Abstract
Objective
The recent development of endotypes to categorize disease variants of chronic rhinosinusitis (CRS) reflects an evolving understanding of the various pathophysiologic and pathogenetic mechanisms that contribute to the clinical heterogeneity of CRS manifestations. This review highlights popular endotype‐based criteria used to define different CRS with nasal polyposis (CRSwNP) subtypes and further discusses the emerging therapeutic advances for each classificatory approach.
Data Sources
PubMed literature review.
Methods
A review of the current literature was conducted to determine present‐day uses of immunologic and molecular profiles in the CRSwNP disease spectrum to identify specific endotypes.
Results
Four distinct but overlapping classification schemes have emerged to define endotypes within the CRSwNP phenotype: 1) type 2 cytokine‐based approach, 2) eosinophil‐based approach, 3) immunoglobulin (Ig)E‐based approach, and 4) cysteinyl based approach. The identification of key inflammatory biomarkers related to these CRSwNP endotypes has broadened the classification of CRS beyond common phenotypic expressions. Furthermore, CRSwNP endotypes may improve the selection of CRSwNP patients who are suitable candidates for biomarker‐specific treatment options, such as anti‐interleukin‐5; anti‐IgE; and platelet‐directed therapies.
Conclusion
Chronic rhinosinusitis endotyping with key biomarker patterns of inflammation allows for improved diagnostic and potentially therapeutic classifications of CRSwNP variants.
Keywords: Chronic rhinosinusitis, nasal polyps, endotypes, phenotypes, asthma, biologic therapy, molecular biomarkers, eosinophils
INTRODUCTION
Chronic rhinosinusitis (CRS) is a broad clinical syndrome that is defined by mucosal inflammation of the nose and paranasal sinuses. The inflammatory condition is commonly divided into two phenotypes based the presence or absence of nasal polyps: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). Current treatment regimens are based on this phenotypic classification. High‐level evidence supports the treatment of CRSwNP with intranasal steroids, oral steroids, and saline irrigations, while the common medical regimen recommended for CRSsNP includes intranasal steroids, saline irrigations, and macrolide antibiotics.1 However, about 38% to 51% of CRS patients still fail to respond to these recommended medical therapies.2, 3
The limitations of currently recommended pharmacotherapy highlight the clinical variability that characterizes CRS as a whole, even within the CRSwNP and CRSsNP phenotypes. Increasing evidence suggests the heterogeneity in CRS manifestations may be explained by a variety of disparate molecular and cellular pathways that result in the mucosal inflammation of CRS. The improved understandings of different pathophysiologic mechanisms in CRS have allowed for the identification of disease variants as endotypes.4 While by convention, CRS phenotyping differentiates disease variants with observable clinical features, endotyping relies on immunohistologic biomarkers involved in disease pathophysiology to create defined subtypes.5, 6 Compared to phenotyping, endotyping provides a more comprehensive approach to classify CRS variants because it emphasizes the upstream pathophysiologic factors that determine and influence the clinical manifestations of disease.
The current interest in CRS endotypes draws from prior research efforts in asthma, a similarly heterogeneous inflammatory disorder involving epithelium of the lower airway.7, 8 Over the last few years, the development of asthma endotypes based on the biologic mechanisms of inflammation have not only enhanced descriptive diagnostic schemes, but have also streamlined the application of targeted biologic treatments for patients with disease refractory to conventional therapy.7, 8 Biologic therapy, primarily through monoclonal antibodies, provides highly effective treatment alternatives for severe and resistant asthma due to the targeting of specific biomarkers and thus the underlying causes of inflammation in certain endotypes.9 The similarities in inflammatory mechanisms between asthma and CRS have thus raised the possibility of using biologic therapy for certain CRS endotypes.10, 11
The goal of this review is to highlight specific molecular and cellular biomarkers related to potential CRSwNP endotypes, as well as therapeutic opportnities for targeted treatment. Overall, we propose four distinct, but overlapping, classification schemes for identifying endotypes within the CRSwNP phenotype: 1) Type 2 cytokine based approach, 2) eosinophil‐mediated approach, 3) immunoglobulin (Ig)E‐based approach, and 4) cysteinyl leukotriene (CysLT)‐based approach.
