Abstract
BACKGROUND
The number of survivors of breast cancer aged ≥65 years (“older”) is growing, but to the authors' knowledge, little is known regarding the cognitive outcomes of these individuals.
METHODS
A cohort of cognitively intact older survivors with nonmetastatic, invasive breast cancer was recruited from 78 sites from 2004 through 2011; approximately 83.7% of the survivors (1280 survivors) completed baseline assessments. Follow‐up data were collected at 6 months and annually for up to 7 years (median, 4.1 years). Cognitive function was self‐reported using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30); scores ranged from 0 to 100, with a higher score indicating better function. Group‐based trajectory modeling determined trajectories; women were assigned to a trajectory group based on the highest predicted probability of membership. Multinomial logistic regression evaluated the association between receipt of chemotherapy (with or without hormonal treatment) and trajectory group.
RESULTS
Survivors were aged 65 to 91 years; approximately 41% received chemotherapy. There were 3 cognitive trajectories: “maintained high” (42.3% of survivors); “phase shift” (50.1% of survivors), with scores slightly below but parallel to maintained high; and “accelerated decline” (7.6% of survivors), with the lowest baseline scores and greatest decline (from 71.7 [standard deviation, 19.8] to 58.3 [standard deviation, 21.9]). The adjusted odds of being in the accelerated decline group (vs the maintained high group) were 2.1 times higher (95% confidence interval, 1.3‐3.5) for survivors who received chemotherapy (with or without hormonal therapy) versus those treated with hormonal therapy alone. Greater comorbidity and frailty also were found to be associated with accelerated decline.
CONCLUSIONS
Trajectory group analysis demonstrated that the majority of older survivors maintained good long‐term self‐reported cognitive function, and that only a small subset who were exposed to chemotherapy manifested accelerated cognitive decline. Future research is needed to determine factors that place some older survivors at risk of experiencing cognitive decline. Cancer 2016;122:3555–3563. © 2016 American Cancer Society
Keywords: breast cancer, chemotherapy, cognition, older, survival, trajectory
Short abstract
Among older survivors of breast cancer who were followed for up to 7 years, approximately 42% reported maintaining high cognitive function, but receipt of chemotherapy (with or without hormonal therapy) appeared to double the odds of being in the group that reported accelerated cognitive decline (vs maintaining high function), compared with receiving hormonal treatment alone. Further research is needed to determine factors that place some older survivors at risk of experiencing cognitive decline.
INTRODUCTION
There are > 3 million survivors of breast cancer in the United States.1, 2 Greater than 50% of these survivors are aged ≥65 years (“older”), and the absolute number of older survivors is rapidly increasing with the population aging, the association between breast cancer and increasing age, the greater use of early detection methods, and improvements in survival.2, 3, 4
There is a growing body of evidence that cancer and its systemic treatments can have adverse effects on cognition in some, but not all, survivors of breast cancer.5, 6, 7 Older survivors may be especially vulnerable to cancer‐related decrements in cognitive function due to decreases in reserve.5 Cognitive issues can go unrecognized in clinical encounters,8 but can limit the ability of older survivors to conduct daily activities. Cognitive deficits also can lead to social isolation due to difficulty driving or organizing social activities, or attempts to conceal these deficits.9, 10, 11 Declines in cognitive function also can adversely affect survivorship care due to difficulty tracking medications, following instructions for surveillance, and coordinating care across multiple providers.5, 10, 12
Self‐report is commonly used to measure cognitive function,6, 13 and is considered a gold standard in patient‐reported outcomes research. Self‐reported cognitive function generally correlates with objective testing,6, 13 predicts impairment and dementia,14, 15, 16 and relates to abnormalities on neuroimaging even in the absence of objective deficits.17 Unfortunately, to the best of our knowledge, little is known regarding cognitive function outcomes and their time course in older survivors of breast cancer because past research has focused on younger patients, had few older survivors, did not include baseline function, and/or lacked long‐term follow‐up.18, 19, 20, 21, 22
The current study presents up to 7 years of data from a prospective cohort of older survivors of breast cancer to determine trajectories of self‐reported cognitive function, and to test the effects of chemotherapy on cognitive trajectories. We also evaluated associations between self‐reported cognitive and physical function trajectories. These self‐report results are intended to stimulate future research to identify older survivors who are at risk of long‐term cognitive issues.
MATERIALS AND METHODS
The study was conducted at 78 Cancer and Leukemia Group B (CALGB) sites (currently the Alliance for Clinical Trials in Oncology) to determine the preferences of older women with regard to chemotherapy.23 We used follow‐up data to conduct secondary analysis. Each participant signed an Institutional Review Board‐approved informed consent.
Setting and Population
Survivors were newly diagnosed with breast cancer between January 1, 2004 and April 1, 2011 and had available follow‐up data to April 1, 2015. Prior reports included earlier subsets of enrollees and/or shorter‐term follow‐up.24, 25, 26, 27 To the best of our knowledge, the current study is the first report using the final cohort and their complete follow‐up data. Eligible participants were aged ≥65 years; diagnosed with invasive, nonmetastatic breast cancer; spoke English or Spanish; passed an entry cognitive screen using the Blessed Orientation‐Memory‐Concentration test28; and were within 20 weeks of surgery.
