Abstract
Aims
Evaluate magnitude and duration of subjective and physiologic responses to neutral and marijuana (MJ)–related cues in cannabis dependent volunteers.
Methods
33 volunteers (17 male) who met DSM-IV criteria for Cannabis Abuse or Dependence were exposed to neutral (first) then MJ-related visual, auditory, olfactory and tactile cues. Mood, drug craving and physiology were assessed at baseline, post-neutral, post-MJ and 15-min post MJ cue exposure to determine magnitude of cue- responses. For a subset of participants (n=15; 9 male), measures of craving and physiology were collected also at 30-, 90-, and 150-min post-MJ cue to examine duration of cue-effects.
Results
In cue-response magnitude analyses, visual analog scale (VAS) items craving for, urge to use, and desire to smoke MJ, Total and Compulsivity subscale scores of the Marijuana Craving Questionnaire, anxiety ratings, and diastolic blood pressure (BP) were significantly elevated following MJ vs. neutral cue exposure. In cue-response duration analyses, desire and urge to use MJ remained significantly elevated at 30-, 90- and 150-min post MJ-cue exposure, relative to baseline and neutral cues.
Conclusions
Presentation of polysensory MJ cues increased MJ craving, anxiety and diastolic BP relative to baseline and neutral cues. MJ craving remained elevated up to 150-min after MJ cue presentation. This finding confirms that carry-over effects from drug cue presentation must be considered in cue reactivity studies.
Keywords: Marijuana cue reactivity, craving, magnitude, duration
1. INTRODUCTION
Marijuana is the most commonly used illicit drug in the United States and primary problem among substance abuse treatment seekers (SAMHSA, 2014). Psychosocial interventions are partially efficacious, but most cannabis dependent patients in treatment do not achieve abstinence (Copeland et al., 2001; Marijuana Treatment Project Research Group, 2004). Currently there are no FDA-approved medications for treating cannabis use disorder (CUD).
Craving is a central feature of drug abuse (O’Brien, 2005) associated with motivating use (O’Brien et al., 1998; Wolfling et al., 2008; Preston et al., 2009) and relapse (Childress et al., 1988; Lowman et al., 2000). As a symptom of CUD (American Psychiatric Association, 2013) and cannabis withdrawal (Budney et al., 2004; Haney, 2005; Vandrey et al., 2008), craving can be considered a significant target in studies of treatment efficacy.
The cue reactivity paradigm offers a means to induce and quantify craving in a controlled environment. Substance-abusing individuals are exposed to drug-related cues (e.g., paraphernalia, videos depicting drug preparation or taking) and neutral cues (e.g., wood chips, pencils, water). Craving and physiologic responses to these different cues are compared. An extensive literature demonstrates cue-induced craving across various substances including nicotine, alcohol, cocaine, heroin (reviewed by Carter and Tiffany, 1999), and marijuana (Haughey et al., 2008; Wolfling et al., 2008; Gray et al. 2008; 2011; Lundahl and Johanson, 2011; McRae-Clark et al., 2011; Nickerson et al., 2011; Charboneau et al., 2013; Lundahl and Greenwald, 2015). Cue-induced craving for marijuana has been found to be population-, cue-, and drug-specific. That is, only marijuana smokers (but not marijuana-naïve controls) responded to marijuana-related cues (but not neutral cues) with increased self-reported craving, and with increased craving only for marijuana but not nicotine (Lundahl and Johanson, 2011).
This paradigm has been used to evaluate potential anti-craving medications for cocaine (Kranzler and Bauer, 1992; Robbins et al., 1992; Hersh et al., 1995; Berger et al., 1996; Ehrman et al., 1996; LaRowe et al., 2007; Reid and Thakkar, 2009), nicotine (Reid et al., 2007; Rohsenow et al., 2008; Franklin et al., 2011; Ditre et al., 2012), alcohol (Rohsenow et al., 2000; Hutchison et al., 2001), and marijuana (Lundahl and Greenwald, 2015). Across studies, drug-related cues reliably induced drug-specific craving despite variable efficacy of the potential medications tested. In general, cue reactivity procedures provide a valuable set of outcomes for screening putative anti-craving medications, and may identify mechanisms relevant to pharmacotherapy even in the absence of medication efficacy (Berger et al., 1996).
