SUMMARY
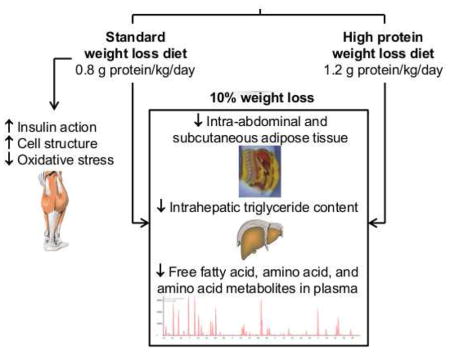
High-protein (HP) intake during weight loss (WL) therapy is often recommended because it reduces the loss of lean tissue mass. However, HP intake could have adverse effects on metabolic function because protein ingestion reduces postprandial insulin sensitivity. In this study, we compared the effects of ~10% WL with a hypocaloric diet containing 0.8 g protein/kg/day and a hypocaloric diet containing 1.2 g protein/kg/day on muscle insulin action in postmenopausal women with obesity. We found that HP intake reduced the WL-induced decline in lean tissue mass by ~45%. However, HP intake also prevented the WL-induced improvements in muscle insulin signaling and insulin-stimulated glucose uptake, and the WL-induced adaptations in oxidative stress and cell structural biology pathways. Our data demonstrate that the protein content of a WL diet can have profound effects on metabolic function and underscore the importance of considering dietary macronutrient composition during WL therapy for people with obesity.
Keywords: Calorie restriction, insulin sensitivity, protein, amino acids, skeletal muscle
Graphical Abstract
INTRODUCTION
Insulin-resistant glucose metabolism is the most common metabolic complication associated with obesity and a key risk factor for developing type 2 diabetes (T2D) and coronary heart disease (Kirk and Klein, 2009). Weight loss induced by dietary energy restriction is the cornerstone of therapy for people who are obese because it improves or even normalizes insulin sensitivity and related comorbidities (Klein, 2001). However, diet-induced weight loss also decreases lean tissue (including muscle) mass (Wycherley et al., 2012, Leidy et al., 2015), which could have adverse effects on physical function, particularly in populations who are at increased risk of sarcopenia, such as postmenopausal women (Samson et al., 2000, Phillips et al., 1993). Although increased protein intake during diet-induced weight loss is often recommended because it helps preserve lean tissue mass (Wycherley et al., 2012, Leidy et al., 2015), data from a series of studies suggest that high protein (HP) intake could have detrimental metabolic effects; acute intravenous amino acid infusion or protein ingestion reduces insulin sensitivity (Smith et al., 2015, Krebs et al., 2002, Robinson et al., 2014) and habitual HP intake is associated with insulin resistance and an increased risk of developing T2D (Linn et al., 1996, Sluijs et al., 2010, Tinker et al., 2011). The effect of increased protein intake per se on weight-loss induced changes in insulin sensitivity and glucose homeostasis are not known because of the confounding effects of differences in weight loss and food selection and overall diet composition (e.g., consumption of dairy and meat products and saturated and unsaturated fatty acids) between groups in studies that compared HP with standard protein diets (Rietman et al., 2014, Wycherley et al., 2012, Schwingshackl and Hoffmann, 2013).
The major purpose of the present study was to conduct a randomized, controlled trial (RCT) to determine whether increasing protein intake as part of a macronutrient-balanced, hypocaloric diet attenuates both the weight loss-induced reduction in lean tissue mass and the beneficial effect of a targeted 8%–10% weight loss on insulin action. Postmenopausal women with obesity were randomized to one of three interventions: 1) a weight loss (WL) group who consumed a hypocaloric diet containing 0.8 g protein/kg body weight per day, 2) a weight loss, high-protein (WL-HP) diet group who consumed a hypocaloric diet containing 1.2 g protein/kg body weight per day, and 3) a weight-maintenance (WM) control group. Subjects in the WL and WL-HP groups were studied before and after they lost 8%–10% of their initial body weight and were weight-stable (<2% change in body weight) for 3–4 weeks, whereas subjects in the WM group were studied after a time-matched (~6 months) weight maintenance period. Insulin-sensitivity was assessed by using the hyperinsulinemic-euglycemic clamp procedure (HECP) in conjunction with stable isotope labeled glucose tracer infusion and by evaluating muscle AKT phosphorylation. The rate of insulin stimulated glucose uptake (glucose rate of disappearance during the HECP) was the primary outcome. In addition, we evaluated the muscle global transcriptome and a series of factors that can influence insulin action, including: i) the concentrations of amino acids and their metabolites (C3[proprionyl]- and C5[isovaleryl]-acylcarnitine) in plasma and the phosphorylation of intramyocellular amino acid targets (mTOR and its downstream effector, 4E-BP1, and upstream regulator, AMPK) (Newgard et al., 2009, Schooneman et al., 2013, Krebs et al., 2007, Tsai et al., 2015, Tremblay et al., 2005, Saha et al., 2011); ii) plasma free fatty acid (FFA) concentration (Boden and Chen, 1995, Roden et al., 1996), palmitate rate of appearance in plasma as an index of adipose tissue lipolytic activity (Mittendorfer et al., 2003a), and the expression of selected genes involved in lipogenesis, and fatty acid oxidation and mitochondrial function in muscle; iii) the gene expression of key enzymes involved in oxidative stress defense in muscle; iv) plasma fibroblast growth factor 21 (FGF21) (Camporez et al., 2013, Mashili et al., 2011, Xu et al., 2009, Markan et al., 2014, Laeger et al., 2014); and v) the plasma concentration and muscle gene expression of selected inflammatory markers (Pedersen, 2007, Kirk and Klein, 2009).
RESULTS
Baseline subject characteristics, dietary compliance and duration of the intervention
Baseline characteristics of the study subjects in the WM, WL, and WL-HP groups were not different from each other (Table 1). During the intervention, which lasted 27.8 ± 2.8, 26.4 ± 2.9, and 27.4 ± 1.2 weeks in the WL, WL-HP, and WM groups, respectively, protein intake (assessed by food records) closely matched the prescribed amounts of 0.8 g per kg body weight per day in the WL and 1.2 g per kg body weight per day in the WL-HP groups; urinary nitrogen excretion rate was ~50% greater (P < 0.01) in the WL-HP than the WL group (Table 1). The contribution of carbohydrates and fat to total energy intake was only minimally different (<6%) in the two weight loss groups and the contribution of carbohydrates and fat to non-protein energy intake was the same (Table 1).
Table 1.
Subject characteristics at screening, energy and macronutrient intake during the dietary intervention, and diet-induced change in urinary urea nitrogen excretion rate.
| WM | WL | WL-HP | |
|---|---|---|---|
| Subject characteristics | |||
| Age (years) | 60 ± 1 | 58 ± 1 | 58 ± 1 |
| Body mass index (kg/m2) | 36 ± 2 | 35 ± 1 | 36 ± 1 |
| Body mass (kg) | 98 ± 7 | 95 ± 2 | 93 ± 2 |
| Fat-free mass (kg) | 51 ± 2 | 49 ± 1 | 46 ± 1 |
| Body fat (%) | 49 ± 2 | 48 ± 1 | 50 ± 1 |
| Intrahepatic triglyceride content (%) | 10.2 ± 3.4 | 6.6 ± 1.2 | 8.3 ± 2.8 |
| Intra-abdominal adipose tissue volume (cm3) | 1,596 ± 279 | 1,404 ± 138 | 1,341 ± 205 |
| Plasma concentrations | |||
| Glucose (mg/dL)* | 95 ± 5 | 96 ± 2 | 94 ± 3 |
| Glucose - 2 h post OGTT (mg/dL) | 143 ± 16 | 124 ± 8 | 134 ± 8 |
| Triglycerides (mg/dL)* | 148 ± 19 | 136 ± 25 | 95 ± 12 |
| Total cholesterol (mg/dL)* | 215 ± 20 | 215 ± 14 | 203 ± 10 |
| HDL-cholesterol (mg/dL)* | 56 ± 5 | 60 ± 6 | 59 ± 2 |
| LDL-cholesterol (mg/dL)* | 129 ± 19 | 128 ± 11 | 124 ± 9 |
| Diet composition and diet-induced change in urinary urea nitrogen excretion rate | |||
| Energy (kcal/day) | 1,743 ± 146 | 1,345 ± 57† | 1,389 ± 72† |
| Carbohydrates (% total energy) | 47 ± 1 | 49 ± 2 | 43 ± 1†# |
| Carbohydrates (% non-protein energy intake) | 58 ± 2 | 63 ± 2 | 63 ± 2 |
| Fat (% total energy) | 34 ± 1 | 29 ± 2 | 26 ± 1† |
| Fat (% non-protein energy intake) | 42 ± 2 | 37 ± 2 | 37 ± 2 |
| Protein | |||
| % total energy | 19 ± 1 | 22 ± 1 | 31 ± 1†# |
| grams/day | 81 ± 8 | 73 ± 4 | 105 ± 3†# |
| grams/kg body weight/day | 0.79 ± 0.05 | 0.85 ± 0.04 | 1.25 ± 0.03†# |
| grams/kg ideal body weight‡/day | 1.18 ± 0.11 | 1.09 ± 0.06 | 1.64 ± 0.07†# |
| Urinary urea nitrogen excretion rate (g/d) | |||
| Before the diet intervention | 10 ± 2 | 11 ± 1 | 10 ± 1 |
| After the diet intervention | 8 ± 1 | 10 ± 1 | 15 ± 2†# |
Data are expressed as mean ± SEM.
