Summary
Background
Grains are high in FODMAPs (Fermentable Oligo‐, Di‐, Monosaccharides And Polyols) and often considered as triggers of IBS symptoms.
Aim
To evaluate if rye bread low in FODMAPs would be better tolerated than regular rye bread in subjects with IBS.
Methods
The study was conducted as a randomised double blind controlled cross‐over study (n = 87). Participants were supplied with both regular rye bread and low‐FODMAP rye bread for 4 weeks. Symptoms were measured with a symptom severity scoring system (IBS‐SSS) and visual analogue scale (VAS) assessments of individual symptoms. Quality of life was monitored. Colonic fermentation was measured by the breath hydrogen test and dietary intake by food diaries.
Results
Dietary fibre intake increased during both study periods compared to baseline. Many signs of IBS i.e. flatulence, abdominal pain, cramps and stomach rumbling were milder on the low‐FODMAP rye bread (P‐values: 0.04; 0.049; 0.01 and 0.001). The mean of VAS measurements was favourable towards LF bread [−3 (95% CI): −6 to −1, P = 0.02] but no differences were detected in IBS‐SSS or quality of life. The AUC of breath hydrogen values was significantly lower during the low‐FODMAP bread period (median 52.9 vs. 72.6; P = 0.01).
Conclusions
Low‐FODMAP rye bread helps IBS patients to control their symptoms and reduces gastrointestinal gas accumulation. However, replacing regular rye bread by low‐FODMAP bread without concomitant broader dietary changes does not improve quality of life or IBS‐SSS. Nonetheless, inclusion of low‐FODMAP rye bread in diet might be one way that IBS patients could increase their fibre intake.
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder. It affects, on average, 11% of the adult population in the developed countries.1 IBS causes substantial human suffering as well as having financial consequences.2
The role of diet in triggering IBS symptoms has long been recognised but only limited amounts of dietary research have been performed. IBS patients typically report intolerance of wheat, milk, onion, garlic, apples, alcohol, coffee, chili and beans.3, 4 The role of colonic fermentation of indigestible carbohydrates has attracted considerable research interest. Australian researchers from Monash University have introduced the concept of Fermentable Oligo‐, Di‐, Monosaccharides and Polyols (FODMAPs).5 FODMAPs are simple carbohydrates which are unabsorbed in small intestine but rapidly and extensively fermented in the upper part of the colon. In the presence of impaired handling of intestinal gas, abnormalities in bowel motility and visceral sensitivity, it is the rapid fermentation which is postulated to trigger IBS symptoms. It is now widely accepted that FODMAPs are at least one of the culprits behind the so‐called subjective gluten or wheat sensitivity.6
Recent clinical studies have provided support for the FODMAP theory. There are now several randomised controlled trials and observational studies demonstrating the beneficial effect of a low‐FODMAP diet in IBS.7 Generally, in these studies, the entire dietary pattern has been modified to achieve a broad restriction of dietary FODMAPs. In contrast, the role of single food items high in FODMAPs in IBS is poorly understood.
Whole grain rye bread is considered as a healthy food, mainly because of its high fibre content (11–14%).8 In Finland, rye is the single most important source of dietary fibre, accounting for 28–35% of fibre intake of working age adults.9 In a Danish prospective study, a high consumption of rye bread was associated with a lower risk of death in men.10 Rye bread increases satiety,11 reduces the insulin response compared to that encountered after an isocaloric wheat‐based meal.12 By consuming rye bread, the individual increases his/her intake of cereal fibre which is linked to a reduced risk of colorectal cancer.13 Bread, as such, is important part of the daily diet all over the Western world.
Rye bread is also high in fructans.14 According to a Finnish study, rye grain typically contains 4.4–6.6 g of fructans per 100 g and most of these fructans are classified as fructooligosaccharides (FOS) such as 1‐kestose.8 FOS is a short chain carbohydrate that is not digested by mammalian enzymes and as such can be classified as a FODMAP.
In a retrospective study, 91% of IBS patients reported tolerating small amounts of wheat‐free rye bread despite its high‐FODMAP content.15 However, it is not known whether lowering the fructan content of rye bread would be of any help in controlling IBS symptoms, and would patients be able to increase the amounts of rye bread in their diet if the fructan content was lowered during the baking process. As far as we are aware, there are no randomised studies which would have investigated whether rye can be linked with IBS symptoms.
This study aimed to determine whether a simple substitution of low‐FODMAP rye bread for regular rye bread (high in FODMAPs), without any other concomitant dietary changes, affects the symptoms and gas accumulation or quality of life in IBS patients. Therefore, the effects on gastrointestinal symptoms of low‐FODMAP‐rye bread and regular rye bread alone were compared in a randomised, double‐blinded, cross‐over study in patients with IBS who did not follow strict low‐FODMAP or any other type of elimination diet. Furthermore, we evaluated the intake of dietary fibre at baseline and during the intervention.
Subjects
A total of 87 patients with IBS were recruited via the Internet and from individuals attending a private hospital clinic (Aava Medical Centre, Helsinki, Finland). The two eligibility inclusion criteria were (i) subjects were aged 18–65 years and (ii) had IBS as defined by the Rome III criteria.16 Exclusion criteria included the presence of an organic GI disease, such as inflammatory bowel disease, coeliac disease, major abdominal surgery, any malignancy, pregnancy or breastfeeding, inability to tolerate rye, strict low‐FODMAP diet or other elimination diet, or taking some medication potentially influencing gastrointestinal function.