Although exposure to substance-related cues increases craving for that specific substance, duration of cue-responses has received little attention but is important for several reasons. First, establishing a timeline of craving could inform design of laboratory cue paradigms. To control for potential carry-over effects, it has been recommended (Monti et al., 1987; Hutchison et al., 1999) that the neutral cue should always precede active drug cue presentation; thus neutral and drug-related cues have not been counterbalanced in most cue reactivity studies (Carter and Tiffany, 1999). Few studies have investigated whether there are carry-over effects or when cue-induced craving returns to baseline levels. Heishman et al. (2010) found that male and female smokers responded to tobacco-related imagery and in vivo cues with greater tobacco craving and increased heart rate and blood pressure, elevations that were sustained for 30-min post-exposure (Heishman et al., 2010). If cue-induced craving persists for extended periods of time, then paradigms would need to account for carry-over effects by scheduling washout intervals between cue conditions to avoid confounding. Second, because craving is related to motivating drug use (Wolfling et al., 2008) and relapse (Lowman et al., 2000), from a safety perspective, participants should not leave the laboratory until craving levels return to baseline to minimize risk of iatrogenic drug use. Finally, when using the cue reactivity paradigm in medication development studies, it is essential to know the duration of induced drug craving. Even if a medication acutely attenuates craving, a longer-acting formulation may be necessary to be efficacious.
The present study investigated the magnitude and duration of marijuana cue-induced subjective and physiological reactivity in cannabis-dependent male and female adults. We hypothesized that marijuana cue exposure would increase craving relative to neutral cue exposure. We also examined the time course of marijuana cue-induced exposure until 150-min post-cue.
2. METHODS
2.1. Participant selection
The local IRB approved all study procedures. Candidates from 18 to 44 years old were recruited through local newspaper ads and word-of-mouth referrals. Eligible participants had to be in good health based on history and physical exam, standard laboratory studies, electrocardiogram, and psychiatric interview. Participants were not seeking treatment, met DSM-IV (APA, 1994) criteria for Cannabis Dependence, and submitted a cannabinoid-positive (cutoff ≥ 50 ng/ml) urine sample at screening. Candidates with positive urine tests for non-cannabinoid drugs were excluded. Additional exclusion criteria were any current DSM-IV axis I disorder except Cannabis or Nicotine Dependence (assessed using the Structured Interview for DSM-IV; First et al., 1996); neurologic, cardiovascular, pulmonary or systemic diseases; and cognitive impairment. Females could not be pregnant or lactating, and had to be using medically approved contraceptives. All participants had to provide sober (BAC < 0.02%) informed consent and demonstrate adequate cognitive functioning (i.e., estimated IQ > 85; Zachary, 1986). Participants also completed questionnaires regarding their drug and alcohol use. Volunteers were paid for their participation.
2.2. Design and procedure
2.2.1. General procedures
Participants were admitted to a university-affiliated inpatient unit at 9:00 pm. and spent the evening prior to their session to control for alcohol and drug use for the 12-h prior. After eating breakfast at 7:30 a.m., they were and transported to the laboratory via taxicab with staff escort. Participants provided breath and urine samples for toxicology testing upon their arrival at the laboratory. While on the inpatient unit, participants were offered periodic tobacco cigarette breaks during which they could smoke cigarettes. At the laboratory, cigarette breaks were allowed only when participants were not completing questionnaires or experimental tasks.
2.2.2. Experimental session procedures
Participants were seated in a recliner in a light- and sound-attenuated private testing room each participant underwent the marijuana cue exposure procedure described below. A telemetric (Mini-Mitter Co, Inc., Bend, OR) was used to collect skin temperature and heart rate data, and a blood pressure cuff was fitted to each participant to monitor blood pressure. The experimental session consisted of three, 10-min phases (i.e., baseline, neutral cue, marijuana cue), followed by a 30-min recovery period. Each phase was separated by 10 min. Following advice of Monti et al. (1987), the order of cue presentation was not counterbalanced to avoid possible carryover effects from marijuana cues. All experimental instructions to the participants were delivered via speaker in the chamber to minimize distraction during cue exposures.
Baseline
While seated in the recliner, participants were asked to “relax” for 10-min while pre-cue subjective and physiological measures (see below) were recorded. The neutral phase began immediately after the baseline phase.
Neutral-cue phase
Participants were instructed to remove the inverted opaque cover marked “A”, which revealed a variety of school supplies, including pencils, erasers, a ruler, and floral scented potpourri in a small bowl. Participants were next instructed to handle and smell these items while viewing a videotaped film clip containing nature scenes set to classical music. Following this 10-min cue period, participants were instructed to return the items to the table and to replace the cover. Subjective and physiological measures were recorded. They were then asked to “sit back and relax” for 10-min until the next phase began.
