Abstract
Neural stem cells (NSCs) reside in specialized niches in the adult mammalian brain. The ventricular–subventricular zone (V‐SVZ), adjacent to the lateral ventricles, gives rise to olfactory bulb (OB) neurons, and some astrocytes and oligodendrocytes throughout life. In vitro assays have been widely used to retrospectively identify NSCs. However, cells that behave as stem cells in vitro do not reflect the identity, diversity, and behavior of NSCs in vivo. Novel tools including fluorescence activated cell sorting, lineage‐tracing, and clonal analysis have uncovered multiple layers of adult V‐SVZ NSC heterogeneity, including proliferation state and regional identity. In light of these findings, we reexamine the concept of adult NSCs, considering heterogeneity as a key parameter for analyzing their dynamics in vivo. V‐SVZ NSCs form a mosaic of quiescent (qNSCs) and activated cells (aNSCs) that reside in regionally distinct microdomains, reflecting their regional embryonic origins, and give rise to specific subtypes of OB interneurons. Prospective purification and transcriptome analysis of qNSCs and aNSCs has illuminated their molecular and functional properties. qNSCs are slowly dividing, have slow kinetics of neurogenesis in vivo, can be recruited to regenerate the V‐SVZ, and only rarely give rise to in vitro colonies. aNSCs are highly proliferative, undergo rapid clonal expansion of the neurogenic lineage in vivo, and readily form in vitro colonies. Key open questions remain about stem cell dynamics in vivo and the lineage relationship between qNSCs and aNSCs under homeostasis and regeneration, as well as context‐dependent plasticity of regionally distinct adult NSCs under different external stimuli. WIREs Dev Biol 2016, 5:640–658. doi: 10.1002/wdev.248
For further resources related to this article, please visit the WIREs website.
INTRODUCTION
Adult tissues contain a small number of cells that retain unique developmental properties, called adult stem cells. Adult stem cells undergo self‐renewal and have the ability to differentiate into a variety of postmitotic cells, thereby playing a central role in tissue maintenance under homeostasis and in response to injury. Dissecting the precise identity of adult stem cells is a prerequisite to understand their behavior and function in vivo. Importantly, adult stem cells do not constitute a homogeneous population. Rather they comprise a mosaic of individual cells that differ in their proliferation state, as well as in their responses to physiological inputs. Indeed, deeply quiescent and actively dividing stem cells coexist in many adult organs, regardless of somatic cell turnover rate.1 Quiescence is an actively maintained state that is thought to be important for long‐term stem cell self‐renewal, somatic cell replacement, and DNA integrity.2 A major focus of ongoing research is delineating whether quiescent and activated stem cells are different pools, with quiescent stem cells being a reserve population recruited upon need, or whether they are lineally related and reflect two states of the same cells. In either case, it is important to know if individual quiescent stem cells are capable of long‐term self‐renewal in vivo, and how they contribute to population self‐renewal under homeostasis and regeneration. Interestingly, adult stem cells often present diverse differentiation patterns, with distinct subpopulations giving rise to specific progeny under homeostasis. Whether this heterogeneity is fixed due to intrinsic fate commitment or whether it is dynamically responsive to external changes in the stem cell microenvironment, is still unknown in many systems. These challenging questions likewise apply to the mammalian brain, which also harbors quiescent and activated stem cells.
In the adult vertebrate brain, neural stem cells (NSCs) generate neurons throughout the lifespan. Intriguingly, adult NSCs across phylogeny exhibit hallmark features of glial cells,3, 4 although individual species differ in the levels of neurogenesis and brain areas to which new neurons are added.5 In the adult mammalian brain, stem cells reside in two main germinal regions: the subgranular zone (SGZ) of the dentate gyrus in the hippocampus, and the ventricular–subventricular zone (V‐SVZ) adjacent to the lateral ventricles.6 In this review, we focus primarily on the adult V‐SVZ niche, which gives rise to astrocytes, oligodendrocytes, and olfactory bulb (OB) interneurons throughout life.7 While historically NSCs have largely been studied in vitro as neurosphere‐forming cells,8 recent advances in new tools and technologies, including fluorescence activated cell sorting (FACS) purification and lineage tracing in vivo, now allow the behavior of prospectively defined cells to be illuminated. We outline and integrate recent findings along two principal dimensions of adult NSC heterogeneity, proliferation dynamics and regional identity, and their implications for understanding stem cell behavior and function in vivo. We then address the context‐dependent plasticity exhibited by adult NSCs under different physiological states, especially injury.
V‐SVZ STEM CELL LINEAGE
The V‐SVZ is a thin layer of dividing cells located along the walls of the lateral ventricles (Figure 1(a) and (b)), and is the largest germinal region in the adult rodent brain. V‐SVZ stem cells and their progeny are found between multiciliated ependymal cells, which are arranged as pinwheels along the ventricular surface, and a planar vascular plexus at the interface with the striatum9, 10, 11 (Figure 1(c)). V‐SVZ stem cells predominantly give rise to OB neurons, but also give rise to small numbers of glia.12, 13, 14, 15, 16
Figure 1.
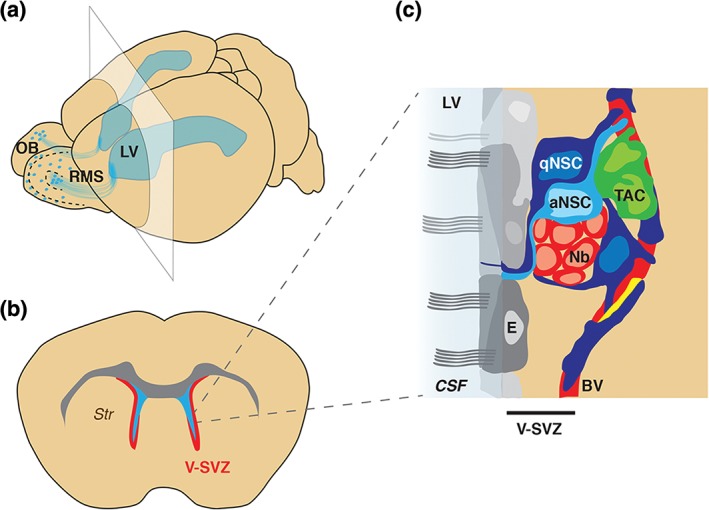
Architecture of the V‐SVZ niche. (a) Schema of the whole mouse brain showing the LVs (blue). The V‐SVZ lies adjacent to the walls of the LV, and generates neurons that migrate along the RMS to the OB. (b) Schema of coronal section at level of the plane shown in (a) showing the V‐SVZ (red) located between the lateral ventricles (light blue) and the striatum (Str). (c) Schema showing V‐SVZ cell types (modified from Ref 10). aNSC, activated neural stem cell; BV, blood vessel; CSF, cerebrospinal fluid; E, ependymal cells; LV, lateral ventricle; Nb, neuroblasts; OB, olfactory bulb; pericytes (yellow); qNSC, quiescent neural stem cell; RMS, rostral migratory stream; V‐SVZ, ventricular–subventricular zone; TAC, transit amplifying cells.
More than 15 years ago, V‐SVZ stem cells were shown to be Type B cells, which exhibit features of astrocytes at both the ultrastructural and molecular level, including expression of the markers glial fibrillary acidic protein (GFAP) and glutamate aspartate transporter (GLAST).17, 18 They have a radial morphology and contact the cerebrospinal fluid (CSF) at the center of ependymal cell pinwheels via a small apical process that exhibits a primary cilium. On their basal side, they extend a long process that frequently terminates on the vascular plexus.9 Type B cells act as stem cells under both homeostasis and during regeneration, and can give rise to multipotent self‐renewing neurospheres in vitro. 18 In vivo, quiescent stem cells become activated and give rise to neuroblasts (Type A cells) via rapidly dividing transit amplifying cells (TACs) (Type C cells) (Figure 1(c)). The neuroblasts then migrate to the OB, where they differentiate into different types of OB interneurons. Importantly, Type B cells also give rise to oligodendrocytes and astrocytes,12, 13, 14, 15, 16 but whether neurons and glia arise from distinct stem cell lineages in vivo is still unknown.
The identification of V‐SVZ NSCs as GFAP+ Type B cells raises important questions about how they differ from other brain astrocytes, and how heterogeneous this population is. In early studies, two types of Type B cells were described at the ultrastructural level. Type B1 cells have a light cytoplasm, contact the ventricle, and are largely quiescent. In contrast, Type B2 cells have a darker cytoplasm, are located closer to blood vessels, and incorporate [3H]‐thymidine.19 At the morphological level, several different types of astrocytes are found in the V‐SVZ.9, 19, 20, 21 Those with a characteristic branched morphology are considered ‘niche’ astrocytes, as opposed to those with a radial shape, which can divide. Antimitotic drug infusion demonstrated that a subset of Type B cells lacking epidermal growth factor receptor (EGFR) is quiescent, survives the treatment, and completely regenerates the V‐SVZ.18, 22 In contrast, activated stem cells express EGFR, are actively dividing, and are eliminated by antimitotic treatment.22 Thus, astrocytes in the V‐SVZ exhibit heterogeneity at the morphological, functional, and molecular levels. Identifying additional markers that resolve this heterogeneity is an ongoing and essential effort to reveal novel NSC subpopulations and understand their functional properties in vivo. As new NSC subpopulations are identified, it will be key to define their regional distribution, and how they map onto previously described ultrastructurally and morphologically distinct subtypes.
