Abstract
Heterotrimeric guanine nucleotide binding proteins (G proteins) transduce signals from cell-surface receptors to intracellular effector proteins. Two forms of stimulatory G protein (Gs) alpha-like subunit have been described in Drosophila melanogaster. To examine the function of these subunits we have used vaccinia virus vectors to express both proteins in cyc- cells, a murine S49 cell line deficient for Gs alpha activity. Receptor-independent activation of each Drosophila Gs alpha has demonstrated that both forms are capable of activating mammalian adenylyl cyclase and thus have the activity expected of stimulatory G proteins. However, the Drosophila Gs alpha subunits interact poorly with mammalian Gs-coupled receptors. These observations have helped to identify a region of high variability in Gs alpha proteins that may be important for receptor interactions.
Full text
PDF

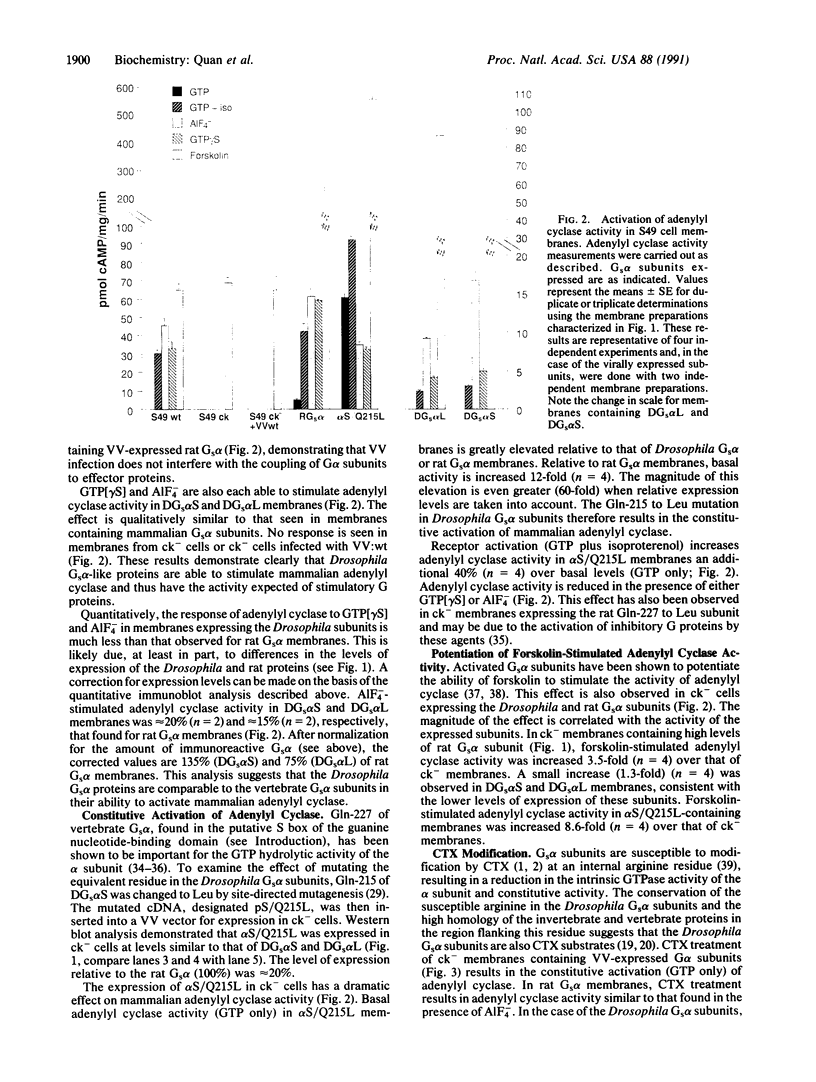

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bender J. L., Neer E. J. Properties of the adenylate cyclase catalytic unit from caudate nucleus. J Biol Chem. 1983 Feb 25;258(4):2432–2439. [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J. 1987 Oct;6(10):2907–2913. doi: 10.1002/j.1460-2075.1987.tb02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Graziano M. P., Gilman A. G. Synthesis in Escherichia coli of GTPase-deficient mutants of Gs alpha. J Biol Chem. 1989 Sep 15;264(26):15475–15482. [PubMed] [Google Scholar]
- Green D. A., Clark R. B. Direct evidence for the role of the coupling proteins in forskolin activation of adenylate cyclase. J Cyclic Nucleotide Res. 1982;8(5):337–346. [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Harris B. A., Robishaw J. D., Mumby S. M., Gilman A. G. Molecular cloning of complementary DNA for the alpha subunit of the G protein that stimulates adenylate cyclase. Science. 1985 Sep 20;229(4719):1274–1277. doi: 10.1126/science.3839937. [DOI] [PubMed] [Google Scholar]
- Holbrook S. R., Kim S. H. Molecular model of the G protein alpha subunit based on the crystal structure of the HRAS protein. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1751–1755. doi: 10.1073/pnas.86.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Thomas G., Herbert E., Franke C. A. Use of vaccinia virus as a neuropeptide expression vector. Methods Enzymol. 1986;124:295–309. doi: 10.1016/0076-6879(86)24022-7. