Abstract
Cardiomyocytes from human pluripotent stem cells (hPSC) are of growing interest as models to understand mechanisms underlying genetic disease, identify potential drug targets and for safety pharmacology as they may predict human relevant effects more accurately and inexpensively than animals or other cell models. Crucial to their optimal use are accurate methods to quantify cardiomyocyte phenotypes accurately and reproducibly. Here, we review current methods for determining biophysical parameters of hPSC‐derived cardiomyocytes (hPSC‐CMs) that recapitulate disease and drug responses. Even though hPSC‐CMs as currently available are immature, various biophysical methods are nevertheless already providing useful insights into the biology of the human heart and its maladies. Advantages and limitations of assays currently available looking toward applications of hPSC‐CMs are described with examples of how they have been used to date. This will help guide the choice of biophysical method to characterize healthy cardiomyocytes and their pathologies in vitro. Stem Cells 2016;34:2008–2015
Keywords: Human, Pluripotent stem cells, Heart, Drug testing, Cardiac disease modelling, Cardiomyocyte maturation, Cardiomyocyte physiology
Significance Statement
With the advent of patient human induced pluripotent stem cell (hiPSC) generation as a relatively routine procedure, many labs get “stuck” in characterizing the disease phenotype. They may sometimes have access to the right equipment, they will often have state‐of‐the‐art optical systems available but do not know exactly how much specialist knowledge or if operator skills are required. Here, we address this and believe that we have provided a useful overview of what is available, how reliable each method is and give examples of the sort of studies that have been done with each of the methods using diseased and healthy human pluripotent stem cells cardiomyocytes.
Introduction
Almost one in three drugs are not used clinically because of side‐effects on the heart 1. Cardiotoxic drugs are often not detected in animal models or cultured cell lines expressing selected cardiac genes because their physiology differs from that of the human heart. In rodents, for example, heart rates are almost 10 times faster than in humans and ion channels, most importantly the Kv7.1 and hERG channels, are differentially expressed 2. Mutations in the KCNQ1 or KCNH2 genes that encode these channels and cause severe cardiac disease in humans thus have little effect in mice. It is therefore not surprising that human relevance for drug responses and modelling cardiac disease is limited.
An emerging method to model the human heart and cardiac disease is using human pluripotent stem cells (hPSCs). hPSCs can be induced to differentiate to various types of cardiomyocytes (hPSC‐CMs) with high efficiency (reviewed in 3), although most protocols to date yield hPSC‐CM with a ventricular phenotype. As a result, most data reported concerns drug responses or the effect of mutations in ventricular‐like cardiomyocytes. hPSCs may be either embryonic (hESCs) or “genetically reprogrammed” adult cells called induced pluripotent stem cells (hiPSCs) 3. hPSC‐CMs are relatively immature compared with cardiomyocytes from adult heart 4 (Table 1) but nevertheless can show drug‐related cardiac responses that reflect QT‐prolongation and arrhythmia evident in electrocardiograms from patients 29. hiPSC‐CMs generated from patients with mutations in cardiac‐relevant genes are already proving useful for understanding mechanisms of disease as they often show expected disease phenotypes 30, 31, 32, 33. Gene targeting is also being used to introduce cardiac disease mutations into hPSCs, and hiPSCs from patients with mutations are being genetically “repaired” to create isogenic pairs that differ only in the genomic region of interest. This allows control over the genetic background used for study 34.
Table 1.
Biophysical parameters of human stem cell‐derived ventricular cardiomyocytes during development and primary cardiomyocytes
| hPSC‐CM | hPSC‐EHT | Primary cardiomyocytes | ||||||
|---|---|---|---|---|---|---|---|---|
| Biophysical parameter | Early | Late | Late | Adult | ||||
| Force of contraction (mN/mm2)a | 0.3 | [5] | 0.5 | [6] | 4.4 | [7] | 51 | [8] |
| Cell aspect ratio (length‐to‐width) | 1:2 | [9] | 1:4 | [10] | Unknown | 1:7 | [10] | |
| Sarcomeric organization | Disorganized | [9] | Organized | [9] | Highly organized | [11] | Highly organized | |
| Sarcomeric distance (µm) | 1.65 | [12] | 1.81 | [12] | Unknown | 2.15 | [8] | |
| Conduction velocity (cm/s) | <2 | [13] | <20 | [14] | <26 | [14] | 100 | [15] |
| Multinucleated cells (%) | 5 | [16] | 20 | [16] | 25 | [17] | 26 | [18] |
| Mitochondria‐to‐cell volume ratio | 0.06 | [19] | 0.09 | [20] | Unknown | 0.3 | [20] | |
| Resting membrane potential (mV) | −50 | [21] | −73.5 | [22] | −50b | [11] | −81.8 | [23] |
| Voltage upstroke velocity (V/s) | <9 | [21] | 26.2 | [22] | 8b | [11] | 215 | [23] |
| Calcium Transient Duration (ms) | Unknown | <370 (CaDT90) | [24] | <375 (CaDT80) | [25] | ∼300 (CaDT90) | [15] | |
| s | ||||||||
| Calcium Transient Rise time (ms) (80%) | Unknown | >10 | [26] | <100 | [25] | Unknown | ||
| Calcium Transient Decay (ms) (80%) | Unknown | >50 | [26] | <150 | [25] | Unknown | ||
| ATP level |
<2,000c
(lum/#cell) |
[27] |
<3,000c
(lum/#cell) |
[27] | Unknown |
5.69
(mmol/kg weight) |
[28] | |
Some differences may be explained by a difference in measured contraction phase (isotonic vs. isometric) or method (beads vs. posts).
Measured on dissociated cardiomyocytes from EHTs.
