal.No special post-surgical care is necessary, but cages should be monitored for the next few days as mortality is highest in the days immediately following surgery, and dams are likely to cannibalize dead or unhealthy pups.
- Long-term recovery: Wean pups (and remove clips, if used) at about 21-23 days of age. House pups in pairs or triads.Animals should not be singly housed before adulthood.
If desired, offspring may be separated into single housing at or after PD 56 (adulthood).
Figure 1.
Photographs illustrating correct placement and taping of a pup on the Styrofoam platform, which is secured in place by the ear bars and tooth bar. The view from directly above (A) illustrates the centering and spreading of the pup on the platform, with the axes of the body placed along the perpendicular guide lines. The scalp has been incised; the sagittal and posterior transverse sinuses are easily visible, and the coronal suture and bregma can be seen (although less easily) in this view. Note how the front piece of tape is used to both secure the front of the head to the platform, and to assist in holding the incision open. The view from the side (B) illustrates how the pup's skull should be horizontally level.
Figure 2.
Photograph illustrating the injection cannula lowered to the target coordinates in the left hemisphere. The arrow indicates the tubing depth marker, the bottom edge of which is touching the skull. The cannula has been lowered and raised back up, returning the skull to its rounded position as described in Steps 14-15 of Basic Protocol 2 above. Note how the electrode block clamp is located low on the injection cannula to keep the needle stable.
SUPPORT PROTOCOL 1
Preparation of the stereotaxic frame and injection cannula
This support protocol describes how to produce a modified stereotaxic apparatus for use with these neonatal surgeries, and how to prepare the injection cannula (namely, placement of a depth marker).
Materials
Small animal stereotaxic frame with ear bars (e.g., Kopf Model 900)
Standard vertical electrode holder with clamp (e.g., Kopf Model 1770)
Section of thick Styrofoam or similar material, sized to fit inside the stereotaxic frame
Circular (Bulls-eye) level (allows leveling in two dimensions)
Injection cannula –26 gauge needle with sharp bevel (e.g., Hamilton 775802)
PE-20 tubing
- Trim the Styrofoam block if needed so that it fits completely inside the stereotaxic frame. It should be at least 2-3 inches thick. The length and width may vary but should nearly fill the available area inside the stereotaxic “U” frame with the ear bars removed. In particular, make sure that the block abuts the two sides of the frame.If the block fits snugly inside the frame and the height is roughly even with the frame, Steps 2-4 may not be necessary. However, following these steps will increase the stability of the platform, which is desirable since ear bars cannot be used to ensure stability of the skull.
Raise the nose clamp as far as it will go.
- Place the bulls-eye level on top of the Styrofoam block. Push the Styrofoam block onto the bottom piece of the tooth bar assembly, keeping the block level as you push. Tighten the nose clamp, allowing it to press into the Styrofoam block (Fig. 1).If the block is not thick enough to slide onto the tooth bar while sitting on the stereotaxic base plate, use another piece of Styrofoam or some other stiff material to prop up the block to the necessary height.
Again keeping the Styrofoam block level, insert each ear bar into the sides of the block as far as they will go. Tighten the ear bars.
To prepare the depth marker for the injection cannula, cut a small (3-5 mm) length of PE tubing and slide it onto the cannula. It should fit tightly.
- Adjust the placement of the tubing so that the bottom end of the tubing is 5 mm from the center of the cannula needle's bevel.Take care not to bend the needle while making this adjustment.
- Clamp the injection cannula onto the vertical electrode holder.Clamp the cannula close to the tip of the bevel to provide a stable attachment, and to avoid having the needle bend when it is inserted into the brain (see Fig. 2).
Using the stereotaxic manipulator to move the injection cannula, locate a point on the Styrofoam block platform that is the approximate center of where the ear bars would meet. Mark this spot on the Styrofoam with a pen.
Move the cannula laterally all the way to the left and mark the spot; repeat on the right. Connect these two points with a straight line that traverses the platform.
- Return the cannula to the center point. Move the cannula to the anterior and then posterior extremes, marking each spot, and then connect those points.You should now have a cross (perpendicular lines) marked on the platform, which will act as guidelines for placing pups on the platform as described in the main protocol.
Before each set of surgeries, check that the platform is level, and that the drawn guide lines still match the anterior-poster and lateral movements of the stereotaxic manipulator. Adjust the platform if necessary by adjusting the nose clamp and/or ear bars.