Among eligible survivors, approximately 83.7% (1280 survivors) completed a baseline interview (Fig. 1).24 Those who completed interviews (vs those who refused) were slightly younger (aged 74.1 years vs 72.7 years; P = .09), had an earlier stage of disease (39.2% vs 45.6% American Joint Committee on Cancer stage I; P = .01), and were more likely to be white than nonwhite (81.3% vs 88.1% white; P = .005). Blessed screening was only performed among those survivors who consented to interviews, and therefore these data were not available for nonparticipants.
Figure 1.
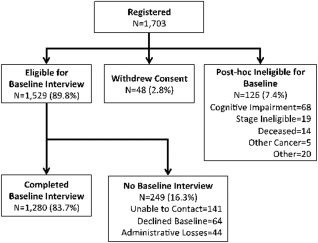
Study sample of older survivors of breast cancer showing study schema for initial enrollment (subsequent follow‐up interviews, disease recurrence, and death events are summarized in Supporting Information Table 2). Compared with an earlier report from this cohort that included 1288 survivors,24 8 women subsequently withdrew consent. The final cohort included 1280 survivors.
Although the goal was to conduct baseline interviews before the initiation of systemic therapy, the mean times from surgery to registration and registration to baseline interview were 8.4 weeks (standard deviation [SD], 4.9 weeks) and 3.9 weeks (SD, 3.0 weeks), respectively, and thus some baseline interviews could have occurred during chemotherapy. Data from those survivors with disease recurrence (132 survivors; 10.4%) were excluded from the point of disease recurrence. Disease recurrence, death, and follow‐up interview data are included in Supporting Information Table 1.
Data Collection
Clinical research associates ascertained patients, confirmed eligibility, obtained permission to contact survivors (received for 95%), obtained consent, and abstracted records. Registration and clinical data collection was managed by the Alliance Statistics and Data Center. Survey data were collected via telephone by centralized staff; follow‐up interviews occurred 6 months and 12 months after the baseline interview, and then annually for up to 7 years.
Outcome Variables
Self‐reported cognitive function was measured using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30) scale,29 which includes 2 Likert‐scored items: 1) “Have you had difficulty in concentrating on things, like reading a newspaper, or watching television?”; and 2) “Have you had difficulty remembering things?” Physical function was based on the EORTC QLQ‐C30 5‐item scale; both scales had 4 possible responses.29, 30 Baseline reliability (Cronbach α) of the cognitive and physical function scales was.52 and.66, respectively, and was similar across time points. Scores were normalized to a range of 0 to 100, in which 100 indicated no problems.
Independent Variables
The primary independent variable was systemic chemotherapy (with or without hormonal therapy), including neoadjuvant treatment. The Observational Assessment Rating Scale (OARS) ascertained the number of preexisting comorbidities31; results were dichotomized at the median. Frailty was based on the Searle index (excluding cognition)32; scores were grouped into 3 categories (robust, pre‐frail, and frail) based on relationships to mortality33 and adherence to treatment.24
Controlling Variables
Several factors were considered as potential confounders of the association between cognitive trajectories and chemotherapy, including age, race (white vs nonwhite), education (≤high school vs > high school), marital status, insurance, stage of disease, surgery (mastectomy vs lumpectomy), estrogen receptor status, premorbid function, and American Joint Committee on Cancer 6th edition stage. Premorbid physical ability, anxiety, depression, vitality, and pain were captured by Medical Outcomes Study Short Form‐12 (MOS SF‐12) scores for the 2 months before diagnosis.34 We also considered settings of care (main member cancer center vs community affiliate) and year of diagnosis.
Statistical Analysis
We used SAS Proc Traj statistical software (SAS Institute Inc, Cary, NC) to conduct group‐based trajectory modeling to identify trajectories of cognitive and physical function.35 This approach fits a discrete, semiparametric mixture model to longitudinal data using maximum likelihood methods to estimate membership probabilities for multiple trajectories, rather than using single‐group means as in linear regression or growth curve models.35, 36 This method allows for the use of all observed data, even if they are missing for some time points. The Bayesian information criterion was used to select the number of trajectories that best fit the data. When identifying trajectories, we considered patterns consistent with aging theories,37 such as the mild decline observed in normal aging, declines that are shifted below but parallel to those noted with normal aging (phase shift), or steeper slopes of decline (accelerated aging).
Survivors were assigned to a trajectory group based on the highest predicted probability of group membership.35Analysis of variance (ANOVA) and chi‐square tests evaluated the bivariate associations between trajectory group and covariates. Covariates with a P<.10 were included in multinomial logistic regression analyses evaluating the association between chemotherapy and cognitive trajectory group; age was retained in all models for face validity. The cognition trajectory group also was evaluated as a predictor of the physical function trajectory group, controlling for covariates.35
We conducted several sensitivity analyses: totally excluding women with disease recurrence; examining diabetes, cardiovascular disease, or frailty levels; using single items from the cognition scale; controlling for baseline cognition; considering different treatment definitions; and evaluating the impact of deaths and attrition on trajectories.38 We also examined relationships between the Blessed and EORTC QLQ‐C30 cognition scores. Finally, we used a mixed effects model that included random effects for intercepts and slopes to compare with results from the group‐based trajectory modeling approach. All analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc).