OGTT: oral glucose tolerance test. WL: weight loss (n = 10). WL-HP: weight loss high-protein (n = 10). WM: weight maintenance (n = 7).
Values were obtained after an overnight fast.
Value significantly different from corresponding value in the WM group (P < 0.05).
Value significantly different from corresponding value in the WL group (P < 0.05).
Ideal body weight is the weight corresponding to a body mass index of 24.9 kg/m2 (IOM, 2005).
See also Figure S4.
Changes in body weight and composition
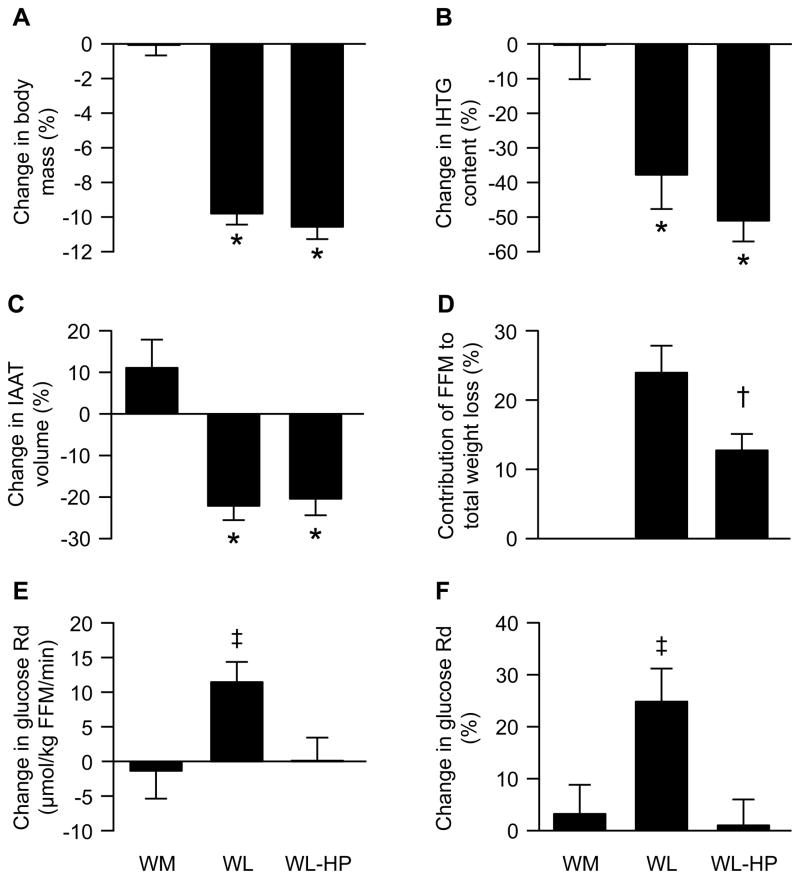
Body weight and body composition in the WM group did not change during the intervention. Both the WL and WL-HP groups lost ~10% of their initial body weight but the contribution of FFM to total weight loss was ~45% less in the WL-HP than the WL group (P = 0.03) (Figure 1). However, the absolute loss of FFM was small, so that only ~700 g of FFM were preserved in the WL-HP compared with the WL group. Intrahepatic triglyceride (IHTG) content and intra-abdominal adipose tissue (IAAT) volume did not change in the WM group, but decreased by ~45% (IHTG) and ~20% (IAAT), respectively, in both the WL and WL-HP groups (P < 0.01 vs the WM group; no difference between the WL and WL-HP groups) (Figure 1).
Figure 1. Changes in body weight and composition and insulin-stimulated glucose uptake.
Percent changes in body mass (A), intra-hepatic triglyceride (IHTG) content (B), and intra-abdominal adipose tissue (IAAT) volume (C), percent contribution of fat-free mass (FFM) to total weight loss (D), and absolute (μmol/kg FFM per min) and relative (percent) changes in insulin-stimulated glucose rate of disappearance (Rd) (E and F) before and after the diet intervention in the weight-maintenance (WM) group (n = 7) and in subjects who consumed either the standard weight loss (WL; n = 10) or weight loss high-protein (WL-HP; n = 10) diets. Data are expressed as mean ± SEM. * Value significantly different from corresponding value in the WM group (P <0.05). † Value significantly different from corresponding value in the WL group (P <0.05). ‡ Value significantly different from value in the WM and WL-HP groups (P <0.05).
Plasma hormone and metabolite concentrations
Basal insulin concentration was the same before and after the intervention in the WM group and decreased to the same extent (~30%) after weight loss in the WL and WL-HP groups (P <0.05 vs the WM group; no difference between the WL and WL-HP groups) (Table 2). Glucose and insulin concentrations achieved during the HECP before and after the intervention were not different from those achieved before the intervention in all three groups (Table 2). Basal FFA concentration did not change in the WM group and decreased to the same extent (~15%) after weight loss in the WL and WL-HP groups. During the HECP, FFA concentration decreased by ~90%, both before and after the interventions, in all three groups (Table 2). Basal branched-chain amino acid concentration was ~8% lower (P <0.05) after weight loss in both the WL and WL-HP groups (no difference between groups) whereas basal total essential (including branched-chain) and non-essential amino acid concentrations were not affected by weight loss in either the WL or WL-HP groups. During the HECP, the sum of all (total) and non-essential amino acid concentrations decreased to the same extent in all three groups before and after the intervention. In contrast, the HECP-induced decreases in branched-chain and total essential (including branched-chain) amino acid concentrations were ~15% greater (P < 0.05) after than before weight loss in both the WL and WL-HP groups (Table 2 and Figure S1). Plasma C3 and C5 acylcarnitine concentrations did not change in the WM group and tended to decrease by ~15% (P = 0.11) after weight loss in both the WL and WL-HP groups (no difference between groups) (Table 2). FGF21 concentrations were not different between groups at baseline and decreased by ~25% in both the WL and WL-HP groups (P < 0.05 vs the WM group; no difference between the WL and WL-HP groups), but did not change in the WM group (Table 2).
Table 2.
Plasma hormone and metabolite concentrations and glucose kinetics before and after the dietary intervention.
