Abstract
The present work demonstrates that yeasts belonging to the Schizosaccharomyces genus release a high quantity of polysaccharides of cell wall origin starting from the onset of the alcoholic fermentation. By the end of the alcoholic fermentation, all of the Schizosaccharomyces yeast strains released a quantity of polysaccharides approximately 3-7 times higher than that released by a commercial Saccharomyces cerevisiae yeast strain under the same fermentative conditions of synthetic juice. A higher content of polysaccharide was found in media fermented by Schizosaccharomyces japonicus with respect to that of Schizosaccharomyces pombe. Some of the strains evaluated were also able to produce high levels of pyruvic acid, which has been shown to be an important compound for color stability of wine. The presence of strains with different malic acid consumption patterns along with high polysaccharide release would enable production of naturally modified wines with enhanced mouth feel and reduced acidity. The chemical analysis of the released polysaccharides demonstrated divergence between the two yeast species S. pombe and S. japonicus. A different mannose/galactose ratio and a different percentage of proteins was observed on the polysaccharides released by S. pombe as compared to S. japonicus. Analysis of the proteins released in the media revealed the presence of a glycoprotein with a molecular size around 32-33 kDa only for the species S. japonicus. Mass spectrometry analysis of carbohydrate moieties showed similar proportions among the N-glycan chains released in the media by both yeast species but differences between the two species were also observed. These observations suggest a possible role of rapid MALDI-TOF screening of N-glycans compositional fingerprint as a taxonomic tool for this genus. Polysaccharides release in the media, in particular galactomannoproteins in significant amounts, could make these yeasts particularly interesting also for the industrial production of exogenous polysaccharide preparations.
Keywords: wine, polysaccharide, mannoprotein, galactomannoprotein, Schizosaccharomyces pombe, Schizosaccharomyces japonicus, MALDI-TOF
1. Introduction
The addition of commercial products containing polysaccharides derived from yeast cells wall to wine (in particular mannoproteins), is becoming a common practice during the winemaking process (Pozo-Bayón et al., 2009). Positive enological properties associated with the polysaccharides and mannoprotein content of wine have been reported: reduction in protein and tartrate instability (Brown et al., 2007; Dupin et al., 2000; Gerbaud et al. 1997; Gonzalez-Ramos et al., 2008; Moine-Ledoux and Dubourdieu,1999; Lubbers et al.,1993; Waters et al., 1994), improvement of mouth-feel (Vidal et al., 2004), increase of sweetness and roundness (Guadalupe and Ayestarán, 2007; Rosi et al., 1998), decrease in astringency (Escot et al., 2001; Quijada-Morín et al., 2014), prevention of tannin aggregation and precipitation (Poncet-Legrand et al., 2007), addition of complexity and aromatic persistence (Chalier et al., 2007; Lubbers et al.,1994), stabilization of the colour of red wines (Fuster and Escot, 2002; Riou et al., 2002), and stability of the foam of sparkling wine (Vanrell et al. 2007). In contrast, Guadalupe and Ayestarán (2008), using commercial mannoprotein-rich preparations in Tempranillo must, observed a reduction of the content of wine proanthocyanidins and wine stable pigments. Thus the nature of the polysaccharide is an important factor in defining the potential impact on the wine.
Guadalupe et al. (2010) suggested that using yeasts during the alcoholic fermentation production that are able to release mannoproteins would be less costly than use of exogenous mannoproteins. However yeast release variable portions of mannoproteins and the typical yeast present during fermentation, S. cerevisiae, releases low amounts of polysaccharides, normally ranging from 50 to 150 mg/L (Rosi et al., 2000). Therefore, an interesting alternative to the addition of these commercial exogenous polysaccharides-based products would be the use of yeasts able to release high quantity of polysaccharides during the alcoholic fermentation. Several studies have shown that non-Saccharomyces yeasts are generally characterized by the capacity to release a high quantity of polysaccharides (Comitini et al., 2011; Domizio et al., 2011a, 2011b, 2014; Giovani et al., 2012; Gobbi et al., 2013). In most of these analyses, the concentration of polysaccharides released by the non-Saccharomyces yeasts was much higher when compared with S. cerevisiae yeasts used as controls under the same conditions.
Schizosaccharomyces yeast strains have found application in winemaking because of their ability to reduce malic acid in grape juice and/or wine (Ciani, 1995; Dharmadhikari and Wilker, 1998; Gao and Fleet, 1995; Magyar and Panyik, 1989; Munyon and Nagel, 1977; Rankine, 1966; Silva et al. 2003; Snow and Gallender, 1979; Thornton and Rodríguez, 1996; Yokotsuka et al., 1993). Recently, Benito et al. (2012, 2014) reported benefits in addition to the demalication activity deriving from using S. pombe yeast in wine fermentation, such as the production of pyruvic acid and the possibility to reduce ethyl carbamate in wine, through the removal of its urea precursor, as a consequence of urease activity. Pyruvic acid seems to be of particular interest for the color stability of the wine. Indeed, a strong correlation between the amount of pyruvic acid released and the formation of vitisin A (a pyranoanthocyanin, a natural polyphenol found in grapes) has been observed (Morata et al., 2003). Moreover, malic acid consumption by Schizosaccharomyces yeasts permit non-bacterial biological deacidification and averting production of amines. In this context, in order to avoid the risk of biogenic amines formation by lactic bacteria during malolactic fermentation, Benito et al. (2015) proposed mixed fermentation by using two different yeasts: S. pombe, to consume malic acid, and Lachancea thermotolerans to produce lactic acid to balance the acidity of wines produced from low acidity musts. S. pombe is also able to utilize D-gluconate as an alternative carbon and energy source for growth during glucose starvation (Tsai et al., 1995). Therefore, the possibility to reduce gluconic acid in wines produced from rotten grapes by using S. pombe strains could represent an interesting approach. However, glucose addition rapidly inhibits gluconate degradation. For this reason, S. pombe use has been proposed as a way to remove gluconic acid from wine to be subsequently subjected to biological aging (Peinado et al 2004). However, in following studies, Peinado et al. (2007, 2009), successfully used glucose-transport-deficient mutants of S. pombe mutant strains to reduce the content in gluconic acid also of grape juice obtained from rotten grapes. Thus this yeast has already been proposed for use in wine production. The ability to release polysaccharides with beneficial effects would also be of interest.