QUIESCENT AND ACTIVATED ADULT NSCs
Prospective Purification of Quiescent and Activated Adult NSCs
Adult NSCs share many features of brain astrocytes, including expression of glial markers. To distinguish NSCs from ‘niche’ astrocytes, and to discriminate between quiescent and activated NSCs, it is essential to combine multiple markers to allow their purification directly from their in vivo niche using FACS. Adult NSCs can be separated from brain astrocytes by using CD133 (prominin) in hGFAP::GFP mice,23 but this strategy does not distinguish between quiescent and activated NSCs (Table 1).
Table 1.
Common Markers of the NSC Lineage Are Often Shared by Different Cell Types in the V‐SVZ Lineage and Niche.
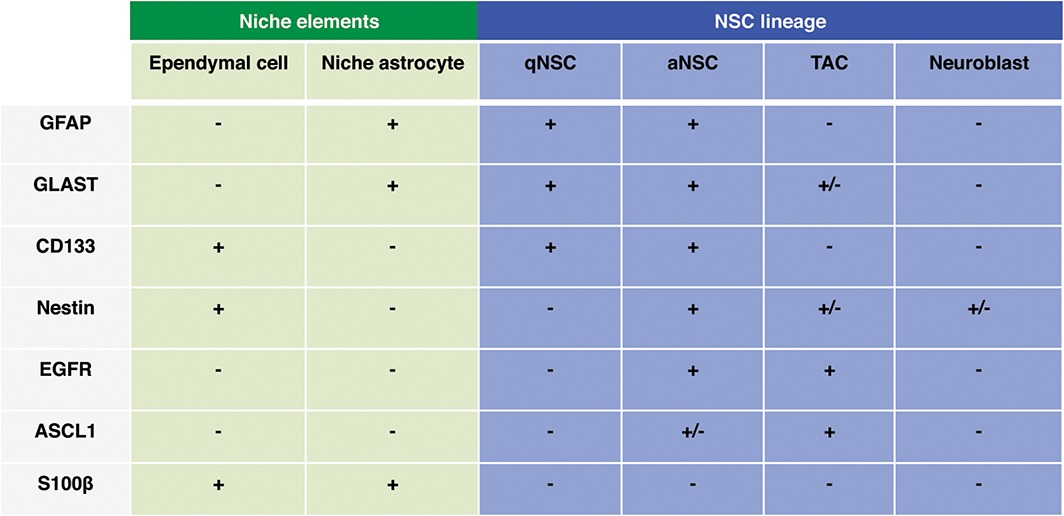
aNSC, activated NSC; EGFR, epidermal growth factor receptor; GFAP, glial fibrillary acidic protein; GLAST, glutamate aspartate transporter; NSC, neural stem cell; qNSC, quiescent NSC.
Multiple combinations of markers have recently been used to prospectively purify quiescent NSCs (qNSCs) from the adult V‐SVZ, and elucidate their functional properties, as well as gene expression profiles, as compared to actively dividing (activated, aNSCs) stem cells (Figure 2(a)). While all strategies use EGFR to distinguish aNSCs from qNSCs, they employ different markers or transgenic mice to prospectively identify the stem cell population, including hGFAP::GFP, CD133, GLAST, Hes5::GFP or LeX (Figure 2(a)).21, 24, 25, 26, 27 Although it is still unclear precisely how overlapping the purified qNSC populations are, they do exhibit common functional properties. qNSCs are slowly dividing based on cell cycle analysis, survive antimitotic treatment, and can regenerate the lineage after depletion of actively dividing stem cells and TACs.21, 25, 26, 27 Transplantation of purified qNSCs revealed that they have slower kinetics of neuron formation than aNSCs.21 Interestingly, qNSCs do not express Nestin, but upregulate EGFR and Nestin upon activation.21 Importantly, qNSCs only extremely rarely form neurospheres, or give rise to adherent colonies,21, 25, 27 highlighting that these in vitro assays are not a good read‐out of stem cell function in vivo 8 (Figure 2(a)). Instead they reflect the potential of actively dividing cells, including TACs, to behave as stem cells in vitro. 8
Figure 2.
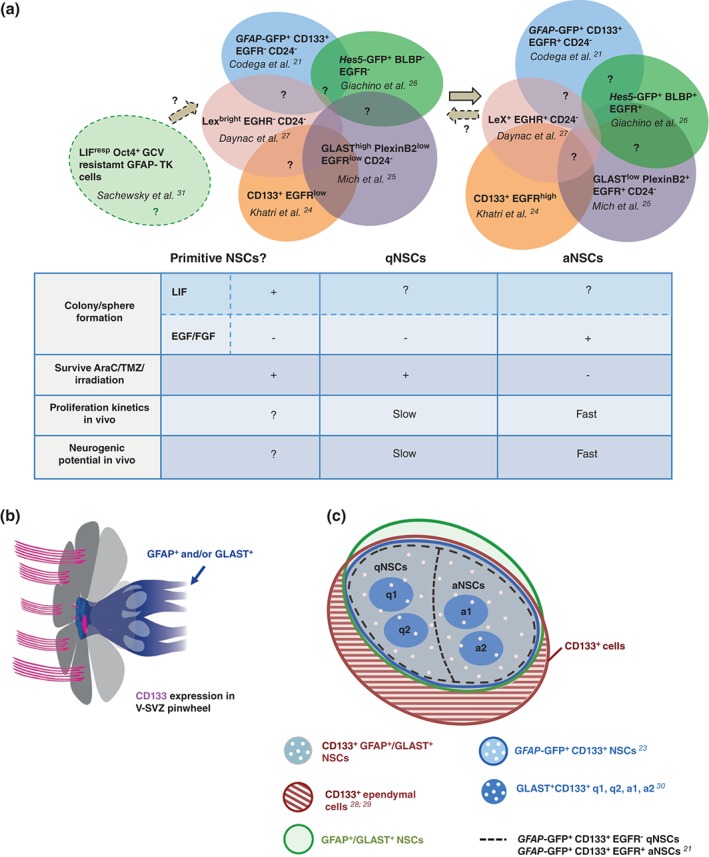
Prospective purification of adult NSCs. (a) Combination of markers used for FACS purification of adult V‐SVZ qNSCs and aNSCs (colored circles). The degree of overlap between the isolated subpopulations is still unclear (question marks). Table shows functional properties of qNSCs and aNSCs. Whether a primitive population (light green circle on the left) lies upstream of GFAP+/GLAST+ qNSCs or is a subpopulation of qNSCs is still unclear. (b) Schema of ependymal cell (grey) pinwheel and CD133 (magenta) expression in ependymal cell cilia, and on the primary cilium of qNSCs and over the apical surface of aNSCs (GFAP+ and/or GLAST+ cells, blue cells). (c) CD133+ cells comprise both ependymal cells (hatched brown) and GFAP+ and/or GLAST+ adult qNSCs and aNSCs (intersection between brown, green and blue circles). Among CD133+ NSCs, q1, q2, a1, and a2 subpopulations have been described. aNSCs, activated NSCs; FACS, fluorescence activated cell sorting; GFAP, glial fibrillary acidic protein; GLAST, glutamate aspartate transporter; NSC, neural stem cell; qNSCs, quiescent NSCs; V‐SVZ, ventricular–subventricular zone.
While ependymal cells have also been proposed to be quiescent stem cells, which can become activated upon injury,28, 29, 32, 33 the markers that were used were not ependymal specific. For instance, CD133 alone28, 29 cannot be used to purify ependymal cells and perform lineage tracing. Indeed, several reports have clearly shown that CD133 is also expressed by qNSCs and aNSCs9, 21, 23, 30 (Figure 2(b) and (c)), making interpretation of these studies difficult.
A ‘primitive’ population of dormant stem cells has also been proposed to lie upstream of GFAP+ qNSCs,31 which is resistant to ganciclovir treatment in Gfap‐Tk mice and forms neurospheres in response to LIF, but not with EGF and FGF (Figure 2(a)). It will be important to determine if these cells are a subpopulation of previously identified qNSCs, which are very slowly dividing and also do not form neurospheres with EGF and FGF (Figure 2(a)), or correspond to a completely separate pool.
Transcriptome Analysis Reveals Molecular Differences Between qNSCs and aNSCs
The ability to FACS‐purify qNSCs and aNSCs has allowed their gene expression profiles to be defined for the first time at both the population and single cell level, and has yielded novel insights into the molecular mechanisms underlying stem cell quiescence and activation.21, 30 Both single cell and population transcriptome analyses of qNSCs highlight that they dynamically integrate signals from the microenvironment, and actively maintain the quiescent state. qNSCs are enriched in gene categories of cell–cell adhesion, extracellular‐matrix‐response and anchorage‐dependent niche signals, as well as cell communication, signaling receptors, transmembrane transporters, and ion channels (Figure 3). In contrast, the aNSC transcriptome is highly enriched in cell cycle and DNA repair related gene categories. Interestingly, qNSCs and aNSCs differ in their energy metabolism, with glycolysis and fatty acid gene sets upregulated in qNSCs, and oxidative phosphorylation enriched in aNSCs. Reflecting their more active state, aNSCs have higher rates of protein synthesis than qNSCs.30
Figure 3.