Type 2 Cytokine‐based approach for endotype classification
CRSsNP and CRSwNP in the United States and Europe have historically been differentiated by distinct inflammatory cytokine profiles. A skewed type 1 inflammation, characterized by the presence of neutrophils, elevated interferon‐γ (IFN‐γ), and T‐helper 1 cells, is generally associated with CRSsNP, while approximately 80% to 85% of CRSwNP patients in the United States and Europe have shown a strong predilection for a skewed type 2 immune response. This type 2 inflammatory pattern is characterized by a high presence of eosinophils, mast cells, basophils, and T‐helper 2 (Th2) cells, and comorbid associations with asthma and atopy.12 Within the type 2 inflammatory milieu, multiple cytokines, including interleukin (IL)−5, IL‐4, and IL‐13, have also been found to drive the immunologic pathways central to CRSwNP pathophysiology. Figure 1 illustrates several identified pathways important in these type 2 immune responses.
Figure 1.
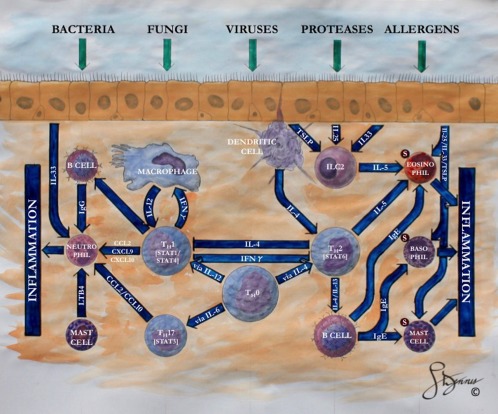
Highlighted cellular and molecular pathways in the type 1 and type 2 inflammatory cascades for patients with chronic rhinosinusitis. The typical chronic rhinosinusitis patients in North America and Europe follow a type 2 inflammatory response with prevalent eosinophilic mucin. Type 1 inflammation, classically see in Asian patients, manifests a predominately neutrophilic response. CCL = chemokine ligand; CXCL = chemokine ligand; ILC2 = type 2 innate lymphoid cell; S = Siglec‐8 receptor; TSLP = thymic stromal lymphopoietin; TH0 5 Undifferentiated T helper cell; TH1 5 T helper 1 cell; TH2 5 T helper 2 cell; TH17 5 T helper 17 cell; IFNγ 5 interferon gamma.
Released by type 2 innate lymphoid cells, Th2 cells, and mast cells, IL‐5 is a common cytokine that coordinates the local influx, maturation, and survival of eosinophils. The important role of IL‐5 in CRSwNP pathophysiology was demonstrated by Tomassen et al.,5 who correlated different phenotypic manifestations of CRS to various patterns of immune cytokines. In this cluster analysis, patients with high levels of IL‐5 had the highest prevalence of nasal polyposis and asthma. In contrast, patients with low IL‐5 consisted of primarily CRSsNP patients.5 Patients with moderate levels of IL‐5 exhibited variable expressions of nasal polyposis and asthma comorbidity. This study suggests that for diagnostic purposes, elevated IL‐5 in CRS universally indicates the presence of nasal polyposis, although nasal polyposis does not necessarily indicate an elevation of IL‐5.