A study comparing the quantity of polysaccharides released at the end of alcoholic fermentation by eighty-nine non-Saccharomyces yeasts strains, found that the only Schizosaccharomyces strain tested released the highest level of polysaccharides (712 mg/L), about 5 times higher than the average of those released by three S. cerevisiae strains, used as controls (Romani et al., 2010). In contrast, Giovani et al. (2012) found that the only S. pombe strain tested released a quantity of polysaccharides (203 mg/L) lower than that released by three S. cerevisiae strains (ranging from 225 to 264 mg/L) when tested under the same conditions. Both of these studies used a single strain of Schizosaccharomyces and the differences could be due to strain effects or to the growth conditions of the studies.
The presence of a high quantity of polysaccharides from the beginning of the alcoholic fermentation process could promote the formation of polysaccharide-tannin complexes that stabilize the reactive tannins and in turn enhance the mouthfeel of red wine as well as enable other types of interactions of benefit to wine stability. Therefore, considering the elevated quantity of polysaccharides released by the strain of Schizosaccharomyces (712 mg/L), as previously reported by Romani et al., (2010) and the variability in release reported in the literature (Giovani et al., 2012), in the present work we evaluated the ability of different strains belonging to the genus Schizosaccharomyces to release polysaccharides during the alcoholic fermentation.
2. Materials and methods
2.1. Yeast strains
Nine yeast strains belonging to the genus Schizosaccharomyces from the yeast culture collection of the Department of Agricultural, Food and Forestry Systems (GESAAF, University of Florence, Italy) and the Department of Viticulture & Enology University of California-Davis, (Davis) were used (Table 1). Six strains were ascribed to the species Schizosaccharomyces pombe and three to the species Schizosaccharomyces japonicus by D1-D2 domain analysis.
Table 1. Origin and source of the Schizosaccharomyces strains used in the present study.
| CODE | Species | Strain | Origin | Source |
|---|---|---|---|---|
| # 1 | Schizosaccharomyces japonicus | 13 | GESAAF a | Wine |
| # 2 | Schizosaccharomyces pombe | 227 | UCD b | Unkown |
| # 3 | Schizosaccharomyces pombe | 582 | UCD b | Sherry wine |
| # 4 | Schizosaccharomyces pombe | 583 | UCD b | Sherry wine |
| # 5 | Schizosaccharomyces pombe | 584 | UCD b | Wine |
| # 6 | Schizosaccharomyces pombe | 687 | UCD b | Wine |
| # 7 | Schizosaccharomyces pombe | 807 | UCD b | Unkown |
| # 8 | Schizosaccharomyces japonicus | 2096 | UCD b | Wine |
| # 9 | Schizosaccharomyces japonicus | 2489 | UCD b | Wine |
Dipartimento di Gestione dei Sistemi Agrari, Alimentari e Forestali, Università degli Studi di Firenze, Italy
Department of Viticulture & Enology, University of California-Davis, Davis
A commercial strain, Lalvin EC1118 (Lallemand Inc., Montreal, Canada), was used as reference strain for S. cerevisiae and for comparison determinations.
2.2. Fermentation trials
The fermentations were carried out in duplicate at 27°C in 200 mL Erlenmeyer flasks containing 150 mL of a synthetic grape juice medium “Minimal Must Medium” (MMM) (Spiropoulos et al., 2000). The medium was sterilized by filtration. The flasks were inoculated at optical density of 0.1 (OD600 nm), with 48-h pre-cultures grown in 10 mL of YPD medium (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose) (Oxoid Unipath Ltd, Hampshire, UK), at 25 °C in a roller drum. The levels of sugar and total assimilable nitrogen were 220 g/L and 208 mg/L, respectively. The assimilable nitrogen concentration was obtained by using 0.2 g/L of L-arginine and 0.5 g/L of ammonium phosphate. The flasks, continuously agitated at 150 rpm, were stoppered with silicone vent bungs (Ferm-Rite), allowing the CO2 to escape. The flasks were weighed daily until the end of fermentation (defined as a constant weight for two consecutive days) to monitor the fermentation kinetics.
2.3. Biomass determination
Samples were taken from each flask during the alcoholic fermentation to monitor the growth kinetics by OD600 nm. At the end of the fermentation, the dry weight biomass was determined gravimetrically by filtering 5 ml of sample from each culture on pre-weighed 0.45 μm nitrocellulose membranes. Membranes were washed twice with distilled water and placed in oven at 100 °C for 24 h before being weighed again, and the difference in starting and final weight was used to calculate biomass.
2.4. Analytical determinations of the fermentation products
Ethanol and residual sugars were determined by high performance liquid chromatography (HPLC) using an Agilent 1100 series HPLC system (Agilent, Palo Alto, CA, USA) coupled with a refractive index (Hewlett Packard HP-1047A) detector. After filtration through 0.45 μm nitrocellulose membranes and after appropriate dilution in water, 20 μL of each sample was injected into the HPLC apparatus. Isocratic separation was performed at 75 °C on a (300 × 7.7 mm) Hi-Plex H column (Agilent, Palo Alto, CA, USA). The mobile phase was 4 mM H2SO4 at a flow rate of 0.4 mL/min. The compounds were identified and quantified by comparisons with external calibration curves for each compound.
The areas of the peaks of interest were integrated using the ChemStation Data Analysis System, version A.10.2 (1757) (Agilent, Palo Alto, CA, USA). All the analyses were carried out in duplicate.