| WM | WL | WL-HP | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Plasma hormone and metabolite concentrations | ||||||
| Glucose (mg/dL) | ||||||
| Basal | 96.2 ± 4.1 | 94.7 ± 4.3 | 96.1 ± 3.1 | 92.9 ± 1.8 | 92.2 ± 1.3 | 91.9 ± 1.9 |
| HECP | 102.3 ± 1.3* | 102.4 ± 0.9* | 101.2 ± 1.0* | 102.5 ± 1.0* | 100.5 ± 0.9* | 101.6 ± 1.4* |
| Insulin (μU/mL) | ||||||
| Basal | 8.2 ± 1.5 | 7.0 ± 1.5 | 5.9 ± 1.1 | 3.5 ± 0.5† | 7.7 ± 1.3 | 5.0 ± 0.8† |
| HECP | 56.6 ± 6.7* | 57.0 ± 2.2* | 53.3 ± 5.3* | 55.2 ± 5.2* | 66.3 ± 5.1* | 63.1 ± 5.4* |
| Free fatty acids (mM) | ||||||
| Basal | 0.71 ± 0.06 | 0.70 ± 0.04 | 0.64 ± 0.04 | 0.54 ± 0.04† | 0.71 ± 0.06 | 0.61 ± 0.02† |
| HECP | 0.10 ± 0.02* | 0.07 ± 0.01* | 0.06 ± 0.01* | 0.04 ± 0.01* | 0.05 ± 0.01* | 0.03 ± 0.01* |
| Branched-chain amino acids (μM) | ||||||
| Basal | 377 ± 19 | 385 ± 18 | 375 ± 8 | 339 ± 15† | 386 ± 13 | 361 ± 8† |
| HECP | 237 ± 29* | 235 ± 25* | 209 ± 14* | 159 ± 12*† | 216 ± 13* | 183 ± 12*† |
| Essential amino acids (μM) | ||||||
| Basal | 907 ± 75 | 942 ± 75 | 845 ± 25 | 818 ± 44 | 830 ± 31 | 856 ± 41 |
| HECP | 661 ± 87* | 664 ± 77* | 558 ± 45* | 468 ± 52*† | 580 ± 41* | 533 ± 32*† |
| Non-essential amino acids (μM) | ||||||
| Basal | 1,006 ± 90 | 998 ± 98 | 921 ± 48 | 881 ± 53 | 954 ± 36 | 882 ± 53 |
| HECP | 846 ± 91* | 799 ± 85* | 709 ± 49* | 616 ± 55* | 770 ± 43* | 684 ± 39* |
| Total amino acids (μM) | ||||||
| Basal | 1,913 ± 164 | 1,940 ± 172 | 1,766 ± 69 | 1,699 ± 92 | 1,784 ± 61 | 1,738 ± 87 |
| HECP | 1,507 ± 174* | 1,463 ± 158* | 1,268 ± 86* | 1,083 ± 104* | 1,349 ± 80* | 1,217 ± 64* |
| Sum of C3 and C5 acylcarnitines (μM) | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.39 ± 0.03 | 0.32 ± 0.02 | 0.36 ± 0.03 | 0.32 ± 0.02 |
| Fibroblast growth factor 21 (pg/mL) | 219 ± 46 | 247 ± 52 | 177 ± 36 | 148 ± 24† | 168 ± 25 | 116 ± 13† |
| Glucose kinetics | ||||||
| Basal glucose Ra | ||||||
| (μmol/min) | 761 ± 15 | 780 ± 30 | 789 ± 36 | 736 ± 25† | 784 ± 31 | 713 ± 26† |
| (μmol/kg FFM/min) | 15.7 ± 0.6 | 16.0 ± 0.6 | 16.3 ± 0.7 | 15.8 ± 0.6 | 17.2 ± 0.7 | 16.0 ± 0.6 |
| GIR during the HECP | ||||||
| (μmol/min) | 2,619 ± 554 | 2,601 ± 429 | 2,512 ± 197 | 2,986 ± 164†# | 2,893 ± 337 | 2,798 ± 289 |
| (μmol/kg FFM/min) | 55.7 ± 13.2 | 55.0 ± 10.6 | 52.6 ± 4.9 | 64.6 ± 4.5†# | 63.2 ± 6.9 | 62.8 ± 6.3 |
| Glucose Rd during the HECP | ||||||
| (μmol/min) | 2,741 ± 537 | 2,681 ± 383 | 2,649 ± 214 | 3,108 ± 184†# | 2,976 ± 317 | 2,905 ± 270 |
| (μmol/kg FFM/min) | 58.2 ± 12.9 | 56.5 ± 9.6 | 55.5 ± 5.3 | 67.3 ± 5.0†# | 65.0 ± 6.5 | 65.2 ± 5.9 |
Data are expressed as mean ± SEM.
FFM: fat-free mass. GIR: glucose infusion rate. HECP: hyperinsulinemic-euglycemic clamp procedure. Ra: rate of appearance. Rd: rate of disappearance. WL: weight loss (n = 10). WL-HP: weight loss high-protein (n = 10). WM: weight maintenance (n = 7).
Value significantly different from corresponding basal value (P < 0.05).
Value significantly different from corresponding value in the WM group (P < 0.05).
Value significantly different from corresponding value in the WL-HP group (P < 0.01).
See also Figure S1.
Glucose kinetics
Basal glucose rate of appearance (Ra) and glucose rate of disappearance (Rd) during the HECP in the WM group did not change during the intervention (Table 2). Basal glucose Ra decreased by ~6% after weight loss in both the WL and WL-HP groups (P <0.05 vs the WM group; no difference between the WL and WL-HP groups) (Table 2). Glucose Ra during the HECP was almost completely (by 85 ± 2 %) suppressed in all studies, both before and after weight loss (main effect of clamp, P < 0.001; no significant interactions and no significant main effects of either group or time) (Table 2). Glucose Rd during the HECP increased by 25.3 ± 6.5% (P <0.01) after weight loss in the WL group, whereas glucose Rd during the HECP after weight loss was not different from before weight loss in the WL-HP group (Figure 1).
By chance, mean glucose Rd during the clamp procedure was 12% higher in the WL-HP than the WL and WM groups. This difference was largely driven by one person whose baseline glucose Rd was 5,528 μmol/min (nearly double the mean value). The average value in the remaining 9 subjects was 2,693 ± 158 μmol/min and nearly identical to the average glucose Rd values in the other two groups (2,741 ± 537 and 2,649 ± 214 μmol/min in the WM and WL groups, respectively). Excluding this subject from the statistical analysis does not affect the results (i.e., HP intake eliminates the weight-loss induced improvement in insulin-mediated glucose Rd regardless of whether this person is or is not included in the analysis).
Intramyocellular signaling elements
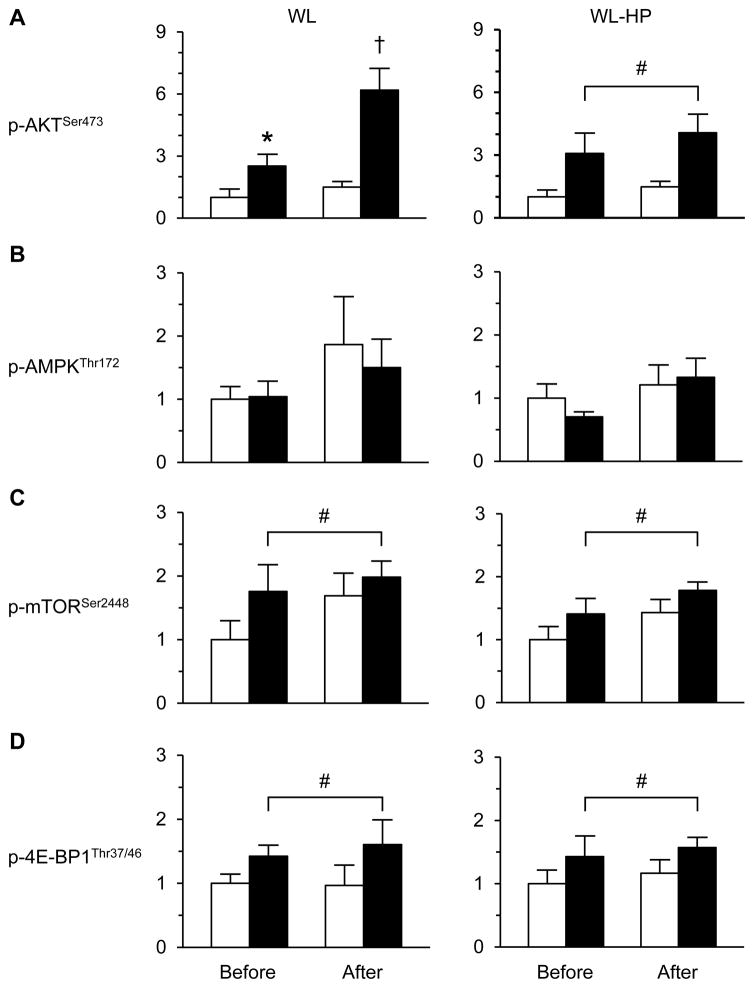
During the HECP, muscle p-AKTSer473, p-mTORSer2448, and p-4E-BP1Thr37/46 contents increased by ~50–150% above basal values in the WM, WL, and WL-HP groups (Figure 2 and Figure S2). The increase in p-AKTSer473 during the HECP was greater after than before weight loss (P <0.05) in the WL group, but did not change in the WL-HP and WM groups (Figure 2 and Figure S2). The increases in p-mTORSer2448 and p-4E-BP1Thr37/46 during the HECP after weight loss/maintenance in the WL, WL-HP, and WM groups were not different from the increases observed before the intervention (Figure 2 and Figure S2). Muscle p-AMPKThr172 was not affected by the HECP or by weight loss/maintenance in the WL, WL-HP, and WM groups (Figure 2 and Figure S2).
Figure 2. Intramyocellular signaling elements before and after weight loss.