Relative values. Abbreviations: ATP, Adenosine triphosphate; EHT, engineered heart tissue; hPSC, human pluripotent stem cells; hPSC‐CM, human pluripotent stem cell‐derived cardiomyocytes.
Improving the maturity of hPSC‐CMs is one of the main priorities in the field. To assess the state of maturation as well as effects of drugs and disease, improved biophysical methods for cardiomyocyte analysis are still needed. These should be able to measure dynamic parameters such as force of contraction, calcium handling, and electrical activity and preferably, should not require specialist electrophysiology or mechanobiology facilities so that they can easily be used in many laboratories. Of note though, physical stimuli, such as mechanical stress and electrical pacing, might also be necessary for cardiomyocyte maturation 35 so some specialist expertise may be required. Here, we review measurement techniques currently available for phenotyping and stimulating contractile dynamics of hPSC‐CMs in vitro and discuss ways to modulate the physical environment to best mimic human physiological and pathological states. Cardiomyocytes resulting from different differentiation protocols can vary in their stage of maturation but in general ion currents, sarcomeric structure, and calcium handling stabilize 20 to 30 days after initiation of differentiation. The methods described in this review have generally been used once this stability has been established. We provide examples of how these approaches have been used on healthy and diseased hPSC‐CMs.
Finally, assuming limitations with respect to maturity of hPSC‐CM in vitro and assay methods are overcome, we look forward toward future use of these human models in drug discovery and clinical translation.
Characterizing Dynamics of hPSC‐CMs
Action Potential
The cardiac action potential (AP) is shaped by tightly regulated ion currents, most importantly the Na+, Ca2+, and K+ current. hPSC‐CMs are considered electrically immature: in contrast to their adult counterparts ventricular hPSC‐CMs show spontaneous beating and more depolarized resting membrane potentials (Table 1) caused by high pacemaker current (I f) and low inwardly rectifying K+ current (I K1), respectively. Low expression of Na+ channels causes slow upstroke velocity in the AP (Table 1) compared to adult ventricular cardiomyocytes; recent in silico analysis based on previously published hiPSC‐CM electrophysiological data shows an increased sensitivity to L‐type Ca2+ block due to overexpression of the Na+/Ca2+ exchanger 36. For a complete overview of ion current differences between hPSC‐derived and adult cardiac cells, we refer the reader to 22. AP characteristics are distinct for each cardiomyocyte subtype. The subtype formed during differentiation can be directed by timed addition of cytokines and hormones: retinoic acid, for example, directs formation of atrial‐like cardiomyocytes 37 and a Smoothened Agonist in combination with insulin‐like growth factor‐1 induces pacemaker‐like cardiomyocytes 6. Three experimental approaches are available to measure ion currents and APs in hPSC‐CMs: patch clamp electrophysiology, voltage sensitive sensors and multielectrode array (MEAs). Each has its own advantages and disadvantages.
Patch‐clamp electrophysiology allows precise measurements of ion currents and membrane potentials by voltage and current clamping, respectively. It can be used to determine the identity of the cardiac subtype and specific drug responses. For example, atrial and ventricular cells from hPSC and their differential responses to the atrial specific drugs vernakalant and XEN‐D0101 27 to 30 days after initiation of differentiation were evident in patch clamp assays 37. As every cardiomyocyte is “clamped” individually and a well‐sealed pipette‐membrane interface is essential, the procedure is labor intensive and dependent on operator skills. To address this, Scheel et al. used automated whole‐cell patch clamp (CytoPatch 2) and measured responses to nifedipine, cisapride and TTX in commercially‐supplied ventricular hiPSC‐CMs that would be expected from clinical data and conventional patch clamp 38. Similarly, using the IonWorks Barracuda system (Molecular Devices, Sunnyvale, CA, www.moleculardevices.com), a group of 353 compounds that included known hERG channel blockers, largely gave predicted pharmacological responses in HEK293 and CHO‐K1 cells expressing hERG channels 39. However, these automated whole‐cell patch clamp systems measure APs by patching the cardiomyocytes while they are in suspension and not attached to a substrate. Under these conditions, cardiomyocyte lifespan is limited so that experiments need to be complete within a short time window. Automated patch clamp is designed for high throughput phenotyping of many cells under different conditions but manual patch clamp is at present more accurate.
A less invasive and labor intensive method is based on electrochromic fluorescent voltage‐sensitive dyes (VSDs) 24, such as di‐4‐ANEPPS. VSDs are fast‐response fluorescent probes that intercalate between lipid bilayers; they respond to changes in electrical field by fluctuations in fluorescence intensity that can be recorded optically. In standard (low‐speed) optical systems, the major limitation is the sampling frequency of the optical recorder. The upstroke velocity, the fastest kinetic parameter of the AP, is about 285 V/s in adult ventricular cardiomyocytes 23, 40 causing a positive change in the membrane potential of about 130 mV. The minimum sample frequency of this near linear transient is around 8.8 kHz. hPSC‐CMs have smaller upstroke velocities (10–100 V/s) so that such high frequencies might not be required. Genetically encoded fluorescent voltage sensors, first used to map neural signalling pathways 41, are also potentially useful optical alternatives. Although they have improved since first introduced and have been stably expressed in hPSC‐CMs, they have longer response times than VSDs, limiting their utility (Supporting Information Table S1).