RESULTS
Older survivors of breast cancer ranged in age from 65 to 91 years (Table 1).30, 34 The median follow‐up was 4.1 years (range, 0.5‐7 years) (see Supporting Information Table 1). Nearly 41% of survivors received chemotherapy; anthracyclines were the predominant regimen (60.4%). Among those survivors treated with hormonal therapy, 77.8% received aromatase inhibitors.
Table 1.
Characteristics of Older Survivors of Breast Cancer by Cognitive Function Trajectory Groupa
| Cognitive Function Trajectory | ||||||
|---|---|---|---|---|---|---|
| Total | Accelerated Decline | Phase Shift | Maintain High | |||
| Characteristic | N=1280 | N=97 | N=641 | N=542 | P | |
| Covariate | No. (%) or mean (SD) | |||||
| Baseline cognitionb | Mean (SD) | 92.6 (13.3) | 71.7 (19.8) | 89.9 (13.2) | 99.4 (3.8) | <.001 |
| Cognitive screen scorec | Mean (SD) | 3.1 (3.4) | 4.1 (3.8) | 3.2 (3.3) | 2.7 (3.3) | <.001 |
| Age, y | Mean (SD) | 72.7 (5.9) | 73.2 (6.6) | 72.9 (5.9) | 72.3 (5.9) | .10 |
| Age group, y | 65‐69 | 474 (37.0%) | 38 (39.2%) | 222 (34.6%) | 214 (39.5%) | .15 |
| 70‐74 | 356 (27.8%) | 23 (23.7%) | 184 (28.7%) | 149 (27.5%) | ||
| 75‐79 | 267 (20.9%) | 16 (16.5%) | 136 (21.2%) | 115 (21.2%) | ||
| >80 | 183 (14.3%) | 20 (20.6%) | 99 (15.4%) | 64 (11.8) | ||
| Comorbidity | ≤2 illnesses | 565 (44.5%) | 22 (23.4%) | 257 (40.5%) | 286 (52.8%) | <.001 |
| >2 illnesses | 706 (55.5%) | 72 (76.6%) | 378 (59.5%) | 256 (47.2%) | ||
| Frailty | Frail | 64 (5.1%) | 15 (16.1%) | 41 (6.5%) | 8 (1.5%) | <.001 |
| Pre‐frail | 231 (18.3%) | 35 (37.6%) | 129 (20.4%) | 67 (12.4%) | ||
| Robust | 970 (76.7%) | 43 (46.2%) | 463 (73.1%) | 464 (86.1%) | ||
| Physical health prediagnosisd | Mean (SD) | 51.2 (7.7) | 48.4 (9.0) | 50.2 (8.7) | 52.9 (5.5) | <.001 |
| Physical function trajectory | Accelerated | 407 (31.8%) | 59 (60.8%) | 222 (34.6%) | 126 (23.2%) | <.001 |
| Phase shift | 489 (38.2%) | 31 (32.0%) | 262 (40.9%) | 196 (36.2%) | ||
| Maintain high | 384 (30.0%) | 7 (7.2%) | 157 (24.5%) | 220 (40.6%) | ||
| Mental health prediagnosisd | Mean (SD) | 56.7 (5.3) | 53.9 (6.8) | 56.4 (5.8) | 57.5 (4.2) | <.001 |
| Race | Nonwhite | 152 (11.9%) | 13 (13.4%) | 81 (12.6%) | 58 (10.7%) | .53 |
| White | 1128 (88.1%) | 84 (86.6%) | 560 (87.4%) | 484 (89.3%) | ||
| Education | ≤High school | 539 (42.1%) | 36 (37.1%) | 277 (43.2%) | 226 (41.8%) | .51 |
| >High school | 740 (57.9%) | 61 (62.9%) | 364 (56.8%) | 315 (58.2%) | ||
| Insurance | Medicare | 315 (24.6%) | 23 (23.7%) | 148 (23.1%) | 144 (26.6%) | .38 |
| Medicare Plus | 965 (75.4%) | 74 (76.3%) | 493 (76.9%) | 398 (73.4%) | ||
| Setting | Cancer center | 366 (28.6%) | 24 (24.7%) | 191 (29.8%) | 151 (27.9%) | .52 |
| Community affiliate | 914 (71.4%) | 73 (75.3%) | 450 (70.2%) | 391 (72.1%) | ||
| AJCC 6th edition stage of disease | I | 584 (45.6%) | 37 (38.1%) | 285 (44.5%) | 262 (48.3%) | .19 |
| IIA | 399 (31.2%) | 35 (36.1%) | 195 (30.4%) | 169 (31.2%) | ||
| ≥IIB | 297 (23.2%) | 25 (25.8%) | 161 (25.1%) | 111 (20.5%) | ||
| Disease recurrence | No | 1148 (89.7%) | 83 (85.6%) | 578 (90.2%) | 487 (89.9%) | .38 |
| Yes | 132 (10.3%) | 14 (14.4%) | 63 (9.8%) | 55 (10.1%) | ||
| Surgery | BCS | 864 (67.6%) | 64 (66.0%) | 437 (68.3%) | 363 (67.0%) | .84 |
| Mastectomy | 415 (32.4%) | 33 (34.0%) | 203 (31.7%) | 179 (33.0%) | ||
| ER status | Negative | 216 (16.9%) | 21 (21.6%) | 107 (16.7%) | 88 (16.2%) | .42 |
| Positive | 1062 (83.1%) | 76 (78.4%) | 532 (83.3%) | 454 (83.8%) | ||
| Systemic treatmente | Chemotherapy (with or without hormonal therapy) | 519 (40.5%) | 49 (50.5%) | 253 (39.5%) | 217 (40.0%) | .07 |
| AC‐based | 313 (60.4%) | 27 (55.1%) | 162 (64.0%) | 124 (57.4%) | .25 | |
| Non‐AC | 205 (39.6%) | 22 (44.9%) | 91 (36.0%) | 92 (42.6%) | ||
| Hormonal only | 687 (53.7%) | 41 (42.3%) | 352 (54.9%) | 294 (54.2%) | ||
| Tamoxifen | 225 (22.2%) | 22 (30.6%) | 113 (22.2%) | 90 (20.8%) | .19 | |
| AI | 789 (77.8%) | 50 (69.4%) | 397 (77.8%) | 342 (79.2%) | ||