Study candidates were pre‐screened by a study dietician. Those meeting the preliminary inclusion criteria were subsequently referred to a screening consultation with a gastroenterologist to assess further their eligibility for participating in the study. Blood count, sedimentation rate, transglutaminase antibodies for coeliac disease and thyroid function tests were performed at the physician's screening visit, if these tests had not already been performed within the past 12 months. Laboratory tests were done in order to screen eligible patients. Patients were provided with the obligatory written, informed consent forms which they all signed.
The study protocol was approved by the ethics committee of the Hospital District of Helsinki and Uusimaa. The trial was registered at the US National Institutes of Health (Clinical Trials.gov) #NCT02161120.
Methods
Study design
This study was a randomised, double blind, 2 × 2 cross‐over study with a 1‐week run‐in period on usual diet, followed by two 4‐week treatment periods during which the patients received either the low‐FODMAP rye bread or the regular rye bread, and a washout period of ≥4 weeks between these two periods. Randomisation was performed in blocks of four as a nonconcealed allocation. An independent person who was not involved with the study generated the randomisation list. The randomisation was blinded for both the participants and the investigators.
Study breads
The breads were developed and supplied by Fazer Bakeries (Vantaa, Finland). Both rye bread recipes contained the same amount of wholegrain rye flour, wheat flour and other ingredients. The breads had a similar appearance, taste and were packaged in transparent plastic pouches (Figure S1). The control bread was prepared using traditional rye sourdough whereas the low‐FODMAP rye bread was prepared using a specific sourdough system which resulted in rye bread with a clearly lower FODMAP (fructan and mannitol) content (Table 1).
Table 1.
Nutritional composition of the breads
| Low‐FODMAP rye breada/100 g | Regular rye breada/100 g | |
|---|---|---|
| Energy, kJ (kcal) | 1024 (245) | 1033 (247) |
| Protein, g | 9.2 | 9.3 |
| Fat, g | 1.3 | 1.2 |
| Carbohydrates, g | 43.6 | 44.0 |
| Dietary fibre, g | 10.2 | 10.5 |
| Sodium, g | 0.4 | 0.4 |
| Fructans, g | 0.3 | 1.1 |
| Mannitol, g | 0.1 | 0.3 |
| LMWDF, g | 1.9 | 2.6 |
| Insoluble HMWDF, g | 5.9 | 5.4 |
| Soluble HMWDF, g | 2.5 | 2.4 |
| Resistant starch, g | 0.9 | 0.8 |
LMWDF, low molecular weight dietary fibre; HMWDF, high molecular weight dietary fibre.
Participants were supplied and instructed to consume 3.5–4 slices (105–120 g) of each bread/day during the first week of the study and 7–8 slices (210–240 g) during the weeks 2–4.
The nutrient composition was analysed by Eurofins scientific Finland, Raisio (Food & Agro), Finland. The dietary fibre content of the breads was determined using the AOAC method 2011.25, discriminating soluble and insoluble, low and high molecular weight dietary fibres. Resistant starch was determined using AOAC 2002.02 method and mannitol by HPAEC method used by Eurofins Food &Agro, Lidköping, Sweden. Fructan content was measured using the AOAC 999.03 method (Megazyme assay kit K‐FRUC; Megazyme international Ireland Ltd, Bray, Ireland). A Lactobacillus strain was identified in the sourdough which efficiently consumed fructans and prevented mannitol accumulation during proofing, and thus resulting in lower FODMAP content of low‐FODMAP rye bread.
Subjects were asked to follow their usual diet, and they were asked to keep 4‐day food records during the run‐in and during both treatment periods. Patients were advised to consume 7–8 slices of rye bread per day depending on the individual energy need during 2nd, 3rd and 4th week. During the first week of treatment periods, the targeted rye bread dose was half, i.e. 3.5–4 slices. Adherence to bread consumption was evaluated by patient entries into a tick‐box diary for each treatment period and also by food records.
IBS‐SSS
The primary outcome variable was IBS‐SSS (IBS‐Symptom Severity Score) as described by Francis et al.17 The individual symptoms were assessed at the baseline (week 0) and at weeks 2 and 4 during the study periods. The overall IBS‐SSS score (=abdominal pain + number of days of abdominal pain during the last 10 days ((number of days with abdominal pain)*10) + abdominal distension + satisfaction of defecatory behaviour + interference of IBS symptoms) was calculated for weeks 2 and 4 and for both study periods. The possible range was then 0–500.15 The mean values of weeks 2 and 4 were calculated for the individual symptoms and for the overall IBS‐SSS score.
Weekly IBS symptoms
IBS‐SSS includes only some aspects of the gastrointestinal symptoms experienced by IBS patients. Therefore, we constructed an additional questionnaire to assess other symptoms related to IBS. This questionnaire was also based on visual analogue scale (VAS) and assessed the severity of diarrhoea, constipation, abdominal cramps and pain, flatulence, stomach rumbling, heartburn, dyspepsia, incomplete feeling of defecation and urgency in defecation.
A total of 10 symptoms were recorded (VAS 0–100 mm for each) at the baseline (week 0) and during study weeks 1, 2, 3 and 4 during both study periods. A total symptom score (= mean of all ten symptoms) was calculated for each week. The mean values of weeks 1, 2, 3 and 4 were calculated for the individual symptoms and for the total symptom score. We did not formally validate the questionnaire containing 10 different symptoms but it follows the practise and idea as described by Francis et al.17 A similar scoring system has been used previously in other diet studies in IBS.18
Quality of life
Each study subject also filled in a Finnish language version of the validated IBS‐QoL19 as a further secondary outcome. The quality of life questionnaire was filled in at the baseline (week 0), and after the both study periods at week 4.