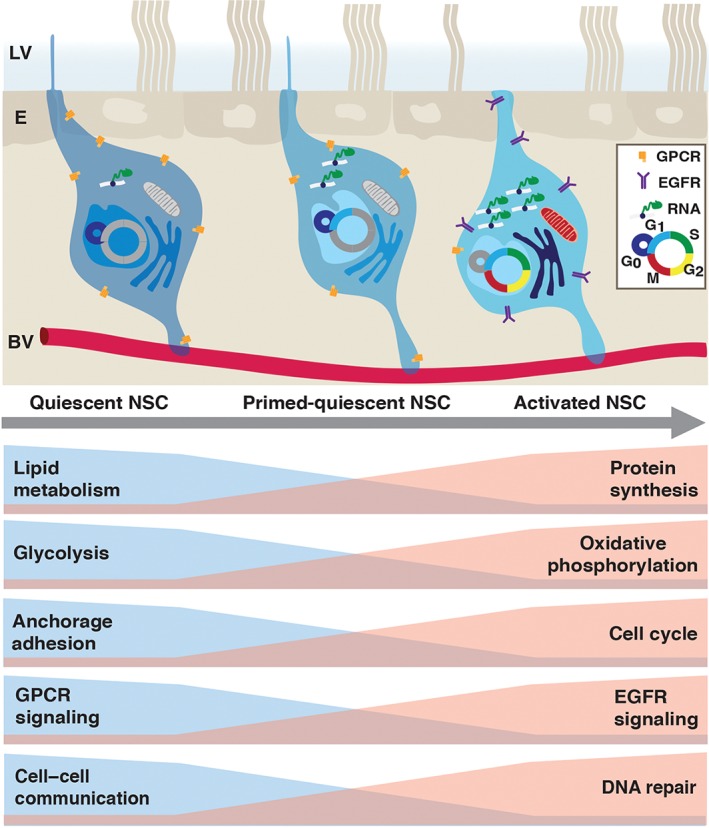
Molecular changes upon stem cell activation. Top: Schema of quiescent (left), primed‐quiescent (middle), and activated (right) adult V‐SVZ NSCs, located between the ependymal cell layer (E) lining the lateral ventricle (LV), and the vascular plexus (BV). Summary of transcriptome data of purified qNSCs and aNSCs at the population and single cell level. EGFR, epidermal growth factor receptor; GPCR, G‐protein coupled receptor; NSC, neural stem cell.
Single cell profiling of GLAST+ CD133+ V‐SVZ NSCs has provided further insight into adult NSC heterogeneity within the qNSC and aNSC populations, as well as into the qNSC–aNSC lineage transition (Figure 3). Within the quiescent pool, two states were identified: dormant (q1) and primed qNSCs (q2)30 (Figures 2(c) and 3). Cells in the primed state have slightly higher ribosomal activity and lower glial marker expression than dormant stem cells, but still lack cell cycle markers. Injury results in an increased proportion of primed NSCs. Interestingly, single cell analysis of SGZ stem cells revealed a similar pattern of deeply quiescent, primed and actively dividing NSCs.34 Although bioinformatic analysis suggests that qNSC activation follows a linear trajectory, it will be important to functionally test whether dormant, primed and activated stem cells are lineally related or whether they correspond to distinct NSC subpopulations, that are differentially recruited and respond to specific external stimuli.
Tracking the in vivo Dynamics of Adult NSCs over Time
A key step in defining adult NSC behavior in vivo is to determine their proliferation and lineage dynamics under homeostasis or regeneration, including their long‐term neurogenic or gliogenic potential, and whether they persist or become exhausted over time.
Lineage‐tracing in the adult V‐SVZ has been performed using a variety of different inducible Cre drivers, such as Gfap,25 Glast,25, 35 Nestin,36, 37 Dlx1,25 Ascl1,25, 38, 39 Gli1,16, 25 Sox1,40 Sox2,41 Fgfr3,42 Tlx,43 Musashi1,44 Id1,45 and Hes5 26 (Table 2). While the vast majority of cells produced in the V‐SVZ under normal conditions are neurons, Gfap +, Gli1 +, Nestin +, and Glast + NSCs also give rise to oligodendrocytes and astrocytes under homeostatic conditions (Table 2).12, 13, 14, 15, 16 However, whether NSCs are tripotent in vivo, or whether there are separate neurogenic and gliogenic stem cells or progenitors remains unclear. Importantly, the different Cre drivers used for lineage tracing are largely not specific to stem cells, and are either also expressed by cells later in the lineage or by cells in the surrounding niche (Table 1). Moreover, they do not allow the selective targeting of qNSCs and aNSCs in vivo to elucidate their respective long‐term behavior under different physiological conditions. To date, most lineage analyses have been characterized after 1 month, with 3‐month time points considered long‐term neurogenic23, 25, 26, 40, 43, 44 (Table 2). Although occasional studies have performed analysis at 6 or 13–15 months chase16, 36, 37, 38, 39, 42 (Table 2), detailed characterization of NSC long‐term behavior is still largely lacking.
Table 2.
Summary of In Vivo Lineage Tracing of Adult V‐SVZ NSCs
Despite these current limitations, important insights into adult NSC lineage dynamics in vivo have been gained from population‐based fate mapping. For the purposes of this review, we define analyses performed at one month as short‐term lineage tracing, and long term neurogenic activity as the presence of newly generated neuroblasts in the V‐SVZ and RMS, or increasing numbers of neurons in the OB two months or more after Cre induction. Based on these criteria, Hes5, Gfap, Gli1, and Glast lineages have long‐term neurogenic potential, while Dlx1, Ascl1, and Sox1 lineages are mainly short‐term (Table 2). In addition to single marker based lineage‐tracing, intersectional fate‐mapping using viral split‐Cre complementation demonstrated that GFAP+ CD133+ V‐SVZ cells sustain neurogenesis for 3 months,23 although this approach does not discriminate between qNSCs and aNSCs. To date, the only way to selectively target qNSCs is to perform lineage tracing during regeneration. This has revealed that initially labeled Glast +, Gfap +, Gli1 +, and Id1 + adult NSCs can replenish the V‐SVZ in vivo after depletion of actively dividing cells.16, 20, 25, 26, 45 Recent studies have suggested that qNSCs that are recruited during regeneration are set aside between embryonic day 12.5 and 15.5.46 Moreover, these cells largely remain quiescent until they become activated in the adult.16, 47 However, whether the quiescent pool recruited during regeneration in the adult is the same as the quiescent pool under homeostasis is still unknown.
Another key question is whether individual adult NSCs undergo self‐renewal throughout life shuttling between quiescent and activated states as they can in vitro, 21, 48 or whether they become exhausted after a few rounds of generating progeny in vivo. Clonal analysis allows dissecting the behavior of single NSCs and their progeny, thereby revealing their self‐renewal capacity or exhaustion over time. Two recent clonal studies, using either Glast‐CreERT2; Confetti reporter mice or embryonic retroviral library bar‐coding, suggest that there is no systematic self‐renewal of NSCs throughout life.35, 47 Instead, individual stem cells become activated, and transiently sustain fast clonal expansion of the neurogenic lineage.35 In both studies, many clones contained only olfactory‐bulb neurons at late time points, but some clones were detected that contained both neurons and a radial stem cell in the V‐SVZ. Although rare, these events provide evidence that at least some adult NSCs may undergo self‐renewal and revert back to a quiescent state.