The strong association between IL‐5 and CRSwNP has drawn attention to IL‐5 as a possible therapeutic target for biologic therapies used primarily in asthma.5 Studies in CRS have focused on two humanized anti‐IL‐5 antibodies: reslizumab and mepolizumab. A pilot study with reslizumab showed efficacy for CRSwNP patients with significant reductions in polyp size; a strong correlation between high IL‐5 levels in nasal secretions and a positive treatment response was also noted.13 Likewise, a second study on mepolizumab found that a majority of CRSwNP patients demonstrated significant decreases in both polyp size and Lund‐McKay scores.14 Notably, the effects of mepolizumab on polyp size reduction were sustained after a 1‐year interval. Additionally, benralizumab is an anti‐IL‐5 receptor monoclonal antibody that has not yet been studied in CRSwNP, although there have been promising results for eosinophilic asthma. In study patients, benralizumab was found to improve a disease‐specific quality‐of‐life score and decrease the number of asthmatic exacerbations.15
Like IL‐5, IL‐4 and IL‐13 are defining cytokines of the type 2 inflammatory milieu and share many overlapping functionalities, such as driving the differentiation of Th2 cells. As such, IL‐4 and IL‐13 both actively promote adaptive Th2 responses through the activation of B cells and the local production of immunoglobulins, especially IgE. In these patients, local IgE levels may be elevated without evidence of elevated systemic IgE levels.16 Given the importance of IL‐4 and IL‐13 in promoting local IgE synthesis and recruiting type 2 effector cells, dupilumab—a monoclonal antibody targeting a shared alpha subunit on the IL‐4 and IL‐13 receptors—has received attention for its theoretical ability to suppress both IL‐4 and IL‐13 downstream pathways.12 In CRSwNP patients, dupilumab has shown significant reductions in nasal polyp burden and Lund‐Mackay scores when compared to a placebo group. These effects have additionally been sustained for at least 2 months after treatment completion.
Over the last decade, epithelial cells have been recognized as an active component of the immune response. In addition to serving as a physical barrier between the environment and the underlying mucosa, epithelial cells are capable of responding to environmental triggers by releasing cytokines capable of coordinating type 2 inflammatory responses. These epithelial cell‐derived cytokines, which include IL‐25, IL‐33, and thymic stromal lymphopoietin, assist in activating the adaptive Th2 cascade, as well as stimulating basophils, mast cells, eosinophils, and type 2 innate lymphoid cells (ILC2s).17 Increased populations of ILC2s have been observed in CRSwNP versus CRSsNP. Due to the cytokine‐producing propensity of these cells, ILC2s are thought to be integral in the type 2 immune response.18 The importance of epithelial cell‐derived cytokines in type 2 inflammation suggests the potential targeting of these cytokines for biologic pharmacotherapy, but most ongoing clinical trials on biologic therapy targeting the epithelial cell‐derived cytokine pathways are directed toward asthma populations.
Eosinophil‐based approach for endotype classification
Another means of endotyping CRS patients is through the identification of predominant immune cells in the inflamed sinonasal mucosa. One such proposed categorization of CRSwNP inflammation has involved eosinophilic versus neutrophilic predominance. In Caucasian CRSwNP patients, the inflamed sinonasal mucosa is characterized by a prominence of eosinophils, whereas CRSwNP from East Asia it is associated with a neutrophilic predominance.19 Numerous potential molecular pathways may modulate eosinophil‐mediated CRSwNP (Fig. 1). The increasing use of eosinophilic enzymes as a surrogate marker for eosinophil count has increased the facility of using eosinophilic content to classify CRSwNP endotypes. In all, eosinophils express more than 30 cytokines and chemokines, which are rapidly released following cellular activation and lead to a unique inflammatory signature.20 Any of these eosinophil‐associated chemokines may thus serve as a sufficiently specific serum biomarker for endotyping CRSwNP, eliminating the current necessity of obtaining tissue for its diagnosis.
Eosinophilic mucin is strongly associated with the nasal polyposis in allergic fungal rhinosinusitis (AFRS), aspirin‐exacerbated respiratory disease (AERD), and not‐otherwise‐categorized eosinophilic rhinosinusitis (EMCRS). These phenotypes, however, vary in their patient demographics, comorbidity with asthma, and clinical responses to therapy. In a study that compared differential expressions of IL‐4, IL‐5, and IL‐13 between EMCRS and AERD, Steinke et al.20 found that EMCRS was defined by significantly elevated levels of IL‐5 and IL‐13, whereas AERD demonstrated a greater elevation of IL‐4. As a result, while an eosinophilic predilection is noted in several phenotypically distinct CRSwNP variants, additional molecular endotyping is necessary to further identify inflammatory patterns that more precisely differentiate these CRSwNP variants.