2.5. Polysaccharide purification
After thirteen days of alcoholic fermentation, the fermented juice was centrifuged (8000 g, 4°C, 10 min) to separate the yeast cells. The purification of the polysaccharidic fraction was performed by ethanol precipitation, as previously described (Domizio et al., 2014). The dried pellets obtained during this step were then rehydrated with nanopure water for the successive analytical determinations.
2.6. Polysaccharide characterization
2.6.1. Monosaccharide composition
After acid hydrolysis of the rehydrated pellets, as previously reported (Domizio et al., 2014), the hydrolyzed samples were neutralized with Ba(OH)2, as proposed by François (2006), with some modifications. Briefly, sulfate ions were precipitated by the addition of saturated Ba (OH)2 until neutral pH was reached (checked with pH paper). The total volume was adjusted with nanopure water to 1 ml and BaSO4 precipitates were pelleted by centrifugation at 3700 g for 5 min. The supernatant was removed and left at 4°C overnight to allow precipitation of the remaining sulfate ions which was removed by a second centrifugation at 3700 g for 5 min. The monosaccharide composition of the polysaccharides was then determined by high-performance anion exchange chromatography with pulsed amperometric detection (Thermo Scientific HPAEC-PAD ICS-5000, Sunnyvale, CA) equipped with a detector/chromatography module including a pulsed amperometry electrochemical detector, an electrochemical cell with a disposable gold working electrode, a pH-Ag/AgCl reference electrode, an autosampler and a single pump. After filtration through a 0.22-μm membrane (Pall, Port Washington, NY) and after appropriate dilution in nano pure water, 25 μL of each sample was injected into the CarboPacPA200 analytical column (3 × 250 mm, Dionex, Sunnyvale, CA) and a CarboPacPA200 Guard Column (3 × 50 mm, Dionex). The separation was performed with isocratic elution (18 mM NaOH;1.2 mL/min; 25 °C). Quantification of the monosaccharides was performed in comparison with an external calibration curve with concentrations of each compound ranging from 1 to 60 mg/L.
2.6.2. Protein quantification
The protein concentration was determined by dye-binding Bradford assay (Bradford, 1976) using Bio-safe Coomassie blue G-250 dye reagent (Bio-Rad). Bovine serum albumin (BSA) (Sigma-Aldrich) was used to constitute the external calibration curve.
2.6.3. Protein profiling by gel electrophoresis
Electrophoresis was performed by using 10% Sodium Dodecyl Sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970) to characterize the released proteins and mannoproteins. Based on the protein concentration, the rehydrated pellets obtained from the precipitated polysaccharides were further diluted with ultrapure water to have a protein concentration ranging from 0.2 to 0.3 mg/mL. Then 20 μL of the diluted sample was treated with 6.65 μL of 4X Laemmli buffer (Bio-Rad) and 2.75 μL of 1M dithiothreitol (DTT) (Acros Organics) and heated at 95 °C for 5 min. After this treatment, 15 μL of the mixture was loaded onto the gel. The CandyCane molecular weight standard (a mixture of glycosylated and un-glycosylated protein standards) (Invitrogen, Carlsbad, CA, USA) at a concentration of 750 ng/lane, was used. In addition, 10 μL of Blue precision plus protein standard (Bio-Rad) was loaded.
The SDS-PAGE was developed in a Mini Protean II apparatus (Bio-Rad) at 45 V within the stacking gel and at 104 V in the developing gel (until the tracking dye bromophenol blue ran off the gel). Gels were stained with a Pro-Q Emerald 488 glycoprotein stain (Molecular Probes, Inc., Eugene, OR, USA) to detect the glycoproteins according to the manufacturer's protocol. The resulting green-fluorescent signal produced by the Pro-Q Emerald 488 dye was visualized by a TyphoonTrio Plus variable mode imager (GE Healthcare) with a 488 nm excitation laser and a 520 nm long-pass emission filter. Following detection of glycoproteins, total protein profiles were subsequently displayed using the Bio-Safe Coomassie G-250 stain (Bio-Rad).
2.6.4. N- glycan isolation and MALDI-TOF analysis
Analysis of the N-glycans released by PNGase F from the glycoprotein was performed as previously described (Domizio et al., 2014) in order to characterize the mannoproteins released in the media at the end of alcoholic fermentation by the different Schizosaccharomyces yeast strains. The N-glycans released from the pool of proteins were detected according to the method reported in Bordiga et al. (2012), with some modifications (Domizio et al., 2014). MALDI-TOF analysis was performed on sodiated species in the m/z scan range from 1600 Da to 4000 Da on a Bruker Ultraflex II MALDI-TOF (BrukerDaltonics, Bremen, Germany) in positive ion mode. Each sample was spotted in triplicate from biological duplicate extractions and the mass spectra were collected by using 100 laser shots. Resulting mass spectra were analyzed using the Flex-Analysis software from Bruker Daltonics (Bremen, Germany).