Weight loss-induced changes in p-AKTSer473 (A), p-AMPKThr172 (B), p-mTORSer2448 (C), and p-4E-BP1Thr37/46 (D) in muscle during basal, postabsorptive conditions (white bars) and the hyperinsulinemic-euglycemic clamp (black bars) in subjects consuming the standard weight loss (WL) and weight loss high protein (WL-HP) diets. Data (n = 6–8) are expressed as mean ± SEM. * Value significantly different from corresponding basal value (P < 0.05). † Value significantly different from all other values (P <0.05). # Significant main effect of clamp (P <0.05). See also Figure S2.
Palmitate kinetics and the expression of selected genes involved in lipogenesis, and fatty acid oxidation and mitochondrial function in muscle
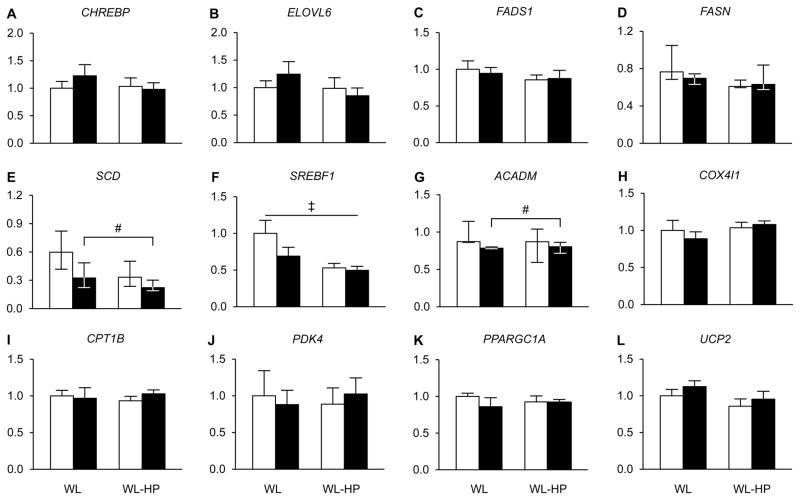
Basal palmitate Ra was not different among the WM, WL and WL-HP groups at baseline (131 ± 15, 119 ± 8, and 123 ± 9 μmol/min, respectively) and was ~15 % lower (P <0.01) after than before the interventions in all groups (118 ± 12, 100 ± 8, and 108 ± 12 μmol/min, respectively). The expression of most of the analyzed genes involved in lipogenesis (CHREBP, ELOVL6, FADS1, FASN, and SREBF1 but not SCD) and fatty acid oxidation and mitochondrial function (COX4/1, CPT1B, PDK4, PPARGC1A, and UCP2 but not ACADM) in muscle did not change with weight loss in either the WL or WL-HP groups (Figure 3). Muscle gene expression of SCD and ACADM was lower after than before weight loss in both the WL and WL-HP groups (Figure 3).
Figure 3. Expression of genes involved in lipogenesis, and fatty acid oxidation and mitochondrial function in muscle.
Expression of genes involved in lipogenesis [A: carbohydrate response element binding protein (CHREBP), B: elongation of very long-chain fatty acids protein 6 (ELOVL6), C: fatty acid desaturase 1 (FADS1), D: fatty acid synthase (FASN), E: stearyl Co-A desaturase (SCD), and F: sterol regulatory element binding transcription factor 1 (SREBF1)], and fatty acid oxidation and mitochondrial function [G: acyl-Coenzyme A dehydrogenase (ACADM), H: cytochrome C oxidase subunit IV (COX4/1), I: carnitine palmitoyl transferase 1 (CPT1B), J: pyruvate dehydrogenase kinase 4 (PDK4), K: peroxisome proliferator activated receptor gamma coactivator 1 alpha (PPARGC1A), and L: uncoupling protein 2 (UCP2)] in muscle before (white bars) and after (black bars) weight loss in subjects consuming the standard weight loss (WL) and weight loss high-protein (WL-HP) diets. Data (n = 6–9) are expressed relative to the housekeeping gene and presented as mean ± SEM, except for ACADM, FASN1 and SCD, which are expressed as median (quartiles). # Significant main effect of weight loss (P <0.05). ‡ Significant main effect of group (P <0.05). See also Table S3.
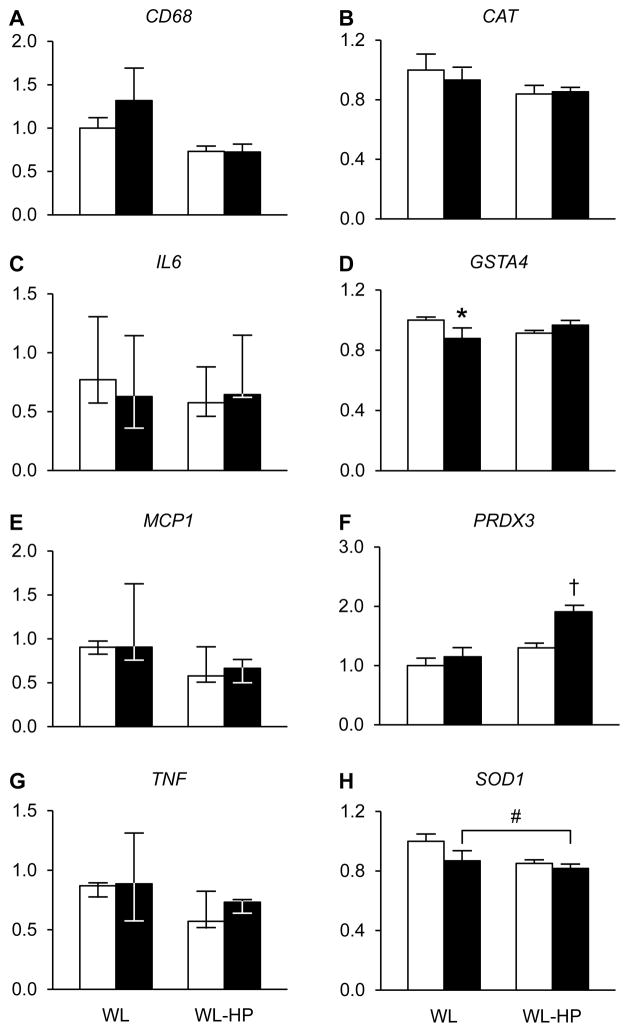
Inflammatory and oxidative stress defense markers in plasma and muscle
C-reactive protein and interleukin-6 concentrations in plasma (Figure S3) and muscle CD68, IL6, MCP1, and TNF gene expression did not change during the interventions (Figure 4). Muscle GSTA4 gene expression decreased in the WL group and PRDX3 gene expression increased in the WL-HP group; SOD1 gene expression decreased after weight loss in both the WL and WL-HP groups and CAT gene expression did not change in either the WL or WL-HP groups (Figure 4).
Figure 4. Expression of genes involved in inflammatory and oxidative stress defense pathways in muscle.
Expression of genes involved in inflammatory [A: cluster of differentiation 68 (CD68), C: interleukin-6 (IL6), E: monocyte chemoattractant protein 1 (MCP1), and G: tumor necrosis factor (TNF)]) and oxidative stress defense [B: catalase (CAT), D: glutathione S-transferase alpha 4 (GSTA4), F: peroxiredoxin 3 (PRDX3), and H: superoxide dismutase 1 (SOD1)] pathways before (white bars) and after (black bars) weight loss in subjects consuming the standard weight loss (WL) and weight loss high-protein (WL-HP) diets. Data (n = 6–9) are expressed relative to the housekeeping gene and presented as mean ± SEM, except for IL6, MCP1 and TNF, which are expressed as median (quartiles). * Value significantly different from corresponding basal value (P <0.05). † Value significantly different from all other values (P <0.05). # Significant main effect of weight loss (P <0.05). See also Table S3 and Figure S3.