The role of fungi in the pathophysiology of eosinophil‐mediated CRSwNP remains controversial. The presence of fungi within sinonasal mucus is largely dependent on the techniques used for the isolation, treatment, and culture of the collected specimens. Alternatively, sensitization of the immune response to fungi has been illustrated in CRSwNP patients through a peripheral blood‐based assay that demonstrated fungi induce IL‐4 production.21, 22 In addition, other markers such as periostin, a proinflammatory mediator, have been found to be significantly elevated in AFRS patients.23 Therefore, various other biomarkers related to AFRS can be needed to further characterize eosinophil‐based CRSwNP should targeting eosinophils alone prove an insufficient treatment goal.
Application of biologic therapy targeting IL‐5 is relevant to eosinophil‐based CRSwNP due to the overlapping upstream inflammatory mechanisms. It is suspected that anti‐IL‐5 therapy will most significantly affect local eosinophil levels given its role in the infiltration, maturation, and stabilization of eosinophils. Another means of addressing the elevated eosinophils within the inflamed mucosa is by targeting eosinophils directly. Siglec‐8 is a receptor found predominately on the surface of type 2 immune cells, including eosinophils, mast cells, and basophils. Engaging the Siglec‐8 receptor has been shown to induce apoptosis of eosinophils and inhibition of mast cell degranulation. As such, ligands for Siglec‐8 have been suggested as a therapeutic strategy for suppressing sinonasal inflammation in eosinophil‐mediated CRSwNP.24
IgE‐based approach for endotype classification
Elevated levels of IgE are seen in all forms of CRSwNP, except AERD, and may thus serve as a broad approach to endotype CRSwNP.25 In particular, local IgE may be a stronger driver of disease pathophysiology than systemic IgE; a study by Kim et al. found no correlation between systemic IgE levels and the presence of eosinophils in polyp tissue.26 Other studies attribute local sinonasal production of IgE as one of many contributing drivers of nasal polyp development and may signify a greater prevalence of comorbid asthma.27 Interestingly, the production of Staphylococcus aureus enterotoxin‐specific IgE has been found to be correlated with some of the highest local concentrations of IgE and asthma prevalence.5, 19, 28
Due to the high mucosal IgE concentrations present in nasal polyposis, a targeted approach to IgE may be appropriate. Omalizumab, a monoclonal antibody to free IgE, has been approved for the treatment of severe allergic asthma. It has also been investigated in multiple randomized control trials for CRSwNP with comorbid asthma in allergic and nonallergic patients.12 The most recent study showed that omalizumab significantly decreased total nasal polyp score and sinus opacification on computed tomography scan, and improved nasal symptoms in both allergic and nonallergic subjects,28 thus supporting the role of local IgE in CRSwNP. An earlier study that included CRSwNP and CRSsNP found no significant improvement in outcomes with omalizumab versus placebo.29 The conflicting results may suggest variability in local IgE levels within CRS subtypes.12
Due to the relatively localized nature of most CRS disease and topical accessibility, locally administered therapies would be preferable to decrease systemic risks. One possible target is GATA‐3, which is the transcription factor controlling the production of IL‐4, IL‐5, and IL‐13 in Th2 cells.12 Because GATA‐3 is overexpressed in patients with asthma, nasal polyps, and atopic eczema, inhibition of GATA‐3 has the potential to greatly reduce the Th2 burden. A GATA‐3 DNAzyme applied through inhalation or spray is currently being investigated. Initial studies have shown mucosal lymphocyte uptake and decreased GATA‐3 RNA, which have led to decreased IL‐5 production.12
CysLT‐based approach for endotype classification