REAGENTS AND SOLUTIONS
Artificial cerebrospinal fluid (aCSF)
This recipe makes 1 L of physiologic aCSF (pH=7.4) with 150 mM Na, 3.0 mM K, 1.4 mM Ca, 0.8 mM Mg, 1.0 mM P, and 155 mM Cl.
- Mix Solution A as follows:
- 500 ml sterile distilled water
- 8.66 g NaCl
- 0.224 g KCl
- 0.206 g CaCl2 * 2H2O
- 0.163 g MgCl2 * 6H2O
- Mix Solution B as follows:
- 500 ml sterile distilled water
- 0.214 g Na2HPO4 * H2O (dibasic) or 0.358 g Na2HPO4 * 7H2O
- 0.027 g NaH2PO4 * H2O (monobasic)
Combine Solutions A and B.
- Storage:
- aCSF may be stored at 4° C (refrigerated) for up to a week.
- Aliquots of aCSF (recommended volume = 10-15 ml) may be stored at −80° C indefinitely.
Ibotenic acid
This recipe makes about 500 μl of a 10 μg/μl solution of ibotenic acid.
Begin with a 5 mg vial of ibotenic acid (CAS 2552-55-8), e.g., Sigma Aldrich I2765-5MG. Make the solution in the original purchased vial, thus avoiding the need to weigh out the ibotenic acid.
Add 480 μl of aCSF to the vial of ibotenic acid powder.
Add 20 μl of 1 N NaOH to the vial. Check to see if the ibotenic acid has dissolved. Use pH paper to check the pH. The target pH is 7.6.
Add 1N NaOH as needed in small (5-10 μl) increments, checking the vial and the pH each time, until all of the acid has dissolved and the pH=7.6.
- Storage:
- Ibotenic acid should be divided into 20-50 μl aliquots in microcentrifuge tubes and stored covered (i.e., wrap aliquots in foil) at −80° C.
- When an aliquot is removed to be used for a set of surgeries, it should be kept on ice and protected from light.
COMMENTARY
Background Information
The complexity of clinical schizophrenia has presented a challenge to the development of useful animal models. It is unrealistic to expect that an animal model can reproduce every clinical symptom of a given disorder, but the NVHL model possesses several advantages that make it an attractive manipulation for modeling certain aspects of schizophrenia (Tseng et al., 2009). Importantly, it is a neurodevelopmental manipulation, now considered to be a primary feature of schizophrenia (Lewis and Levitt, 2002). It induces damage that leads not simply to localized cell loss but to disorganization of forebrain pathways and downstream targets, which is more likely to model the disruption of neural circuits in clinical schizophrenia (i.e., patients with schizophrenia do not simply have lesions in the hippocampus). Finally, many of the behavioral abnormalities associated with the NVHL model do not manifest until late adolescence or adulthood, paralleling the clinical presentation of schizophrenia.
Many other animal models of schizophrenia exist; one estimate puts the number at 150 (http://www.schizophreniaforum.org/res/models/default.asp). The NVHL model is, at the least, competitive with many of these other models in terms of its construct validity. It may be considered more robust than models which do not incorporate a developmental component, for example, pharmacological models based on dopamine hyperactivity or glutamate insufficiency (O'Donnell, 2012; Tseng et al., 2009). From a translational perspective, a major weakness of the NVHL model is its failure to incorporate a genetic component, which is clearly a factor in the etiology of clinical schizophrenia. However, the NVHL model remains a valid approach to modeling a subset of the neurobiological, behavioral, and cognitive aspects of schizophrenia.
Critical Parameters and Troubleshooting
Positioning of the animal
Perhaps the most critical aspect of the surgical procedure is the placement of the adhesive tape to secure the pup to the platform. The pup must be centered, level, and held secure by the tape. The first piece needs to be placed securely and with enough force to slightly compress the pup's torso. When placing the second piece of tape, after the skull has been incised, it can be helpful to gently pull the head forward with one hand while holding the incision open with the other hand, to “stretch” the head out and ensure that the skull is level and straight. Once the tape pieces are in place, and especially after bregma has been located, take care to avoid jostling the pup out of place under the tape. Keep the Styrofoam platform dry to improve adhesion of the tape. It is helpful to pat the pup dry after removing it from the ice, prior to placing it on the platform, to aid in tape adhesion.
Since even the best tape placement will not keep the pup completely secure, another critical aspect of the surgery is to hold the head steady when inserting and removing the cannula. Hold the head firmly with the thumb and forefinger placed approximately over the ears. Note that the incision can also be held open at the same time, although it is preferred if the incision is wide enough (and centered) to stay open on its own, to avoid having the sides of the scalp push on the injection cannula when it is in place.