Marijuana-cue phase
Immediately following the neutral cue exposure, participants were asked to remove the inverted opaque cover marked “B”, which revealed various marijuana-related paraphernalia, including a recently used bong, pipe, rolling papers, hollowed-out blunts, and a roach clip. Participants were instructed to handle and smell these items while viewing a videotaped film clip depicting of individuals smoking marijuana. Set to dance music, video scenes depicted preparing marijuana for smoking (i.e., rolling joints and blunts), and smoking marijuana in a variety of ways (i.e., joint, blunt, bong, pipe) in different settings (i.e., party, on a date, in a living room). At the end of the 10-min exposure, participants were asked to return the items to the table and replace the opaque cover over the items. Subjective and physiological measures were recorded. Marijuana was not made available to the participants at any time.
Post-cue recovery period
Immediately after the marijuana-cue phase, participants were escorted to a recreation room where they could read or watch movies (screened to exclude marijuana content) for a 150-min recovery period. Subjective and physiologic measures were collected at 15-min (magnitude sample) and at 15-, 30-, 90-, and 150-min (duration sample) during this period.
2.3. Measures
2.3.1 Physiologic Dependent Variables
Heart rate and skin temperature were monitored continuously and recorded at 1-min intervals throughout the session. Systolic and diastolic blood pressure (BP) values were measured at baseline, immediately following neutral cue and marijuana cue presentation, and every 30-min during the recovery period.
2.3.2 Subjective Effects Questionnaires
Mood
A series of computerized visual analog scales (VAS) was presented and participants were instructed to place a vertical mark on a 100-mm line anchored on the left by the phrase not at all and on the right by the word extremely that corresponded with their responses to the following mood-related items: “How (“Anxious”, “Upset”, “High”, “Happy”, “Irritable”, “Sedated”, “Down”, “Uncomfortable”, “Stimulated”) do you feel right now?”
Craving
Craving was assessed using three VAS items that included the phrase, “How strong is your…” followed by “desire to smoke marijuana right now?”, “urge to smoke marijuana right now?”, and “craving for marijuana right now?” Participants were asked to respond identically to the mood VAS items as described above.
Marijuana craving also was assessed using the Marijuana Craving Questionnaire-Brief Form (MCQ-BF; Singleton et al., 2002), a 17-item instrument that represents four domains of marijuana craving characterized in the original 47-item MCQ (Heishman et al., 2001). These four constructs are: (1) Compulsivity, the inability to control marijuana use; (2) Emotionality, marijuana use for relief from withdrawal or negative affect; (3) Expectancy, anticipation of positive consequences from smoking marijuana; and, (4) Purposefulness, intention and planning of marijuana use for positive consequences.
2.4. Statistical analyses
Data were analyzed using SPSS version 22 for Mac (IBM, 2013). Gender differences in screening measures were examined using one-way ANOVA and nonparametric tests. The ten, 1-min samples immediately preceding the first cue phase were averaged to yield baseline heart rate and skin temperature values. Heart rate and skin temperature data were averaged over each 10-min cue exposure period to yield a mean value for each condition. To control for movement artifact during the first minute of each cue exposure period (when participants leaned forward and removed the opaque covers from the boxes containing the tactile cues), the 1-min data point was excluded both from the heart rate and skin temperature data average during neutral and marijuana cue conditions. To assess magnitude of cue response, subjective and physiologic data were analyzed using one-way repeated measures ANOVAs, with condition (Baseline/Neutral/Marijuana/Recovery) as the repeated factor. To assess duration of cue response, subjective and physiologic data were analyzed using one-way repeated measures ANOVAs, with time (Baseline, Neutral, Marijuana, and 15-, 30-, 90-, and 150-min post-marijuana cue exposure) as the repeated factor. Significant main effects were examined using Tukey post hoc tests. All effects were tested at the .05 level of significance.
3. RESULTS
3.1. Participant characteristics
In the full sample (response magnitude analysis), all 33 (17 male) participants met DSM-V criteria for Cannabis Use Disorder and provided cannabinoid-positive urine samples. Almost all participants were African American (91%), with the remainder Caucasian (3%) or multiracial (6%). Participant demographics and drug use measures are reported in Table 1a. Males and females did not differ on any screening measures.
Table 1.