AERD presents a unique category within the CRSwNP phenotype due to its inherent association with two other comorbid features: 1) asthma, and 2) intolerance to aspirin and other medications that inhibit the cyclooxygenase (COX)−1 enzyme. From a clinical standpoint, AERD accounts for 9.7% of all CRSwNP patients1 and also represents a more severe and recalcitrant form of disease involving both the upper and lower airways. The clinical manifestations of CRSwNP and asthma in AERD typically present during the third or fourth decade of life, which contrasts with the early childhood onset of CRSwNP and asthma in aspirin‐tolerant patients.20, 25 Additionally, AERD tends to occur more commonly in patients who do not demonstrate an atopic history and are female.30
From a pathophysiologic standpoint, AERD has been linked to enzymatic defects in eicosanoid metabolism, including a functional reduction of COX enzymes and an upregulation of the 5‐lipoxygenase and leukotriene C4 (LTC4) synthase pathways. This metabolic imbalance results in a decreased production of anti‐inflammatory prostaglandin E2, whereas the proinflammatory CysLT produced from the 5‐lipoxygenase and LTC4 synthase pathways is markedly elevated.25 Elevated levels of CysLT are attributed to the downstream activation of important effector cells, including eosinophils and mast cells, which stimulate the inflammatory response within the sinonasal and respiratory mucosa. Other cytokines composing the inflammatory milieu of AERD include IL‐4, IL‐33, and IFN‐γ, thus demonstrating mechanistic overlap of AERD with CRSwNP in aspirin‐tolerant patients.20, 30
The significance of CysLT in the pathophysiology of AERD and possibly other CRSwNP patients, highlights its use as a biomarker for a distict CRS endotype.25, 31 Because CysLT is metabolized and excreted through the urine, the 24‐hour urinary measurement of LTC4 has been suggested as a means to identify CRSwNP patients who have the AERD variant.32 The classification of CysLT‐mediated CRS may then provide a guide toward therapy utilizing leukotriene modifiers and aspirin desensitization.20 Two common groups of leukotriene modifiers have included leukotriene receptor antagonists (i.e., montelukast and zafirlukast) and a 5‐lipoxygenase inhibitor (i.e., zileuton), both of which have shown efficacy in improving pulmonary function, stabilizing nasal polyposis, and alleviating quality‐of‐life in AERD patients.33 Aspirin desensitization has proven an effective treatment option in that patients undergoing long‐term treatment have shown a downregulation of CysLT and IL‐4 levels, with concurrent improvements in nasal and pulmonary symptoms.34 Aspirin desensitization, however, is associated with a 15% to 50% dropout rate due to adverse gastrointestinal effects and the need for specialist availability.25
CysLT‐mediated CRS may further benefit from another targeted treatment modality: platelet‐targeted therapy. Recent research has found that activated platelets stimulate the inflammatory state in AERD patients by forming aggregates with circulating leukocytes and recruiting the platelet‐adherent leukocytes to extravascular tissue.35 High levels of platelet‐adherent leukocytes, furthermore, are increasingly recognized as inducers of LTC4 synthase expression in CysLT‐mediated CRS.35, 36 The recent observations that activated platelets may strongly enhance the generation of CysLT in AERD have resulted in several clinical trials that are evaluating the efficacy of platelet‐targeted therapies in appropriate patients. Current therapies under investigation include prasugrel and ifetroban, which selectively inhibit the purinergic receptors P2Y12 and T prostanoid receptors, respectively.
CONCLUSION
An improved understanding of the different pathophysiologic pathways of CRS has increasingly clarified the disease's varied phenotypic expressions. Current attempts to endotype CRSwNP variants are based on these distinctive pathophysiologic pathways and have enhanced the precision of diagnostic descriptors for CRSwNP. Type 2 cytokine, eosinophils, IgE, and CysLT provide the basis of four popular but overlapping methods to classify endotypes within the CRSwNP phenotypes. The use of specific inflammatory biomarkers to endotype different CRSwNP variants furthermore highlights the potential opportunities for the development and application of biomarker‐directed biologic therapies in these well‐defined CRSwNP populations.
Acknowledgment
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Science or the National Institutes of Health.