Other outcome variables
Breath hydrogen was measured with Gastrolyzer (Bedfont Scientific Ltd, Kent, UK) at the baseline and 12 times at 30 min intervals during the 6 h after eating the study breads. To minimise variables that might affect breath hydrogen production, subjects were asked to restrain from vigorous physical activity and instructed to consume a standard breakfast containing three slices of the study breads, spread, cheese or ham, tomato and/or cucumber and water. Coffee and tea were allowed. Subjects were asked to refrain from eating anything more until 4 h from the beginning of the test, i.e. at lunch time, and to eat a similar lunch, if any, during the two study periods. Breath hydrogen was evaluated as a marker of colonic fermentation.
Patients were allowed to use OTC‐dimeticone (Cuplaton; Orion Oy, Espoo, Finland) as a rescue medication if their symptoms required relief. Dimeticone was provided free‐of‐charge to the subjects and its purpose was to reduce the likelihood of premature withdrawals from the study.
The timing of the different measurements is depicted in Table S1.
Statistical analysis
The overall IBS‐SSS score was the primary outcome variable and the weekly IBS symptoms, the weekly symptom scores and the IBS‐QOL score were the secondary outcome variables. The sample size calculation for the present study was based on the primary variable IBS‐SSS. Suitable previously published data were not available to be used in calculations. Thus, we assumed that the difference between study breads would be at least 50 points on the 500‐point IBS‐SSS score and that the standard deviation of that difference would be 150 points. In that case, a sample size of 73 would have 80% power to detect this 50 point difference of using a paired t‐test with a 0.05 two‐sided significance level. The anticipated drop out was 15–20% and therefore 84–88 patients were targeted for this cross‐over study.
The patient characteristics are expressed as mean (range) for continuous variables and as number of patients (%) for categorical variables. The primary and the secondary outcome variables were analysed using the repeated measures anova for cross‐over design. The effects of treatment (low‐FODMAP rye bread vs. regular rye bread), the time effect and the carry‐over effect were tested and the differences between study breads are expressed as mean (95% CI).
Area under curve estimates of breath hydrogen test were calculated using the absolute breath hydrogen values (AUC0–360 min). The trapezium rule was applied for AUC calculations. The number of slices of study breads was assessed at week 1 and during weeks 2–4. The weight of patients was inquired at the baseline and after both study periods. The Wilcoxon signed‐rank test was used with respect to breath hydrogen level and the number of consumed slices of study breads and paired samples t‐test was used to compare weight between the low‐FODMAP rye bread vs. regular rye bread. The Friedman's two‐way anova was used to compare dietary intake during the study periods. Pairwise comparisons between study periods were conducted only if the global test was significant. A P ≤ 0.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS Statistics for Windows (versions 22.0 and 23.0; IBM Corp, Armonk, NY, USA).
Results
A total of 87 patients with IBS were recruited for the study. Seven patients withdrew from the study during the run‐in period. Of remaining 80 patients, three patients withdrew during the first period and four during the wash out period. One patient withdrew from the trial when consuming the low‐FODMAP rye bread and two patients during regular rye bread. The reasons for discontinuation are detailed in Table 2; Figure 1 depicts the flow chart for the study.
Table 2.
The reasons for discontinuing the study
| Reason | N | % of all randomised patients | % of discontinued patients |
|---|---|---|---|
| Other AE (other than abdominal symptoms) | 6 | 6.9 | 42.9 |
| Abdominal symptoms | 4 | 4.6 | 28.6 |
| Lack of compliance | 2 | 2.3 | 14.3 |
| Reason not known | 2 | 2.3 | 14.3 |
| Total | 14 | 16.1 | 100.0 |
Figure 1.
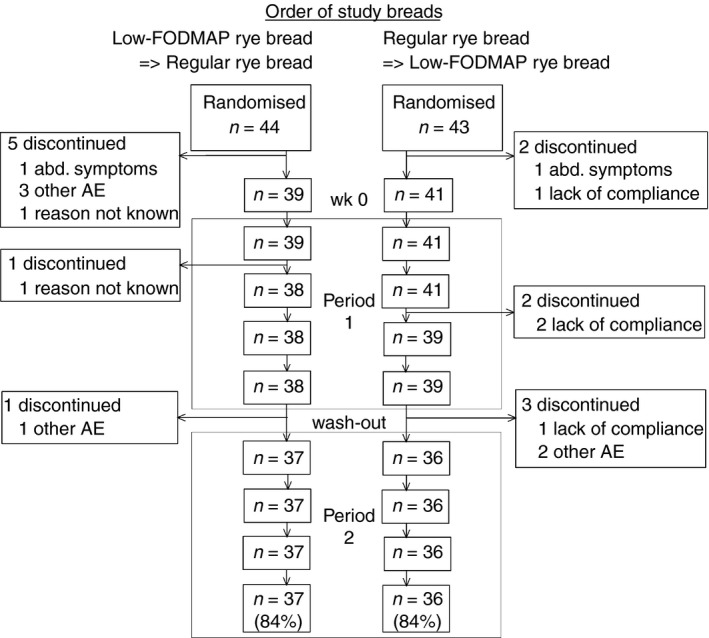
Flow chart for IBS patients who were randomised in the rye bread cross‐over study. Other AE indicates adverse events other than abdominal symptoms.
The majority of patients were females (91.3%). Patients had a mean age of 42.9 (range 21–64) years and median body mass index of 23.4 (range 17.3–36.6) kg/m2. The median consumption of any bread daily at baseline was 3.0 slices (approximately 84.1 g/day) and 62.5% of patients reported the regular use of rye bread. See Table 3 for details. Mean IBS‐SSS score was 228 (range 80–430) at baseline. Most patients (62.5%) had mixed type of IBS, 32.5% had diarrhoea predominant IBS and 5% had unspecified symptoms.