Multielectrode arrays (MEAs) are medium throughput, noninvasive alternatives that requires less operator training and specialized equipment compared to patch clamp. These are glass culture substrates containing electrodes that measure external electrical activity by transducing the extracellular ion flux into an electrical current readout of field potential. Several studies have shown that near‐equivalents of QT‐prolongation and changes in upstroke velocities can be measured as external field potentials. hESC‐CMs exposed to compounds affecting AP duration 29 for example, or hiPSC‐CMs from long QT patients with KCNQ1 mutations showed significant responses in MEA‐recordings 20 to 30 days after initiation of differentiation 42. Spira et al. recently manufactured MEAs with sharp electrodes that can penetrate the cell membrane and directly access the cytosol, much like patch‐clamp 43; however, variation in access resistance of the electrode and poor seal formation, as in the automated systems, limited their use as an alternative to conventional patch‐clamp.
VSDs and MEAs have an additional advantage: cardiac conduction velocities can be measured as the wave propagation velocity in hPSC‐CM monolayer cultures; this can reveal defective electrical conduction in the heart. Thompson et al., for example, used VSDs to show improved AP conduction after engrafting ventricular‐like hESC‐CMs in a neonatal rat ventricular‐cell model of arrhythmogenic cardiac tissue 44.
Calcium Flux
The inflow of Ca2+ ions during the cardiac AP affects the AP itself and the shape of the contraction transient in hPSC‐CMs. The kinetics of the Ca2+ flux provide information on calcium handling which is carefully orchestrated by the L‐type channels, RYR2‐channels, and sarco/endoplasmic reticulum Ca2+‐ATPase (SERCA); this is of utmost importance in revealing disease phenotypes 32 and in predicting drug responses, toxicity, and cardiac safety 45, 46. Fluorescent probes that bind Ca2+ are the method of choice for calcium transient measurements (reviewed in 47). Gepstein et al., for example, used fluo 4 to investigate calcium handling by hESC‐CMs. He showed an increase in sarcoplasmic reticulum calcium load as a function of time in vitro and predicted Ca2+ storage release in response to caffeine and ryanodine 26.
In general, fluorescent calcium sensitive detectors (CSDs) are categorized as ratiometric or nonratiometric, the most important difference being their ability to measure absolute versus relative concentrations of Ca2+. Ratiometric dyes allow bound and unbound dye molecules to be determined; from these values the absolute concentration of Ca2+ can be calculated 48. This ratio of bound to unbound dye molecules is not known when using nonratiometric CSDs; while changes in fluorescence intensity reflect transient changes in cytosolic Ca2+ concentration these values cannot be translated to the absolute Ca2+ concentration without calibration. Additionally, there is interassay variability as the intracellular dye concentration is dependent on loading time, temperature, cell permeability, and cell access (e.g., cell cluster size) rather than the loading concentration 47. This makes keeping the intracellular dye concentration constant between experiments very difficult. The change in Ca2+ concentration is often calculated as a pseudo‐ratio dF/F 48. Although this pseudo‐ratio adequately relates the change in signal to the change in Ca2+ concentration, it is sometimes incorrectly interpreted as the maximum value depends on loading conditions. Relative changes are best calculated and compared within a zero‐to‐one scale defined by the baseline conditions, eliminating confusing values for the maximum amplitude.
In hPSC‐CMs, the relative change in Ca2+ response resulting from disease mutations or drugs is often more important than absolute levels. For example, Kosmidis et al. recently showed Ca2+ handling increased relatively in response to the synthetic glucocorticoid dexamethasone in ventricular hESC‐CMs 28 to 30 days after initiation of differentiation 41. It has also been shown that reduced caffeine‐induced NCX currents in hiPSC‐CMs from patients with catecholaminergic polymorphic ventricular tachycardia were evident as similar decreases of fluorescence intensity of the CSD 49. A recent review on calcium signaling in hPSC‐CMs 50 concluded that well‐characterized hPSC‐CMs are often reliable models for human cardiomyocyte calcium handling, as all calcium parameters of cardiac signaling are present and functional: calcium current induced Ca2+ release, Ca2+ sparks, NCX currents, SERCA2a, and ß‐adrenergic regulation. We would like to add that although CSDs are among the most valuable tools for evaluating hPSC‐CMs, caution is needed in interpreting data as not all calcium regulating organelles are necessarily fully developed in vitro (e.g., t‐tubules) possibly impacting conclusions.
Genetically encoded CSDs are relatively new; they were initially developed for neuroscience applications in vivo and have outperformed synthetic Ca2+ dyes in their sensitivity 51. However, as for the genetically encoded VSDs, slow on‐and‐off kinetics currently limit their use for cardiac mapping 52. Both ratiometric and nonratiometric detectors can easily be transduced and stably expressed in hPSC‐CMs but variability in expression levels introduces similar interassay variation as the loading conditions of dyes.
Force of Contraction
Heart failure is associated with decreased contraction force or a change in its kinetics 53. Bray et al. showed that myocyte geometry dictates sarcomeric alignment 54; in ventricular hPSC‐CMs this was optimal at width‐to‐length (or “aspect”) ratios of 1:7 and that it determined the net force of contraction 55. The maximum contraction forces reported for hPSC‐CMs are always an order of magnitude lower than for adult cardiomyocytes (see Table 1). Contraction of cardiac muscle is characterized by isotonic and isometric phases, where tension is developed with and without the cell being able to shorten, respectively, for exapmle, in free space or attached. Generally, the isotonic phase of hPSC‐CM contraction has been assessed most often 5 and although techniques have been developed that assess the isometric phase of single cardiomyocytes 56, they have not yet been used on hPSC‐CMs.