Abbreviations: AC, anthracycline; AI, aromatase inhibitors; AJCC, American Joint Committee on Cancer; BCS, breast‐conserving surgery; ER, estrogen receptor; SD, standard deviation.
Year, marital status, and health maintenance organization were not found to be related to trajectories or chemotherapy (data not shown).
Group‐based trajectory modeling identified trajectories; survivors were assigned to trajectories based on the highest predicted probability of group membership. Associations between trajectories and covariates were assessed using chi‐square and analysis of variance tests.
Cognition scores were derived from the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30) (version 3.0); scores ranged from 0 to 100, with a higher score indicating better function.30
Higher scores indicated worse cognitive function. Survivors with scores > 11 (suggesting ≥ mild cognitive impairment) were excluded; remaining scores ranged from 0 to 11.
The Medical Outcomes Study Short Form (SF)‐12 was obtained at baseline for the 2 months before diagnosis. Scores included physical ability, anxiety, depression, vitality, and pain and had a mean of 50 (standard deviation, 10).34
Survivors with missing treatment data (74 survivors) were excluded in subsequent analyses.
The best fit to the cognition data was provided by 3 trajectory groups (Fig. 2): 42.3% of survivors who began with near‐perfect scores (mean, 99.4; SD, 3.8) and maintained this level (maintained high), 50.1% of survivors with scores shifted slightly below (mean, 89.9; SD, 13.2) but declining in parallel with the high group (phase shift), and 7.6% of survivors who had the lowest baseline scores (mean, 71.7; SD, 19.8) and a steeper rate of decline (accelerated decline).
Figure 2.
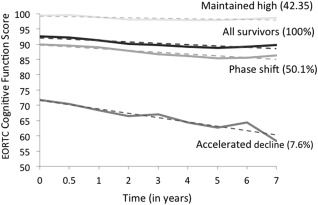
Trajectories of long‐term, self‐reported cognitive function in older survivors of breast cancer. The graph shows the mean self‐reported cognition scores over time for survivors assigned to the 3 trajectory groups plus the average scores for all survivors. There were 42.3% in the “maintained high,” 50.1% in the “phase shift,” and 7.6% in the “accelerated decline” trajectory groups. Time zero represents the baseline assessment (which may have been mid‐treatment); subsequent time periods are indicated in years. Data points (indicated by solid lines) and trend lines (indicated by dotted lines) are shown for each trajectory group. EORTC indicates European Organization for Research and Treatment of Cancer.
The adjusted odds of being in the accelerated decline group (vs the maintained high group) were 2.1 times higher (95% confidence interval [95% CI], 1.3‐3.5) for survivors who received chemotherapy (with or without hormonal therapy) versus those treated with hormonal therapy alone (Table 2). Higher premorbid emotional or physical function decreased the odds of being in the accelerated decline group (vs the maintained high group).
Table 2.
Adjusted Odds of Membership to Self‐Reported Cognitive Function Trajectory Groups Among Older Survivors of Breast Cancer Over 7 Years After Treatmenta
| Variable | Accelerated Decline (Versus Maintain High) | Phase Shift (Versus Maintain High) | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | Overall P | |
| Age (per 1‐y increase) | 1.00 (0.96‐1.05) | .92 | 1.00 (0.98‐1.03) | .87 | .99 |
| Chemotherapy (with or without hormonal therapy) versus hormonal therapy | 2.1 (1.3‐3.5) | .005 | 1.1 (0.8‐1.4) | .48 | .02 |
| Comorbidity (≥2 illnesses vs < 2 illnesses) | 3.0 (1.7‐5.4) | <.001 | 1.4 (1.0‐1.8) | .02 | <.001 |
| Mental health prediagnosis (per 1‐point increase) | 0.90 (0.87‐0.93) | <.001 | 0.95 (0.93‐0.98) | .001 | <.001 |
| Physical health prediagnosis (per 1‐point increase) | 0.93 (0.91‐0.96) | <.001 | 0.95 (0.93‐0.97) | <.001 | <.001 |
| Model fit (Hosmer‐Lemeshow test; value closer to 1 indicates a better fit) | .40 | ||||
Abbreviations: 95% CI, 95% confidence interval; OR, odds ratio.