Table 3.
Baseline characteristics of patients with irritable bowel syndrome (N = 80)
| Characteristics | |
|---|---|
| Females, n (%) | 73 (91.3) |
| Age (years), mean (range) | 42.9 (21–64) |
| BMI (kg/m2), median (range) | 23.4 (17.3–36.6) |
| Weight (kg), median (range) | 69.0 (42–102) |
| Self‐reported daily consumption of any bread (no. of slices), median (range) | 3.0 (0–8) |
| Regular rye bread consumers, n (%) | 50 (62.5) |
| IBS symptom severity (SSS score), mean (s.d.; range) | 228 (76; 80–430) |
| IBS subgroup, n (%) | |
| IBS‐M, mixed | 50 (62.5) |
| IBS‐D, diarrhoeal | 26 (32.5) |
| IBS‐U, unspecified | 4 (5) |
There was no significant difference in IBS‐SSS values between the study breads. The estimated mean was 199 (95% CI 179–220) during the low‐FODMAP rye bread vs. 207 (187–227) during the regular rye bread. The difference between breads was −8 (95% CI −27–11), P = 0.40, see Figure 2.
Figure 2.
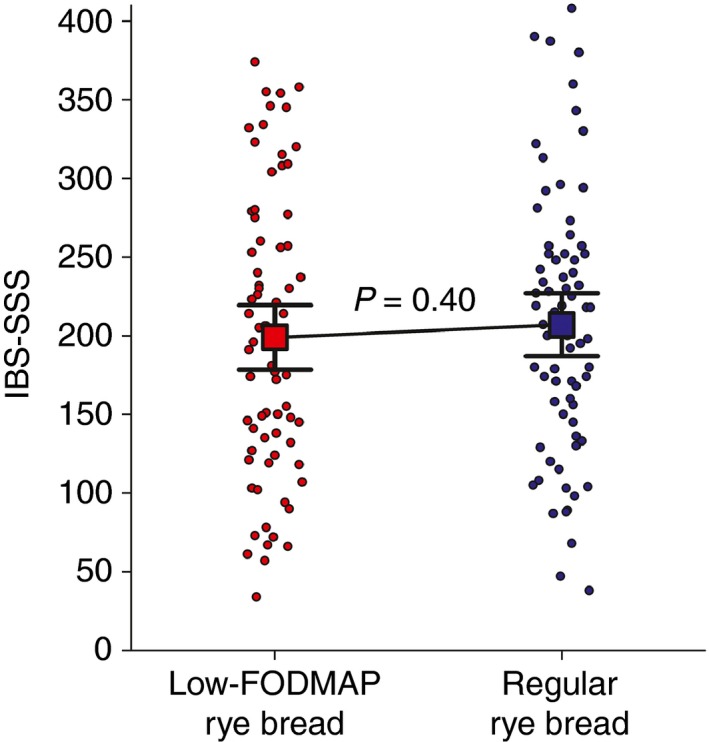
Individual IBS‐SSS symptom scores as dots during the low‐FODMAP rye bread and the regular rye bread, using the mean of IBS‐SSS scores of weeks 2 and 4. Only subjects with valid IBS‐SSS score for both study breads are included (n = 73). Squares with error bars indicate means (95% CI), 199 (179–220) during the low‐FODMAP rye bread vs. 207 (187–227) during the regular rye bread (P = 0.40, repeated measures anova for cross‐over design).
No significant difference was detected between the study breads in the assessment of quality of life. The mean (95% CI) was 29.2 (25.0–33.5) during the low‐FODMAP rye bread vs. 30.0 (25.5–34.5) during the regular rye bread. The difference was −0.8 (−2.9–1.3), P = 0.45.
Weekly VAS measurements of individual symptoms revealed statistically significant differences between the breads when the mean values of weeks 1, 2, 3 and 4 were analysed (Table 4). Flatulence, abdominal pain, cramps and stomach rumbling were milder when the subjects were eating the low‐FODMAP rye bread (P‐values: 0.04; 0.049; 0.01 and 0.001 respectively). There was also a significant difference between the breads in the total symptom score, i.e. in the mean of all 10 symptoms, 30 (95% CI 27–30) during the low‐FODMAP rye bread vs. 33 (95% CI 30–37) during the regular rye bread. The mean difference was −3 (−6 to −1), P = 0.02) favouring low‐FODMAP rye bread. A carry‐over effect was observed with respect to cramps but not in any of the other symptoms. A time‐dependent effect was observed in dyspepsia.
Table 4.
Individual symptoms (VAS 0–100 mm) and total symptom score using the mean value of weeks 1, 2, 3 and 4 (n = 73)
| Symptom | Low‐FODMAP rye bread | Regular rye bread | Low‐FODMAP vs. Regular rye bread | P‐valuea |
|---|---|---|---|---|
| Flatulence | 47 (43–52) | 52 (47–56) | −4 (−8 to −0) | 0.04 |
| Diarrhoea | 23 (19–27) | 25 (21–30) | −2 (−7 to 2) | 0.31 |
| Constipation | 24 (19–29) | 25 (20–30) | −1 (−5 to 3) | 0.66 |
| Abdominal pain | 34 (29–38) | 38 (33–43) | −5 (−9 to −0) | 0.049 |
| Intestinal cramps | 19 (16–23) | 25 (20–29) | −6 (−10 to −2) | 0.01 |
| Rumbling | 33 (28–37) | 39 (34–44) | −6 (−10 to −3) | 0.001 |
| Heartburn | 22 (18–27) | 21 (17–25) | 2 (−2 to 5) | 0.34 |
| Dyspepsia | 31 (25–36) | 34 (29–40) | −4 (−8 to +0) | 0.06 |
| Incomplete defecation | 40 (35–46) | 41 (36–47) | −1 (−5 to 3) | 0.66 |
| Urgency in defecation | 29 (25–33) | 33 (27–38) | −4 (−8 to 1) | 0.11 |
| Total symptom score | 30 (27–33) | 33 (30–37) | −3 (−6 to −1) | 0.02 |
Results are given as mean (95% CI).