REGIONAL HETEROGENEITY IN THE V‐SVZ
Regional heterogeneity is emerging as a key component in adult V‐SVZ stem cell identity and behavior, and is a function of both embryonic origin and niche patterning. While it has long been known that V‐SVZ stem cells generate both granule and periglomerular OB interneurons, fate‐mapping experiments are revealing the remarkable diversity of olfactory interneuron subtypes that are generated in the adult. Viral lineage tracing targeting different rostro‐caudal or dorso‐ventral areas of the postnatal and adult V‐SVZ, and Cre‐lox fate mapping, reveal that the V‐SVZ comprises a mosaic of stem cells that occupy different domains, many of which correlate with the regional expression of specific transcription factors (TFs).49, 50, 51, 52, 53, 54, 55, 56, 57
The above findings raise the important question of how and when adult NSCs acquire their regional identity. Interestingly, postnatal NSCs become regionally specified as early as embryonic day E15.5.47 The different regions of the adult V‐SVZ arise from discrete germinal areas in the embryo. Cre‐loxP fate‐mapping of cells in the embryo based on regional expression of TFs has shown that the pallium, lateral/medial ganglionic eminences, and septum give rise to the dorsal, lateral and medial walls of the adult V‐SVZ, respectively (Figure 4(a)).52 Moreover, regional expression of some TFs in the embryo is conserved in the postnatal and adult V‐SVZ (Figure 4(a) and (b)). A major difference in the adult is the thickness of the V‐SVZ, which decreases dramatically, especially the dorsal region, as compared to the embryonic and postnatal brain (Figure 4(a)).52
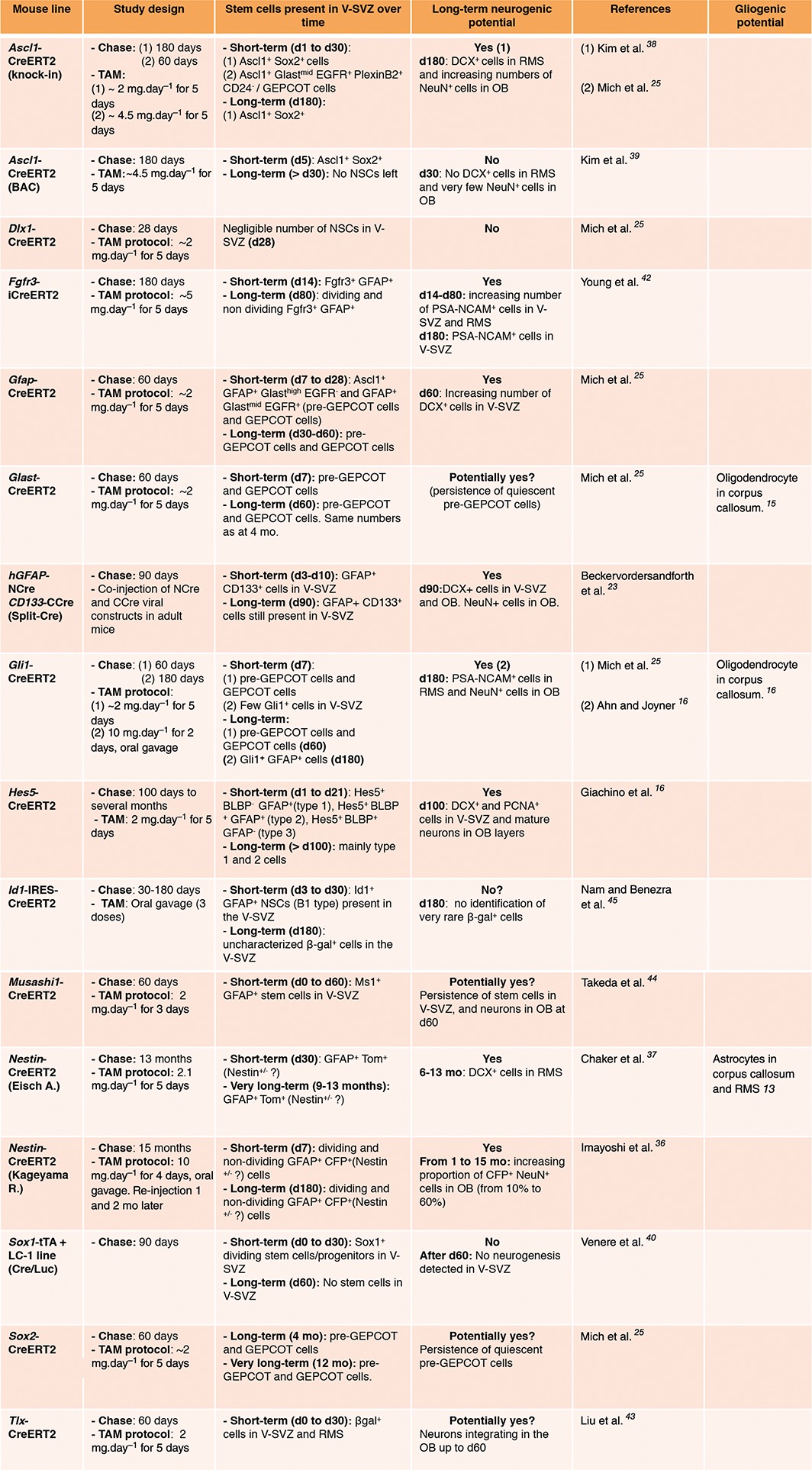
Figure 4.
Regional identity of adult NSCs. (a) Left shows schema of coronal section of the embryonic brain highlighting germinal layers that have been fate mapped based on transcription factor expression to different domains in the adult V‐SVZ (right). Color code depicts the correspondence between embryonic and adult neurogenic zones. LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; Sp, septum. (b) Schema shows transcription factor microdomains in a three‐dimensional representation of the lateral ventricle walls. Lower ventricle shows the expression pattern of transcription factors along the lateral wall, and the upper ventricle shows the expression pattern of transcription factors along the medial wall. (c) DAPI image of coronal section of the olfactory bulb showing different subtypes of olfactory bulb interneurons derived from regionally distinct stem cells. The color code of the interneurons reflects the color code of transcription factor domains in the V‐SVZ walls in panel (b). Interneurons integrate into the superficial and deep granule cell layer (inner dashed line), as well as in the glomerular layer (outer dashed line). GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; IPL, internal plexiform layer; GCL, granule cell layer.
Fate mapping in the adult V‐SVZ, using CreERT2‐loxP lines, viral targeting or loss of function mutants, has revealed that NSCs located in different TF domains generate specific subtypes of morphologically and molecularly distinct granule neurons (GNs), and periglomerular neurons (PGNs) (Figure 4(b) and (c)).53, 54, 55, 56, 57 These interneuron subtypes integrate into distinct layers of the OB and interact with different components of the circuitry, thereby potentially differentially modulating olfactory information processing. Superficial GNs predominantly arise from NSCs in the dorsal aspect of the lateral wall of the V‐SVZ, whereas deep GNs arise from NSCs in the ventral portion. The dorsal and ventral portions of the lateral wall largely correlate with the broad expression of Gsx2 and Gli1, respectively, and give rise to both GNs and PGNs (Figure 4(b) and (c)). Nested within these larger domains, fine microdomains have been identified ventrally, in which NSCs produce small populations of novel OB interneuron subtypes, including Type 1–4 neurons (Figure 4(c)) that populate different OB layers. Type 1 neurons are located in the granule cell layer, and are smaller and more ramified than typical new‐born GNs. Type 2 and 3 cells integrate into the mitral cell layer, and Type 4 cells into the external plexiform layer (Figure 4(c)). The Nkx6.2 microdomain gives rise to Type 1–4 interneurons (Figure 4(b) and (c)), whereas the Zic microdomain only generates Type 1 and 3 neurons.55 Importantly, the Nkx2.1 domain is not a source of Type 1–4 neurons, but specifically gives rise to deep GN and some PGN, highlighting the fine regional specification of neuron subtypes. Dorsally, adult NSCs derived from the embryonic Emx1‐expressing domain (Figure 4(a)), give rise to both superficial GN and PGN (Figure 4(b) and (c)).57 In contrast, PAX6 and SP8 domains (Figure 4(b)), predominantly generate PGN subtypes.53, 56, 58 Finally, some glutamatergic juxtaglomerular interneurons are formed by dorsal Tbr2‐ and Neurog2‐expressing progenitors59 (Figure 4(b) and (c)). How these different TF domains result in the specification of heterogeneous OB neuron subtypes, as well as their significance for olfactory function, still need to be elucidated.
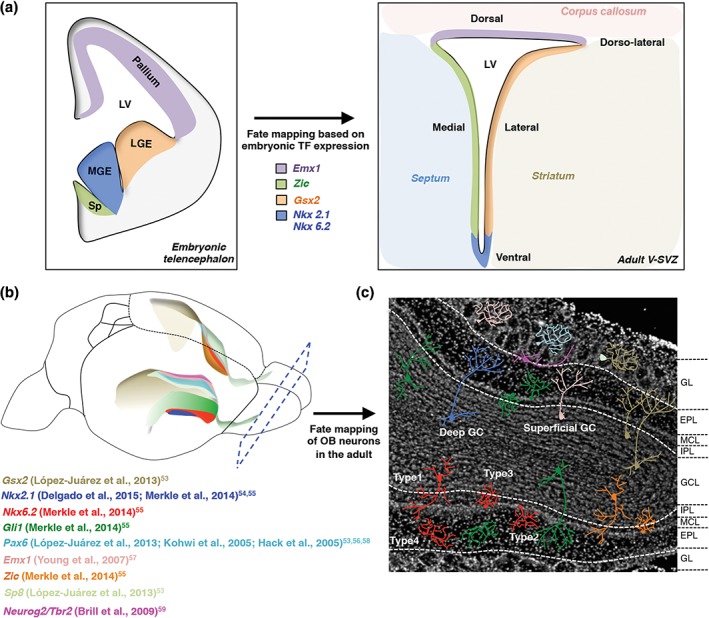
In addition to their neurogenic activity, adult NSCs also produce oligodendrocytes and astrocytes under normal conditions (Figure 6(a)). In vitro time‐lapse imaging revealed that some stem cells or progenitors are committed to oligodendrocyte or neuronal lineages.15 In vivo, the precise identity of stem cells generating oligodendrocytes and astrocytes is still unknown, but gliogenic activity is enriched in dorsal and dorso‐lateral V‐SVZ. V‐SVZ‐derived oligodendrocytes largely come from dorsal NSCs in a sonic hedgehog (SHH)‐ and WNT‐dependent manner, and settle in the corpus callosum (CC) in both the postnatal,69, 70 and adult brain.12, 15 Similarly, adult‐born astrocytes are mainly found in the CC and rostral migratory stream, likely also originating from dorsal NSC subpopulations.13
Figure 6.
Adult NSC plasticity in response to injury. (a) Under normal conditions, regionally distinct NSC subpopulations give rise to specific OB neurons (blue arrow). Gliogenic activity is also detected in the adult V‐SVZ: generation of oligodendrocytes in the corpus callosum (green), and astrocytes in the RMS/corpus callosum (red). (b) Multiple responses of V‐SVZ cells to stroke or ischemia. Neuroblasts are redirected to injury site (orange arrows); NSC migration to injury site and differentiation into reactive astrocytes (RA) (red arrows); migration of NSC‐derived astrocytes to injury site (blue arrows); and oligodendrocyte production at injury site (green arrows). The site of injury can be striatum, cortex or corpus callosum. (c) Response of V‐SVZ cells to demyelination. Adult NSCs predominantly give rise to oligodendrocytes in the corpus callosum. (d) qNSC response to injury. After irradiation, aNSCs (green circles) are depleted, resulting in recruitment of dormant qNSCs (purple circles) to a primed qNSC state and eventually to an aNSC state. qNSCs are also recruited via a primed qNSC state after ischemia.