Stereotaxic coordinates
It takes some practice to visualize bregma on the neonate skull, and there is variation among strains; e.g., bregma is typically more difficult to visualize on Long-Evans pups than on Sprague-Dawley. Unlike in the adult rat, the skull sutures and bregma do not become more prominent as time passes, because of the lack of blood flow, and the opposite may even be true; thus it is advised to locate bregma quickly after the incision. The sagittal sinus is easily observed and may be used as a proxy for the sagittal suture. The coronal suture is usually visualized as a thick white band that is often difficult to distinguish from the underlying skull; notice that it is barely visible in Figures 1 and 2. Experiment with different lighting options and angles to optimize the quick location of bregma after the scalp is incised.
Although the coordinates given in this protocol have been successfully used by this author and others for many years (Chambers and Lipska, 2011), it is essential to perform some pilot experiments to confirm the coordinates by a new investigator, under different laboratory conditions, with different equipment, etc. Use dye (e.g., methylene blue) instead of toxin/aCSF, and remove the brains immediately upon finishing the surgery, i.e., before resuscitating. Since the brains are taken so close in time to the surgery, mechanical damage from the injection cannula may also be visible upon histological preparation (such mechanical damage is no longer visible in histology from the adult brain).
Premature loss of anesthesia
Sometimes pups begin to show signs of awakening (limb movement, attempts at respiration) before the surgical procedure has finished, particularly with new/inexperienced surgeons who may still be somewhat slow. Use an ice-filled laboratory glove placed on the pup's torso to maintain hypothermia as needed during the surgery. Remove the supplemental ice as soon as possible, and do not leave it on the pup for more than 5 minutes. If premature awakening is happening consistently, consider lengthening the initial time that pups are placed on ice before beginning the procedure.
Mortality
The NVHL manipulation tends to have a higher mortality rate than adult stereotaxic surgery. In this author's experience, losses of 20-30% of attempted lesions are not uncommon. Assuming that all equipment is clean and functional, and solutions are not contaminated, there are two typical reasons that animals may not survive the surgery.
Animals are overly hypothermic. If lesion and sham animals are being lost at equal rates, this is the most likely explanation. Similarly, if pups expire without showing any behavioral signs of recovery at the conclusion of surgery, it is most likely due to extreme hypothermia. Decrease the time that animals are left on ice before the first checkpoint, and limit the use of supplemental ice to only when it is absolutely necessary.
- The toxin is acting outside of the hippocampus; e.g., it has possibly entered the ventricular system. If lesion animals are exclusively being lost at rates exceeding 20-30%, this is the most likely explanation. Also, if pups show some signs of waking before dying, including limb seizures and/or rigidity, it is most likely due to the toxin. There are several potential strategies to address this mortality.
- Ensure that coordinates are correct, through use of pilot surgeries and immediate brain extraction and histology (see above).
- Ensure that the pup's head is placed level and has not moved during the surgery.
- Allow more time between surgeries to attend more closely to each pup during the immediate post-operative period.
- Replace the aCSF and toxin with new aliquots.
- Replace the tubing, injection cannula and depth marker.
Anticipated Results
Histology
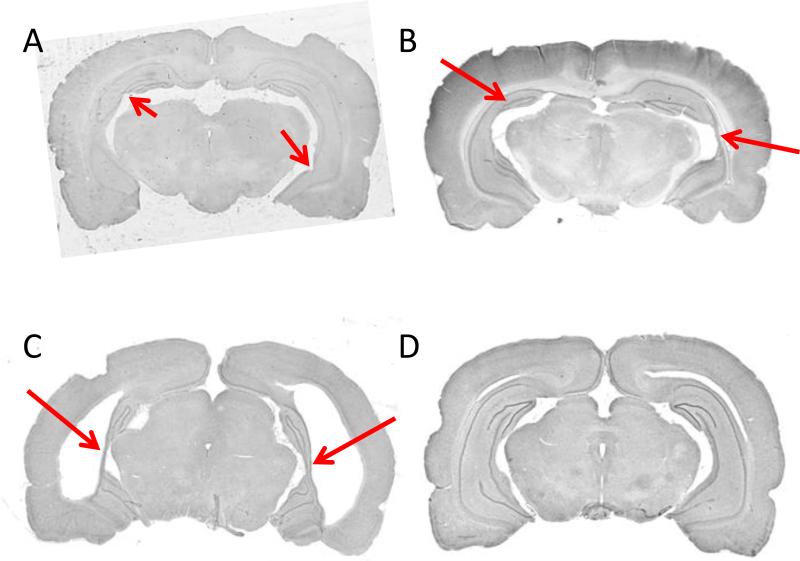
Like any stereotaxic surgical procedure, the NVHL manipulation will likely not yield 100% successful lesions. A realistic expectation is that 70-80% of attempted lesions will be successful (i.e., show some degree of cell loss or disorganization). Histological processing of neural tissue is essential at the conclusion of the experiment to confirm that damage and/or disorganization both exists in and is confined to the hippocampus. Representative examples of damage are shown in Figure 3; as depicted, damage may range from minimal to extensive. A simple rating system may be used to score lesion size in each hemisphere: 0 = no damage; 1 = slight cell loss or disorganization; 2 = moderate cell loss or disorganization; 3 = extensive cell loss or disorganization (Sams-Dodd et al., 1997).