Repeated measures anova for cross‐over design.
The breath hydrogen level was significantly lower during the low‐FODMAP rye bread as compared to the regular rye bread. The median (inter‐quartile range) AUC was 52.9 (32.4–76.8) ppm during low‐FODMAP rye bread vs. 72.6 (46.7–114.9) ppm during the regular rye bread (P = 0.01, Wilcoxon signed‐rank test). The results are illustrated in Figure 3. Only subjects for whom we had complete breath hydrogen measurements during both study breads are included (n = 60).
Figure 3.
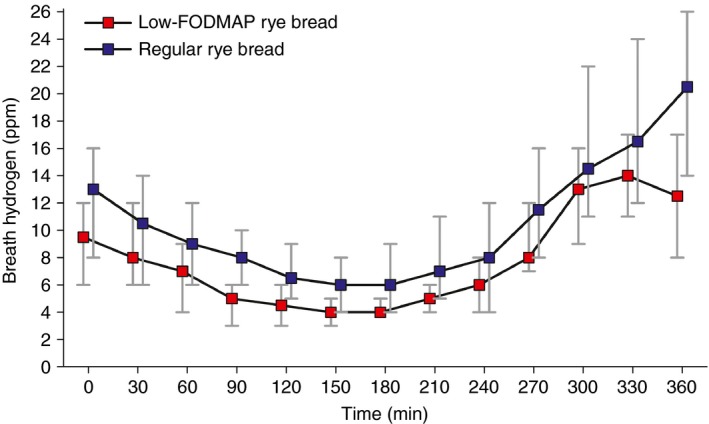
Breath hydrogen concentration (ppm) before the meal (0 min) and during 6 h after eating the meal containing either the low‐FODMAP rye bread or the regular rye bread. Only subjects with complete breath hydrogen measurements during both study breads are included (n = 60). The median AUC 0–360 min was 52.9 ppm·min during the low‐FODMAP rye bread vs. 72.6 ppm·min during the regular rye bread (P = 0.01, Wilcoxon signed‐rank test).
The weight of the patients differed significantly after the treatments. The mean difference was −0.5 kg (95% CI −0.9 to −0.0, P = 0.03, t‐test for paired samples). After the low‐FODMAP rye bread period, the patients' mean weight was 70.6 kg; it was slightly more after the regular rye bread period, 71.1 kg.
Dietary intakes are reported in Table 5. The median intake of energy was 8103 kJ/day during baseline, 7994 kJ/day during low‐FODMAP rye bread and 7873 kJ/day during regular rye bread. There were no statistically significant differences in energy intake between the periods. Furthermore, there were no statistically significant differences in the intake of carbohydrates, protein or fat between the three periods. Fibre intake increased by 6 g/day during the low‐FODMAP rye bread period and by 8 g/day during the regular rye bread period as compared to the baseline period (P < 0.001 for both vs. baseline), but there were no statistically significant differences between the two treatment periods (P = 0.09).
Table 5.
Dietary intake during the baseline and during the study breads
| Baseline N = 79 | Low‐FODMAP rye bread N = 67 | Regular rye bread N = 73 | P‐valuea | |
|---|---|---|---|---|
| Energy, kJ/day | 8103 (6653–9298) | 7994 (6425–9450) | 7873 (6207–9536) | 0.35 |
| Energy, kcal/day | 1935 (1589–2222) | 1910 (1535–2258) | 1881 (1483–2278) | |
| Carbohydrates, g/day | 192 (143–235) | 185 (153–218) | 198 (150–239) | 0.31 |
| Protein, g/day | 81 (68–97) | 81 (63–98) | 81 (70–104) | 0.37 |
| Fat, g/day | 77 (59–93) | 72 (60–88) | 73 (57–86) | 0.56 |
| Total fibre, g/day | 21 (17–25) | 27 (22–32) | 29 (23–34) | <0.001b |
Results are given as median (inter‐quartile range).
Overall comparison between study periods assessed by Friedman's two way analysis of variance.
The differences between individual periods were investigated only if the overall comparison between study periods was statistically significant: Low‐FODMAP rye bread vs. Baseline P < 0.001, Regular rye bread vs. Baseline P < 0.001 and: Low‐FODMAP rye bread vs. Regular rye bread P = 0.09
Median intake of FODMAPs from low‐FODMAP bread was 0.42 g/day and regular rye bread 1.47 g/day during the first week of the study (P < 0.001) and during the study weeks 2–4, it was 0.60 g/day and 2.21 g/day (P < 0.001) respectively. Table 6 details the intake of mannitol, fructans and total FODMAPs during the study.
Table 6.