All variables were adjusted for the remaining variables in the table.
Associations between group membership and covariates were determined using multinomial logistic regression.
Age was not found to be related, but having >2 (vs ≤ 2) comorbid illnesses was associated with trajectories, with a stronger association with the accelerated group (odds ratio [OR], 3.0; 95% CI, 1.7‐5.4) than the phase shift group (OR, 1.4; 95% CI, 1.0‐1.8) (vs the maintained high group). Among specific comorbidities, trajectory group membership was only found to be related to cardiovascular disease (P = .04), but this was not significant in multivariable analyses (data not shown).
Frailty demonstrated a similar relationship to trajectory group as comorbidity, with frail (vs robust) survivors having a significantly higher adjusted odds of being in the accelerated cognitive decline group (OR, 19.9; 95% CI, 7.8‐50.8) or phase shift group (OR, 4.5; 95% CI, 2.1‐9.8) (vs the maintained high group) (data not shown).
The results for the association between chemotherapy and the cognitive trajectories were unchanged if education or race, which were not found to be related to trajectory in bivariate analyses, were retained in the models; if women who had disease recurrences were totally excluded from analyses; when single items from the cognition scale were used; if controlling for baseline cognition; when separating treatment into 3 groups (chemotherapy alone, chemotherapy and hormonal therapy, or hormonal therapy alone); or if we accounted for dropout due to death or loss to follow‐up (data not shown). The mixed effects model confirmed that chemotherapy was associated with the slope of decline in cognitive function (P = .05).
The Blessed screening scores were correlated with baseline cognition scores (correlation coefficient, ‐0.12; P<.001) and cognitive trajectories (P<.001), but not chemotherapy (P = .42). Last, cognition trajectories were found to be related to physical function trajectories (see Supporting Information Fig. 1). The odds of being in the accelerated physical function decline group (vs maintained high) were 9.5 times higher (95% CI, 3.6‐25.5) for survivors in the accelerated cognitive decline group (vs the maintained high group), controlling for covariates (see Supporting Information Table 2).
DISCUSSION
To the best of our knowledge, the current study is the first prospective study of long‐term, self‐reported cognitive trajectories of older survivors of breast cancer. The majority of survivors reported maintaining high cognitive function, but a small group reported accelerated declines. Chemotherapy, comorbidity (or frailty), and low prediagnosis function increased the odds of accelerated cognitive decline. Accelerated cognitive decline was, in turn, associated with a declining physical function.
Group‐based trajectory modeling has been used to identify outcome patterns for disability at the end of life36; frailty,39 depression,40 and hypertension41 in general populations; and short‐term depression in young survivors of breast cancer.42, 43 To the best of our knowledge, the current study is the first application of this statistical method to long‐term outcomes in older breast cancer survivors. This technique is particularly useful because it has the advantage of identifying trajectories, rather than modeling the mean, which may obscure differences between groups of individuals.35, 44, 45
The majority of older survivors reported maintaining excellent cognitive function. However, there was variation in baseline values, with those most likely to be in the accelerated decline group having a mean baseline value that was 21 points below that of the cohort. Moreover, the accelerated decline group experienced a 13.4‐point decline over time. This represents a clinically meaningful change,46, 47, 48 especially because those with extant cognitive problems were excluded from analysis. The baseline assessment could have occurred mid‐treatment for survivors undergoing chemotherapy, and therefore it is not possible to determine whether the accelerated decline group had low cognitive scores before therapy or experienced decrements early during treatment. However, all women passed eligibility cognitive screening and their scores began within the range of normative values on the EORTC QLQ‐C30 (ie, 82.6 [SD, 21.1] for survivors of breast cancer aged 60‐69 years and 81 [SD, 23.5] for those aged ≥ 70 years).30 Additional research is needed that includes noncancer controls to determine the early course of cognitive decline compared with that expected with aging, and to explore whether early interventions can improve cognitive function among survivors who demonstrate a decline during therapy.
Indicators of physiological age, such as comorbidity or frailty, were found to be related to cognitive trajectories, whereas age was not. Comorbidity has been associated with both pretreatment and posttreatment cognitive issues in other settings.49 Thus, chronological age alone may not be useful in determining the risk of cognitive issues.
Precancer emotional function, including anxiety and depression, was found to be associated with cognitive trajectories, but did not affect the relationship between cognition and treatment. To the best of our knowledge, past studies of the relationship between emotional factors and cognitive outcomes among survivors of breast cancer have been inconsistent and none examined older survivors.6, 50, 51, 52 If confirmed as risk factors, anxiety and depression screening and early intervention might improve cognitive outcomes.