Participant demographic and drug use variables (n=33)
| Magnitude Sample (n = 33) |
Duration Sample (n = 15) |
|
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (yr) | 27.5 (4.7) | 27.9 (4.9) |
| Education (yr) | 12.7 (1.7) | 12.1 (0.7) |
| Age at first use of marijuana (yr) |
15 (2.9) | 13.3 (2.1) |
| No. blunts smoke per day |
4.7 (3.9) | 4.5 (2.1) |
| Duration of daily cannabis use (mo) |
97.2 (60) | 115.2 (55.9) |
| Estimated # lifetime episodes of MJ use |
11,847 (9867) | 13,391 (9782) |
| Regular tobacco use |
91% | 94% |
| Regular alcohol use |
18% | 24% |
| # times used MDMA |
0.3 (.7) | 0.4 (.9) |
| # times used cocaine |
1.6 (7.1) | 0 (0) |
All of the 15 (9 male) participants in the subset sample (response duration analysis) were African American. Participant demographics and drug use variables are presented in Table 1b.
3.2 Magnitude of cue reactivity
3.2.1. Physiological responses
A Condition main effect was found on diastolic BP, F(3,93) = 3.03, p < .04. Post hoc comparisons indicated that diastolic BP was significantly elevated following marijuana relative to neutral cue exposure (Fig 1, left panel). There were no significant differences in skin temperature, heart rate or systolic BP.
Figure 1.
Marijuana (MJ) vs. neutral (Neu) cue presentation significantly increased diastolic blood pressure (left panel) and VAS ratings of Anxiety (right panel).
3.2.2. Subjective responses
There was a significant Condition main effect for VAS “anxious”, F(3,93) = 3.48, p < .03 Fig 1, right panel), “craving”, F(3,93) = 18.58, p < .001, “desire to smoke”, F(3,93) = 17.02, p < .001 (Fig 2, left panel), “urge to use marijuana”, F(3,93) = 21.36, p < .001, and for MCQ Compulsivity, F(3,93) = 7.69, p < .001 (Fig 2, right panel), and MCQ Total Score, F(3,93) = 4.82, p < .01. Post hoc comparisons indicated that, for all measures except “anxious” and MCQ Total Score, ratings during marijuana cues and recovery were significantly elevated relative both to baseline and during neutral cues. Ratings of “anxious” were significantly higher during marijuana cues and recovery compared to neutral cues. MCQ Total Scores were significantly elevated during marijuana cues compared to both baseline and neutral cues, and greater during recovery compared to neutral cues. There were no other significant differences.
Figure 2.
Marijuana (MJ) vs. neutral (Neu) cue presentation significantly increased VAS ratings of “Desire to Smoke Marijuana” (left panel) and Marijuana Craving Questionnaire (MCQ) Compulsivity (but not other MCQ) subscale scores (right panel).
3.3 Duration of cue reactivity
3.3.1. Physiological responses
The increase in diastolic BP observed immediately and 15-min post-marijuana cue exposure was not sustained at later time points. There were no significant differences on any other physiological measures.
3.3.2. Subjective responses
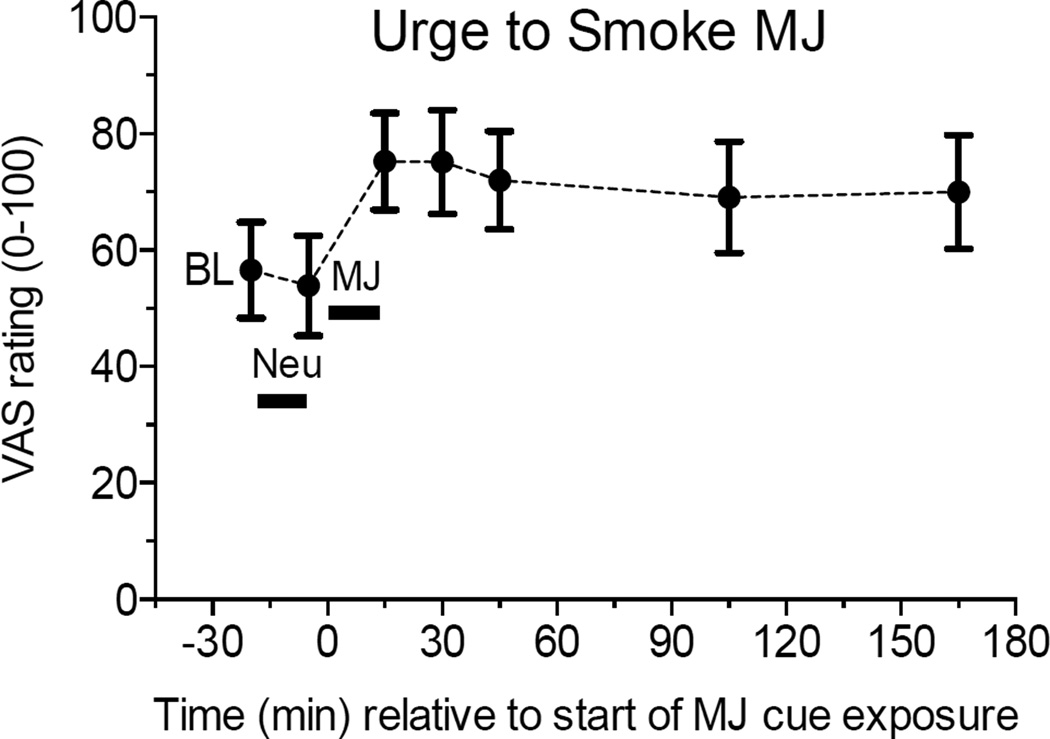
A Time main effect was found for “craving for marijuana”, F(6,84) = 3.45, p < .03, “desire to smoke marijuana”, F(6,84) = 5.66, p < .01, and “urge to smoke marijuana”, F(6,84) = 6.98, p < .001 (Fig 3). Post hoc comparisons indicated that the significant increases in self-reported “desire to smoke marijuana” and “urge to smoke marijuana” during marijuana cues remained significantly elevated at 15-, 30-, 90-, and 150-min post marijuana cues, relative to both baseline and neutral cues. “Craving for marijuana” was significantly greater immediately after and at 15-, 30-, and 90-min post-marijuana cues relative to baseline, and greater immediately after and at 15-min post-marijuana cues compared to neutral cues. There were no other significant differences.