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Masters S. B., Bourne H. R., Reed R. R. Biochemical characterization of three stimulatory GTP-binding proteins. The large and small forms of Gs and the olfactory-specific G-protein, Golf. J Biol Chem. 1990 Feb 15;265(5):2671–2676. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989 May 19;244(4906):790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Masters S. B., Miller R. T., Chi M. H., Chang F. H., Beiderman B., Lopez N. G., Bourne H. R. Mutations in the GTP-binding site of GS alpha alter stimulation of adenylyl cyclase. J Biol Chem. 1989 Sep 15;264(26):15467–15474. [PubMed] [Google Scholar]
- Masters S. B., Stroud R. M., Bourne H. R. Family of G protein alpha chains: amphipathic analysis and predicted structure of functional domains. Protein Eng. 1986 Oct-Nov;1(1):47–54. [PubMed] [Google Scholar]
- Masters S. B., Sullivan K. A., Miller R. T., Beiderman B., Lopez N. G., Ramachandran J., Bourne H. R. Carboxyl terminal domain of Gs alpha specifies coupling of receptors to stimulation of adenylyl cyclase. Science. 1988 Jul 22;241(4864):448–451. doi: 10.1126/science.2899356. [DOI] [PubMed] [Google Scholar]
- Navon S. E., Fung B. K. Characterization of transducin from bovine retinal rod outer segments. Participation of the amino-terminal region of T alpha in subunit interaction. J Biol Chem. 1987 Nov 15;262(32):15746–15751. [PubMed] [Google Scholar]
- Neer E. J., Pulsifer L., Wolf L. G. The amino terminus of G protein alpha subunits is required for interaction with beta gamma. J Biol Chem. 1988 Jun 25;263(18):8996–8970. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter T., King S. M., Witman G. B. A two-step procedure for efficient electrotransfer of both high-molecular-weight (greater than 400,000) and low-molecular-weight (less than 20,000) proteins. Anal Biochem. 1987 May 1;162(2):370–377. doi: 10.1016/0003-2697(87)90406-4. [DOI] [PubMed] [Google Scholar]
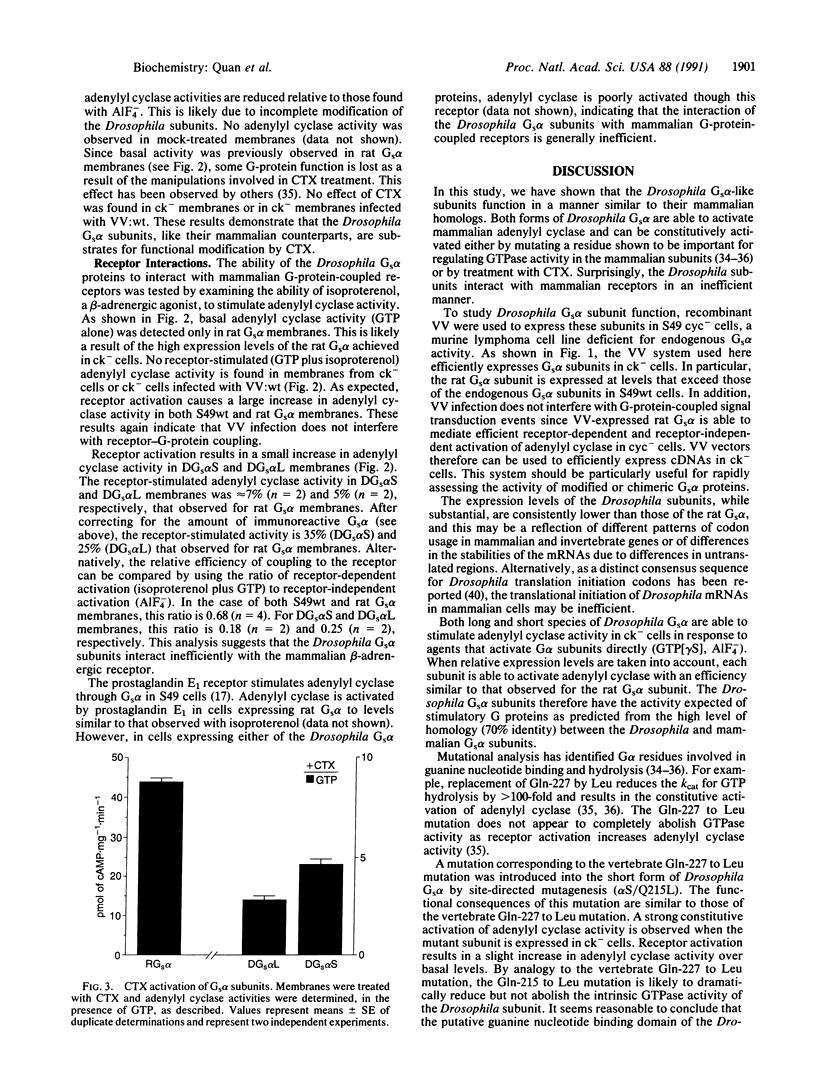
- Quan F., Forte M. A. Two forms of Drosophila melanogaster Gs alpha are produced by alternate splicing involving an unusual splice site. Mol Cell Biol. 1990 Mar;10(3):910–917. doi: 10.1128/mcb.10.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan F., Wolfgang W. J., Forte M. A. The Drosophila gene coding for the alpha subunit of a stimulatory G protein is preferentially expressed in the nervous system. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4321–4325. doi: 10.1073/pnas.86.11.