Chemotherapy has been associated with short‐term and long‐term18, 53 cognitive decrements in many settings and populations, but the effects are not universal.37, 54 The results of the current study suggest that the majority of older survivors of breast cancer maintain high self‐reported cognitive function, but that chemotherapy (with or without hormonal treatment), which was received by the healthiest older survivors,23 can have adverse long‐term effects in a small number of survivors. This finding was independent of emotional and other factors and, if confirmed, highlights the importance of integrating cognitive function assessment into survivorship care for older survivors. The strong association noted between cognitive and physical function trajectories underscores the relevance of the results. This latter finding could signal a need for supportive care interventions to maintain function, because together, cancer and cognitive decline account for the majority of morbidity, mortality, and health care costs and ability to live independently among older individuals.55, 56, 57
Although the current study reported on a large cohort followed for a long period of time and used a novel approach to examine outcomes, there were several limitations. First, because this was an unplanned, secondary analysis, we relied on available self‐report data and did not have information from neuropsychological testing. However, the validity of self‐report is supported by correlation with the objectively measured Blessed Orientation‐Memory‐Concentration test in the current study sample and the results of past research and neuroimaging studies,6, 13, 14, 15, 16, 17 including reports of correlations between the EORTC cognition and Functional Assessment of Cancer Therapy‐Cognitive Function scales.46 Moreover, the analytic procedure makes assumptions regarding the equality of error variances across trajectories that could produce optimistic P values.35 Given the strong relationship between chemotherapy and accelerated decline (P = .005), the result is likely to be robust despite this potential procedural limitation. Another limitation is that the EORTC cognition scale only evaluates memory and concentration, and current recommendations are to include multiple domains.58 Furthermore, the EORTC cognition scale had low reliability, but we observed comparable reliability as reported in the original EORTC validation29 and other studies.54, 59 Overall, the EORTC is the most frequently reported subjective cognitive measure among patients with breast cancer, and its limits likely bias results toward the null.
Second, we did not have longitudinal measures of fatigue, pain, activities of daily living, and instrumental activities of daily living or information regarding substance abuse, but the association between chemotherapy and the cognitive trajectory was independent of premorbid depression, anxiety, physical ability, vitality, and pain. Furthermore, we did not examine cognitive outcomes separately by regimens because there was limited treatment variability within each adjuvant modality. The majority of survivors were largely from community sites, but they may not represent all older survivors because participants were recruited from cooperative group settings and prescreened to exclude cognitive impairment. These factors should underestimate cognitive declines in the general older population of individuals with breast cancer.
Overall, the results of the current study suggest that there may be different long‐term trajectories of cognitive function among older survivors of breast cancer, but only a small subset may experience early and accelerated decline with associated decrements in physical function. These findings suggest that further research is needed to determine risk factors for cognitive decline to identify those individuals most likely to benefit from cognitive monitoring during survivorship care.
FUNDING SUPPORT
Supported by National Cancer Institute (NCI)/National Institutes of Health (NIH) grants CA84131 and CA127617 (to Jeanne S. Mandelblatt). Research also was supported in part by NCI grants CA197289, CA129769, CA124924, and CA96940 (to Jeanne S. Mandelblatt); grant CA166342 (to Leigh Anne Faul); and the Biostatistics and Bioinformatics Shared Resources at Georgetown‐Lombardi Comprehensive Cancer Center funded by the NCI/NIH under grant P30CA51008. The research for Cancer and Leukemia Group B (CALGB) 369901 also was supported in part by NCI/NIH grant CA31946 to the CALGB (Monica Bernagnoli, MD, Chair) and grant CA33601 to the CALGB Statistical Center (Stephen George, PhD). Earlier portions of the research also were funded in part by a grant to support patient accrual from Amgen Pharmaceuticals to the CALGB Foundation.
CONFLICT OF INTEREST DISCLOSURES
Robert Stern has acted as an advisory board member for Biogen, Avanir Pharmaceuticals, and Amarantus BioScience Holdings; has received grants from Avid Radiopharmaceuticals and Amarantus BioScience Holdings; has acted as a paid consultant for Quest; and has received royalties from Psychological Assessment Resources for authorship of neuropsychological tests for work performed outside of the current study. Paul Jacobsen has acted as a paid consultant for Bayer Inc and Carevive Systems Inc and received research support from Pfizer Inc and Carevive Systems Inc for work performed outside of the current study. Hyman Muss has acted as an unpaid consultant for Pfizer Inc for work performed outside of the current study. Arti Hurria has received research funding from Celgene, Novartis, and GlaxoSmithKline and has acted as a paid consultant for Boehringer Ingelheim Pharmaceuticals, Carevive Systems Inc, Sanofi, GTx Inc, and On Q Health for work performed outside of the current study. Claudine Isaacs has received a grant and personal fees from Genentech, a grant from Novartis, and a grant and personal fees from Pfizer Inc for work performed outside of the current study.