Figure 3.
Marijuana (MJ) relative to neutral (Neu) cue presentation significantly increased VAS ratings of “Urge to Smoke Marijuana” for more than 2.5 hours afterward.
4. DISCUSSION
This is the first study to examine the duration of craving in response to marijuana related cue exposure. Following exposure to polysensory marijuana cues, robust increases in self-reported marijuana craving, anxiety, and MCQ Compulsivity subscale and total scores, were observed in this sample of individuals with cannabis use disorder. All these measures were significantly greater than at baseline levels and during neutral cue exposure for at least 15-min after marijuana cue exposure. Including the baseline time point enabled us to confirm that neutral cues did not increase marijuana craving relative to baseline (i.e., craving was not a response to the novelty of cue exposure or other factors). Thus, we are confident that increased cue reactivity observed during and after marijuana cue exposure was in fact due to the marijuana cues. Notably, two craving indices remained elevated for at least 2.5 h post-marijuana cue exposure. These persistent (longer than anticipated) elevations confirm that carry-over effects from drug cue exposure must be considered in cue reactivity studies.
While several measures were significantly greater 15-min after marijuana cues, only increased “desire” and “urge” to smoke marijuana were sustained for 2.5 h after exposure. “Craving” for marijuana was significantly greater immediately and 15-min after marijuana relative to neutral cues, and at later time points compared to baseline but not neutral cues. It is possible that “craving” connotes a stronger experience than “urge” or “desire” and some participants may be less likely to endorse it over time. Thus, asking about urge or desire to use may better capture longer-lasting cue reactivity than asking about craving.
The long-lasting duration of marijuana craving evident in this sample of individuals with cannabis use disorder indicates that cue-induced marijuana craving may not be a transient state. It may also be that over the course of the day, these heavy marijuana users started to experience acute withdrawal, although they did not report time-related changes in mood that were measured (e.g., irritable, uncomfortable) that would be consistent with cannabis withdrawal. It is possible that time of day was a confound, as participants were in the lab almost an entire day, and the last data collection time point in the late afternoon might have coincided with naturalistic marijuana smoking patterns. Unfortunately, it is difficult to disentangle the effect of time spent in the laboratory from cue exposure on craving responses, and this is beyond the scope of this study. Of importance is the observation that craving, once experienced and reported, persists for a significant period of time after the cues are removed.
These results support findings by Heishman et al. (2010), who found that tobacco craving increased following tobacco cue exposure and smoking imagery and remained unchanged for 30-min. Data collection in that study ended at 30-min post-cue exposure, so is unknown how long craving might have continued.