Measurements of isotonic tension on hPSC‐CMs have been done on single cells, or on two‐dimensional (2D) and three‐dimensional (3D) multicellular cultures. Rape et al. developed polyacrylamide‐coated coverslips containing fluorescent beads, with 20 µm wide microcontact‐printed gelatine lines to guide orientation and elongation of single cells 57. We used this technique (Fig. 1A, D) recently to show that a commercially available culture medium for cardiomyocyte maturation increased contraction force of ventricular hESC‐CM 33 days after initiation of differentiation compared to human fetal ventricular cardiomyocytes 5. Another study by Rodriguez et al. using an array of small polydimethylsiloxane (PDMS) microposts showed higher contractile velocities in single ventricular hiPSC‐CMs on a surface coated with laminin compared to fibronectin and collagen IV 17. Of note though, the position in the z‐axis of the post to which the cardiomyocyte attached affected its deflection so that correction for this underestimation is needed. Also, since the stiffness of the substrate on which hPSC‐CMs are plated influences the force they generate 60, direct comparison of absolute values from different assays (i.e. with different substrates) is not meaningful.
Figure 1.
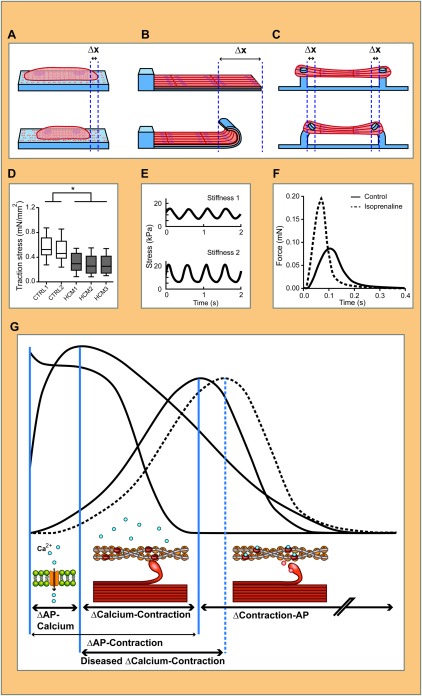
Methods for measuring contraction force in human pluripotent stem cells‐derived cardiomyocytes (hPSC‐CMs) in single cell, two‐dimensional (2D) and three‐dimensional (3D) constructs. hPSC‐CM cultured as single cells, 2D muscular thin film (MTF) or 3D engineered heart tissue (EHT) and corresponding methods for contraction force measurements. In (A), fluorescent beads are present in the elastic substrate, their displacement caused by the contraction of a single hPSC‐CM is measured and used to calculate the resulting stress. In (D), this method is used to show a difference in maximum force of contraction in hiPSC‐CMs with MYBPC3 mutations (HCM1, HCM2, and HCM3) compared to control (CTRL1 and CTRL2) (adapted from 58). Similarly, the deflection of MTFs can be used to calculate the force (B). The stress generated on different substrates with varying elasticity (E) (adapted from 59) can be used to calculate the actual force of contraction. EHTs are fabricated around silicon rubber posts that deflect during contraction (C) and can be related to the force of contraction. This has been used to show the change in force of contraction in response to isoprenaline (F) (adapted from 25). In (G), the solid traces show an example of simultaneous measurement of AP, calcium flux (Ca2+), and contraction, which allows correlation between these biophysical parameters in time (vertical blue lines) to gain mechanistic insights in diseases and drug responses. Aspects of disease or drug responses reflected in the traces that might be compromised in hPSC‐CMs are shown below: influx of calcium ions (blue circles) to the cytosol, binding of the myosin head to the actin filament, and the release of the myosin head by binding of calcium to the filament. The dotted trace indicates an example of a diseased phenotype: if the calcium binding site on the filament is compromised in a diseased hPSC‐CM one would expect the delay between Ca2+ and contraction to be longer, while the delay between AP and Ca2+ remains unaffected. Abbreviation: AP, action potential.
Muscular thin films (MTFs) or biohybrid films are 2D constructs first developed for use with neonatal rat ventricular cardiomyocytes by Feinberg et al. (Fig. 1B). They showed that greater tissue alignment resulted in a higher peak systolic stress 61. Since then, MTFs have been proposed as in vitro contractility assays for drug screening and disease modeling 62. However, Hinson et al. recently showed that 2D constructs do not recapitulate the structured architecture of native cardiac tissue well enough to capture the diseased phenotype in MTFs from patient hiPSC‐CMs with a mutation in the sarcomeric protein titin. In contrast, 3D cardiac tissues generated from the same patient‐derived iPSC line clearly showed reduced contraction 63.
Other approaches for measuring force of contraction include Engineered Heart Tissue (EHTs), cardiac microtissues, and cardiac microwires. These are millimeter‐sized biomaterial 3D structures seeded with cardiomyocytes in vitro, which mimic cardiac myobundles (Fig. 1C). EHTs are attached to PDMS posts and their deflection is directly related to the force exerted by the cell. hPSC‐CMs incorporated in EHTs have been reported to be more mature than conventional 2D cultures, exhibiting highly organized sarcomeres, myocyte elongation, and multinucleation (Table 1). For this reason and because of cell population heterogeneity that includes many fibroblasts, EHTs are strong candidates as models of mature functional myocardium. EHTs induce clinically relevant drug responses, such as the positive inotropic effects of isoprenaline, in ventricular hiPSC‐CMs from day 25 on after initiation of differentiation 25. Despite their exceptional myocardial maturity, the immediate utility of hPSC‐CM‐EHTs in high‐throughput screening is limited by the large numbers of cells they require, drug penetration and challenges with imaging. However, they are particularly amenable to analysis using classical physiology techniques such as “skinning” often used on explanted primary heart tissue, in which cytoplasm and membranes are removed leaving just the sarcomeric structures intact for force measurement 8.