AUTHOR CONTRIBUTIONS
Jeanne S. Mandelblatt: Conception and design, data interpretation, article writing, and final approval of the article. Jonathan Clapp: Conception and design, data analysis, data interpretation, article writing, and final approval of the article. Gheorghe Luta: Data analysis, data interpretation, article writing, and final approval of the article. Leigh Anne Faul: Data interpretation, article writing, and final approval of the article. Michelle Tallarico: Collection and assembly of data, data interpretation, article writing, and final approval of the article. Trina McClendon: Collection and assembly of data, data interpretation, article writing, and final approval of the article. Jessica Whitley: Collection and assembly of data, data interpretation, article writing, and final approval of the article. Ling Cai: Collection and assembly of data, data interpretation, article writing, and final approval of the article. Tim A. Ahles: Conception and design, data interpretation, article writing, and final approval of the article. Robert A. Stern: Conception and design, data interpretation, article writing, and final approval of the article. Paul B. Jacobsen: Conception and design, data interpretation, article writing, and final approval of the article. Brent J. Small: Data analysis, data interpretation, article writing, and final approval of the article. Brandelyn Pitcher: Collection and assembly of data, data interpretation, article writing, and final approval of the article. Estrella Dura‐Fernandis: Data interpretation, article writing, and final approval of the article. Hyman Muss: Provision of study materials and patients, data interpretation, article writing, and final approval of the article. Arti Hurria: Conception and design, provision of study materials and patients, data interpretation, article writing, and final approval of the article. Harvey J. Cohen: Conception and design, provision of study materials and patients, data interpretation, article writing, and final approval of the article. Claudine Isaacs: Provision of study materials and patients, data interpretation, article writing, and final approval of the article.
Supporting information
Additional supporting information may be found in the online version of this article
Supporting Information
We thank Drs. Richard Schilsky, Clifford Hudis, and Eric Winer for their support of this study; the physicians and patients who participated in the research, especially Dr. Steven Sugarman from Memorial Sloan Kettering Cancer Center for accrual; the clinical research associates who contributed to the data collection; and Adrienne Ryans for article preparation.
The content of this article is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute at the National Institutes of Health or the Cancer and Leukemia Group B. Cancer and Leukemia Group B is currently part of the Alliance for Clinical Trials in Oncology.
Alliance for Clinical Trials in Oncology (formerly Cancer and Leukemia Group B) Protocol #369901
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2. Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol. 2014;32:2662–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Census Bureau . Total population by age and sex for the United States: 2012. www.census.gov/age/data/2012comp.html. Accessed July 13, 2015.
- 5. Mandelblatt JS, Jacobsen PB, Ahles T. Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J Clin Oncol. 2014;32:2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganz PA, Petersen L, Castellon SA, et al. Cognitive function after the initiation of adjuvant endocrine therapy in early‐stage breast cancer: an observational cohort study. J Clin Oncol. 2014;32:3559–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hurria A, Naylor M, Cohen HJ. Improving the quality of cancer care in an aging population: recommendations from an IOM report. JAMA. 2013;310:1795–1796. [DOI] [PubMed] [Google Scholar]
- 10. Institute of Medicine . Delivering High‐Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: The National Academies Press; 2013. http://www.nap.edu/catalog/18359/delivering-high-quality-cancer-care-charting-a-new-course-for. Accessed January 1, 2015. [PubMed]
- 11. Montejo P, Montenegro M, Fernandez MA, Maestu F. Memory complaints in the elderly: quality of life and daily living activities. A population based study. Arch Gerontol Geriatr. 2012;54:298–304. [DOI] [PubMed] [Google Scholar]
- 12. Lange M, Rigal O, Clarisse B, et al. Cognitive dysfunctions in elderly cancer patients: a new challenge for oncologists. Cancer Treat Rev. 2014;40:810–817. [DOI] [PubMed] [Google Scholar]
- 13. Von Ah D, Tallman EF. Perceived cognitive function in breast cancer survivors: evaluating relationships with objective cognitive performance and other symptoms using the Functional Assessment of Cancer Therapy‐Cognitive Function instrument. J Pain Symptom Manage. 2015;49:697–706. [DOI] [PubMed] [Google Scholar]
- 14. Piras F, Piras F, Orfei MD, Caltagirone C, Spalletta G. Self‐awareness in mild cognitive impairment: quantitative evidence from systematic review and meta‐analysis. Neurosci Biobehav Rev. 2016;61:90–107. [DOI] [PubMed] [Google Scholar]
- 15. Howieson DB, Mattek N, Dodge HH, Erten‐Lyons D, Zitzelberger T, Kaye JA. Memory complaints in older adults: prognostic value and stability in reporting over time. SAGE Open Med. 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Snitz BE, Small BJ, Wang T, Chang CC, Hughes TF, Ganguli M. Do subjective memory complaints lead or follow objective cognitive change?. A five‐year population study of temporal influence. J Int Neuropsychol Soc. 2015;21:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schultz SA, Oh JM, Koscik RL, et al. Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle‐aged adults at risk for AD. Alzheimers Dement (Amst). 2015;1:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. [DOI] [PubMed] [Google Scholar]
- 19. Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta‐analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39:297–304. [DOI] [PubMed] [Google Scholar]