Figure 3.
Histological (Nissl-stained) examples of NVHL (A-C) and sham (D) brains from adult animals. A, NVHL brain with minimal cell loss and disorganization, indicated by arrows. Anterior-posterior (AP) level is approximately −4.5 mm relative to bregma (Paxinos and Watson, 1988). B, NVHL brain with moderate cell loss and disorganization, indicated by arrows. Approximate AP level, −5.3 mm. C, NVHL brain with extensive cell loss and disorganization, indicated by arrows. Note the enlarged ventricles and slight lateral cortical thinning. Approximate AP level, −5.6 mm. D, Sham-treated brain showing an intact hippocampus. Approximate AP level, −5.6 mm.
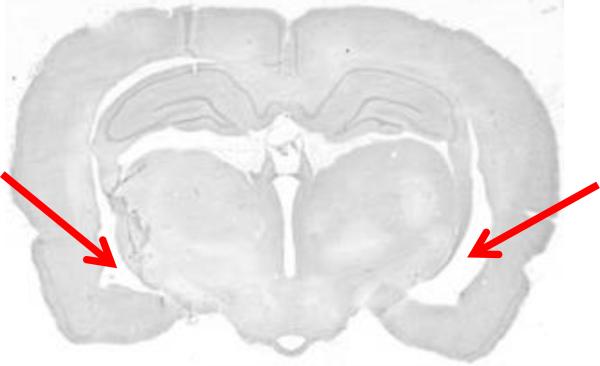
Figure 4 shows an example of an intact hippocampus that may be mistaken for a lesion in a preliminary examination. The arrows do not show an area of cell loss; rather, this section is just anterior to the level where the ventral and dorsal aspects of the hippocampus join (the joining itself can be seen on the left in Figure 3A), and thus the region that appears to include an enlarged ventricle is actually normal. Take care not to mistake this normal, more anterior section for the presence of a lesion, which typically appears more posteriorly as in Figures 3C and 3D.
Figure 4.
Nissl-stained example of a sham-treated brain at a more anterior level (approximate AP = −3.8 mm) that may be mistaken for a lesion. This level is just anterior to the juncture of the dorsal and ventral aspects of the hippocampus; at this level, the lateral ventricle is normally enlarged (arrows). This cavity should not be mistaken for a lesion.
Behavior
There are several relatively simple behavioral assays that show reliable disruptions in NVHL animals, and thus may be used as positive controls in experiments investigating novel behavioral outcomes. Representative data from two of these are presented below.