Although NSC regional identity appears largely intrinsically defined, it can be redirected by ectopic activation of regionally expressed signaling pathways. Indeed, overexpression of SHH signaling pathways dorsally induces dorsal NSCs to generate ventral neuron subtypes.71 Thus, NSCs can exhibit plasticity in their output, raising the important question of how niche signals regulate NSC behavior.
EXTRINSIC NICHE SIGNALS ARE KEY REGULATORS OF NSC BEHAVIOR
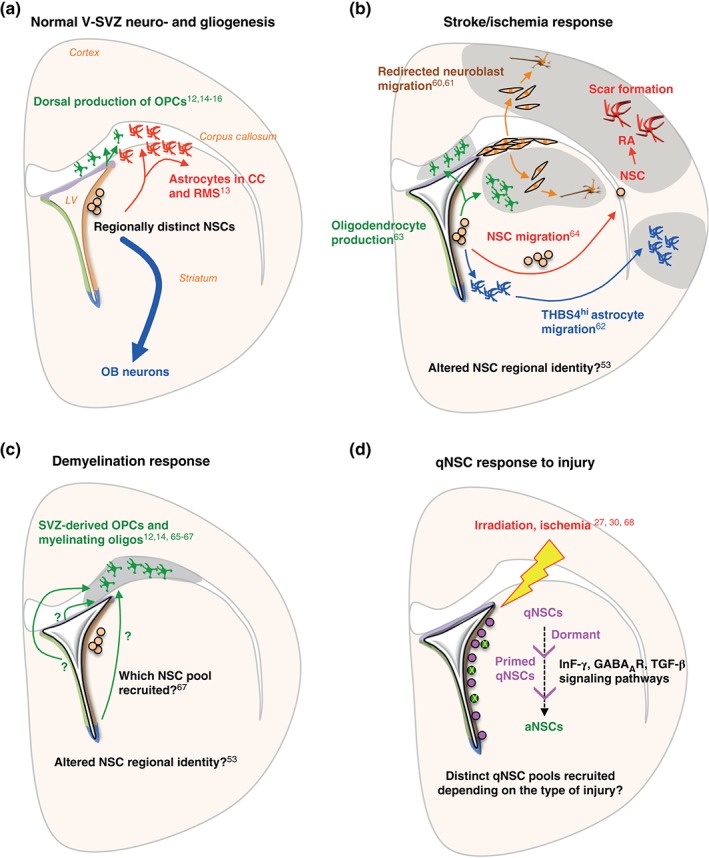
Adult NSCs span different compartments of the stem cell niche and respond dynamically to external signals (Figure 5). On their apical side, NSCs are bathed by the CSF, which flows continuously through the ventricular system.72 On their basal side, NSCs contact the vasculature at specialized sites that lack astrocyte end‐feet.10 They therefore have unique access to contact‐mediated, diffusible, and systemic signals.10, 73, 74, 75, 76, 77 Cell–cell contact between adult NSCs and endothelial cells, or with ependymal cells, promote quiescence, highlighting the importance of anchorage for qNSCs.77, 78, 79, 80 Cell–cell communication between NSCs and other niche cells, such as resident V‐SVZ microglia or ependymal cells, also influence stem cell activation and quiescence.81, 82 Adult NSCs receive feedback signals from cells at later stages in the lineage. aNSCs and TACs send pro‐dormancy signals to qNSCs via the DLL1 Notch ligand, as well as EGFR, thereby controlling stem cell depletion over time.83, 84 In addition, neuroblasts secrete nonsynaptic GABA that binds to GABAAR expressed by B1 cells, and inhibits their proliferation.85, 86, 87 Interestingly, GABA signaling also promotes quiescence in the adult dentate gyrus, suggesting that neuronal activity in the vicinity of NSC niches modulates proliferation behavior.88 Indeed, activity from local cholinergic neurons residing in the niche,89 as well as long‐distance neurotransmitter signals affect cell proliferation in the adult V‐SVZ.90, 91, 92, 93 These findings highlight the importance of both local and long‐distance signals in modulating V‐SVZ neurogenesis.
Figure 5.
V‐SVZ niche components affecting adult NSC behavior. Diverse extrinsic sources of signals regulate NSC behavior in the V‐SVZ niche (light orange), including the lateral ventricle choroid plexus (LVCP), which produces cerebrospinal fluid (CSF), blood vessels (BV), systemic signals, cell–cell interactions, microglia, neuronal innervation, and extracellular matrix (ECM). qNSCs are dark blue, aNSCs light blue, TACs green, and Nb red.
A key open question is how local and long‐range niche components dynamically change in response to external stimuli, and whether these changes impact distinct pools of stem cells. The lateral ventricle choroid plexus (LVCP), which is located in close proximity to the V‐SVZ, is important for brain homeostasis and produces bulk CSF. It also is a key niche compartment that dynamically modulates its secretome to differentially affect multiple V‐SVZ populations and may act as a sensor of different physiological states.94 Interestingly, FACS‐purified qNSCs, aNSCs, and TACs exposed only to LVCP secreted factors give rise to more complex cultures, including oligodendrocyte production, than with EGF. Moreover, more individual qNSCs and aNSCs are recruited, suggesting that the diverse repertoire of LVCP‐secreted factors may support different pools of stem cells.94 Importantly, while adult NSCs have a regional identity, the question of whether the micro‐environment itself is also regionally patterned, and thereby influences the behavior of distinct NSC subpopulations remains open.
ADULT NSCs EXHIBIT PLASTICITY IN RESPONSE TO INJURY
The actual potential of adult NSCs for mammalian brain repair is still largely unknown. Intriguingly, GFAP+ stem cells are also present in the adult human V‐SVZ.95, 96 In contrast to hippocampal neurogenesis,97 OB neurogenesis declines in infancy in humans.98 However, although largely dormant, human V‐SVZ NSCs can be recruited to divide upon injury.99 As such, defining the molecular regulators of stem cell quiescence and activation in other vertebrate brains may yield important insight into how to stimulate human adult V‐SVZ NSCs for brain repair.
The zebrafish model has provided insight into adult NSC behavior as live‐imaging can be performed in vivo. 100, 101 While quiescent radial glia in the zebrafish Pallium (V‐SVZ equivalent) are able to activate after injury,3 radial glial subpopulations exhibit differential sensitivity to the type of stimulus and do not equally reenter cell cycle,3 suggesting that distinct qNSCs are recruited on demand. Interestingly, stimulus‐specific subpopulations of qNSCs have also been identified in the adult mammalian hippocampus,102 suggesting parallels between injury responses in the fish and mammalian brain. Radial glia in zebrafish can migrate long distances to lesioned areas, and neuronal subtypes generated under injury can differ from those produced constitutively.3 Similarly, migration of some neural progenitors to tumor/inflammatory sites has been observed in the adult mouse brain.103, 104
Stroke/ischemia models have been widely used to probe responses of V‐SVZ cells to injury and their capacity to regenerate other brain areas. Stroke elicits major changes in V‐SVZ proliferation and NSC differentiation fate, although it is unknown whether the stem cells recruited under injury originate from specific qNSC reserve pools, or whether all adult NSCs can switch fate upon need. To date, after stroke, three responses of V‐SVZ cells have been described (Figure 6(b)). First, neuroblasts can be redirected to the site of injury and generate appropriate neuronal subtypes.60, 61 Second, stroke can induce a gliogenic response, with the production of V‐SVZ‐derived THBS4high astrocytes,62 or oligodendrocyte progenitors migrating to the site of injury63 (Figure 6(b)). Finally, NSCs themselves can migrate from the V‐SVZ to the injury site, where they give rise to reactive astrocytes that have the potential to be reprogrammed toward a neurogenic fate.64
Upon demyelination injury in the corpus callosum, V‐SVZ stem cells produce oligodendrocyte progenitors, which migrate to the injury site and form myelinating oligodendrocytes (Figure 6(c)).12, 14, 65, 66 Many of these oligodendrocyte progenitors derive from V‐SVZ Gli1 + cells.67 It will be important to define whether this oligodendrocyte production is a result of phenotypic plasticity in normally neurogenic Gli1 + NSCs and/or whether oligodendrocyte committed stem cells are recruited by the injury.67
With the ability to now distinguish qNSCs and aNSCs using FACS, how qNSCs respond to injury and contribute to regeneration is beginning to be examined. After gamma‐irradiation, which kills actively dividing cells and generates a hostile niche environment, qNSCs exit G0 via GABAAR and TGF‐β dependent mechanisms,27, 68 and replenish the V‐SVZ. Similarly, after ischemia, single cell analysis revealed a shift from dormant (q1) to primed (q2) NSCs that is dependent on interferon gamma signaling30 (Figure 6(d)). The primed q2 state is similar to the reversible GAlert state adopted by other adult quiescent stem cells in response to a distant injury, including muscle satellite cells and long‐term hematopoietic stem cells.105 In the adult brain, it is still unclear whether the pool of injury‐recruited qNSCs (q2) is lineally related to or distinct from those contributing to neurogenesis under homeostasis, and whether different injuries trigger recruitment of different pools of stem cells through specific signaling pathways (Figure 6(d)).