Apart from measuring mechanical displacement, the kinetics of contraction force can also be determined from sarcomere movement, motion vector analysis and (as a surrogate) impedance measurements. During contraction, the sarcomere shortens and the distance between Z‐disks is reduced. This can be measured in hiPSC‐CMs as a change in sarcomeric distance which can be monitored using genetically encoded fluorescent alpha‐actinin reporters built into hiPSC or live staining to visualize sarcomeres directly 55. However, imaging systems with high spatial (∼200 nm) and temporal (∼30 ms) resolution are required to assess kinetics accurately. Motion vector analysis by contrast does not require such high spatial resolution, ectopic reporters, or staining methods. It uses transmitted light microscopy to calculate the kinetics of contraction through, for example, edge detection algorithms or, more recently, an algorithm evaluating frame‐to‐frame similarities 64. After taking substrate mechanics into account, this technique compares well with other measurement techniques, illustrating its utility 64. Impedance measurements have also been used as readouts of contractile kinetics 65, but to date these have not been compared quantitatively with established technologies. Impedance measurement detects alterations in resistance of cardiomyocytes as a function of shape changes during contraction. xCELLigence (ACEA Biosciences, San Diego, CA, www.aceabio.com) and CardioExcyte96 (Nanion Technologies, Munich, Germany, www.nanion.de) are among the most widely used devices for measuring impedance. Results are encouraging 66 but require further validation.
Multiplexed Measurements
More valuable are attempts to measure multiple dynamic parameters in hPSC‐CMs simultaneously, as their temporal correlation can provide crucial insights into drug responses or disease mechanisms. For example, diseased hiPSC‐CMs might have a genetically defective contractile apparatus, which delays the interval between the calcium flux and the contraction transient and/or alters drug response (Fig. 1G).
The integration of multiple standard optical assays is an approach now being developed commercially for hPSC‐CMs. Q‐State Biosciences (Cambridge, MA, www.qstatebio.com), for example, is offering a platform to analyse calcium flux and electrical activity simultaneously, although initially on neural cells. Relatively slow genetically encoded dyes may limit use for hPSC‐CMs at present but this will undoubtedly improve (Supporting Information Table S1). Clyde Biosciences (Newhouse, United Kingdom, www.clydebio.com) measure calcium, contraction and AP in the same cells sequentially as a service but at present without temporal correlation. Drawbacks of these multiplexed measurements include their invasiveness, light‐induced generation of reactive oxygen species 67, and chemical interactions of the surface of the substrate with the cardiomyocytes. As an example of invasiveness, the chemical interaction of the calcium indicator with free cytosolic calcium ions will reduce the number of free calcium ions available for the contractile apparatus. When the force of contraction is measured in the presence of a calcium indicator, the reduction in free cytosolic Ca2+ will cause a significantly lower outcome in force. Since interactions between the techniques used for measurement can impact their outcome, Supporting Information Table S2 summarizes and compares the most important features of dynamic measurement techniques qualitatively and scores their applicability in multiplexed systems. This table can be used as a starting point to select combinations of bioassays for hPSC‐CMs that are compatible because they do not show mutual interference.
Applying Biophysical Stimuli
hPSC‐CMs not only exert stress, and strain during contraction but they can also respond to imposed biophysical stimuli.
Mechanical Stimulation
Kensah et al. showed that increased static stress enhances sarcomere alignment, cell‐to‐cell coupling and force of contraction in ventricular hPSC‐CMs 7. Salameh et al. reported on using “cyclic mechanical stretch” (CMS) to induce alignment 68; CMS has also been shown to increase intercellular coupling between cardiomyocytes 69. In addition, manipulating the magnitude or frequency of mechanical stress might also be used to reveal disease phenotypes in vitro that would not otherwise be evident; an example would be hypertrophic cardiomyopathy which in vivo can manifest as sudden death during exercise. Force transducers (as in the Flexcell Tension FX‐series) (Flexcell, Burlington, NC, http://www.flexcellint.com/) or an oscillating vacuum underneath a thin film substrate 70 can be used to induce (uniaxial) stress on substrates. Although PDMS is often the material of choice we, and others 71, have encountered cell detachment during stretching due to the hydrophobic character of the material. Special coatings are currently being developed to solve this problem.
Electric Field and Optical Stimulation
Mature ventricular cardiomyocytes do not generate spontaneous APs but rely on pacemaker activity for contraction that is regulated by the sinus atrial node (SAN). Because hPSC‐CMs are often cultured as isolated ventricular cell populations without SAN cells, electrical stimulation might be key to inducing maturation in vitro 72. In addition, congestive heart failure can be induced in vivo by forcibly “pacing” the heart at increased frequencies (reviewed by Moe and Armstrong 73) and may also be induced in (diseased) hiPSC‐CMs in vitro. Such frequency‐dependent behavior emphasizes the importance of absolute beating frequency control in ventricular hPSC‐CMs, either to exclude interassay variation or to investigate pacing‐induced effects.
Exposing cardiomyocytes to short electric fields of 0.5 to 10 ms, causes the membrane to depolarize and initiates an AP. Long‐term pacing experiments have, however, been limited by electrolysis at the culture medium‐electrode interface. This can be reduced by perfusion or frequently refreshing medium but the issue is circumvented using optogenetics.
One optogenetic approach is based on channelrhodopsin‐2 (ChR2), a light gated sodium ion channel originally found in algae to enable light induced movement. Nagel et al. showed that, when expressed in mammalian cells, the influx of Na+ initiates the AP cascade directly 74. Zhuge et al. demonstrated that ChR2 can be used to depolarize hESC‐CMs in vitro 75. However, hPSC‐CM cultures in vitro often lack adequate concentrations of retinal—essential for the conformational change under the influence of blue light—so that ChR2 channels do not respond; this can be solved by adding retinal to the medium 76.