Prepulse inhibition (PPI)
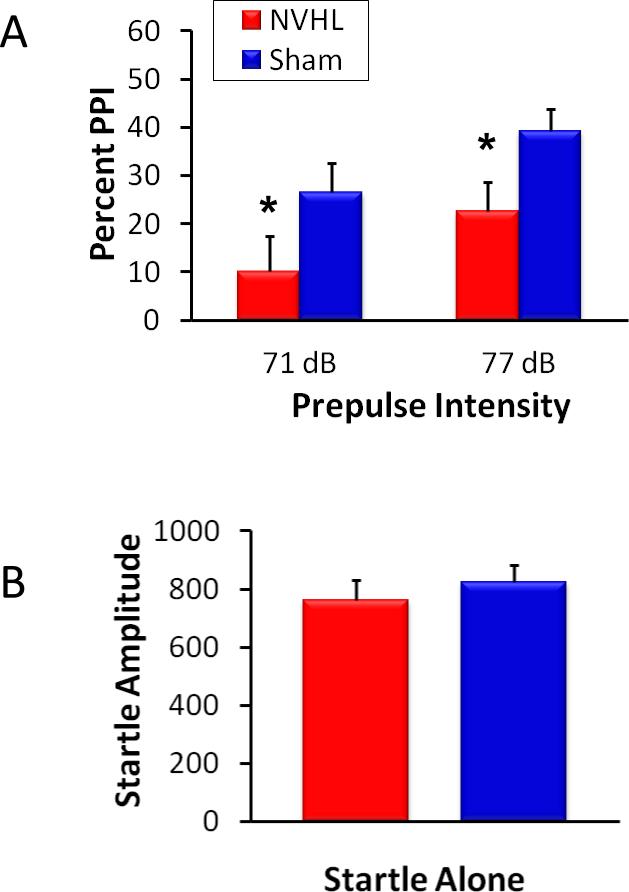
PPI is a measure of sensorimotor gating and is consistently disrupted in NVHL animals (Lipska et al., 1995), as it is in patients with schizophrenia (Swerdlow et al., 1994). Use of the acoustic startle response is the easiest way to demonstrate PPI deficits. Representative data are shown in Figure 5A; NVHL animals typically display a reduction in the percentage of PPI, which represents a failure of the prepulse to inhibit the startle response. The amplitude of the startle response itself is typically unaffected (Lipska et al., 1995), as shown in Figure 5B.
Figure 5.
Prepulse inhibition (PPI) of the acoustic startle response is disrupted in NVHL animals. A, NVHL animals (n=21) showed a lower percent prepulse inhibition (extent to which the prepulse signal was able to inhibit the subsequent startle response to a 120 dB startle stimulus occurring 100 ms later) than sham-treated animals (n=27). This NVHL deficit was present across two different prepulse intensities, as shown. B, The acoustic startle response itself (startle to the 120 dB stimulus alone) was unchanged in NVHL animals. Startle amplitude is depicted in arbitrary units. *p < .05, NVHL vs. sham.
Amphetamine-induced locomotion
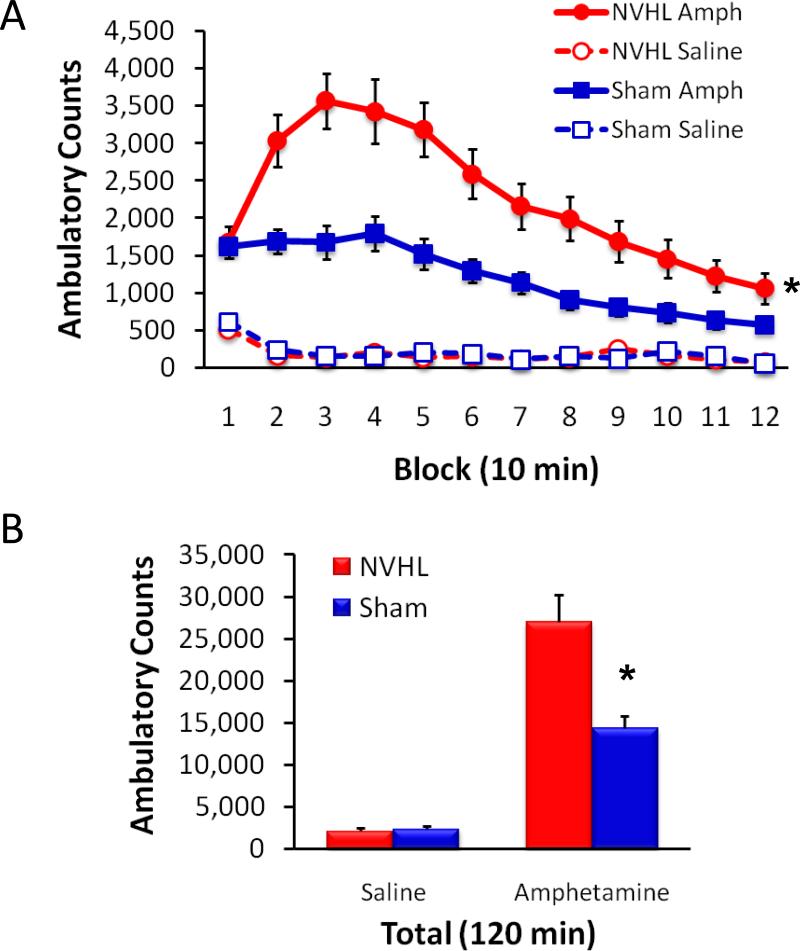
Hyper-responsiveness to amphetamine is also a hallmark of behavioral disruption among NVHL animals (Lipska et al., 1993) and is used as a species-specific manifestation of the hypersensitivity to dopaminergic agonists displayed by patients with schizophrenia (Lipska and Weinberger, 2000). The use of automated activity chambers makes this behavioral assay relatively easy to carry out (Lodge, 2013). As shown in Figure 6, NVHL animals typically display a robust and long-lasting augmentation of the normal increase in locomotor activity following a single, non-stereotypy-inducing dose of amphetamine.