3. Results and Discussion
3.1. Fermentation performance
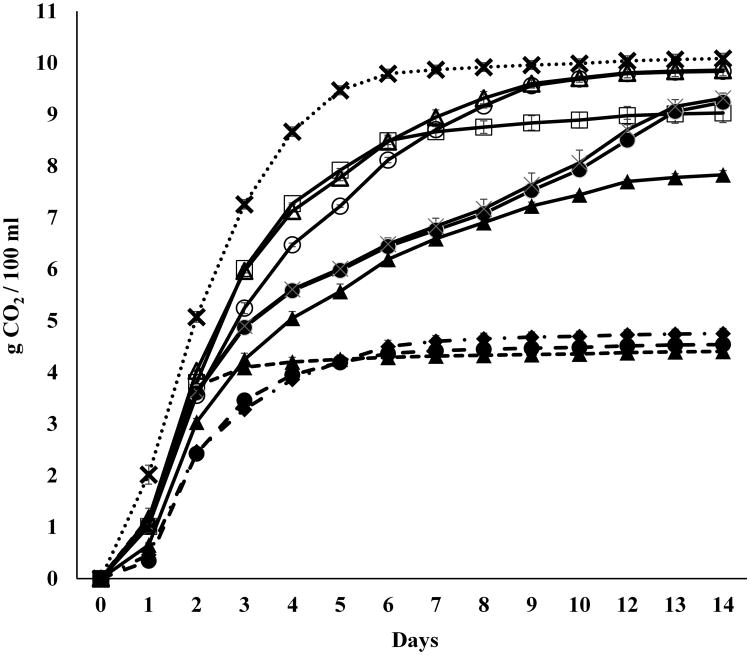
Nine different Schizosaccharomyces yeast strains, belonging to two different species, S. pombe and S. japonicus (Table 1), were selected to be evaluated for polysaccharide production over the course of fermentation using a synthetic grape juice in comparison with a commercial strain of Saccharomyces, Lalvin EC1118. The Schizosaccharomyces yeasts selected were isolated from wines of different origins. Based on the quantity of CO2 released during the alcoholic fermentation (Fig. 1), it was possible to distinguish different fermentative behaviors across the strains. The three strains belonging to S. japonicus were characterized by the low fermentative activity, reaching the maximum quantity of CO2 produced (∼ 4.5g/100 mL) just after two days (strain #1) or after five days (strains #8 and #9) of alcoholic fermentation. After thirteen days of fermentation, the residual sugar concentration ranged between 11-12% (w/v) and the ethanol level was roughly 6% (v/v) (Fig. 2). In contrast, among the six strains of S. pombe two different fermentation patterns were observed (Fig. 1). The first group of S. pombe strains (# 3, #4 and #7) showed an exponential increase in fermentation between days 1 and 3 of the fermentation after which they were characterized by a slower constant production of CO2 until day 9 when fermentation rates increased again for strains #3 and #4 but stayed constant for strain #7. S. pombe strain #7 overall presented the lowest rate of CO2 loss but it still exceeded that of any of the S. japonicus strains. The second group of S. pombe strains (#2, #5 and #6), presented fermentation profiles more similar to that of the strain of S. cerevisiae used as a reference with fermentation rates decreasing sooner between the third and fourth day of fermentation. After 10 days of fermentation, strains #5 and # 6 produced the same quantity of CO2 as that released by the S. cerevisiae strain. Different fermentative behaviors among S. pombe strains have been previously reported (Benito et al., 2012, 2014) that also describe strains with slow and strong fermentative ability. The analysis of glucose and fructose consumption demonstrated that all the Schizosaccharomyces strains are glucophilic, similar to Saccharomyces yeasts (Figure 2).
Figure 1.
Fermentation kinetics of pure cultures of S. japonicus (dash lin
 #1,
#1,
 #8,
#8,
 #9), S. pombe (continuous line:
#9), S. pombe (continuous line:
 #2,
#2,
 #3,
#3,
 #4,
#4,
 #5,
#5,
 #6,
#6,
 #7) and S. cerevisiae (dot lines:
#7) and S. cerevisiae (dot lines:
 ) inoculated in synthetic grape juice. Data are representative of two independent experiments. Error bars represent standard deviation of two independent experiments, each carried out in duplicate.
) inoculated in synthetic grape juice. Data are representative of two independent experiments. Error bars represent standard deviation of two independent experiments, each carried out in duplicate.
Figure 2.
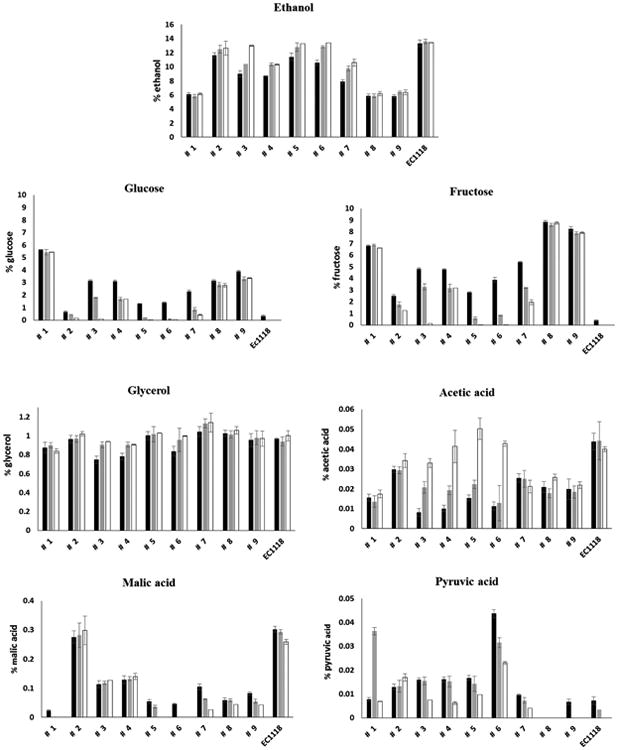
Fermentative performance of the nine Schizosaccharomyces yeast strains and the commercial strain of S. cerevisiae in terms of ethanol production and amounts of residual sugars, glycerol, acetic acid, malic acid and pyruvic acid at 5 ■, 8 ■ and 14 ■ days of alcoholic fermentation. Data are representative of two independent experiments. Error bars represent standard deviation of two independent experiments, each carried out in duplicate.
An inter and intra specific variability of the demalication activity was also observed among the Schizosaccharomyces strains. In particular, within the species S. japonicus, strains # 8 and #9 showed a similar trend in malic acid consumption, with a 73-80% reduction in malate levels after five days of alcoholic fermentation. However, 92% of the malic content was reduced by strain #1 within this same time frame. Among the yeast strains belonging to the species S. pombe, strain # 2 showed no malic consumption. In contrast, almost a complete reduction of malic acid was observed after five days of fermentation for strains #5 and #6, and after eight days for strain #7. Strains #3 and #4 reduced the malic content by approximately 55% after 5 days of fermentation and no further decrease was then observed. Similar variability among Schizosaccharomyces strains in the malic acid degradation has been observed previously by other authors (Gao and Fleet, 1995; Magyar and Panik, 1989; Silva et al., 2003; Snow and Gallander, 1979; Taillandier et al., 1995; Thornton and Rodriguez, 1996). Some strains appeared to consume malate simultaneously with glucose and fructose (strains #5 and #6) consuming all substrates by day ten of the fermentation while others showed a preference for one substrate over the other: malate for strain #1 and glucose and fructose for strain #2, and malate and glucose over fructose for strains #8 and #9 (Figure 2). Such variability could be advantageous during wine production if malate depletion was desired for example or retention of sugar substrates for Saccharomyces.