Intake of FODMAPs of the study breads during the first week and the weeks 2–4. The bread dose was doubled from the second week onwards
| Week | Low‐FODMAP rye breadN = 73 | Regular rye breadN = 74 | P‐valuea | |
|---|---|---|---|---|
| Study bread consumption, slices/dayb | 1 | 3.5 (3.0–4.0) | 3.5 (3.0–4.0) | 0.68 |
| 2–4 | 5.0 (4.0–7.0) | 5.3 (4.0–6.0) | 0.91 | |
| Intake of fructans from the bread, g/day | 1 | 0.32 (0.27–0.36) | 1.16 (0.99–1.32) | <0.001 |
| 2–4 | 0.45 (0.36–0.63) | 1.73 (1.32–1.98) | <0.001 | |
| Intake of mannitol from the bread, g/day | 1 | 0.11 (0.09–0.12) | 0.32 (0.27–0.36) | <0.001 |
| 2–4 | 0.15 (0.12–0.21) | 0.47 (0.36–0.54) | <0.001 | |
| Total FODMAPs from the bread, g/day | 1 | 0.42 (0.36–0.48) | 1.47 (1.26–1.68) | <0.001 |
| 2–4 | 0.60 (0.48–0.84) | 2.21 (1.68–2.52) | <0.001 |
Results are given as median (inter‐quartile range).
Wilcoxon signed‐rank test.
One slice weighed on average 30 g.
Patients reported that they were consuming three slices of bread daily during the run‐in period. There were no differences in the number of slices of study breads consumed during week 1 and during weeks 2–4 between low‐FODMAP rye bread vs. regular rye bread (medians were 3.5 vs. 3.5, P = 0.68 at week 1 and 5.0 vs. 5.3 at weeks 2–4, P = 0.91).
Discussion
As far as we are aware, this is the first randomised study to investigate the exclusive effect of bread in‐patients with the irritable bowel syndrome. We were interested in rye bread because it seems to induce long satiety (second meal effect), offer special benefits in terms of glucose and insulin metabolism, has high‐fibre content and many micronutrients, but on the other hand, is considered as a high‐FODMAP grain.8, 11, 12 The key finding of the study is that the modification of FODMAP content of bread alone does have an observable effect on the symptoms of IBS. Low‐FODMAP rye bread caused evidently less symptoms as there was statistically significant difference in abdominal pain, flatulence, stomach rumbling, intestinal cramps as well as in the total score of symptoms measured by weekly VAS, favouring the low‐FODMAP rye bread. All of these symptoms can be considered as typically associated with the colonic fermentation of FODMAPs. No differences were observed in other individual symptoms that are more likely linked to the stomach and rectum sections of the digestive system (heartburn, dyspepsia, diarrhoea, constipation, incomplete defecation, urgency in defecation). These findings in individual symptoms are in line with the observed difference in breath hydrogen excretion, i.e. less hydrogen was excreted during the low‐FODMAP rye bread period confirming the lower level fermentation of FODMAPs in colon and reduced gas accumulation. These results also suggest that modifying the FODMAP content of just one regularly consumed food item may have significant effects on certain symptoms of IBS.
Rye bread is an important source of dietary fibre in Finland. The fibre composition of rye bread is complex as it contains both soluble and insoluble fibre. Moreover, it has different types of fibre components; of these, arabinoxylan is the most prominent followed by fructan. In this study, both of the study breads had high‐fibre content. The main difference between the breads was the fructan content; the regular rye bread contained more than three times the amount of fructan than the low‐FODMAP rye bread.
We detected a substantial increase in dietary fibre intake during both treatment periods. In fact, the fibre intake at baseline was at the same level as in general female population of this age in Finland (22 g in our study vs. 21 g in FinnDiet 2012 study).9 During the low‐FODMAP rye bread period, the intake of fibre was 27 g slightly less than the 29 g during the regular rye bread period (P = 0.09).
Despite the results in individual symptoms, we did not observe any effect in IBS‐SSS, which was our primary end point. To the best of our knowledge, IBS‐SSS has been used only once in a randomised IBS study on FODMAPs.20 In a 6‐week trial, IBS‐SSS symptom scores were significantly lower when patients consumed a holistic low‐FODMAP diet compared to the situation when they adhered to their usual diet. In our study, the intake of FODMAPs (mannitol and fructans) from the study breads differed on average by 1.5 g/day, of which 1.2 g was fructans. In comparison, the low‐FODMAP cut‐off value used by the research team at Monash University for oligosaccharides [total fructans plus galacto‐oligosaccharides (GOS)] from core grain/cereal/nut/seed products is 0.3 g per sitting (Jane Muir personal communication). On the other hand, when a holistic low‐FODMAP diet is prescribed, the intake of all FODMAPs seems to be substantially reduced, by 12–40 g/day from the level of 30–50 g/day.21, 22 Hence, the overall daily reduction in FODMAPs in our study was probably too small to induce major differences in IBS‐SSS. This explanation is also likely to apply to quality of life, where similarly we could detect no difference between the two bread periods.
The fact that we found a difference in gas accumulation, and individual symptoms as well as in total score of symptoms measured by weekly VAS but not with IBS‐SSS (twice per treatment period) might also be explained by the differences between assessment tests. IBS‐SSS is an instrument driven by pain and overall satisfaction with bowel function; it does not measure individual symptoms other than bloating and abdominal pain. Our total score of VAS symptoms consisted of 10 different symptoms of which only one (abdominal pain) was included in the IBS‐SSS. Measurements of individual symptoms and their composite end point may be more sensitive to changes than IBS‐SSS, which reflects more overall satisfaction and pain during the last 10 days. In fact, measurements of individual symptoms seem to be more frequently used in low‐FODMAP diet studies than IBS‐SSS measurements.7 Moreover, gas accumulation, measured as bread hydrogen concentration, is an objective way to measure the extent of colon fermentation.