Figure 6.
NVHL animals are hyper-responsive to amphetamine. A, Following a single injection of amphetamine (1.5 mg/kg, i.p.), NVHL animals (n=21) exhibited a pronounced, long-lasting increase in ambulatory counts compared to sham-treated animals (n=27). Open-field ambulation was measured in automated activity chambers for two hours following injection of amphetamine or saline, which was preceded by a 60 min acclimation period with no injection (data not shown). B, Total ambulatory counts over the two hour post-injection period. *p < .001, NVHL vs. sham.
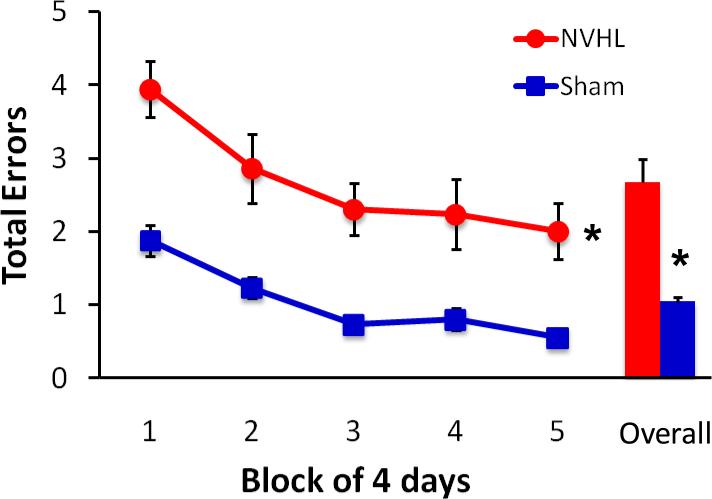
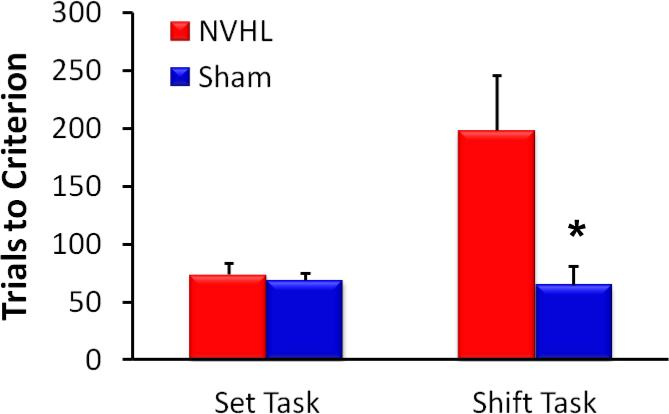
More complex cognitive behaviors are also reliably disrupted in the NVHL animal, including working memory and behavioral flexibility (Brady et al., 2010; Chambers et al., 1996; Gruber et al., 2010; Lipska et al., 2002; Marquis et al., 2008; Placek et al., 2013). Representative data showing sustained NVHL deficits in spatial memory in a radial arm maze are depicted in Figure 7 (Brady et al., 2010). In addition, while NVHL animals are not impaired at initially acquiring a conditional discrimination, following a rule shift they have difficulty suppressing the original response strategy and shifting to a new strategy (Placek et al., 2013), as shown in Figure 8.
Figure 7.
Animals with a NVHL manipulation are impaired at spatial working memory in a radial arm maze. NVHL animals (n=14) consistently made more errors than sham-treated animals (n=14) in the test phase of a delayed win-shift task, in which animals were required to visit previously unrewarded arms of a radial arm maze after a 30 min delay. Total errors declined for all animals across the 20 days of testing, but NVHL animals remained less accurate than sham animals (Overall = mean performance collapsed across blocks). Figure modified from (Brady et al., 2010). *p < .001, NVHL vs. sham.
Figure 8.
Behavioral flexibility (strategy shifting) is impaired in NVHL animals. NVHL animals (n=10) exhibited normal acquisition of a simple position-based discrimination rule (Set Task), but required significantly more trials than sham-treated animals (n=8) to successfully shift to a new visual-based discrimination rule (Shift Task) on the following day. Figure modified from (Placek et al., 2013). *p < .05, NVHL vs. sham.