Financial Disclosure: a.l. is supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Science. The Department receives research funding from Intersect ENT, Allakos, Amgen, ENTvantageDx, and Otonomy. A.L. serves as a consultant/scientific advisor for 480 Biomedical, Aerin Medical, ENTvantageDx, Laurimed, and Medtronic.
BIBLIOGRAPHY
- 1. Orlandi, R.R. , Kingdom T.T., and Hwang P.H., International Consensus Statement on Allergy and Rhinology: Rhinosinusitis Executive Summary. Int Forum Allergy Rhinol, 2016. 6 Suppl 1: p. S3–S21. [DOI] [PubMed] [Google Scholar]
- 2. Lal, D. , Scianna J.M., and Stankiewicz J.A., Efficacy of targeted medical therapy in chronic rhinosinusitis, and predictors of failure. Am J Rhinol Allergy, 2009. 23(4): p. 396–400. [DOI] [PubMed] [Google Scholar]
- 3. Baguley, C. , et al., The fate of chronic rhinosinusitis sufferers after maximal medical therapy. Int Forum Allergy Rhinol, 2014. 4(7): p. 525–32. [DOI] [PubMed] [Google Scholar]
- 4. Akdis, C.A. , et al., Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol, 2013. 131(6): p. 1479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tomassen, P. , et al., Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol, 2016. 137(5): p. 1449–1456. [DOI] [PubMed] [Google Scholar]
- 6. Hamilos, D.L. , Chronic rhinosinusitis endotyping: Sharpening the focus on inflammation. J Allergy Clin Immunol, 2016. 137(5): p. 1457–1459. [DOI] [PubMed] [Google Scholar]
- 7. Lotvall, J. , et al., Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol, 2011. 127(2): p. 355–60. [DOI] [PubMed] [Google Scholar]
- 8. Wenzel, S. , Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy, 2012. 42(5): p. 650–8. [DOI] [PubMed] [Google Scholar]
- 9. Fajt, M.L. and Wenzel S.E., Biologic therapy in asthma: entering the new age of personalized medicine. J Asthma, 2014. 51(7): p. 669–76. [DOI] [PubMed] [Google Scholar]
- 10. Lam, K. , Kern R.C., and Luong A., Is there a future for biologics in the management of chronic rhinosinusitis? Int Forum Allergy & Rhinol, 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pauwels, B. , Jonstam K., and Bachert C., Emerging biologics for the treatment of chronic rhinosinusitis. Expert Rev Clin Immunol, 2015. 11(3): p. 349–61. [DOI] [PubMed] [Google Scholar]
- 12. Bachert, C. , Zhang L., and Gevaert P., Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol, 2015. 136(6): p. 1431–40. [DOI] [PubMed] [Google Scholar]
- 13. Gevaert, P. , et al., Nasal IL‐5 levels determine the response to anti‐IL‐5 treatment in patients with nasal polyps. J Allergy Clin Immunol, 2006. 118(5): p. 1133–41. [DOI] [PubMed] [Google Scholar]
- 14. Gevaert, P. , et al., Mepolizumab, a humanized anti‐IL‐5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol, 2011. 128(5): p. 989–95 e1–8. [DOI] [PubMed] [Google Scholar]
- 15. Castro, M. , et al., Benralizumab, an anti‐interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose‐ranging study. Lancet Respir Med, 2014. 2(11): p. 879–90. [DOI] [PubMed] [Google Scholar]
- 16. Bakhshaee, M. , et al., The presence of fungal‐specific IgE in serum and sinonasal tissue among patients with sinonasal polyposis. Eur Arch Otorhinolaryngol, 2014. 271(11): p. 2871–5. [DOI] [PubMed] [Google Scholar]
- 17. Matsushita, K. , et al., Proallergic cytokines and group 2 innate lymphoid cells in allergic nasal diseases. Allergol Int, 2015. 64(3): p. 235–40. [DOI] [PubMed] [Google Scholar]
- 18. Shaw, J.L. , et al., IL‐33‐responsive innate lymphoid cells are an important source of IL‐13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med, 2013. 188(4): p. 432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ba, L. , et al., The association between bacterial colonization and inflammatory pattern in Chinese chronic rhinosinusitis patients with nasal polyps. Allergy, 2011. 66(10): p. 1296–303. [DOI] [PubMed] [Google Scholar]