With respect to acetic acid, strains #4, #5 and #6 of S. pombe produced the highest levels ranging from 0.4 to 0.5 g/L. All the other strains of Schizosaccharomyces produced a lower concentration of acetic acid, ranging from 0.17 g/L to 0.34 g/L, which was consistent also with their lower fermentation capacity. High levels of acetic acid (ranging from 0.86 g/L to 1 g/L) have been often reported for S. pombe yeasts during the alcoholic fermentation of grape juice and represents one of the major problems observed among these types of yeast (Benito et al., 2012). In order to find strains with positive enological potential, Benito et al. (2014) evaluated one hundred strains of Schizosaccharomyces and found only five strains producing less than 0.4 g/l of acetic acid, about 20% of strains producing a concentration of acetic acid ranging from 0.3 g/L to 0.6 g/L and most of the strains producing a quantity around 0.8 g/L. In a recent study, Benito et al. (2016) evaluated 75 strains of S. pombe and found that only 8% of the strains produced less than 0.4 g/L of acetic acid, while the majority of the strain (65%) produced acetic acid ranging from 0.4 g/L to 0.7 g/L and 15 % between 0.8 and 1.4 g/L of acetic acid. In general, we observed lower levels of acetic acid production in the present study; however this may be impacted by medium composition and further studies using actual winemaking conditions and juices are warranted.
All S. pombe strains except strain #8 produced more pyruvic acid than the Saccharomyces yeast during the course of fermentation. In particular, strain # 6 produced 0.43 g/L of pyruvate after five days of fermentation, six times higher than that produced by the S. cerevisiae strain (0.072 g/L) and this level remained high at the end of fermentation (Figure 2). A lower quantity of pyruvic was found in the media fermented by strain #9 of S. japonicus (0.066 g/L) and pyruvate was undetectable at day 14. A high spike in pyruvate formation was observed at eight days of fermentation, for strain #1 of S. japonicus. The results here obtained are consistent with those previously published (Benito et al., 2012, 2014), where strains of S. pombe were reported to be able to produce levels of pyruvic acid ranging from 0.2 to 0.42 g/L during the alcoholic fermentation.
High variability among the Schizosaccharomyces strains was also observed for the final glycerol content, ranging from 8.3 g/L to 10.5 g/L for the S. japonicus strains and from 9 g/L to 11.4 g/L for the S. pombe strains (Fig. 2) and was not significantly different with respect to glycerol production by the S. cerevisiae strain (9.9 g/L). Similar levels of glycerol have been reported by Benito et al (2016) with values ranging from 8.14 to 8.9 g/L. Also in this case, the values were similar to those obtained for the two S. cerevisiae strains used as reference.
3.2. Polysaccharide release and cell growth
The release of polysaccharides for each strain was compared to the optical density observed at the time of assay (Figure 3). Four strains were flocculent, strain #2 and all three S. japonicus strains (#1, #8, and #9). However, S. japonicus strains started to de-flocculate after about five days of fermentation. Hence, when the strains were flocculent, it was not possible to obtain accurate assessments of biomass using optical density. All Schizosaccharomyces strains started growth immediately upon transfer and released polysaccharides throughout fermentation with the greatest release ranging from 70% and 86% with respect to the final content occurring before day 3 suggesting that release of polysaccharides in these strains accompanies active growth and cell division. A slightly lower percentage of polysaccharide (63%-67%) was detected for strains #2, #6 and #7. The flocculent strains also released polysaccharides immediately upon transfer to the medium with the bulk of the release occurring by day 2. At the end of the alcoholic fermentation, the level of polysaccharides detected in the media ranged from 542 mg/L to 786 mg/L for all strains of S. pombe with the exception of strain # 6 (900 mg/L). The level of polysaccharides for the three strains of S. japonicus ranged from 930 mg/L to 1386 mg/L. In any case, at the end of the alcoholic fermentation all the Schizosaccharomyces yeast strains released a higher quantity of polysaccharides as compared to the commercial S. cerevisiae yeast EC1118 strain and, specifically, 2.6 - 4.3 times higher for S. pombe yeast species and 4.4 - 6.6 times higher for S. japonicus yeast species.
Figure 3.
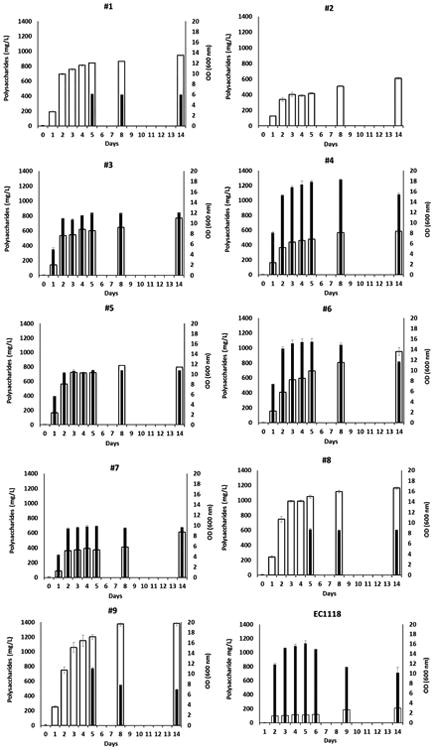
Growth (■) and total polysaccharide release (■) by Schizosaccharomyces and S. cerevisiae during the alcoholic fermentation. Data are representative of two independent experiments. Error bars represent standard deviation of two independent experiments, each carried out in duplicate.