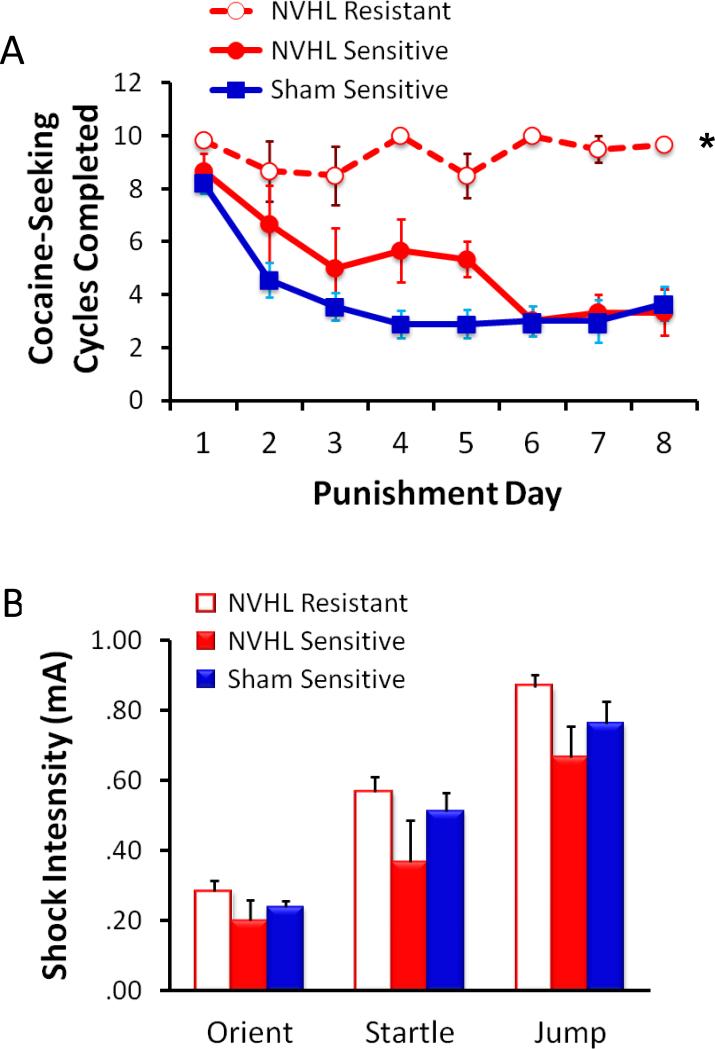
Finally, NVHL animals also show enhanced addictive behavior, leading to the suggestion that the NVHL manipulation may be especially useful as a model for dual diagnosis schizophrenia (Brady et al., 2008; Chambers et al., 2001; Chambers and Self, 2002). NVHL animals show escalated self-administration of cocaine, methamphetamine, and nicotine (Berg et al., 2014; Brady et al., 2008; Chambers and Self, 2002; Karlsson et al., 2013; Palacorolla et al., 2013). As shown in Figure 9, NVHL animals are also more resistant to punishment, continuing to seek cocaine despite footshock punishment (Palacorolla et al., 2013), an addiction phenotype that closely models compulsive drug-seeking behavior in humans (Pelloux et al., 2007).
Figure 9.
NVHL animals are vulnerable to compulsive drug-seeking (Palacorolla et al., 2013). A, NVHL animals (n=9) trained to self-administer cocaine on a seeking-taking cycle were dramatically more likely to be resistant to punishment (footshock delivered instead of cocaine) than sham-treated animals (n=9). Over eight days of being punished for cocaine-seeking, six of nine NVHL animals continued to seek cocaine at near-maximal levels (Resistant phenotype), while all nine sham-treated animals quickly attenuated their cocaine-seeking (Sensitive phenotype). B, The differences in sensitivity to punishment were not explained by group differences in sensitivity to unconditioned shock. Thresholds to orient, startle, and jump did not differ among the three lesion/phenotype groups. *p < .001, Group by Day interaction.
Time Considerations
The NVHL surgical manipulation itself is rapid. After the ibotenic acid and modified stereotaxic frame are prepared, each surgical procedure takes only about 15 min per animal. Of course, there is time before and after for anesthesia induction and recovery, respectively, but animals can be staggered (i.e., place the next animal on ice while one is finishing up the surgical procedure) to maximize time efficiency. With a single stereotaxic frame, where lesion (ibotenic acid) surgeries and sham (aCSF) surgeries are completed in two consecutive batches, a set of about 8-10 surgeries can easily be completed in one day.