Although craving remained elevated after marijuana cue exposure, this was not as clear for physiological reactivity. Diastolic blood pressure (DBP) was significantly elevated after marijuana cue exposure relative to neutral cues, but not baseline. A decrease in DBP was observed from baseline to neutral cues, which might be explained by a possible calming effect of the neutral cues, which included viewing nature scenes set to classical music. Thus, the increase in DBP following marijuana cues must be interpreted with caution, as it is not clear whether the increase was due to marijuana cue exposure, or to the decrease during neutral cue exposure. Carter and Tiffany (1999) concluded from their meta-analytic review of 41 cue-reactivity studies (none of which focused on marijuana) that unreliable physiologic responses to drug-related cues are due to large variance and lack of cue specificity. Marijuana cue reactivity studies generally have not found cue-induced changes in BP, heart rate, or skin temperature (Gray et al., 2008, 2011; Lundahl and Johanson, 2011; Nickerson et al., 2011; Lundahl and Greenwald, 2015). It may be that, similar to other abused substances, cue-elicited physiologic changes are less reliable than self-reported craving.
The current study has several limitations. First, the sample size was modest and almost exclusively African American, which reduces generalizability of the findings. Second, participants were only included if they reported no interest in treatment for their marijuana use. As alleviation of craving is a reasonable treatment target, further studies are needed to establish validity of the cue-reactivity model for treatment-seeking cannabis dependent individuals. Another limitation shared with nearly all cue reactivity studies is that order of cue presentation was fixed and not counterbalanced. Neutral cues were always presented first to reduce potential confound of carry-over effects (Monti et al., 1987). Counterbalancing cue presentation is one way to control for carry-over effects, but results of the current study show that intercue interval following marijuana cue exposure might need to be at least 2.5 h. However, this approach may be less feasible to test medication effects because the model may have to accommodate medications with relatively short duration of action. In such cases it may be preferable to conduct neutral and drug-related cue sessions on separate days. Finally, cannabis withdrawal symptoms were not assessed. Several subjective VAS items might be considered proxies for cannabis withdrawal symptoms (i.e., “anxious”, “irritable”, “uncomfortable”), however, and there were no significant changes in these variables over the session. Future studies should assess cannabis withdrawal symptoms at baseline and throughout cue exposure sessions to disentangle craving effects of cue exposure from cannabis withdrawal. Tiffany and Wray (2014) distinguish between tonic craving, which results from abstinence or withdrawal, and phasic craving, which arises from drug cue exposure either in the laboratory or the natural environment and is characterized by rapid onset and relatively short duration. The extent to which these two types of craving are associated is currently unclear.
In conclusion, this study demonstrated that marijuana polysensory cues elicited robust increases in marijuana craving that continued at least 2.5 h post-cue exposure. Given that subjective craving responses of almost all participants failed to return to baseline levels by the end of the session, it remains unclear how long cue-induced craving persists. These results indicate that carryover effects from marijuana cues need to be considered when using a cue-reactivity model. Although this possibility has been suggested (i.e., Monti et al., 1987; Rohsenow et al., 2000, 2001), this is the first study to examine cue-induced response duration effects. Studies using this model, particularly those examining the ability of a medication to block craving, should carefully consider the time course of cue-induced craving when attempting to detect a signal of a medication’s efficacy in reducing craving. These results also suggest that participants should remain in the laboratory until craving has dissipated, for safety reasons. Such studies may elect to utilize a relapse prevention or coping with craving intervention at the conclusion of the study session to prevent post-session drug use. Finally, integrating information about duration of cue-induced craving with intensity of craving may help inform treatment strategies designed to alleviate craving.
Table 2.
Correlation coefficients among VAS craving and MCQ scales (n=33)
| VAS Craving Items | MCQ Scales | |||||||
|---|---|---|---|---|---|---|---|---|
| VAS Items | Craving | Desire | Urge | Compulsivity | Emotionality | Expectancy | Purposefulness | Total Score |
| Craving | 1.00 | .99** | .967** | .660** | .03 | .36* | .41* | .55** |
| Desire | 1.00 | .987** | .68** | .00 | .36* | .44* | .56** | |
| Urge | 1.00 | .69** | .00 | .33 | .43* | .56** | ||
| MCQ Scales | ||||||||
| Compulsivity | 1.00 | .47** | .54** | .55** | .91** | |||
| Emotionality | 1.00 | .02 | .33 | .57** | ||||
| Expectancy | 1.00 | .34* | .63** | |||||
| Purposefulness | 1.00 | .75** | ||||||
| Total Score | 1.00 | |||||||
p < .05
p < .01
Highlights.
Examined effects of marijuana cues on mood, craving, and physiological indices.
Marijuana cue exposure increased marijuana craving, anxiety, and blood pressure.
Marijuana craving remained elevated up to 150-min post marijuana cue exposure.
Drug cue exposure carry-over effects must be considered in cue reactivity studies.