Postmitotic ependymal cells have also been proposed to retain neurogenic potential, and become activated in response to injury.28, 29, 33 These studies used CD133 or cilia‐related proteins (such as FoxJ1) as unique lineage markers, although it is now well‐acknowledged that a large proportion of adult NSCs possess a single cilium and express CD1339, 21, 23, 30 (Figure 2(b)). Thus, there is no clear evidence yet that ependymal cells are a reserve population of stem cells.
REGULATION OF NSC BEHAVIOR DURING AGING: IMPLICATIONS FOR POPULATION HETEROGENEITY
Olfactory bulb (OB) neurogenesis and proliferation in the V‐SVZ decline dramatically with aging, due to both intrinsic and extrinsic changes.106, 107 Both the number of qNSCs and aNSCs, as well as the architecture of the niche are altered with aging.108 However, it is unclear how stem cell heterogeneity changes in the aging brain, and whether biases occur in specific NSC pools due to clonal cell competition.109 To date, very few aging studies have taken the existence of qNSCs and aNSCs, or their regional diversity, into account. While some findings indicate that aging may hasten the transition from quiescence to activation under control of longevity‐related genes,110, 111, 112 others show that the number of aNSCs decreases over age and that remaining stem cells enter a deeply dormant state26 (Figure 7). This state can be terminal, as it has been proposed in the aging muscle, where senescent satellite cells switch from reversible quiescence to irreversible senescence.113
Figure 7.
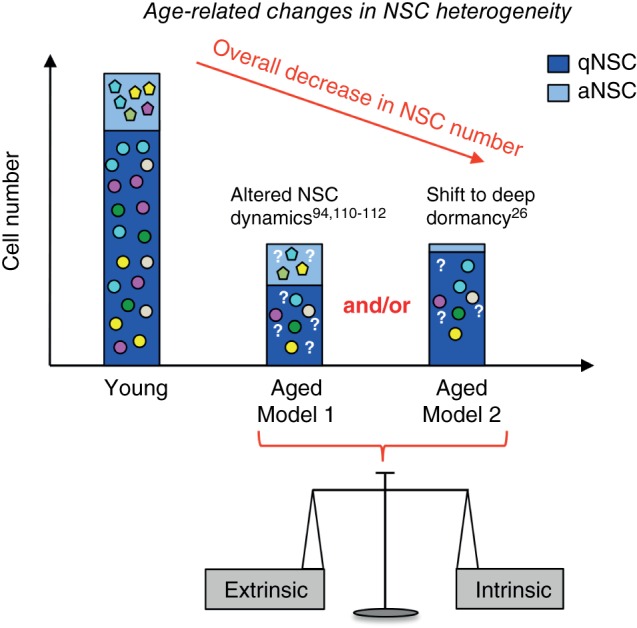
Aging‐related changes in V‐SVZ NSCs. NSC number decreases with aging due to a balance between intrinsic and extrinsic signals. Few studies have examined the effect of aging on qNSCs and aNSCs. Two nonexclusive models may explain the age‐related depletion of stem cells. (1) Model 1 suggests that NSC dynamics are altered with aging. This can be due to multiple factors including changes in NSC recruitment and/or division rate. (2) Model 2 proposes a progressive shift of adult NSCs toward deep quiescence. Whether NSC subpopulations (colored shapes) are depleted unevenly with aging, and whether neuronal and glial differentiation is altered, is unknown (question marks).
The specific intrinsic and/or extrinsic mechanisms underlying stem cell aging are just beginning to be elucidated in many adult stem cell niches.114, 115, 116 During aging, stem cells undergo intrinsic changes at the genetic and epigenetic level that alter their behavior, including cell cycle kinetics, segregation of cellular components and mode of cell division. In the adult brain, aNSCs undergo a progressive lengthening of G1 phase that already starts at 6 months of age.117 In addition, during stem cell division, cellular components and damaged proteins are asymmetrically segregated between mother and daughter cell, through a lateral diffusion barrier of the ER membrane in hippocampal NSCs,118 which becomes disrupted with aging. Conversely, extrinsic signals in the aging brain milieu also play an important role.94, 119, 120, 121 The V‐SVZ has specialized access to signals in the circulation.10 Systemic factors in the blood can influence NSC behavior. Heterochronic parabiosis models, in which young and old animals are surgically conjoined and share the same blood circulation, show that circulating factors from the young animal can rejuvenate adult neurogenesis in the aged mouse, as well as enhance oligodendrocyte differentiation and remyelination capacity in the context of injury.121, 122 The LVCP is another niche compartment that undergoes aging‐related changes, with aNSCs being especially sensitive to alterations in the LVCP secretome (LVCPsec).94 Heterochronic exposure of FACS‐purified aNSCs to old versus young LVCPsec shows that stem cell recruitment, proliferation state and differentiation are highly dependent on LVCP‐borne signals.94 Together with heterochronic transplantation experiments,120 these findings reveal that extrinsic niche signals are key modulators of stem cell function. Whether regionally distinct pools of NSCs are equally sensitive to aging in terms of recruitment, proliferation, and neurogenic and gliogenic potency is still unknown (Figure 7).
CONCLUSION
Stem cell heterogeneity is an increasingly appreciated parameter that needs to be considered to fully understand adult NSC biology, and eventually to harness their potential in vivo. While NSCs exhibit pronounced regional heterogeneity inherited from their embryonic origins, supporting a deterministic model of NSC identity, they also exhibit striking context‐dependent plasticity. Future work will provide insight into how NSC heterogeneity in the V‐SVZ may affect cell competition within the niche, and whether such heterogeneity may be a form of population plasticity that allows adaptation to different environmental changes.
Time is another key dimension of adult NSC heterogeneity, in addition to proliferation dynamics and regional identity. First, within individual NSC populations, mRNA levels oscillate over short time scales,123 which can affect single cell gene expression analysis. Second, at the population level, the generation of distinct subtypes of OB interneurons is highly orchestrated over embryonic and adult periods.124 Third, both embryonic neural progenitors and adult NSCs may undergo changes in chromatin structure that make them differentially sensitive to external cues within specific time windows.125 This raises the exciting possibility that NSCs keep an epigenetic imprint of their own cell history, which may be reversible or irreversible. Along the same line, imprinted genes play a key role in the regulation of adult neurogenesis, highlighting epigenetics as an additional layer of NSC heterogeneity, as are noncoding RNAs.126, 127, 128
In the future, unraveling the multiple layers of adult NSC heterogeneity and its functional consequences will have important ramifications for understanding normal brain function, plasticity and repair.
ACKNOWLEDGMENTS
We apologize to all those whose work we could not cite due to space limitations. We thank V. Silva‐Vargas for comments on the manuscript. This work was supported by University of Basel, and NIH NINDS R01NS074039 to FD.
Conflict of interest: The authors have declared no conflicts of interest for this article.
References
- 1. Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010, 327:542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orford KW, Scadden DT. Deconstructing stem cell self‐renewal: genetic insights into cell‐cycle regulation. Nat Rev Genet 2008, 9:115–128. [DOI] [PubMed] [Google Scholar]
- 3. Than‐Trong E, Bally‐Cuif L. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia 2015, 63:1406–1428. [DOI] [PubMed] [Google Scholar]
- 4. Kriegstein A, Alvarez‐Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009, 32:149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paredes MF, Sorrells SF, Garcia‐Verdugo JM, Alvarez‐Buylla A. Brain size and limits to adult neurogenesis. J Comp Neurol 2016, 524:646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell 2015, 17:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim DA, Alvarez‐Buylla A. Adult neural stem cells stake their ground. Trends Neurosci 2014, 37:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pastrana E, Silva‐Vargas V, Doetsch F. Eyes wide open: a critical review of sphere‐formation as an assay for stem cells. Cell Stem Cell 2011, 8:486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mirzadeh Z, Merkle FT, Soriano‐navarro M, García JM, Alvarez‐buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008, 3:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tavazoie M, Van der Veken L, Silva‐Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia‐Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen Q, Wang Y, Kokovay E, Lin G, Chuang S, Susan K, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell‐cell interactions. Cell Stem Cell 2009, 3:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menn B, Garcia‐Verdugo JM, Yaschine C, Gonzalez‐Perez O, Rowitch D, Alvarez‐Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 2006, 26:7907–7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sohn J, Orosco L, Guo F, Chung SH, Bannerman P, Mills Ko E, Zarbalis K, Deng W, Pleasure D. The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice. J Neurosci 2015, 35:3756–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nait‐Oumesmar B, Decker L, Lachapelle F, Avellana‐Adalid V, Bachelin C, Baron‐Van Evercooren A. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci 1999, 11:4357–4366. [DOI] [PubMed] [Google Scholar]
- 15. Ortega F, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol 2013, 15:602–613. [DOI] [PubMed] [Google Scholar]
- 16. Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature 2005, 437:894–897. [DOI] [PubMed] [Google Scholar]
- 17. Doetsch F. The glial identity of neural stem cells. Nat Neurosci 2003, 6:1127–1134. [DOI] [PubMed] [Google Scholar]
- 18. Doetsch F, Caillé I, Lim DA, García‐Verdugo JM, Alvarez‐Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97:703–716. [DOI] [PubMed] [Google Scholar]
- 19. Doetsch F, García‐Verdugo JM, Alvarez‐Buylla A. Cellular composition and three‐dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 1997, 17:5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia ADR, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP‐expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 2004, 7:1233–1241. [DOI] [PubMed] [Google Scholar]
- 21. Codega P, Silva‐Vargas V, Paul A, Maldonado‐Soto A, DeLeo AM, Pastrana E, Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 2014, 82:545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pastrana E, Cheng L‐C, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci USA 2009, 106:6387–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beckervordersandforth R, Tripathi P, Ninkovic J, Bayam E, Lepier A, Stempfhuber B, Kirchhoff F, Hirrlinger J, Haslinger A, Lie DC, et al. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell 2010, 7:744–758. [DOI] [PubMed] [Google Scholar]