A set of surgeries may be completed even more efficiently if two stereotaxic frames and injection cannula assemblies are used, with one devoted to lesion surgeries and one to sham surgeries. If two setups are used in this way, which is recommended, stagger start times so that two animals are undergoing the surgical protocol concurrently with minimal overlap of active procedures. With two stereotaxic frames, a set of about 15-20 surgeries is easily doable in a single day.
It is important to note that overall, substantial long-range planning is necessary, especially if the animals are not to be tested until adulthood (8 weeks). In this case, timed pregnant dams must be ordered about 10 weeks prior to the desired testing time. Once the pregnant dams are in the colony, close watch must be kept so that the date of birth (PD 0) is accurately recorded.
Significance Statement.
The use of animal models has been instrumental in advancing the understanding of psychiatric disease. Although no animal model can fully reproduce the entire spectrum of symptoms of a human psychiatric disorder, such models are useful in investigating particular behavioral and/or neurobiological components of a disease state, as well as evaluating treatment efficacy. This protocol describes a surgical rodent model of schizophrenia that reproduces many of the abnormal behaviors and neural disruptions seen in patients with schizophrenia. Importantly, this model has a developmental component, adding to its construct validity given converging evidence suggesting that schizophrenia is a neurodevelopmental disorder.
ACKNOWLEDGEMENT
Thanks and appreciation are due to Barbara Lipska and Andy Chambers for the development and refinement of the techniques described here (Chambers and Lipska, 2011), and to Patricio O'Donnell for providing a supportive laboratory environment. Portions of the research described in this article were supported by the National Institutes of Health (DA14020 to P. O'Donnell).
Footnotes
KEY REFERENCE (optional)
None
INTERNET RESOURCES (optional)
None
LITERATURE CITED
- Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addict Biol. 2014;19:1020–1031. doi: 10.1111/adb.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, McCallum SE, Glick SD, O'Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology (Berl) 2008;200:205–215. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, Saul RD, Wiest MK. Selective deficits in spatial working memory in the neonatal ventral hippocampal lesion rat model of schizophrenia. Neuropharmacology. 2010;59:605–611. doi: 10.1016/j.neuropharm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol.Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Lipska BK. A method to the madness: producing the neonatal ventral hippocampal lesion rat model of schizophrenia. In: O'Donnell P, editor. Animal Models of Schizophrenia and Related Disorders, Neuromethods. Vol. 59. Humana Press; New York: 2011. pp. 1–24. [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danneman PJ, Mandrell TD. Evaluation of five agents/methods for anesthesia of neonatal rats. Lab Anim Sci. 1997;47:386–395. [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O'Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Kircher DM, Shaham Y, O'Donnell P. Exaggerated cue-induced reinstatement of cocaine seeking but not incubation of cocaine craving in a developmental rat model of schizophrenia. Psychopharmacology (Berl) 2013;226:45–51. doi: 10.1007/s00213-012-2882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu.Rev.Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. Subchronic treatment with haloperidol and clozapine in rats with neonatal excitotoxic hippocampal damage. Neuropsychopharmacology. 1994;10:199–205. doi: 10.1038/npp.1994.22. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Lodge DJ. The MAM rodent model of schizophrenia. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley ... [et al.] 2013 doi: 10.1002/0471142301.ns0943s63. Chapter 9:Unit9 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis JP, Goulet S, Dore FY. Neonatal ventral hippocampus lesions disrupt extra-dimensional shift and alter dendritic spine density in the medial prefrontal cortex of juvenile rats. Neurobiol Learn Mem. 2008;90:339–346. doi: 10.1016/j.nlm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacology & therapeutics. 2012;133:19–25. doi: 10.1016/j.pharmthera.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Palacorolla HL, Gyawali U, Jarrin SE, Smith NK, Brady AM. Compulsive cocaine-seeking in the neonatal ventral hippocampal lesion model of schizophrenia. Society for Neuroscience 2013 Abstract Viewer/Itinerary Planner. 2013:151.103. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. Academic Press; San Diego: 1988. [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Placek K, Dippel WC, Jones S, Brady AM. Impairments in set-shifting but not reversal learning in the neonatal ventral hippocampal lesion model of schizophrenia: Further evidence for medial prefrontal deficits. Behav Brain Res. 2013;256C:405–413. doi: 10.1016/j.bbr.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch.Gen.Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behavioral Brain Research. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol.Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]