Outlook
Rapid technological advances in hPSC biology over the past 5 years are poised to transform disease modelling and drug discovery for the heart. hPSC‐CMs capture genetic variation among healthy individuals and patients with cardiac disease. The development of CRISPR/CAS9 for efficient genetic modification of hPSCs now allows the generation of isogenic pairs—or even series—of diseased and healthy hPSC‐CMs, differing only in the gene of interest 42. Shortcomings remain, the most significant being hPSC‐CM maturity: they are not yet adult cardiomyocyte mimics. Improved and defined culture conditions, heterogeneous cell populations and 3D tissue engineering are, however, contributing to finding solutions and in at least two cases—hypertrophic myopathy caused by mutations in MYBC3 [58] and titin 63—these have been crucial in revealing the disease phenotype. Further improvements in (bio)physical conditions may be expected to allow more cardiac phenotypes in hPSC‐CMs to be revealed with greater differences in experimental and control values.
Improved methods to assess responses and drivers of physiological changes in hPSC‐CMs are still essential to derive full benefit from the opportunities presented by patient cells. New multiplexed systems will soon allow temporal correlation between multiple cardiac features in various contexts of cell population composition, tissue architecture and culture media so that these models accurately mimic physiological and/or pathological characteristics of cardiac tissue. As these models become more complex, they are likely to require more sophisticated measurement techniques as readouts. For example, all cell types in the heart, not just those that are contractile, could be assessed separately by introducing different genetically encoded fluorescent probes into cell type specific loci. Combined with multiplexed optical systems, it would then be possible to assess and correlate the different cell type responses, providing insight into their interactions and identifying the major cellular culprit in cardiac disease. The whole range of single cell and multicellular heterogeneous cell population assays will likely be necessary depending on specific requirements for research on underlying disease mechanisms to drug discovery and repurposing.
In order for Pharma to adopt hPSC‐CM models in their drug development trajectory, these integrated measurement techniques will have to be scalable and high‐throughput. This will reduce the costs and make the “price per data point” acceptable in a business model. Multidisciplinary collaboration between engineers, stem cell biologists, physicists, materials scientists is the way forward in implementing hPSCs in the next generation of cardiac drug discovery and disease research.
Author Contributions
B.J.v.M.: conception and design, manuscript writing; L.G.J.T.: conception and design, manuscript editing; C.L.M.: conception and design, manuscript editing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
C.L.M. is co‐founder of Pluriomics b.v.
Supporting information
Supporting Information
Acknowledgements
We apologize to those authors whose many papers we have not cited due to length constraints. We thank Bas Blankevoort for figure design. Work in the Mummery lab is supported by the European Research Council (ERCAdG 323182 STEMCARDIOVASC) and the GSK‐NC3R CrackIt programme INPULSE.
Available online without subscription through the open access option.
References
- 1. Laverty HG, Benson C, Cartwright EJ et al. How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines? Br J Pharmacol 2011;163:675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Redfern WS, Carlsson L, Davis AS et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: Evidence for a provisional safety margin in drug development. Cardiovasc Res 2003;58:32–45. [DOI] [PubMed] [Google Scholar]
- 3. Mummery CL, Zhang J, Ng ES et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods Overview. Circ Res 2012;111:344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Veerman CC, Kosmidis G, Mummery CL et al. Immaturity of human stem‐cell‐derived cardiomyocytes in culture: Fatal flaw or soluble problem? Stem Cells Dev 2015;24:1035–1052. [DOI] [PubMed] [Google Scholar]
- 5. Ribeiro MC, Tertoolen LG, Guadix JA et al. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro—correlation between contraction force and electrophysiology. Biomaterials 2015;51:138–150. [DOI] [PubMed] [Google Scholar]
- 6. Birket MJ, Ribeiro MC, Verkerk AO et al. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat Biotechnol 2015;33:970–979. [DOI] [PubMed] [Google Scholar]
- 7. Kensah G, Lara AR, Dahlmann J et al. Murine and human pluripotent stem cell‐derived cardiac bodies form contractile myocardial tissue in vitro. Eur Heart J 2013;34:1134–1146. [DOI] [PubMed] [Google Scholar]
- 8. van der Velden J, Klein LJ, van der Bijl M et al. Force production in mechanically isolated cardiac myocytes from human ventricular muscle tissue. Cardiovasc Res 1998;38:414–423. [DOI] [PubMed] [Google Scholar]
- 9. Snir M, Kehat I, Gepstein A et al. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell‐derived cardiomyocytes. Am J Physiol Heart Circ Physiol 2003;285:H2355–H2363. [DOI] [PubMed] [Google Scholar]
- 10. McCain ML, Parker KK. Mechanotransduction: The role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch Eur J Physiol 2011;462:89–104. [DOI] [PubMed] [Google Scholar]
- 11. Schaaf S, Shibamiya A, Mewe M et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One 2011;6:e26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundy SD, Zhu W‐Z, Regnier M et al. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 2013;22:1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehta A, Chung YY, Ng A et al. Pharmacological response of human cardiomyocytes derived from virus‐free induced pluripotent stem cells. Cardiovasc Res 2011;91:577–586. [DOI] [PubMed] [Google Scholar]
- 14. Zhang D, Shadrin IY, Lam J et al. Tissue‐engineered cardiac patch for advanced functional maturation of human ESC‐derived cardiomyocytes. Biomaterials 2013;34:5813–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boron WF. Medical Physiology. 2nd ed Elsevier, Health Sciences Division, 2009. [Google Scholar]