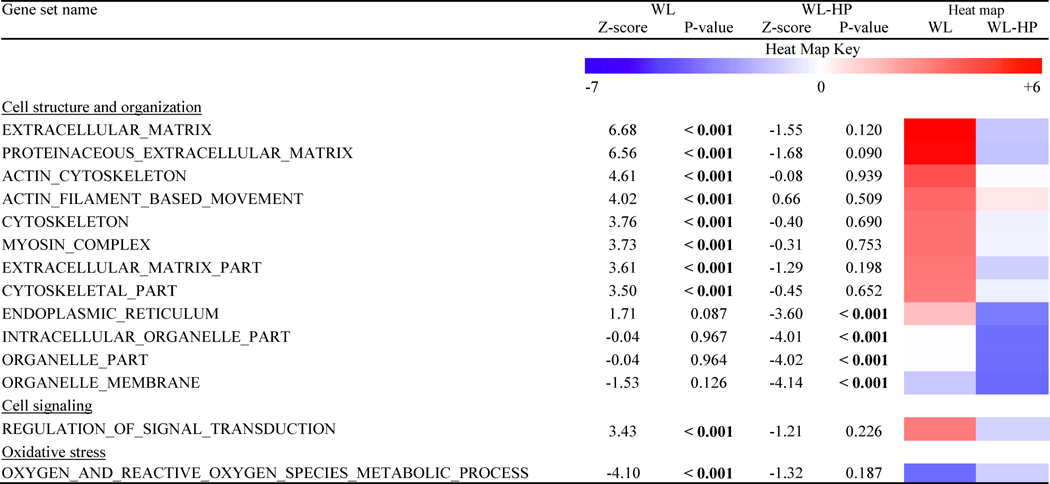
Global muscle gene expression profiling by using the microarray technique
Twenty-six gene sets were similarly affected by weight loss in the WL and WL-HP groups and thirty-four were differently affected by WL and WL-HP (Table 3 and Tables S1 and S2). Of those, several that were related to cell structure and organization were upregulated in the WL group and not affected, or even downregulated, by weight loss in the WL-HP group. A pathway related to the regulation of signal transduction was upregulated and an oxidative stress gene set pathway was downregulated in the WL but not the WL-HP group.
Table 3.
Key gene set pathways that were differently affected by WL and WL-HP.
WL: weight loss (n = 6); WL-HP: weight loss high-protein (n = 9). See also Tables S1 and S2.
DISCUSSION
Although many studies have evaluated the effect of HP diets on metabolic function, the results from several systematic reviews and meta-analyses indicate it is not possible to determine the effect of HP intake per se on insulin sensitivity because of differences in overall diet composition (food selection and nutrient composition) and amount of weight loss between the HP-intervention and control groups (Rietman et al., 2014, Wycherley et al., 2012, Schwingshackl and Hoffmann, 2013). We therefore conducted a RCT to evaluate the effect of dietary protein intake on body composition and insulin sensitivity by providing a protein supplement to subjects during weight loss to minimize the potential confounding influences of differences in overall diet composition on our outcome measures. In addition, participants who were given a standard-protein hypocaloric diet (0.8 g protein/kg per day) and those given a HP hypocaloric diet (1.2 g protein/kg per day) were assessed before and after matched (~10%) weight loss. We found that the WL-HP diet blunted the weight loss-induced decline in FFM by ~45%. However, the clinical importance of this effect is unclear because it represents a very small difference in loss of FFM between groups (~700 g or 1.5 % of total FFM). The beneficial effect of 10% weight loss on muscle insulin action (assessed as glucose disposal rate and phosphorylation of AKT in muscle during a HECP) was eliminated by HP intake. The failure to improve muscle insulin sensitivity in the WL-HP group is clinically important, because it reflects a failure to improve a major pathophysiological mechanism involved in the development of T2D (Groop, 1999, Petersen and Shulman, 2002), and indicates more insulin is required in the WL-HP than the WL group to dispose of a given amount of glucose. In summary, our data therefore demonstrate that the protein content of a weight loss diet can have profound effects on metabolic function and underscore the importance of considering dietary macronutrient composition in conjunction with energy content itself in weight loss therapy for people with obesity.
The mechanism(s) responsible for the adverse effect of HP intake on insulin action are unclear. Failure to improve insulin-stimulated glucose uptake in the WL-HP compared with the WL group occurred in the absence of any major differences in body weight, body composition, plasma FFA availability, and inflammatory markers in plasma or muscle in the two groups. Data from studies conducted in cultured myotubes, isolated rat skeletal muscles and transgenic mice have demonstrated that amino acids, particularly the branched-chain amino acid leucine, can impair insulin-mediated glucose uptake by AMPK-mediated mTOR phosphorylation and subsequent negative feedback inhibition of PI3K-AKT signaling (Iwanaka et al., 2010, Saha et al., 2011) or possibly downstream signaling to 4E-BP1 (Tsai et al., 2015). In contrast, we recently found these pathways were not involved in the inhibition of insulin-mediated glucose uptake that occurred with acute protein ingestion in people (Smith et al., 2015). In the current study, we also found that chronic HP intake impaired the weight loss-induced improvement in insulin-mediated glucose uptake in the absence of differences in p-AMPKThr172, p-mTORSer2448 and p-4E-BP1Thr37/46 in muscle. Data from several recent studies have implicated other amino acids (e.g., glycine and tryptophan) and the amino acid metabolites C3- and C5-acylcarnitine in the development of insulin resistance as well (e.g., Schooneman et al., 2013, Hattersley et al., 2014), but we found no differences in the plasma amino acid profile between the WL and WL-HP groups. Weight loss reduced basal plasma branched-chain amino acid concentrations and tended to decrease the plasma concentrations of the amino acid metabolites C3- and C5 acylcarnitine in both the WL and WL-HP groups without a difference between groups; the decline in both branched-chain and total essential (including branched-chain) amino acid concentrations during the HECP was also greater after than before weight loss in both the WL and WL-HP groups without a difference between groups. These findings suggest that the adverse effect of HP intake on insulin action during weight loss was not due to differences in circulating amino acids or their metabolites.
It is also unlikely that the adverse effects of HP intake on insulin action were mediated by FGF21, which has been shown to increase insulin sensitivity in rodent models (Camporez et al., 2013, Mashili et al., 2011, Xu et al., 2009, Markan et al., 2014). Although plasma FGF21 concentration increases after severe protein (Laeger et al., 2014) and calorie restriction (Galman et al., 2008, Fazeli et al., 2015), we found that moderate weight loss decreased basal FGF21 concentration and had no effect on plasma FGF21 concentration during the HECP in both the WL and WL-HP groups.
An imbalance in cellular redox status is considered a key mechanism for the development of muscle insulin resistance in persons with obesity (Muoio and Neufer, 2012, Anderson et al., 2009). Our microarray analysis results suggest that oxidative stress-related metabolic processes in muscle decreased after weight loss in the WL but not the WL-HP group and weight loss in the WL but not WL-HP group also decreased the gene expression of GSTA4. GSTA4 is a member of the glutathione S-transferase family and its expression is a marker of oxidative stress burden (Frohnert et al., 2014, Raza et al., 2002). Weight loss in the WL-HP group, on the other hand, increased the gene expression of PRDX3 in muscle. PRDX3 is a member of the peroxiredoxin family of antioxidants within mitochondria and experimentally-induced oxidative stress in animal models increases PRDX3 tissue expression (Schroder et al., 2008); in people, gene expression of PRDX3 is also increased in conditions that are associated with oxidative stress, such as cancer (Whitaker et al., 2013, Kim et al., 2009). These results suggest that the adverse effect of HP intake on insulin action during weight loss therapy may have been mediated through its effects on oxidative stress because it prevented the WL-induced decrease, and even increased, metabolic pathways involved in oxidative stress response in muscle.
Our microarray analysis also identified a series of gene set pathways related to intracellular and extracellular structure and organization that were upregulated by weight loss in the WL group, but not changed, or even decreased, in the WL-HP group. The intracellular cytoskeleton and extracellular matrix of muscle cells are important in regulating muscle insulin action by providing a scaffold that serves as a binding site for signaling molecules and for transporting GLUT4 from intracellular vesicles to the plasma membrane (Liu et al., 2013, Klip et al., 2014, Asrih et al., 2011, Bose et al., 2002, Zaid et al., 2008, Brozinick et al., 2007, Chen et al., 2007). Our data therefore suggest that adaptations in pathways related to tissue structural biology are involved in the weight loss-induced improvement in muscle insulin action, which were prevented by HP intake.
Although we found that HP intake during weight loss attenuates the beneficial effect of moderate weight loss on muscle insulin action, this does not mean a HP weight loss diet necessarily results in a diminished improvement in plasma glucose homeostasis. 24-hour glycemic control is determined by both postabsorptive and postprandial plasma glucose concentrations, which are determined by glucose Ra into (from endogenous and exogenous/dietary sources) and glucose Rd from plasma. Both endogenous glucose Ra and glucose Rd are regulated by insulin and the metabolic response to insulin across the range of physiological concentrations (basal, postabsorptive to peak postprandial) differs among organs. Endogenous (mostly hepatic) glucose production is much more sensitive to insulin than is muscle glucose uptake and is nearly completely suppressed at plasma insulin concentrations that only minimally stimulate muscle glucose uptake (Conte et al., 2012). The decline in basal plasma insulin concentration after weight loss without a change in plasma glucose concentration and a slight reduction in basal glucose Ra suggests weight loss in both the WL and WL-HP may have improved hepatic insulin sensitivity. On the other hand, our use of the hyperinsulinemic-euglycemic clamp procedure in conjunction with stable isotopically labeled glucose tracer infusion and an insulin infusion rate that results in systemic plasma insulin concentrations within the postprandial range, allowed us to measure muscle insulin sensitivity and our data demonstrate that HP intake prevented the WL-induced improvement in muscle insulin sensitivity. Dietary protein is a potent insulin secretagogue (Floyd et al., 1966, Ang et al., 2012, Manders et al., 2014), which may overcome the adverse effect of protein on insulin sensitivity by increasing the secretion of insulin. In addition, protein causes greater satiation and has a greater thermogenic effect of feeding than carbohydrate and fat, which can lead to greater weight loss with a HP than a standard protein diet (Wycherley et al., 2012, Leidy et al., 2015). Therefore, the adverse effect of dietary protein on muscle insulin action could be offset by its effect on hepatic insulin sensitivity, insulin secretion and energy balance.