Acknowledgments
The authors thank Ken Bates for recruiting participants, and Cheryl Aubie, Kelty Berardi, Robert Kender, Heather Durdle, Manny Tancer, MD, Michael Eadie, MD, Laura Strathdee, Deborah Kish, and staff at the Psychiatric and Addiction Research Center. We also thank Chris-Ellyn Johanson, PhD for her advice and guidance.
Role of funding source
NIH R21 DA019236 from the National Institute on Drug Abuse (to LHL), a research grant (Joe Young, Sr./Helene Lycaki Funds) from the State of Michigan, and the Detroit Wayne Mental Health Authority supported this research. Data for this study were obtained under NIH clinical trials NCT 00218504 and NCT 00218478.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Both authors declare no conflict of interest with respect to the conduct or content of this work.
Contributors
LHL was responsible for designing the study, psychiatric screening, performing data analyses, drafting the manuscript, and producing the data tables. MKG helped conceptualize data analysis, edited the manuscript, and created the figures. Both authors have reviewed content and approved the final version for publication.
REFERENCES
- American Psychiatric Association. Diagnostic And Statistical Manual Of Mental Disorders. 4th. Washington, DC: APA; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic And Statistical Manual Of Mental Disorders. 5th. Washington, DC: APA; 2013. [Google Scholar]
- Berger SP, Hall S, Mickalian JD, Reid MS, Crawford CA, Delucchi K, Carr K, Hall S. Haloperidol antagonism of cue-elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch. Gen. Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am. J. Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Charboneau EJ, Dietrich MS, Park S, Cao A, Watkins TJ, Blackford JU, Benningfield MM, Martin PR, Buchowski MS, Cowan RL. Cannabis cue-induced brain activation correlates with drug craving in limbic and visual salience regions: preliminary results. Psychiatry Res. 2013;214:122–131. doi: 10.1016/j.pscychresns.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Learning Factors In Substance Abuse, NIDA Res. Monogr. Vol. 94. Washington, DC: U.S. Gov. Printing Office; 1988. Classically Conditioned Responses In Opioid And Cocaine Dependence: A Role In Relapse? pp. 25–43. [PubMed] [Google Scholar]
- Copeland J, Swift W, Rees V. Clinical profile of participants in a brief intervention program for cannabis use disorder. J. Subst. Abuse Treat. 2001;20:45–52. doi: 10.1016/s0740-5472(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Oliver JA, Myrick H, Henderson S, Saladin ME, Drobes DJ. Effects of divalproex on smoking cue reactivity and cessation outcomes among smokers achieving initial abstinence. Exp. Clin. Psychopharmacol. 2012;20:293–301. doi: 10.1037/a0027789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N. Sex differences in smoking cue reactivity: craving, negative affect, and preference for immediate smoking. Am. J. Addict. 2014;23:211–217. doi: 10.1111/j.1521-0391.2014.12094.x. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW, Childress AR, O'Brien CP. Failure of ritanserin to block cocaine cue reactivity in humans. Drug Alcohol Depend. 1996;42:167–174. doi: 10.1016/s0376-8716(96)01278-1. [DOI] [PubMed] [Google Scholar]
- Elsohly. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders - Patient Edition (SCID-I/P, Version 2.0) Washington, DC: APA; 1996. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O'Brien CP, Childress AR, et al. Effects of varenicline on smoking cue–triggered neural and craving responses. Arch. Gen. Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Upadhyaya HP. Cue reactivity in young marijuana smokers: a preliminary investigation. Psychol. Addict. Behav. 2008;22:582–586. doi: 10.1037/a0012985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Watson NL, Carpenter MJ. Reactivity to in vivo marijuana cues among cannabis-dependent adolescents. Addict. Behav. 2011;36:140–143. doi: 10.1016/j.addbeh.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr. Psychiatric Rep. 2005;7:360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Saha S, Singleton EG. Imagery-induced tobacco craving: duration and lack of assessment reactivity bias. Psychol. Addict. Behav. 2004;18:284–288. doi: 10.1037/0893-164X.18.3.284. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Lee DC, Taylor RC, Singleton EG. Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: effects of tobacco deprivation and sex. Exp. Clin. Psychopharmacol. 2010;18:245–256. doi: 10.1037/a0019401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh D, Bauer LO, Kranzler HR. Carbamazepine and cocaine-cue reactivity. Drug Alcohol Depend. 1995;39:213–221. doi: 10.1016/0376-8716(95)01165-3. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Exp. Clin. Psychopharmacol. 1999;7:250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology. 2001;155:27–34. doi: 10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- IBM. Version 22.0 for Mac OS X. Chicago: IBM, Inc.; 2013. Statistical Package for the Social Sciences. [Google Scholar]