- 24. Khatri P, Obernier K, Simeonova IK, Hellwig A, Hölzl‐Wenig G, Mandl C, Scholl C, Wölfl S, Winkler J, Gaspar JA, et al. Proliferation and cilia dynamics in neural stem cells prospectively isolated from the SEZ. Sci Rep 2014, 4:3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mich JK, Signer RAJ, Nakada D, Pineda A, Burgess RJ, Vue TY, Johnson JE, Morrison SJ. Prospective identification of functionally distinct stem cells and neurosphere‐initiating cells in adult mouse forebrain. eLife 2014, 2014:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giachino C, Basak O, Lugert S, Knuckles P, Obernier K, Fiorelli R, Frank S, Raineteau O, Alvarez‐Buylla A, Taylor V. Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells 2014, 32:70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daynac M, Chicheportiche A, Pineda JR, Gauthier LR, Boussin FD, Mouthon M‐A. Quiescent neural stem cells exit dormancy upon alteration of GABAAR signaling following radiation damage. Stem Cell Res 2013, 11:516–528. [DOI] [PubMed] [Google Scholar]
- 28. Luo Y, Coskun V, Liang A, Yu J, Cheng L, Ge W, Shi Z, Zhang K, Li C, Cui Y, et al. Single‐cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell 2015, 161:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Fan G, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA 2008, 105:1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Llorens‐Bobadilla E, Zhao S, Baser A, Saiz‐Castro G, Zwadlo K, Martin‐Villalba A. Single‐cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell 2015, 17:329–340. [DOI] [PubMed] [Google Scholar]
- 31. Sachewsky N, Leeder R, Xu W, Rose KL, Yu F, van der Kooy D, Morshead CM. Primitive neural stem cells in the adult mammalian brain give rise to GFAP‐expressing neural stem cells. Stem Cell Rep 2014, 2:866–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999, 96:25–34. [DOI] [PubMed] [Google Scholar]
- 33. Carlén M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabé‐Heider F, Yeung MSY, Naldini L, et al. Forebrain ependymal cells are Notch‐dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci 2009, 12:259–267. [DOI] [PubMed] [Google Scholar]
- 34. Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming G, et al. Single‐cell RNA‐Seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell 2015, 17:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calzolari F, Michel J, Baumgart EV, Theis F, Götz M, Ninkovic J. Fast clonal expansion and limited neural stem cell self‐renewal in the adult subependymal zone. Nat Neurosci 2015, 18:490–492. [DOI] [PubMed] [Google Scholar]
- 36. Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 2008, 11:1153–1161. [DOI] [PubMed] [Google Scholar]
- 37. Chaker Z, Aïd S, Berry H, Holzenberger M. Suppression of IGF‐I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Aging Cell 2015, 14:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE. Ascl1 (Mash1) defines cells with long‐term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One 2011, 6:e18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci 2007, 27:12764–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Venere M, Han Y‐G, Bell R, Song JS, Alvarez‐Buylla A, Blelloch R. Sox1 marks an activated neural stem/progenitor cell in the hippocampus. Development 2012, 139:3938–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kang W, Hebert JM. A Sox2 BAC transgenic approach for targeting adult neural stem cells. PLoS One 2012, 7:e49038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young KM, Mitsumori T, Pringle N, Grist M, Kessaris N, Richardson WD. An Fgfr3‐iCreERT2 transgenic mouse line for studies of neural stem cells and astrocytes. Glia 2010, 58:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu H, Belz T, Bock D, Liu H, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, et al. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone service. Genes Dev 2008, 22:2473–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takeda H, Koso H, Tessarollo L, Copeland NG, Jenkins NA. Musashi1‐CreERT2: A new cre line for conditional mutagenesis in neural stem cells. Genesis 2013, 51:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nam HS, Benezra R. High Levels of Id1 Expression Define B1 Type Adult Neural Stem Cells. Cell Stem Cell 2009, 5:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Furutachi S, Miya H, Watanabe T, Kawai H, Yamasaki N, Harada Y, Imayoshi I, Nelson M, Nakayama KI, Hirabayashi Y, et al. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat Neurosci 2015, 18:657–665. [DOI] [PubMed] [Google Scholar]
- 47. Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, Alvarez‐Buylla A. Embryonic origin of postnatal neural stem cells. Cell 2015, 161:1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martynoga B, Mateo JL, Zhou B, Andersen J, Achimastou A, Urban N, van den Berg D, Georgopoulou D, Hadjur S, Wittbrodt J, et al. Epigenomic enhancer annotation reveals a key role for NFIX in neural stem cell quiescence. Genes Dev 2013, 27:1769–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci 2007, 27:4297–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Merkle F, Mirzadeh Z, Alvarez‐Buylla A. Mosaic organization of neural stem cells in the adult brain. Science 2007, 317:381–384. [DOI] [PubMed] [Google Scholar]
- 51. Kelsch W, Mosley CP, Lin CW, Lois C. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol 2007, 5:2501–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fiorelli R, Azim K, Fischer B, Raineteau O. Adding a spatial dimension to postnatal ventricular‐subventricular zone neurogenesis. Development 2015, 142:2109–2120. [DOI] [PubMed] [Google Scholar]
- 53. López‐Juárez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, Waclaw R, Sun Y‐Y, Yang D, Kuan C‐Y, et al. Gsx2 controls region‐specific activation of neural stem cells and injury‐induced neurogenesis in the adult subventricular zone. Genes Dev 2013, 27:1272–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Delgado RN, Lim DA. Embryonic Nkx2.1‐expressing neural precursor cells contribute to the regional heterogeneity of adult V‐SVZ neural stem cells. Dev Biol 2015, 407:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez‐Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci 2014, 17:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kohwi M. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci 2005, 25:6997–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci 2007, 27:8286–8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery‐Padan R, Lledo P‐M, Götz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci 2005, 8:865–872. [DOI] [PubMed] [Google Scholar]
- 59. Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascón S, Erdelyi F, Szabo G, et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci 2009, 12:1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002, 8:963–970. [DOI] [PubMed] [Google Scholar]
- 61. Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho K‐L, Morshead C, Chopp M. Activated neural stem cells contribute to stroke‐induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab 2004, 24:441–448. [DOI] [PubMed] [Google Scholar]
- 62. Benner EJ, Luciano D, Jo R, Abdi K, Paez‐Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature 2013, 497:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang RL, Chopp M, Roberts C, Jia L, Wei M, Lu M, Wang X, Pourabdollah S, Zhang ZG. Ascl1 lineage cells contribute to ischemia‐induced neurogenesis and oligodendrogenesis. J Cereb Blood Flow Metab 2011, 31:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Faiz M, Sachewsky N, Gascón S, Bang KWA, Morshead CM, Nagy A. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell 2015, 17:624–634. [DOI] [PubMed] [Google Scholar]
- 65. Brousse B, Magalon K, Durbec P, Cayre M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol Open 2015, 4:980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xing YL, Röth PT, Stratton JAS, Chuang BHA, Danne J, Ellis SL, Ng SW, Kilpatrick TJ, Merson TD. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci 2014, 34:14128–14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Samanta J, Grund EM, Silva HM, Lafaille JJ, Fishell G, Salzer JL. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature 2015, 526:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pineda JR, Daynac M, Chicheportiche A, Cebrian‐Silla A, Sii Felice K, Garcia‐Verdugo JM, Boussin FD, Mouthon MA. Vascular‐derived TGF‐β increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol Med 2013, 5:548–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Azim K, Fischer B, Hurtado‐Chong A, Draganova K, Cantu C, Zemke M, Sommer L, Butt A, Raineteau O. Persistent Wnt/b‐catenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells 2014, 32:1301–1312. [DOI] [PubMed] [Google Scholar]
- 70. Tong CK, Fuentealba LC, Shah JK, Lindquist RA, Ihrie RA, Guinto CD, Rodas‐Rodriguez JL, Alvarez‐Buylla A. A dorsal SHH‐dependent domain in the V‐SVZ produces large numbers of oligodendroglial lineage cells in the postnatal brain. Stem Cell Rep 2015, 5:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ihrie RA, Shah JK, Harwell CC, Levine JH, Guinto CD, Lezameta M, Kriegstein AR, Alvarez‐Buylla A. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron 2011, 71:250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Silva‐Vargas V, Crouch E, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol 2013, 23:935–942. [DOI] [PubMed] [Google Scholar]
- 73. Gómez‐Gaviro MV, Scott CE, Sesay AK, Matheu A, Booth S, Galichet C, Lovell‐Badge R. Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proc Natl Acad Sci USA 2012, 109:1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ramírez‐Castillejo C, Sánchez‐Sánchez F, Andreu‐Agulló C, Ferrón SR, Aroca‐Aguilar JD, Sánchez P, Mira H, Escribano J, Fariñas I. Pigment epithelium‐derived factor is a niche signal for neural stem cell renewal. Nat Neurosci 2006, 9:331–339. [DOI] [PubMed] [Google Scholar]
- 75. Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 2010, 7:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Delgado AC, Ferrón SR, Vicente D, Porlan E, Perez‐Villalba A, Trujillo CM, D'Ocón P, Fariñas I. Endothelial NT‐3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 2014, 83:572–585. [DOI] [PubMed] [Google Scholar]