- 20. Steinke, J.W. , et al., Prominent role of IFN‐gamma in patients with aspirin‐exacerbated respiratory disease. J Allergy Clin Immunol, 2013. 132(4): p. 856–65 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Porter, P.C. , et al., Seeking common pathophysiology in asthma, atopy and sinusitis. Trends Immunol, 2011. 32(2): p. 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porter, P.C. , et al., Airway surface mycosis in chronic TH2‐associated airway disease. J Allergy Clin Immunol, 2014. 134(2): p. 325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laury, A.M. , et al., Periostin and receptor activator of nuclear factor kappa‐B ligand expression in allergic fungal rhinosinusitis. Int Forum Allergy Rhinol, 2014. 4(9): p. 716–24. [DOI] [PubMed] [Google Scholar]
- 24. Schleimer, R.P. , Schnaar R.L., and Bochner B.S., Regulation of airway inflammation by Siglec‐8 and Siglec‐9 sialoglycan ligand expression. Curr Opin Allergy Clin Immunol, 2016. 16(1): p. 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sommer, D.D. , et al., A novel treatment adjunct for aspirin exacerbated respiratory disease: the low‐salicylate diet: a multicenter randomized control crossover trial. Int Forum Allergy Rhinol, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Kim, J.W. , et al., Histological and immunological features of non‐eosinophilic nasal polyps. Otolaryngol Head Neck Surg, 2007. 137(6): p. 925–30. [DOI] [PubMed] [Google Scholar]
- 27. Ahn, C.N. , et al., Local production of antigen‐specific IgE in different anatomic subsites of allergic fungal rhinosinusitis patients. Otolaryngol Head Neck Surg, 2009. 141(1): p. 97–103. [DOI] [PubMed] [Google Scholar]
- 28. Gevaert, P. , et al., Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol, 2013. 131(1): p. 110–6 e1. [DOI] [PubMed] [Google Scholar]
- 29. Pinto, J.M. , et al., A randomized, double‐blind, placebo‐controlled trial of anti‐IgE for chronic rhinosinusitis. Rhinology, 2010. 48(3): p. 318–24. [DOI] [PubMed] [Google Scholar]
- 30. Steinke, J.W. and Wilson J.M., Aspirin‐exacerbated respiratory disease: pathophysiological insights and clinical advances. J Asthma Allergy, 2016. 9: p. 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pezato, R. , et al., Systemic expression of inflammatory mediators in patients with chronic rhinosinusitis and nasal polyps with and without Aspirin Exacerbated Respiratory Disease. Cytokine, 2016. 77: p. 157–67. [DOI] [PubMed] [Google Scholar]
- 32. Divekar, R. , et al., Diagnostic Utility of Urinary LTE4 in Asthma, Allergic Rhinitis, Chronic Rhinosinusitis, Nasal Polyps, and Aspirin Sensitivity. J Allergy Clin Immunol Pract, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cingi, C. , et al., Antileukotrienes in upper airway inflammatory diseases. Curr Allergy Asthma Rep, 2015. 15(11): p. 64. [DOI] [PubMed] [Google Scholar]
- 34. Spies, J.W. , et al., The role of aspirin desensitization in patients with aspirin‐exacerbated respiratory disease (AERD). Braz J Otorhinolaryngol, 2015. 82(3): p. 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laidlaw, T.M. , et al., Cysteinyl leukotriene overproduction in aspirin‐exacerbated respiratory disease is driven by platelet‐adherent leukocytes. Blood, 2012. 119(16): p. 3790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cai, Y. , Bjermer L., and Halstensen T.S., Bronchial mast cells are the dominating LTC4S‐expressing cells in aspirin‐tolerant asthma. Am J Respir Cell Mol Biol, 2003. 29(6): p. 683–93. [DOI] [PubMed] [Google Scholar]