In summary, the results from our study demonstrate that HP intake during weight loss helps preserve FFM but eliminates the beneficial effect of weight loss on skeletal muscle insulin action. he mechanisms responsible for the adverse effect of HP intake on muscle insulin action are not clear. ur data suggest that HP intake causes alterations in muscle cell structure and organization and oxidative stress that are involved in preventing the therapeutic effect of weight loss on muscle insulin action, whereas changes in circulating amino acids, including branched-chain amino acids and their metabolites, plasma FGF21 concentration, muscle mTOR signaling, and inflammatory pathways in muscle are not involved.
EXPERIMENTAL PROCEDURES
Subjects and Study Design
Thirty-four sedentary (<1.5 h exercise/week) and weight-stable (<2 kg change for at least 6 months), 50–65 year old postmenopausal women with obesity were included in this study (ClinicalTrials.gov number NCT01538836), which was approved by the Human Research Protection Office at Washington University School of Medicine in St. Louis, MO. Written, informed consent was obtained from all subjects before their participation in the study. We specifically chose to study postmenopausal women with obesity only, because we wanted to study a population for whom weight loss with increased protein intake is often recommended to reduce the risk of sarcopenia (Houston et al., 2009, Wolfe et al., 2008).
The flow of study subjects is shown in Figure S4. All subjects were evaluated by a history and physical examination, a resting 12-lead electrocardiogram, standard blood tests, and an oral glucose tolerance test. None of the subjects had evidence of chronic illness or significant organ dysfunction (e.g., diabetes mellitus, liver cirrhosis), or were taking medications (including hormone replacement therapy) that could affect insulin or glucose metabolism, and none consumed tobacco products or reported regular consumption of >115 g of alcohol per week or scored >2 points (out of a possible 22) on the Michigan Alcohol Screening Test.
After subjects completed body composition analyses and a HECP, they were randomized to one of three intervention groups: 1) a WM group; 2) a (WL group, who consumed an energy-reduced diet containing 0.8 g protein/kg body weight per day; and 3) a WL-HP group, who consumed an energy reduced diet containing 1.2 g protein/kg body weight per day. All outcomes were evaluated before and after subjects randomized to the weight loss groups lost 8%–10% of their body weight or a time-matched weight maintenance period for those randomized to the WM group. Twenty seven subjects completed the study and were included in the analysis; their characteristics are shown in Table 1. The baseline characteristics of the 7 subjects who dropped out of the study (Figure S4) were not different from those who completed the study (data not shown).
Outcomes assessment
Body composition
Fat mass and FFM were determined by using dual energy X-ray absorptiometry (DXA, Lunar iDXA, GE Healthcare Lunar, Madison, WI), and IAAT volume and IHTG content were determined by using magnetic resonance imaging/spectroscopy (1.5-T superconducting magnet; Siemens, Iselin, NJ) as previously described (Frimel et al., 2007, Magkos et al., 2007).
Insulin action – HECP
Subjects were instructed to refrain from vigorous physical activities for 3 days before being admitted to the Clinical Research Unit, where they consumed a standard 800 kcal dinner (50% CHO, 30% fat, 20% protein) between 1800 h and 1900 h. In addition, all subjects consumed a 100 kcal liquid meal supplement (Ensure®, Abbott Laboratories, Abbott Park, IL, USA, containing 15% of energy as protein, 55% as carbohydrate and 30% as fat) at baseline (before the dietary intervention); after the intervention, subjects in the WM and WL groups consumed the same liquid meal supplement and subjects in the WL-HP group consumed a 100 kcal whey protein solution (Unjury®, ProSynthesis Laboratories, Inc, Reston, VA) containing 21 g protein. Subjects then fasted, except for water, until the next morning. At 0600 h, catheters were inserted into an arm vein for the infusion of stable isotope labeled tracers, and later insulin and dextrose, and into a radial artery for blood sampling. At ~0645 h, a constant infusion of [U-13C16]palmitate (infusion rate: 6 nmol·kg body wt−1·min−1) and a primed, constant infusion of [6,6-2H2]glucose (priming dose: 22 μmol·kg body wt−1, infusion rate: 0.22 μmol·kg body wt−1·min−1), both purchased from Cambridge Isotope Laboratories Inc. (Andover, MA), were started and maintained for 4 h. Upon completion of the basal period, a HECP was initiated with two 5-minute priming doses (first 200 mU·m−2 body surface area (BSA)·min−1, then 100 mU·m−2 BSA·min−1) of human insulin (Novolin R, Novo Nordisk, Princeton, NJ) followed by constant infusion of insulin at a rate of 50 mU·m−2 BSA·min−1. Euglycemia (at blood glucose concentration ~100 mg/dl) was maintained by variable rate infusion of 20% dextrose (Baxter, Deerfield, IL) enriched to 2.5% with [6,6-2H2]glucose. Blood samples to determine plasma metabolite and hormone concentrations and glucose and palmitate kinetics were obtained immediately before starting the tracer infusions and every 6–7 min during the last 20 min of the basal period and the HECP; additional blood samples were obtained every 10 min during the HECP to monitor blood glucose concentration. Muscle tissue from the quadriceps femoris was obtained by using a Tilley-Henkel forceps 60 min after starting the glucose tracer infusion (basal period) and 180 min after starting the insulin infusion. The basal and HECP biopsies were taken through separate incisions (~5 cm apart) from the right leg before and after the intervention.
Blood and tissue sample processing and analyses
Plasma glucose concentration was determined by using an automated glucose analyzer (Yellow Spring Instruments Co, Yellow Springs, OH). Enzyme-linked immunosorbent assays (ELISA) were used to determine insulin (EMD Millipore, St Charles, MO), C-reactive protein, interleukin-6, and FGF21 (all R&D Systems Inc, Minneapolis, MN) concentrations. Plasma amino acid concentrations were determined by using the EZ:faast physiological (free) amino acid kit (Phenomenex, Torrence, CA) and gas-chromatography/mass-spectrometry (GC-MS; Hewlett- Packard MSD 5973 system with capillary column) analysis per manufacturer instructions. Total plasma free fatty acid concentration was quantified by using an enzymatic colorimetric assay (Wako Diagnostics, Richmond, VA). Plasma glucose and palmitate tracer-to-tracee ratios (TTR) were determined by using GC-MS as previously described (Smith et al., 2015, Mittendorfer et al., 2003b). C3- and C5-acylcarnitine concentrations in plasma were quantified by using liquid chromatography-tandem mass spectrometry (LC-MS/MS, Applied Biosystems Sciex 4000QTRAP with Eclipse C18 column) after adding known amounts of propionyl-L-carnitine (N-trimethyl-d3) and isovaleryl-L-carnitine (N,N,N-trimethyl-d9) (both purchased from Cambridge Isotope Laboratories Inc) as internal standards and their conversion to methylesters as described (with minor modifications) by Forni et al. (2010).
Western analysis was used to quantify the contents of p-AKTSer473, p-mTORSer2448, p-4E-BP1Thr37/46, and p-AMPKThr172 in muscle. Frozen muscle tissue was homogenized in ice-cold Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA) and proteins were extracted as previously described (Yoshino et al., 2012). Thirty μg of protein from each sample were loaded onto polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA), separated by SDS-PAGE, and transferred to Immun-Blot polyvinylidene difluoride membranes (Bio-Rad). The blotted membranes were incubated with the following primary antibodies (all from Cell Signaling Technology, except for TUBULIN, which was purchased from Sigma, St. Louis, MO): rabbit monoclonal anti-phospho-AKT (Ser473) and anti-total-AKT, rabbit polyclonal anti-phospho-mTOR (Ser2448) and anti-total-mTOR, rabbit polyclonal anit-phospho-4E-BP1 (Thr37/46) and anti-total-4E-BP1, rabbit monoclonal anti-phospho-AMPKa (Thr172), rabbit polyclonal anti-total-AMPKa, and mouse monoclonal anti-a-TUBULIN. All (except for TUBULIN) blots were incubated with a horseradish peroxidase-conjugated secondary antibody from Cell Signaling Technology; the a-TUBULIN blot was incubated with a horseradish peroxidase-conjugated secondary antibody from Santa Cruz, Biotechnology. Blots were developed by using the Amersham ECL Select Western Blotting Detection Reagent (GE Healthcare Life Sciences, Piscataway, NJ). The contents of p-AKTSer473, p-mTORSer2448, p-4E-BP1Thr37/46 and p-AMPKThr172 were expressed relative to a single sample loading control and relative to total AKT, mTOR, 4E-BP1, AMPK or TUBULIN (p-AKTSer473 only). The results were the same, irrespective of the control protein used.