At the end of the alcoholic fermentation, the amount of polysaccharides released in the media was normalized to cell biomass (polysaccharides / cell dry weight (mg/g)) (Fig. 4). This ratio was also calculated for the three strains of S. japonicus, which was possible because after the fifth day until the end of the fermentation, cells were no longer flocculent and dry weight could be determined. Strain #2 of S. pombe remained flocculent throughout and as a consequence it was not possible to determine the polysaccharide/dry weight ratio. The yeast strain of S. cerevisiae EC1118 produced the lowest ratio (68 mg/g), confirming our previous results (Domizio et al., 2014). In contrast, the three strains of S. japonicus produced the highest amounts of polysaccharides, ranging from 265 to 411 mg/g, while all the S. pombe strains produced an intermediate quantity, ranging from 98 to 208 mg/g (Fig. 4).
Figure 4.
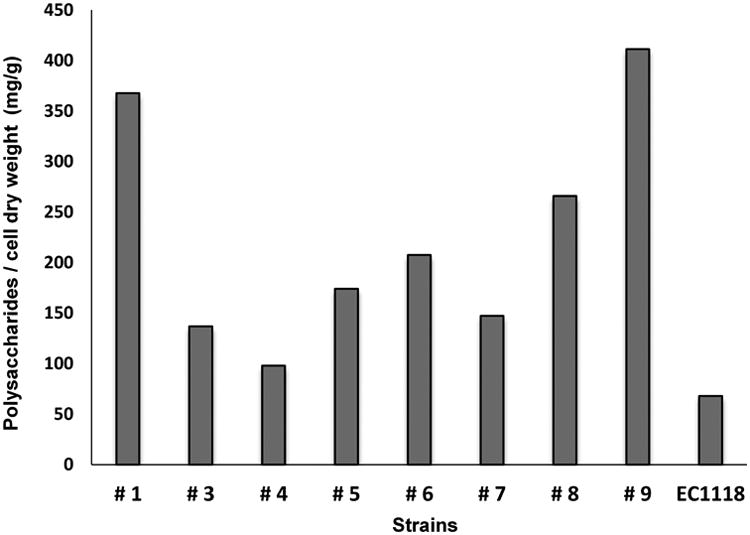
Ratio of polysaccharides (mg) to grams of cell dry weight (mg/g) of the nine Schizosaccharomyces strains and of the commercial strain of S. cerevisiae
High levels of polysaccharides released within the first days of alcoholic fermentation have been observed for other non-Saccharomyces yeasts and have been related to the cell response to external stimuli, causing cell wall stress, or to the cell growth (Domizio et al., 2014). In the budding yeast Saccharomyces polysaccharide release during the alcoholic fermentation has been related to the production of beta-glucanases during the growth phase which serves to weaken the cell wall and allow bud emergence (Fleet, 1991). Yeasts belonging to the genus Schizosaccharomyces divide instead by binary fission with the formation of a septum between the cells. This septum is a three-layered structure composed of a middle layer named the primary septum, bordered at both sides by the secondary septum (Johnson et al., 1973; Sipiczki, 2007). Cell separation represents the last step of cytokinesis and requires degradation by glucanases of the primary septum and the contiguous cell wall, which presents an outer layer rich in galactomannoproteins (Cortés et al., 2012; Horisberger and Rouvet-Vauthey, 1985; Pérez, and Ribas, 2004). Therefore, the polysaccharides found in the media likely derive from the cell wall degradation by the action of glucanase during the separation process between the daughter cells (Dekker et al., 2004; Garcia et al., 2005) thus explaining the association of growth and polysaccharide release. The higher level of polysaccharide released in the media by all the Schizosaccharomyces yeast strains, compared to that released by the S. cerevisiae strain, could be due to their osmophilic character and, as consequence, characterized by a cell wall richer of resistance elements, likely polysaccharides, useful to support high osmotic pressures (Kopeckà et al., 1995). Moreover, a greater proportion of the cell wall surface is likely involved during the binary division of Schizosaccharomyces yeast with respect to that occurring during budding. Indeed, the erosion of outer surface of old cell wall covers the entire cell diameter around the edge of the primary septum, while in budding yeast it concerns only the thin mother-bud neck.
Based on this last observation, we can also speculate that the higher content of polysaccharide found in media fermented by S. japonicus with respect to that of S. pombe is due to the fact that S pombe is characterized by a smaller cell size with respect to S. japonicus (Hironori, 2014). Moreover, the higher level of polysaccharide after the third day of fermentation released by S. japonicus versus S. pombe, could also be due to the faster generation time characterizing yeasts belonging to the species S. japonicus as compared to S. pombe (Klar, 2013).
The increased polysaccharide release of S. japonicus could alternately be a consequence of the sexual cycle that this species undergoes during normal growth. Microscopic observation of S. japonicus cells during the alcoholic fermentation revealed that after two days most of the cell had already started a conjugation process, resulting in the ascospore formation (Fig. 5). Indeed, in fission yeasts meiosis proceeds after karyogamy and zygotes give rise to asci immediately. After a few days the ascus wall surrounding the ascospores dissolves, releasing the ascospores into the media. All the three strains of S. japonicus started to flocculate after one or two days of fermentation and de-flocculate after about five days of fermentation. Calleja et al. (1971, 1977) report that the flocculation phenomenon in S. pombe represents an initial and essential step in the process of sexual conjugation. After cell flocculation, covalent bonds (copulation) are formed between the two cells in order to stabilize the floes. Following floe formation a series of events occur (conjugation tube initiation, cross-wall formation and 2-layer cross-wall erosion, expansion of contact area, conjugation-tube expansion, perforation of 2-layer cross wall, crimping at site of union) permitting nuclear migration and fusion, with the consequent zygote formation. At the end of this process, almost all of the cross-wall is gone and a discontinuity along the site of union between the walls of copulating cells remains until the walls are repaired during a de-differentiation process of site of union. This last step consists in the deposition of new wall material in the gap at the periphery of the contact area of the walls or in the removal of the protein-rich outer surface which fills the gap (Calleja et al., 1977). In this latter case, the galactomannoproteins, participating in the cell wall structure, could be released, contributing to further increase the quantity of polysaccharides in the media. After cell fusion and zygote formation, cells normally start the sporulation process that ends in the formation of asci containing mature ascospores (Tanaka and Hirata, 1982). After that, as final step of the sexual cycle, the ascus wall surrounding the ascospores dissolves, releasing the ascospores and permitting their dispersion into the media. The spore release from the ascus is assured by the action of hydrolytic enzymes such as α-glucanase and β-glucanase (Dekker et al., 2007; Dedo et al., 2009). Since the ascus wall corresponds to the cell wall of conjugating haploids or to the cell wall of the diploid cell, it is predicted to have a composition similar to that of the vegetative cell wall. Therefore, the high content of polysaccharide released in the media by the three strains of S. japonicus could be a natural consequence of the cell conjugation process as well as of the spore release from the ascus.