We did not include predominantly constipated IBS (IBS‐C) patients into our study. We thought initially that IBS‐C patients would respond positively to both breads in terms of bowel movements, leading to reduced constipation and thus improved well‐being. Indeed, in a direct comparison of rye and white wheat bread, the consumption of rye bread achieved increased bowel movements, eased defecation and reduced intestinal transit time in healthy subjects.23 For this reason, we thought that this might introduce heterogeneity in the response and a subsequent dilution of the overall effect in the whole study population. In retrospect, the exclusion of IBS‐C was probably unnecessary, because we found no effect on constipation; similarly in other studies, the effect of grain bran on IBS‐related constipation has been modest.24, 25, 26
The major strength of this study is its double‐blinded randomised setting. All the participants, the principal investigator, the patient contact subject and the statistician were blinded. Keeping patients and contact persons blinded remains a major challenge in dietary IBS studies. In fact, a recent systematic review showed that the most low‐FODMAP diet studies scored poorly on blinding.27 This study is one of the rare blinded studies on FODMAP concept, thus further confirming the role of the FODMAP approach in IBS.
We included all the available participants into the analyses, whether they were low users of bread or fully compliant. Most participants were not able to consume the seven to eight slices of bread as instructed and recommended in Finnish dietary guidelines, and some participants consumed very low amounts (0–2 slices daily) throughout the study. Therefore, the results presented here are intention‐to‐treat analyses that usually are considered more robust than per protocol analyses.
However, our study has some limitations. It may not be possible to generalise the study results to patients with IBS‐C or to patients who are subjectively strictly intolerant to rye or other grains as we excluded these individuals from the study. One distinct difference between our study and many other FODMAP studies is that we did not control the background diet, i.e. the participants were not asked to adhere to a holistic FODMAP restricted diet. Nonetheless, we argue that the lack of strict FODMAP restriction is actually more likely to cause regression to mean in our study. Therefore, the design of the study might cause dilution of the actual effects of low‐FODMAP rye bread rather than exacerbation of the difference in symptoms.
We asked the patients to keep their diet as constant as possible and to avoid from any elimination diets during the study. We think that the design of our study allowed us to model a realistic and natural setting. Furthermore, we have examined a scenario where a patient would substitute one common food item in his/her shopping basket for another similar product, only differing in its FODMAP content. This is a much more patient‐friendly option than a complete change in diet. Participants kept food diaries, but as there is no database in Finland on the FODMAP content of foods, especially data on fructans and polyols is lacking, we were not able to calculate the total amount of FODMAPs consumed during the periods. Some participants may have either reduced or increased the intake of other FODMAPs accidently or purposefully during the study despite instructions to adhere to their normal diet.
We did not analyse the amount of GOS in the study breads. However, we performed a post hoc analysis on a commercial sourdough rye bread which is very similar to our regular rye bread (our control). Its raffinose content was 0.03%. There is no reason to believe that the fermentation process used by us caused increase in the amount of GOS in the low‐FODMAP rye bread when compared to this commercial rye bread. It is unlikely that differences in the amount of GOS in the study breads could have had a significant effect on the study outcomes.
Prevalence of colon cancer is increasing in many countries. According to World Cancer Research Fund's meta‐analyses (2010), both the low intake of cereal fibre and the high intake of processed and red meat are risk factors for colorectal cancer.28 It is postulated that one of the key mechanisms how red and processed meat is associated with the risk of colorectal cancer is the fermentation of unabsorbed protein in colon, especially when concomitant dietary fibre is lacking.29 In this context, low‐FODMAP rye bread could provide a means to increase fibre intake and thereby to reduce colonic fermentation of unabsorbed protein also among IBS patients. However, a possible decrease in protein fermentation during a diet containing plenty of low‐FODMAP rye bread needs to be confirmed and addressed in specific clinical trials.
In conclusion, this study showed that the low‐FODMAP rye bread caused less fermentation in colon, less flatulence, less abdominal pain, less cramps and less stomach rumbling than regular rye bread. The low‐FODMAP rye bread was well accepted by study patients. Moreover, the low‐FODMAP rye bread was a feasible way to increase dietary fibre intake to the recommended level in IBS patients. Since it was the sole change in diet, simply switching from regular rye bread to low‐FODMAP bread is not likely to be sufficient for most patients to achieve clinically relevant reductions of symptoms, as suggested by our measurements with IBS‐SSS and quality of life. Nonetheless, the present study reveals that low‐FODMAP rye bread is feasible and palatable bread for IBS patients who want to follow a healthy, fibre‐rich low‐FODMAP diet. More randomised studies will be needed to clarify the effects of FODMAPs in wholegrain foods, especially rye‐based products and as contributory factors in IBS.
Authorship
Guarantor of the article: Reijo Laatikainen.
Author contributions: Reijo Laatikainen, Riitta Korpela, Sanna‐Maaria Hongisto, Jussi Loponen, Jari Koskenpato and Markku Hillilä contributed to the design of the study. Laatikainen was the principal investigator of this research and drafted the manuscript. Riitta Korpela, Sanna‐Maria Hongisto, Jussi Loponen, Tuija Poussa, Jari Koskenpato and Markku Hillilä critically revised the manuscript. Laatikainen was involved in the data collection. Laatikainen and Poussa conducted the data analyses.
All authors have approved the final version of the manuscript.
Supporting information
Figure S1. Appearance of bread packages in the study. C = low‐FODMAP rye bread and D = regular rye bread.
Table S1. Use and timing of different measurement instruments during the study.