The expression of genes involved in inflammatory (CD68, IL6, MCP1, and TNF), oxidative stress defense (CAT, GSTA4, SOD1, and PRDX3), lipogenic (CHREBP, ELOVL6, FADS1, FASN, SCD, SREBF1), and fatty acid oxidation and mitochondrial function (ACADM, COX4/1, CPT1B, PDK4, PPARGC1A, and UCP2) pathways in muscle were analyzed by using the real-time polymerase chain reaction (RT-PCR) technique after total RNA was isolated from frozen muscle samples by using Trizol reagent (Invitrogen, Carlsbad, CA), quantified spectrophotometrically (NanoDrop 1000, Thermo Scientific, Waltham, MA), and reverse transcribed (High-Capacity cDNA Reverse Transcription Kit, Invitrogen). Gene expression was determined by using an ABI 7500 RT-PCR system (Invitrogen) and SYBR Green Master Mix (Invitrogen) as previously described (Smith et al., 2014, Yoshino et al., 2014). The expression of each gene was determined by normalizing the cycle threshold value of each sample to the housekeeping control gene, ribosomal protein RPLP0. Primer details are listed in Table S3.
Microarray analyses were performed with the GeneChip Human Gene 1.0 ST array (Affymetrix, Santa Clara, CA, USA) and pathways that were significantly altered by the dietary interventions were identified by using the R statistical software package and parametric analysis of gene set enrichment (PAGE) as previously described (Fabbrini et al., 2015, Yoshino et al., 2011, Kim and Volsky, 2005). Gene sets used in PAGE were obtained from http://www.broad.mit.edu/gsea/msigdb/msigdb_index.html (C5: GO gene sets collection). Z scores and P-values were calculated for each gene set. Microarray results were deposited in the NCBI Gene Expression Omnibus (GEO) database (accession number GSE73525).
Glucose and palmitate kinetics calculations
The Ra of unlabeled glucose in plasma, which represents the endogenous glucose production rate during basal conditions, was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR during the last 20 min of the basal period and the last 20 min of the HECP. During the HECP, total glucose Ra, which equals glucose Rd from plasma, was calculated as the sum of endogenous glucose production plus the rate of infused glucose (dextrose plus tracer). Palmitate Ra in plasma was calculated by dividing the tracer infusion rate by the average plasma palmitate TTR during the last 20 min of the basal period.
Diet intervention
Subjects attended weekly sessions led by an experienced weight management dietician to ensure compliance with the diet prescription, monitor body weight, and counsel subjects throughout the dietary intervention. The energy content of the initial packed out meals given to the weight loss groups was targeted to provide 30% fewer calories than each person’s estimated total daily energy expenditure, based on their measured resting metabolic rate (RMR) multiplied by an activity factor of 1.4 (Black et al., 1996)); subsequent meals and dietary intake were adjusted weekly as needed to achieve a 0.5%–1% weight loss per week until 8%–10% was achieved. Once the targeted weight loss goal was achieved, dietary energy intake was modified to maintain a stable body weight for 3–4 weeks before the testing procedures performed at baseline were repeated. Protein intake and macronutrient distribution of the diet were kept constant in accordance with the initial diet prescription throughout the intervention period. In the WM group, each subject’s energy intake was adjusted as needed to maintain body weight within 2% of the initial body weight. Target protein intake for the WL group was 0.8 g protein/kg body weight per day and 1.2 g protein/kg body weight per day for subjects in the WL-HP group.
All subjects were provided with a base diet of frozen entrees (eLiving meals, Morrison Healthcare, Atlanta, GA; Lean Cuisine, Nestlé USA, Solon, OH; Revel Kitchen, St. Louis, MO) for lunch and dinner. For breakfast, subjects consumed two energy bars (NuGo Nutrition, Oakmont, PA) per day. Subjects in the WL-HP diet group also consumed two servings of a whey protein isolate (Unjury®, ProSynthesis Laboratories, Inc, Reston, VA) per day whereas subjects in the WL group consumed snacks that provided mostly carbohydrates and fat (in proportion to their contribution to total non-protein dietary energy content of the base diet; i.e., ~63 and 37%, respectively) instead. Additional calories needed to meet each subject’s total energy and macronutrient requirements were consumed as fruits, vegetables, dairy products and starches. Dietary compliance was monitored by having subjects record their dietary intake every day by using the www.myfitnesspal.com computer app; the study dietician reviewed diet records weekly. In addition, 24-h urinary urea nitrogen excretion was evaluated before and during the final week of the dietary intervention.
Statistical analyses
All data sets were tested for normality by using the Kolmogorov-Smirnov test. One-way analysis of variance was used to compare basic characteristics of the study subjects, their macronutrient intake, and baseline metabolic characteristics in each of the three groups. Analysis of covariance with the baseline value as covariate was used to evaluate the effect of the dietary interventions on study outcomes that were measured during basal or HECP conditions only. Analysis of variance with group (WL vs WL-HP), condition (basal vs clamp) and time (pre vs post) as factors as appropriate was used to evaluate the effects of dietary interventions on outcomes that were measured during basal conditions and during the HECP, and significant interactions were followed by Tukey’s post-hoc procedure to locate significant mean differences. A P-value of ≤0.05 was considered statistically significant for all data, except our microarray data. Treatment-induced changes in gene set pathway expression identified by microarray analysis were considered significant if P was ≤0.001, and a difference in Z-scores >1.96 was considered a significant difference between the WL and WL-HP groups. Data are presented as mean ± SEM unless indicated otherwise.
Supplementary Material
Acknowledgments
The authors thank Emily Lake, Janet Winkelmann, Lynda Bowers and Kathryn Gratza for help with subject recruitment, scheduling and testing; Kelly Stromsdorfer, Freida Custodio, and Jennifer Shew for their technical assistance; the staff of the Clinical Research Unit for their help in performing the studies; and the study subjects for their participation.
This publication was made possible by NIH grants DK94483, DK56341 (Nutrition and Obesity Research Center), DK20579 (Diabetes Research Center), GM103422 (Biomedical Mass Spectrometry Resource) and UL1TR000448 (Clinical Translational Science Award) including KL2 sub-award TR000450, a Central Society for Clinical and Translational Research Early Career Development Award, and a grant from the Longer Life Foundation.
Footnotes
Supplemental information includes 3 tables and 4 figures.