- Kamboj SK, Massey-Chase R, Rodney L, Das R, Almahdi B, Curran HV, Morgan CJ. Changes in cue reactivity and attentional bias following experimental cue exposure and response prevention: a laboratory study of the effects of D-cycloserine in heavy drinkers. Psychopharmacology. 2011;217:25–37. doi: 10.1007/s00213-011-2254-z. http://dx.doi.org/10.1007/s00213-011-2254-z. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Bauer LO. Bromocriptine and cocaine cue reactivity in cocaine-dependent patients. Br. J. Addict. 1992;87:1537–1548. doi: 10.1111/j.1360-0443.1992.tb02661.x. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-acetylcysteine? Am. J. Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111:120–127. doi: 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman C, Hunt WA, Litten RZ, Drummond DC. Research perspectives on alcohol craving: an overview. Addiction. 2000;95(Suppl. 2):S45–S54. doi: 10.1080/09652140050111636. [DOI] [PubMed] [Google Scholar]
- Lundahl LH, Greenwald MK. Effect of oral THC pretreatment on marijuana cue-induced responses in cannabis dependent volunteers. Drug Alcohol Depend. 2015;149:187–193. doi: 10.1016/j.drugalcdep.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl LH, Johanson CE. Cue-induced craving for marijuana in cannabis-dependent adults. Exp. Clin. Psychopharmacol. 2011;19:223–230. doi: 10.1037/a0023030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J. Consult. Clin. Psychol. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, Giarla K, Nicholas K, Brady KT. Stress and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology. 2011;218:49–58. doi: 10.1007/s00213-011-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J. Abnorm. Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Abrams DB. Alcohol cue reactivity: Effects of detoxification and extended exposure. J. Stud. Alcohol. 1993;54:235–245. doi: 10.15288/jsa.1993.54.235. http://dx.doi.org/10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- Nickerson LD, Ravichandran C, Lundahl LH, Rodolico J, Dunlap S, Trksak GH, Lukas SE. Cue-reactivity in cannabis-dependent adolescents. Psychol. Addict. Behav. 2011;25:168–173. doi: 10.1037/a0021117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug use: can they explain compulsion? J. Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology. 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Thakkar V. Valproate treatment and cocaine cue reactivity in cocaine dependent individuals. Drug Alcohol Depend. 2009;102:144–150. doi: 10.1016/j.drugalcdep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Palamar J, Raghavan S, Flammino F. Effects of topiramate on cue-induced cigarette craving and the response to a smoked cigarette in briefly abstinent smokers. Psychopharmacology. 2007;192:147–158. doi: 10.1007/s00213-007-0755-6. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Using cue reactivity to screen medications for cocaine abuse: a test of amantadine hydrochloride. Addict. Behav. 1992;17:491–499. doi: 10.1016/0306-4603(92)90009-k. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. Naltrexone's effects on reactivity to alcohol cues among alcoholic men. J. Abnorm. Psychol. 2000;109:738–742. [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, Abrams DB. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96:1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Miranda R, McGeary JE, Swift RM, Hutchison KE, Sirota AD, Monti PM, et al. Olanzapine reduces urge to smoke and nicotine withdrawal symptoms in community smokers. Exp. Clin. Psychopharmacol. 2008;16:215–222. doi: 10.1037/1064-1297.16.3.215. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (TEDS): 2002–2012. BHSIS Series S-71, HHS Publication No. (SMA) 14-4850. [Google Scholar]
- Singleton EG, Trotman AJ, Zavahir M, Taylor RC, Heishman SJ. Determination of the reliability and validity of the Marijuana Craving Questionnaire using imagery scripts. Exp. Clin. Psychopharmacol. 2002;10:47–53. doi: 10.1037//1064-1297.10.1.47. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM. The clinical significance of drug craving. Ann. N. Y. Acad. Sci. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver BK, McRae-Clark AL, Saladin M, Price KL, Simpson AN, DeSantis SM, Baker NL, Brady KT. Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am. J. Drug Alcohol Abuse. 2010;36:106–113. doi: 10.3109/00952991003686402. [DOI] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J. Consult. Clin. Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur. J. Neurosci. 2008;27:976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, Yao S, Le Foll B, Lu L. Gender and stimulus effects in cue-induced responses in abstinent heroin users. Pharmacol. Biochem. Behav. 2007;86:485–492. doi: 10.1016/j.pbb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised Manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]