- 77. Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH, Parrinello S. Direct cell‐cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol 2014, 16:1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kokovay E, Wang Y, Kusek G, Wurster R, Lederman P, Lowry N, Shen Q, Temple S. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 2012, 11:220–230. [DOI] [PubMed] [Google Scholar]
- 79. Niola F, Zhao X, Singh D, Castano A, Sullivan R, Lauria M, Nam H, Zhuang Y, Benezra R, Di Bernardo D, et al. Id proteins synchronize stemness and anchorage to the niche of neural stem cells. Nat Cell Biol 2012, 14:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Porlan E, Martí‐Prado B, Morante‐Redolat JM, Consiglio A, Delgado AC, Kypta R, López‐Otín C, Kirstein M, Fariñas I. MT5‐MMP regulates adult neural stem cell functional quiescence through the cleavage of N‐cadherin. Nat Cell Biol 2014, 16:629–638. [DOI] [PubMed] [Google Scholar]
- 81. Hamilton LK, Dufresne M, Joppé SE, Petryszyn S, Aumont A, Calon F, Barnabé‐Heider F, Furtos A, Parent M, Chaurand P, et al. Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of Alzheimer's disease. Cell Stem Cell 2015, 17:397–411. [DOI] [PubMed] [Google Scholar]
- 82. Ribeiro Xavier AL, Kress BT, Goldman SA, Lacerda de Menezes JR, Nedergaard M. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci 2015, 35:11848–11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kawaguchi D, Furutachi S, Kawai H, Hozumi K, Gotoh Y. Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nat Commun 2013, 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self‐renewal. Nature 2010, 467:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fernando RN, Eleuteri B, Abdelhady S, Nussenzweig A, Andäng M, Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc Natl Acad Sci USA 2011, 108:5837–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, Monyer H. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell 2012, 10:76–87. [DOI] [PubMed] [Google Scholar]
- 87. Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP‐expressing progenitors. Nat Neurosci 2005, 8:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al. Neuronal circuitry mechanism regulating adult quiescent neural stem‐cell fate decision. Nature 2012, 489:150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Paez‐Gonzalez P, Asrican B, Rodriguez E, Kuo T. Identification of distinct ChAT+ neurons and activity‐dependent control of postnatal SVZ neurogenesis. Nat Neurosci 2014, 17:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Berg DA, Belnoue L, Song H, Simon A. Neurotransmitter‐mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140:2548–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Young SZ, Taylor MM, Bordey A. Neurotransmitters couple brain activity to subventricular zone neurogenesis. J Neurosci 2012, 33:1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Höglinger GU, Arias‐Carrión O, Ipach B, Oertel WH. Origin of the dopaminergic innervation of adult neurogenic areas. J Comp Neurol 2014, 522:2336–2348. [DOI] [PubMed] [Google Scholar]
- 93. Tong CK, Chen J, Cebrián‐Silla A, Mirzadeh Z, Obernier K, Guinto CD, Tecott LH, García‐Verdugo JM, Kriegstein A, Alvarez‐Buylla A. Axonal control of the adult neural stem cell niche. Cell Stem Cell 2014, 14:500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Silva‐Vargas V, Maldonado‐Soto A, Mizrak D, Codega P, Doetsch F. Age‐dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 2016. doi:10.1016/j.stem.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 95. Sanai N, Tramontin AD, Quiñones‐Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel‐García Verdugo J, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004, 427:740–744. [DOI] [PubMed] [Google Scholar]
- 96. van den Berge SA, Middeldorp J, Eleana Zhang C, Curtis MA, Leonard BW, Mastroeni D, Voorn P, van de Berg WDJ, Huitinga I, Hol EM. Longterm quiescent cells in the aged human subventricular neurogenic system specifically express GFAP‐delta. Aging Cell 2010, 9:313–326. [DOI] [PubMed] [Google Scholar]
- 97. Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai H, Wong M, Gupta N, Berger MS, Huang E, Garcia‐Verdugo J‐M, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marti‐Fabregas J, Romaguera‐Ros M, Gomez‐Pinedo U, Martinez‐Ramirez S, Jimenez‐Xarrie E, Marin R, Marti‐Vilalta JL, Garcia‐Verdugo JM. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology 2010, 74:357–365.20054008 [Google Scholar]
- 100. Progatzky F, Dallman MJ, Lo Celso C. From seeing to believing: labelling strategies for in vivo cell‐tracking experiments. Interface Focus 2013, 3:20130001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dray N, Bedu S, Vuillemin N, Alunni A, Coolen M, Krecsmarik M, Supatto W, Beaurepaire E, Bally‐Cuif L. Large‐scale live imaging of adult neural stem cells in their endogenous niche. Development 2015, 142:3592–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jhaveri DJ, O'Keeffe I, Robinson GJ, Zhao Q‐Y, Zhang ZH, Nink V, Narayanan RK, Osborne GW, Wray NR, Bartlett PF. Purification of neural precursor cells reveals the presence of distinct, stimulus‐specific subpopulations of quiescent precursors in the adult mouse hippocampus. J Neurosci 2015, 35:8132–8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci 2006, 26:3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Elvira G, García I, Benito M, Gallo J, Desco M, Penadés S, Garcia‐Sanz JA, Silva A. Live imaging of mouse endogenous neural progenitors migrating in response to an induced tumor. PLoS One 2012, 7:e44466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai C‐R, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert). Nature 2014, 509:393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jung Y, Brack AS. Cellular Mechanisms of Somatic Stem Cell Aging. Curr Top Dev Biol 2014, 107:405–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Capilla‐Gonzalez V, Herranz‐Pérez V, García‐Verdugo JM. The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Front Cell Neurosci 2015, 9:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Conover JC, Shook BA. Aging of the subventricular zone neural stem cell niche. Aging Dis 2011, 2:49–63. [PMC free article] [PubMed] [Google Scholar]
- 109. Goodell MA, Rando TA. Stem cells and healthy aging. Science 2015, 350:1199–1204. [DOI] [PubMed] [Google Scholar]
- 110. Renault VM, Rafalski VA, Morgan AA, Salih DAM, Jamie O, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 2010, 5:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Webb A, Pollina E, Vierbuchen T, Urbán N, Ucar D, Leeman D, Martynoga B, Sewak M, Rando T, Guillemot F, et al. FOXO3 shares common targets with ASCL1 genome‐wide and inhibits ASCL1‐dependent neurogenesis. Cell Rep 2013, 4:477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shook, B. A, Manz, D. H. , Peters, J. J. , Kang, S. & Conover, J. C. Spatiotemporal changes to the subventricular zone stem cell pool through aging. J Neurosci 2012, 32: 6947–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sousa‐Victor P, Gutarra S, García‐Prat L, Rodriguez‐Ubreva J, Ortet L, Ruiz‐Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014, 506:316–321. [DOI] [PubMed] [Google Scholar]
- 114. Rando TA. Stem cells, ageing and the quest for immortality. Nature 2006, 441:1080–1086. [DOI] [PubMed] [Google Scholar]
- 115. Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 2007, 8:703–713. [DOI] [PubMed] [Google Scholar]
- 116. van Wijngaarden P, Franklin RJM. Ageing stem and progenitor cells: implications for rejuvenation of the central nervous system. Development 2013, 140:2562–2575. [DOI] [PubMed] [Google Scholar]
- 117. Daynac M, Morizur L, Chicheportiche A, Mouthon M‐A, Boussin FD. Age‐related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci Rep 2016, 6:21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Moore DL, Pilz GA, Arauzo‐Bravo MJ, Barral Y, Jessberger S. A mechanism for the segregation of age in mammalian neural stem cells. Science 2015, 349:1334–1338. [DOI] [PubMed] [Google Scholar]
- 119. Bouab M, Paliouras GN, Aumont A, Forest‐Bérard K, Fernandes KJL. Aging of the subventricular zone neural stem cell niche: evidence for quiescence‐associated changes between early and mid‐adulthood. Neuroscience 2011, 173:135–149. [DOI] [PubMed] [Google Scholar]
- 120. Piccin D, Tufford A, Morshead CM. Neural stem and progenitor cells in the aged subependyma are activated by the young niche. Neurobiol Aging 2014, 35:1669–1679. [DOI] [PubMed] [Google Scholar]
- 121. Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014, 344:630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ruckh JM, Zhao JW, Shadrach JL, Van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJM. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012, 10:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Isomura A, Kageyama R. Ultradian oscillations and pulses: coordinating cellular responses and cell fate decisions. Development 2014, 141:3627–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Batista‐Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci 2008, 28:3966–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kohwi M, Doe CQ. Temporal fate specification and neural progenitor competence during development. Nat Rev Neurosci 2013, 14:823–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ferrón SR, Charalambous M, Radford E, McEwen K, Wildner H, Hind E, Morante‐Redolat JM, Laborda J, Guillemot F, Bauer SR, et al. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature 2011, 475:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Murao N, Noguchi H, Nakashima K. Epigenetic regulation of neural stem cell property from embryo to adult. Neuroepigenetics 2016, 5:1–10. [Google Scholar]
- 128. Ramos AD, Andersen RE, Liu SJ, Nowakowski TJ, Hong SJ, Gertz CC, Salinas RD, Zarabi H, Kriegstein AR, Lim DA. The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell 2015, 16:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]