- 16. Klug MG, Soonpaa MH Field LJ. DNA synthesis and multinucleation in embryonic stem cell‐derived cardiomyocytes. Am J Physiol 1995;269:H1913–H1921. [DOI] [PubMed] [Google Scholar]
- 17. Rodriguez ML, Graham BT, Pabon LM et al. Measuring the contractile forces of human induced pluripotent stem cell‐derived cardiomyocytes with arrays of microposts. J Biomech Eng 2014;136:051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olivetti G, Cigola E, Maestri R et al. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J Mol Cell Cardiol 1996;28:1463–1477. [DOI] [PubMed] [Google Scholar]
- 19. Birket MJ, Casini S, Kosmidis G et al. PGC‐1α and reactive oxygen species regulate human embryonic stem cell‐derived cardiomyocyte function. Stem Cell Reports 2013;1:560–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piquereau J, Caffin F, Novotova M et al. Mitochondrial dynamics in the adult cardiomyocytes: Which roles for a highly specialized cell? Front Physiol 2013;4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mummery C, Ward‐van Oostwaard D, Doevendans P et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm‐like cells. Circulation 2003;107:2733–2740. [DOI] [PubMed] [Google Scholar]
- 22. Ma J, Guo L, Fiene SJ et al. High purity human‐induced pluripotent stem cell‐derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. AJP Hear Circ Physiol 2011;301:H2006–H2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magyar J, Iost N, Körtvély Á et al. Effects of endothelin‐1 on calcium and potassium currents in undiseased human ventricular myocytes. Pflugers Arch 2000;441:144–149. [DOI] [PubMed] [Google Scholar]
- 24. Lee P, Klos M, Bollensdorff C et al. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell‐derived cardiac myocyte monolayers. Circ Res 2012;110:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stoehr A, Neuber C, Baldauf C et al. Automated analysis of contractile force and Ca2+ transients in engineered heart tissue. AJP Hear Circ Physiol 2014;306:H1353–H1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Satin J, Itzhaki I, Rapoport S et al. Calcium handling in human embryonic stem cell‐derived cardiomyocytes. Stem Cells 2008;26:1961–1972. [DOI] [PubMed] [Google Scholar]
- 27. Rana P, Anson B, Engle S et al. Characterization of human‐induced pluripotent stem cell‐derived cardiomyocytes: Bioenergetics and utilization in safety screening. Toxicol Sci 2012;130:117–131. [DOI] [PubMed] [Google Scholar]
- 28. Beer M, Seyfarth T, Sandstede J et al. Absolute concentrations of high‐energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P‐SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 2002;40:1267–1274. [DOI] [PubMed] [Google Scholar]
- 29. Braam SR, Tertoolen L, Stolpe A et al. Prediction of drug‐induced cardiotoxicity using human embryonic stem cell‐derived cardiomyocytes. Stem Cell Res 2010;4:107–116. [DOI] [PubMed] [Google Scholar]
- 30. Bellin M, Casini S, Davis RP et al. Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long‐QT syndrome. EMBO J 2013;32:3161–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang P, Lan F, Lee AS et al. Drug screening using a library of human induced pluripotent stem cell‐derived cardiomyocytes reveals disease‐specific patterns of cardiotoxicity. Circulation 2013;127:1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin B, Li Y, Han L et al. Modeling and study of the mechanism of dilated cardiomyopathy using induced pluripotent stem cells derived from individuals with duchenne muscular dystrophy. Dis Model Mech 2015;8:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellin M, Marchetto MC, Gage FH et al. Induced pluripotent stem cells: The new patient? Nat Rev Mol Cell Biol 2012;13:713–726. [DOI] [PubMed] [Google Scholar]
- 34. Soldner F, Laganiere J, Cheng AW et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 2012;146:318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruan J‐L, Tulloch NL, Saiget M et al. Mechanical stress promotes maturation of human myocardium from pluripotent stem cell‐derived progenitors. Stem Cells 2015;33:2148–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paci M, Hyttinen J, Rodriguez B et al. Human induced pluripotent stem cell‐derived versus adult cardiomyocytes: An in silico electrophysiological study on effects of ionic current block. Br J Pharmacol 2015;172:5147–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Devalla HD, Schwach V, Ford JW et al. Atrial‐like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial‐selective pharmacology. EMBO Mol Med 2015;7:394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheel O, Frech S, Amuzescu B et al. Action potential characterization of human induced pluripotent stem cell‐derived cardiomyocytes using automated patch‐clamp technology. Assay Drug Dev Technol 2014;12:457–469. [DOI] [PubMed] [Google Scholar]
- 39. Gillie DJ, Novick SJ, Donovan BT et al. Development of a high‐throughput electrophysiological assay for the human ether‐à‐go‐go related potassium channel hERG. J Pharmacol Toxicol Methods 2013;67:33–44. [DOI] [PubMed] [Google Scholar]
- 40. Dangman KH, Danilo P, Hordof AJ et al. Electrophysiologic characteristics of human ventricular and purkinje fibers. Circulation 1982;65:362–368. [DOI] [PubMed] [Google Scholar]
- 41. Siegel MS, Isacoff EY. A genetically encoded optical probe of membrane voltage. Neuron 1997;19:735–741. [DOI] [PubMed] [Google Scholar]
- 42. Zhang M, D'aniello C, Verkerk AO et al. Recessive cardiac phenotypes in induced pluripotent stem cell models of Jervell and Lange‐Nielsen syndrome: Disease mechanisms and pharmacological rescue. Proc Natl Acad Sci USA 2014;111:E5383–E5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spira ME, Hai A. Multi‐electrode array technologies for neuroscience and cardiology. Nat Nanotechnol 2013;8:83–94. [DOI] [PubMed] [Google Scholar]