CONFLICTS OF INTEREST
SK is a shareholder of Aspire Bariatrics and has served on Scientific Advisory Boards for Takeda Pharmaceuticals and NovoNordisk. GIS, JY, SCK, DNR, AO, BWP, and BM declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: BM and SK. Methodology: BM and SK. Validation: BM and JY. Formal Analysis: BM and GIS. Investigation: AO, BM, BWP, DNR, GIS, JY, SCK, and SK. Writing – original draft: BM and GIS. Writing – review and editing: BM, GIS, JY, and SK. Visualization: BM and GIS. Supervision: BM and SK. Project Administration: BM. Funding acquisition: BM and SK.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–81. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang M, Muller AS, Wagenlehner F, Pilatz A, Linn T. Combining protein and carbohydrate increases postprandial insulin levels but does not improve glucose response in patients with type 2 diabetes. Metabolism. 2012;61:1696–702. doi: 10.1016/j.metabol.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Asrih M, Pellieux C, Papageorgiou I, Lerch R, Montessuit C. Role of ERK1/2 activation in microtubule stabilization and glucose transport in cardiomyocytes. Am J Physiol Endocrinol Metab. 2011;301:E836–43. doi: 10.1152/ajpendo.00160.2011. [DOI] [PubMed] [Google Scholar]
- Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest. 1995;96:1261–8. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A, Guilherme A, Robida SI, Nicoloro SM, Zhou QL, Jiang ZY, Pomerleau DP, Czech MP. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature. 2002;420:821–4. doi: 10.1038/nature01246. [DOI] [PubMed] [Google Scholar]
- Brozinick JT, Jr, Berkemeier BA, Elmendorf JS. Actin”g on GLUT4: membrane & cytoskeletal components of insulin action. Curr Diabetes Rev. 2007;3:111–22. doi: 10.2174/157339907780598199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, et al. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154:3099–109. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care. 2012;35:1316–21. doi: 10.2337/dc11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, Fraterrigo G, Okunade AL, Patterson BW, Klein S. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125:787–95. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y, Lee H, Catana C, Klibanski A, Patwari P, et al. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015;125:4601–4611. doi: 10.1172/JCI83349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Insulin secretion in response to protein ingestion. J Clin Invest. 1966;45:1479–86. doi: 10.1172/JCI105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni S, Fu X, Palmer SE, Sweetman L. Rapid determination of C4-acylcarnitine and C5-acylcarnitine isomers in plasma and dried blood spots by UPLC-MS/MS as a second tier test following flow-injection MS/MS acylcarnitine profile analysis. Mol Genet Metab. 2010;101:25–32. doi: 10.1016/j.ymgme.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Frimel TN, Deivanayagam S, Bashir A, O’connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–8. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- Frohnert BI, Long EK, Hahn WS, Bernlohr DA. Glutathionylated lipid aldehydes are products of adipocyte oxidative stress and activators of macrophage inflammation. Diabetes. 2014;63:89–100. doi: 10.2337/db13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008;8:169–74. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Groop LC. Insulin resistance: the fundamental trigger of type 2 diabetes. Diabetes Obes Metab. 1999;1(Suppl 1):S1–7. doi: 10.1046/j.1463-1326.1999.0010s1001.x. [DOI] [PubMed] [Google Scholar]
- Hattersley JG, Pfeiffer AF, Roden M, Petzke KJ, Hoffmann D, Rudovich NN, Randeva HS, Vatish M, Osterhoff M, Goegebakan O, et al. Modulation of amino acid metabolic signatures by supplemented isoenergetic diets differing in protein and cereal fiber content. J Clin Endocrinol Metab. 2014;99:E2599–609. doi: 10.1210/jc.2014-2302. [DOI] [PubMed] [Google Scholar]
- Houston DK, Nicklas BJ, Zizza CA. Weighty concerns: the growing prevalence of obesity among older adults. J Am Diet Assoc. 2009;109:1886–95. doi: 10.1016/j.jada.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D.C: The National Academies Press; 2005. [Google Scholar]
- Iwanaka N, Egawa T, Satoubu N, Karaike K, Ma X, Masuda S, Hayashi T. Leucine modulates contraction- and insulin-stimulated glucose transport and upstream signaling events in rat skeletal muscle. J Appl Physiol. 2010;108:274–82. doi: 10.1152/japplphysiol.00420.2009. [DOI] [PubMed] [Google Scholar]
- Kim K, Yu M, Han S, Oh I, Choi YJ, Kim S, Yoon K, Jung M, Choe W. Expression of human peroxiredoxin isoforms in response to cervical carcinogenesis. Oncol Rep. 2009;21:1391–6. [PubMed] [Google Scholar]
- Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics. 2005;6:144. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EP, Klein S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J Clin Hypertens. 2009;11:761–5. doi: 10.1111/j.1559-4572.2009.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. Outcome success in obesity. Obes Res. 2001;9(Suppl 4):354S–358S. doi: 10.1038/oby.2001.142. [DOI] [PubMed] [Google Scholar]
- Klip A, Sun Y, Chiu TT, Foley KP. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol. 2014;306:C879–86. doi: 10.1152/ajpcell.00069.2014. [DOI] [PubMed] [Google Scholar]
- Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Furnsinn C, Promintzer M, Anderwald C, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–7. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, Nowotny P, Roth E, Waldhausl W, Roden M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913–22. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, Woods SC, Mattes RD. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S–9S. doi: 10.3945/ajcn.114.084038. [DOI] [PubMed] [Google Scholar]
- Linn T, Geyer R, Prassek S, Laube H. Effect of dietary protein intake on insulin secretion and glucose metabolism in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:3938–43. doi: 10.1210/jcem.81.11.8923841. [DOI] [PubMed] [Google Scholar]
- Liu LZ, Cheung SC, Lan LL, Ho SK, Chan JC, Tong PC. Microtubule network is required for insulin-induced signal transduction and actin remodeling. Mol Cell Endocrinol. 2013;365:64–74. doi: 10.1016/j.mce.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–8. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- Manders RJ, Hansen D, Zorenc AH, Dendale P, Kloek J, Saris WH, Van Loon LJ. Protein co-ingestion strongly increases postprandial insulin secretion in type 2 diabetes patients. J Med Food. 2014;17:758–63. doi: 10.1089/jmf.2012.0294. [DOI] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–63. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, Chibalin AV, Moller DE, Kharitonenkov A, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011;27:286–97. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003a;52:1641–8. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr. 2003b;77:573–9. doi: 10.1093/ajcn/77.3.573. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012;15:595–605. doi: 10.1016/j.cmet.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK. IL-6 signalling in exercise and disease. Biochem Soc Trans. 2007;35:1295–7. doi: 10.1042/BST0351295. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci. 1993;84:95–8. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- Raza H, Robin MA, Fang JK, Avadhani NG. Multiple isoforms of mitochondrial glutathione S-transferases and their differential induction under oxidative stress. Biochem J. 2002;366:45–55. doi: 10.1042/BJ20020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietman A, Schwarz J, Tome D, Kok FJ, Mensink M. High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr. 2014;68:973–9. doi: 10.1038/ejcn.2014.123. [DOI] [PubMed] [Google Scholar]
- Robinson MM, Soop M, Sohn TS, Morse DM, Schimke JM, Klaus KA, Nair KS. High insulin combined with essential amino acids stimulates skeletal muscle mitochondrial protein synthesis while decreasing insulin sensitivity in healthy humans. J Clin Endocrinol Metab. 2014;99:E2574–83. doi: 10.1210/jc.2014-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–65. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation? Cell Cycle. 2011;10:3447–51. doi: 10.4161/cc.10.20.17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000;29:235–42. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
- Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder E, Brennan JP, Eaton P. Cardiac peroxiredoxins undergo complex modifications during cardiac oxidant stress. Am J Physiol Heart Circ Physiol. 2008;295:H425–33. doi: 10.1152/ajpheart.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L, Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013;12:48. doi: 10.1186/1475-2891-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs I, Beulens JW, Van Der AD, Spijkerman AM, Grobbee DE, Van Der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care. 2010;33:43–8. doi: 10.2337/dc09-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Yoshino J, Reeds DN, Bradley D, Burrows RE, Heisey HD, Moseley AC, Mittendorfer B. Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. J Clin Endocrinol Metab. 2014;99:256–65. doi: 10.1210/jc.2013-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Yoshino J, Stromsdorfer KL, Klein SJ, Magkos F, Reeds DN, Klein S, Mittendorfer B. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation. Diabetes. 2015;64:1555–63. doi: 10.2337/db14-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker LF, Sarto GE, Howard BV, Huang Y, Neuhouser ML, Mossavar-Rahmani Y, Beasley JM, Margolis KL, Eaton CB, Phillips LS, et al. Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women’s Health Initiative. Am J Clin Nutr. 2011;94:1600–6. doi: 10.3945/ajcn.111.018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Jacques H, Marette A. Modulation of insulin action by dietary proteins and amino acids: role of the mammalian target of rapamycin nutrient sensing pathway. Curr Opin Clin Nutr Metab Care. 2005;8:457–62. doi: 10.1097/01.mco.0000172589.55434.03. [DOI] [PubMed] [Google Scholar]
- Tsai S, Sitzmann JM, Dastidar SG, Rodriguez AA, Vu SL, Mcdonald CE, Academia EC, O’leary MN, Ashe TD, La Spada AR, et al. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J Clin Invest. 2015;125:2952–64. doi: 10.1172/JCI77361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker HC, Patel D, Howat WJ, Warren AY, Kay JD, Sangan T, Marioni JC, Mitchell J, Aldridge S, Luxton HJ, et al. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br J Cancer. 2013;109:983–93. doi: 10.1038/bjc.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27:675–84. doi: 10.1016/j.clnu.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:1281–98. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–9. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Almeda-Valdes P, Patterson BW, Okunade AL, Imai S, Mittendorfer B, Klein S. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J Clin Endocrinol Metab. 2014;99:E1666–70. doi: 10.1210/jc.2014-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–64. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–36. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008;413:201–15. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.