Acknowledgements
Declaration of personal interests: Reijo Laatikainen has written a Finnish book on irritable bowel syndrome and diet. He is also founder and owner of Booston Ltd, which provides IBS‐related dietetic services to IBS patients, healthcare professionals and various organisations. Sanna‐Maria Hongisto and Jussi Loponen are employees of Fazer Bakeries. Tuija Poussa has received consultation fees from Booston Ltd. Others have no personal interests to declare.
Declaration of funding interests: Fazer Bakeries funded the study and provided the breads.
The Handling Editor for this article was Professor Peter Gibson, and it was accepted for publication after full peer‐review.
References
- 1. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nellesen D, Yee K, Chawla A, Lewis BE, Carson RT. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013; 19: 755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simrén M, Månsson A, Langkilde AM, et al Food‐related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001; 63: 108–15. [DOI] [PubMed] [Google Scholar]
- 4. Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self‐reported food‐related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013; 108: 634–41. [DOI] [PubMed] [Google Scholar]
- 5. Gibson PR, Shepherd SJ. Evidence‐based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol 2010; 25: 252–8. [DOI] [PubMed] [Google Scholar]
- 6. De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut 2016; 65: 169–78. [DOI] [PubMed] [Google Scholar]
- 7. Staudacher HM, Irving PM, Lomer MC, Whelan K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat Rev Gastroenterol Hepatol 2014; 11: 256–66. [DOI] [PubMed] [Google Scholar]
- 8. Karppinen S, Myllymäki O, Forssell P, Poutanen K. Fructan content of rye and rye products. Cereal Chem 2003; 80: 168–71. [Google Scholar]
- 9. Helldán A, Raulio S, Kosola M, Tapanainen H, Ovaskainen M‐L, Virtanen S. Finravinto 2012 ‐tutkimus – The National FINDIET 2012 Survey. ISBN 978‐952‐245‐950‐3 (printed); 978‐952‐245‐951‐0 (pdf) THL. Raportti 16/2013, 187 p. Helsinki, Finland 2013.
- 10. Olsen A, Egeberg R, Halkjær J, Christensen J, Overvad K, Tjønneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr 2011; 141: 639–44. [DOI] [PubMed] [Google Scholar]
- 11. Isaksson H, Tillander I, Andersson R, et al Whole grain rye breakfast ‐ sustained satiety during three weeks of regular consumption. Physiol Behav 2012; 105: 877–84. [DOI] [PubMed] [Google Scholar]
- 12. Bondia‐Pons I, Nordlund E, Mattila I, et al Postprandial differences in the plasma metabolome of healthy Finnish subjects after intake of a sourdough fermented endosperm rye bread versus white wheat bread. Nutr J 2011; 10: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aune D, Chan DS, Lau R, et al Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose‐response meta‐analysis of prospective studies. BMJ 2011; 343: d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biesiekierski JR, Rosella O, Rose R, et al Quantification of fructans, galacto‐oligosacharides and other short‐chain carbohydrates in processed grains and cereals. J Hum Nutr Diet 2011; 24: 154–76. [DOI] [PubMed] [Google Scholar]
- 15. Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc 2006; 106: 1631–9. [DOI] [PubMed] [Google Scholar]
- 16. Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. Available at: http://www.romecriteria.org/criteria/ (accessed 24 February 2016).
- 17. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997; 11: 395–402. [DOI] [PubMed] [Google Scholar]
- 18. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014; 146: 67–75. [DOI] [PubMed] [Google Scholar]
- 19. Drossman DA, Patrick DL, Whitehead WE, et al Further validation of the IBS‐QOL: a disease‐specific quality‐of‐life questionnaire. Am J Gastroenterol 2000; 95: 999–1007. [DOI] [PubMed] [Google Scholar]
- 20. Pedersen N, Andersen NN, Végh Z, et al Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol 2014; 20: 16215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staudacher HM, Lomer MC, Anderson JL, et al Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr 2012; 142: 1510–8. [DOI] [PubMed] [Google Scholar]
- 22. Derrick Ong, Mitchell S, Barrett J, et al Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol 2010; 25: 1366–73. [DOI] [PubMed] [Google Scholar]
- 23. Holma R, Hongisto SM, Saxelin M, Korpela R. Constipation is relieved more by rye bread than wheat bread or laxatives without increased adverse gastrointestinal effects. J Nutr 2010; 140: 534–41. [DOI] [PubMed] [Google Scholar]
- 24. Arffmann S, Andersen JR, Hegnhøj J, Schaffalitzky de Muckadell OB, Mogensen NB, Krag E. The effect of coarse wheat bran in the irritable bowel syndrome. A double‐blind cross‐over study. Scand J Gastroenterol 1985; 20: 295–8. [DOI] [PubMed] [Google Scholar]
- 25. Rees G, Davies J, Thompson R, Parker M, Liepins P. Randomised‐controlled trial of a fibre supplement on the symptoms of irritable bowel syndrome. J R Soc Promot Health 2005; 125: 30–4. [DOI] [PubMed] [Google Scholar]
- 26. Moayyedi P, Quigley EM, Lacy BE, et al The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta‐analysis. Am J Gastroenterol 2014; 109: 1367–74. [DOI] [PubMed] [Google Scholar]
- 27. Rao SSC, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP‐restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther 2015; 41: 1256–70. [DOI] [PubMed] [Google Scholar]
- 28. WCRF/AICR Systematic Literature Review Continuous Update Project Report. The Associations between Food, Nutrition and Physical Activity and the Risk of Colorectal Cancer October 2010. Available at: http://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/colorectal-bowel-cancer (accessed 25 April 2016).
- 29. Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther 2015; 43: 181–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Appearance of bread packages in the study. C = low‐FODMAP rye bread and D = regular rye bread.
Table S1. Use and timing of different measurement instruments during the study.