Figure 5.
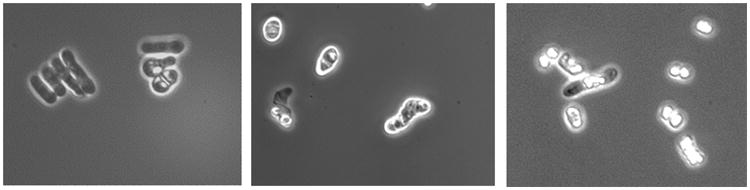
Microscopic observation of the strain #9 of S. japonicus during the alcoholic fermentation of a synthetic grape juice: conjugation process and ascospore formation.
Further, Calleja et al. (1977) observed that, during the conjugation process, a significant portion of cells usually lyse spontaneously, probably as a consequence of faulty fusion or uncontrolled lytic activity during cross-wall removal. This spontaneous lysis of the cells could be another possible reason to explain the high level of polysaccharide found in the media fermented by S. japonicus. In contrast strain #2 of S. pombe, which flocculated during the entire time course of fermentation did not display zygote formation after cell aggregation. Therefore, this was likely a non-sexual flocculation.
The conjugation process of the three strains of S. japonicus started within the first two days of fermentation (as observed via microscopic observation) of the synthetic grape juice. Since S. japonicus cells usually enter into meiosis following the deterioration of nutrient conditions (Hironori, 2014), the early onset of the conjugation process could indicate the lack of specific nutrient. The slower fermentation capacity observed in the three strains of S. japonicus could also be an indication of nutrient limitation. Further investigation is necessary to clarify the fermentation needs of this species.
3.3. Characterization of the released polysaccharides
The nature of the released polysaccharide component was analyzed for the nine yeast strains of Schizosaccharomyces. Proteins and glycoproteins content were characterized by gel electrophoresis and the carbohydrate composition was analyzed by HPAEC-PAD and by mass spectrometry.
The overall pattern of the proteins released in the media as analyzed by SDS PAGE, was similar for the two yeast species. The protein profiles spanned a wide variety of molecular weights, ranging from 25 kDa to greater than 250 kDa for all nine strains (Fig. 6 a-f).
Figure. 6.
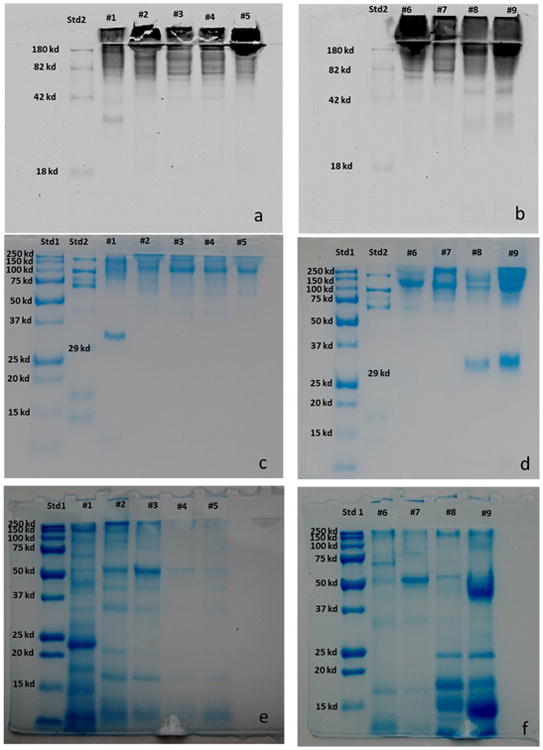
SDS-PAGE electrophoresis of the proteins released into the media from Schizosaccharomyces yeasts. Glycoprotein visualization was performed using a two step procedure. The glycoproteins were first stained on electrophoretic gel with Pro-Q Emerald 488 glycoprotein gel stain kit (6 a-b). Then, the proteins were stained in the same gel with Bio safe Coomassie (6 c-d). The pool of proteins released in the media have been loaded in the gel after enzymatic de-glycosylation with PNGase F and then stained in the gel with Bio safe Coomassie (6 e-f). Std1: Blue precision plus molecular weight standard; Std2: CandyCane molecular weight standard.
The three strains of S. japonicus presented an evident band around 32-33 kDa that corresponded to a glycosylated protein as demonstrated after gel staining with the Pro-Q Emerald 488 gel stain kit (Fig. 6 a-b) that is specific for glycoproteins. To further validate our findings, we applied enzymatic digestion with PNGase F and we observed a molecular size decrease of the 32-33 kDa band that was consistent with the release of glycan moieties (Fig. 6 e-f). Other glycosylated proteins were also identified using this same procedure.