- 20. Jim HS, Phillips KM, Chait S, et al. Meta‐analysis of cognitive functioning in breast cancer survivors previously treated with standard‐dose chemotherapy. J Clin Oncol. 2012;30:3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hurria A, Goldfarb S, Rosen C, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient's perspective. Breast Cancer Res Treat. 2006;98:343–348. [DOI] [PubMed] [Google Scholar]
- 22. Yamada TH, Denburg NL, Beglinger LJ, Schultz SK. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physicians. J Clin Oncol. 2010;28:3146–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheppard VB, Faul LA, Luta G, et al. Frailty and adherence to adjuvant hormonal therapy in older women with breast cancer: CALGB protocol 369901. J Clin Oncol. 2014;32:2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mandelblatt JS, Makgoeng SB, Luta G, et al. A planned, prospective comparison of short‐term quality of life outcomes among older patients with breast cancer treated with standard chemotherapy in a randomized clinical trial vs. an observational study: CALGB #49907 and #369901. J Geriatr Oncol. 2013;4:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mandelblatt JS, Faul LA, Luta G, et al. Patient and physician decision styles and breast cancer chemotherapy use in older women: Cancer and Leukemia Group B protocol 369901. J Clin Oncol. 2012;30:2609–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faul LA, Luta G, Sheppard V, et al. Associations among survivorship care plans, experiences of survivorship care, and functioning in older breast cancer survivors: CALGB/Alliance 369901. J Cancer Surviv. 2014;8:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation‐Memory‐Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. [DOI] [PubMed] [Google Scholar]
- 29. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 30. NK Aaronson, S Ahmedzai, B Bergman, et al. The European Organisation for Research and Treatment of Cancer QLQ‐C30: A quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 31. Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. [DOI] [PubMed] [Google Scholar]
- 32. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long‐term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. [DOI] [PubMed] [Google Scholar]
- 34. Ware J, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 35. Jones BL, Nagin DS. Advances in group‐based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–571. [Google Scholar]
- 36. Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahles TA, Root JC, Ryan EL. Cancer‐ and cancer treatment‐associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30:3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haviland AM, Jones BL, Nagin DS. Group‐based trajectory modeling extended to account for nonrandom participant attrition. Sociol Methods Res. 2011;40:367–390. [Google Scholar]
- 39. Peek MK, Howrey BT, Ternent RS, Ray LA, Ottenbacher KJ. Social support, stressors, and frailty among older Mexican American adults. J Gerontol B Psychol Sci Soc Sci. 2012;67:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melchior M, Chastang JF, Head J, et al. Socioeconomic position predicts long‐term depression trajectory: a 13‐year follow‐up of the GAZEL cohort study. Mol Psychiatry. 2013;18:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. Stroke. 1994;25:40–43. [DOI] [PubMed] [Google Scholar]
- 42. Donovan KA, Gonzalez BD, Small BJ, Andrykowski MA, Jacobsen PB. Depressive symptom trajectories during and after adjuvant treatment for breast cancer. Ann Behav Med. 2014;47:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dunn LB, Cooper BA, Neuhaus J, et al. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol. 2011;30:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagin DS, Odgers CL. Group‐based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 45. Zheng Y, Luo J, Bao P, et al. Long‐term cognitive function change among breast cancer survivors. Breast Cancer Res Treat. 2014;146:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the Functional Assessment of Cancer Therapy: Cognitive Function (FACT‐Cog) in breast cancer patients. J Clin Epidemiol. 2014;67:811–820. [DOI] [PubMed] [Google Scholar]
- 47. Hong F, Bosco JL, Bush N, Berry DL. Patient self‐appraisal of change and minimal clinically important difference on the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 before and during cancer therapy. BMC Cancer. 2013;13:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maringwa J, Quinten C, King M, et al; EORTC PROBE Project and Brain Cancer Group . Minimal clinically meaningful differences for the EORTC QLQ‐C30 and EORTC QLQ‐BN20 scales in brain cancer patients. Ann Oncol. 2011;22:2107–2112. [DOI] [PubMed] [Google Scholar]
- 49. Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32:1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard‐dose systemic chemotherapy in long‐term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. [DOI] [PubMed] [Google Scholar]
- 51. Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695–2701. [DOI] [PubMed] [Google Scholar]
- 52. Seliktar N, Polek C, Brooks A, Hardie T. Cognition in breast cancer survivors: hormones versus depression. Psychooncology. 2015;24:402–407. [DOI] [PubMed] [Google Scholar]
- 53. Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. [DOI] [PubMed] [Google Scholar]
- 54. Freedman RA, Pitcher B, Keating NL, et al; Alliance for Clinical Trials in Oncology . Cognitive function in older women with breast cancer treated with standard chemotherapy and capecitabine on Cancer and Leukemia Group B 49907. Breast Cancer Res Treat. 2013;139:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–761. [DOI] [PubMed] [Google Scholar]
- 56. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010‐2020. J Natl Cancer Inst. 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perna L, Wahl HW, Mons U, Saum KU, Holleczek B, Brenner H. Cognitive impairment, all‐cause and cause‐specific mortality among non‐demented older adults. Age Ageing. 2014;44:455–451. [DOI] [PubMed] [Google Scholar]
- 58. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. [DOI] [PubMed] [Google Scholar]
- 59. Paiva CE, Carneseca EC, Barroso EM, et al. Further evaluation of the EORTC QLQ‐C30 psychometric properties in a large Brazilian cancer patient cohort as a function of their educational status. Support Care Cancer. 2014;22:2151–2160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article
Supporting Information