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Franke C. A., Strauss J. H., Hruby D. E. Expression of Sindbis virus structural proteins via recombinant vaccinia virus: synthesis, processing, and incorporation into mature Sindbis virions. J Virol. 1985 Oct;56(1):227–239. doi: 10.1128/jvi.56.1.227-239.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M. R., Bourne H. R. Novel S49 lymphoma variants with aberrant cyclic AMP metabolism. Mol Pharmacol. 1981 Jan;19(1):109–116. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Liao Y. C., Alborzi A., Beiderman B., Chang F. H., Masters S. B., Levinson A. D., Bourne H. R. Inhibitory and stimulatory G proteins of adenylate cyclase: cDNA and amino acid sequences of the alpha chains. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. A., Miller R. T., Masters S. B., Beiderman B., Heideman W., Bourne H. R. Identification of receptor contact site involved in receptor-G protein coupling. Nature. 1987 Dec 24;330(6150):758–760. doi: 10.1038/330758a0. [DOI] [PubMed] [Google Scholar]
- Ui M., Katada T., Murayama T., Kurose H., Yajima M., Tamura M., Nakamura T., Nogimori K. Islet-activating protein, pertussis toxin: a specific uncoupler of receptor-mediated inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:145–151. [PubMed] [Google Scholar]
- Van Dop C., Tsubokawa M., Bourne H. R., Ramachandran J. Amino acid sequence of retinal transducin at the site ADP-ribosylated by cholera toxin. J Biol Chem. 1984 Jan 25;259(2):696–698. [PubMed] [Google Scholar]
- Van Dop C., Yamanaka G., Steinberg F., Sekura R. D., Manclark C. R., Stryer L., Bourne H. R. ADP-ribosylation of transducin by pertussis toxin blocks the light-stimulated hydrolysis of GTP and cGMP in retinal photoreceptors. J Biol Chem. 1984 Jan 10;259(1):23–26. [PubMed] [Google Scholar]
- Wolfgang W. J., Quan F., Goldsmith P., Unson C., Spiegel A., Forte M. Immunolocalization of G protein alpha-subunits in the Drosophila CNS. J Neurosci. 1990 Mar;10(3):1014–1024. doi: 10.1523/JNEUROSCI.10-03-01014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos A. M., Tong L., Milburn M. V., Matias P. M., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988 Feb 19;239(4842):888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]
- la Cour T. F., Nyborg J., Thirup S., Clark B. F. Structural details of the binding of guanosine diphosphate to elongation factor Tu from E. coli as studied by X-ray crystallography. EMBO J. 1985 Sep;4(9):2385–2388. doi: 10.1002/j.1460-2075.1985.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]