- 44. Thompson SA, Burridge PW, Lipke EA et al. Engraftment of human embryonic stem cell derived cardiomyocytes improves conduction in an arrhythmogenic in vitro model. J Mol Cell Cardiol 2012;53:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim JJ, Yang L, Lin B et al. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J Mol Cell Cardiol 2015;81:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Braam SR, Tertoolen L, Casini S et al. Repolarization reserve determines drug responses in human pluripotent stem cell derived cardiomyocytes. Stem Cell Res 2013;10:48–56. [DOI] [PubMed] [Google Scholar]
- 47. Silei V, Fabrizi C, Venturini G et al. Measurement of intracellular calcium levels by the fluorescent Ca2+ indicator calcium‐green. Brain Res Protoc 2000;5:132–134. [DOI] [PubMed] [Google Scholar]
- 48. Svoboda K, Denk W, Kleinfeld D et al. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 1997;385:161–165. [DOI] [PubMed] [Google Scholar]
- 49. Zhang X‐H, Haviland S, Wei H et al. Ca2+ signaling in human induced pluripotent stem cell‐derived cardiomyocytes (iPS‐CM) from normal and catecholaminergic polymorphic ventricular tachycardia (CPVT)‐afflicted subjects. Cell Calcium 2013;54:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang X‐H, Morad M. Calcium signaling in human stem cell‐derived cardiomyocytes: Evidence from normal subjects and CPVT afflicted patients. Cell Calcium: 2016;59:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen T‐W, Wardill TJ, Sun Y et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013;499:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herron TJ, Lee P Jalife J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res 2012;110:609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davies CH, Davia K, Bennett JG et al. Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure. Circulation 1995;92:2540–2549. [DOI] [PubMed] [Google Scholar]
- 54. Bray M‐AA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton 2008;65:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ribeiro AJS, Ang Y‐S, Fu J‐D et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA 2015;112:12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nishimura S, Yasuda S, Katoh M et al. Single cell mechanics of rat cardiomyocytes under isometric, unloaded, and physiologically loaded conditions. Am J Physiol Heart Circ Physiol 2004;287:H196–H202. [DOI] [PubMed] [Google Scholar]
- 57. Rape AD, Guo W‐HH, Wang Y‐LL. The regulation of traction force in relation to cell shape and focal adhesions. Biomaterials 2011;32:2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Birket MJ, Ribeiro MC, Kosmidis G et al. Contractile defect caused by mutation in MYBPC3 revealed under conditions optimized for human PSC‐cardiomyocyte function. Cell Rep 2015;13:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grosberg A, Alford PW, McCain ML et al. Ensembles of engineered cardiac tissues for physiological and pharmacological study: Heart on a chip. Lab Chip 2011;11:4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hersch N, Wolters B, Dreissen G et al. The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening. Biol Open 2013;2:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feinberg AW, Alford PW, Jin H et al. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials 2012;33:5732–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grosberg A, Nesmith AP, Goss JA et al. Muscle on a chip: In vitro contractility assays for smooth and striated muscle. J Pharmacol Toxicol Methods 2012;65:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hinson JT, Chopra A, Nafissi N et al. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 2015;349:982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kijlstra JD, Hu D, Mittal N et al. Integrated analysis of contractile kinetics, force generation, and electrical activity in single human stem cell‐derived cardiomyocytes. Stem Cell Rep 2015;5:1226–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hayakawa T, Kunihiro T, Ando T et al. Image‐based evaluation of contraction–relaxation kinetics of human‐induced pluripotent stem cell‐derived cardiomyocytes: Correlation and complementarity with extracellular electrophysiology. J Mol Cell Cardiol 2014;77:178–191. [DOI] [PubMed] [Google Scholar]
- 66. Scott CW, Zhang X, Abi‐Gerges N et al. An impedance‐based cellular assay using human iPSC‐derived cardiomyocytes to quantify modulators of cardiac contractility. Toxicol Sci 2014;142:331–338. [DOI] [PubMed] [Google Scholar]
- 67. Lavi R, Shainberg A, Friedmann H et al. Low energy visible light induces reactive oxygen species generation and stimulates an increase of intracellular calcium concentration in cardiac Cells. J Biol Chem 2003;278:40917–40922. [DOI] [PubMed] [Google Scholar]
- 68. Salameh A, Wustmann A, Karl S et al. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res 2010;106:1592–1602. [DOI] [PubMed] [Google Scholar]
- 69. Salameh A, Dhein S. Effects of mechanical forces and stretch on intercellular gap junction coupling. Biochim Biophys Acta Biomembr 2013;1828:147–156. [DOI] [PubMed] [Google Scholar]
- 70. Dekker R, Braam S, Henneken V et al. Living chips and chips for the living. 2012 IEEE Bipolar/BiCMOS Circuits and Technology Meeting (BCTM) . Eden Prairie, MN, IEEE; 2012:1–9. [Google Scholar]
- 71. Wipff P‐J, Majd H, Acharya C et al. The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials 2009;30:1781–1789. [DOI] [PubMed] [Google Scholar]
- 72. Radisic M, Park H, Shing H et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA 2004;101:18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moe GW, Armstrong P. Pacing‐induced heart failure: A model to study the mechanism of disease progression and novel therapy in heart failure. Cardiovasc Res 1999;42:591–599. [DOI] [PubMed] [Google Scholar]
- 74. Nagel G, Szellas T, Huhn W et al. Channelrhodopsin‐2, a directly light‐gated cation‐selective membrane channel. Proc Natl Acad Sci USA 2003;100:13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhuge Y, Patlolla B, Ramakrishnan C et al. Human pluripotent stem cell tools for cardiac optogenetics. Conf Proc IEEE Eng Med Biol Soc 2014;2014:6171–6174. [DOI] [PubMed] [Google Scholar]
- 76. Ullrich S, Gueta R, Nagel G et al. Degradation of channelopsin‐2 in the absence of retinal and degradation resistance in certain mutants. Biol Chem 2013;394:271–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