Treatment of the pool of proteins released by all the S. pombe strains at the end of fermentation with PNGase F enabled de-glycosylation prior to running the gel. Subsequent staining of the gel with Pro-Q Emerald 488 revealed that most of the released proteins were glycosylated. Their greater mobility in the gel compared to the native glycoproteins was evident (Fig. 6 c-f). None of the S. pombe strains had a 32-33 KDa band. These results are in agreement with those of Herrero et al. (1987) who found in comparing the cell wall proteins liberated by Zymolyase from several ascomycetous yeast, that only the S. pombe strain did not present any band in the region of 33 kDa in SDS-polyacrylamide gels. Moine-Ledoux and Dubourdieu (1999) identified a N-glycosylated 32 kDa mannoprotein, corresponding to a parietal invertase fragment of S. cerevisiae. This mannoprotein, released by S. cerevisiae after alcoholic fermentation, was considered responsible for improving protein stability in wine and showed a higher stabilizing effect than other mannoproteins with higher molecular weights. In the fission yeast invertase is a high-molecular-mass glycoprotein (205 Kda) located outside the plasma membrane and, differently from S. cerevisiae, it contains not only mannose but also galactose (Moreno et al., 1990). Further studies are necessary in order to identify the 32-33 Kda protein from S. japonicus and to explore its possible role in wine stability.
The presence of glycoproteins in the stacking gel is likely due to their high molecular masses. In agreement, the existence of a high-molecular-mass glycoprotein component has been observed in the Zymolyase-released glycoprotein material from isolated walls of S. pombe (Herrero et al., 1987).
Bands with high molecular weight were particularly evident in the SDS-polyacrylamide gels for most of the S. pombe strains. This last observation is consistent with the presence of an early elution peak, (retention time of about 6.7 minutes) in the relevant chromatograms of the polysaccharides, analyzed by HPLC, and extracted at the end of the alcoholic fermentation (Fig. 7 a-b). These earlier peaks were particularly evident for the strains # 3, #4, #5 and #6. Indeed, the elution of polysaccharides in HPLC/RI is based on size, with longer polymers eluting before shorter ones. Commercial mannans extracted from S. cerevisiae, and used as reference standard for the quantification of polysaccharide in the present study, showed a peak at a retention time of about 7 minutes (Fig. 7 c), consistent with when most of the polysaccharide fractions extracted from the fermented media of all the Schizosaccharomyces strains have been detected. Palomero et al. (2009) observed a similar early peak in the HPLC chromatograms of the polysaccharides produced by S. pombe. This earlier peak was no evident in the chromatograms of the three strains of S. japonicus (Fig. 7 b). Further research is needed to identify these earlier peaks and to evaluate the influence of these larger size polymers on the chemical properties of wine.
Figure 7.
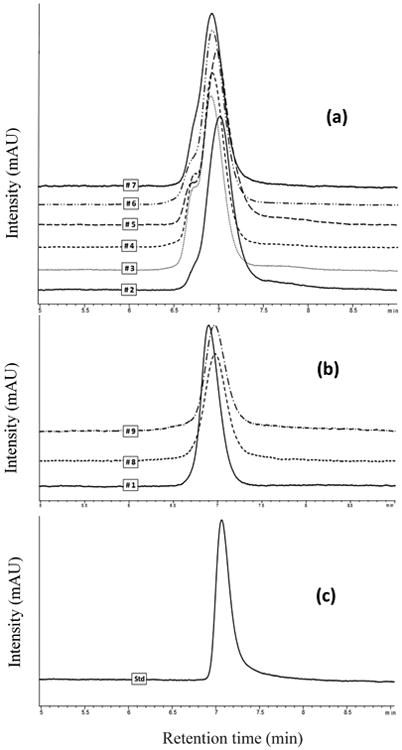
HPLC chromatograms of polysaccharides released in the media by S. pombe strains (a) and S. japonicus (b) at the end of the alcoholic fermentation. and of a standard solution of commercial mannan (c).
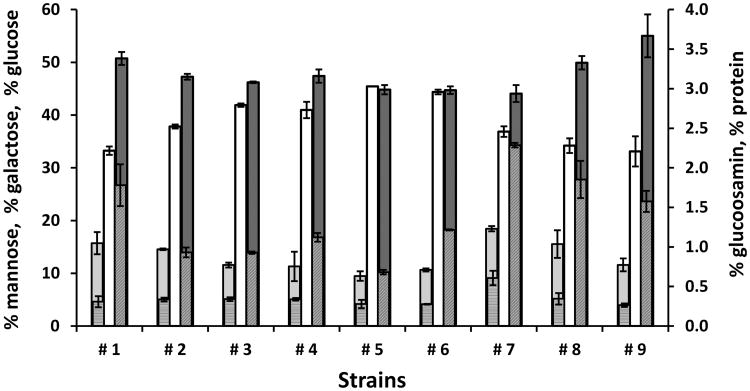
The monosaccharide composition of the total polysaccharide fraction released into the media was also analyzed and revealed high percentages of mannose (ranging from 44% to 55%) and galactose (ranging from 33% to 45%) (Fig. 8). In particular, the S. japonicus strains showed a higher percentage of mannose (ranging from 50% to 55%) and lower percentage of galactose (ranging from 33% to 34%) with respect to S. pombe (ranging from 44% to 47% of mannose and ranging from 36% to 45%, of galactose). The mannose/galactose ratio was higher in the S. japonicus strains (1.45-1.66) versus S. pombe (0.98-1.24). Moreno et al. (1990), during the purification and characterization of the glycoprotein invertase from S. pombe, found equimolar amounts of mannose and galactose consistent with our data. Only yeasts belonging to Schizosaccharomyces genus have been reported to have galactomannans located in the outer layer of the cell wall, which is composed of galactomannan, 9 –14%; alkali-soluble α-1,3- linked glucans, 18–28%; alkali-soluble β-1,3-linked glucans, 24%; β-1,6-linked glucans, 2%; alkali-insoluble β-1,3- linked glucans, 18% (Manners and Meyer, 1977).
Figure 8.
Sugar composition (■ glucose, ■ galactose, ■ mannose,
 glucosamine) and protein
glucosamine) and protein
 contents of polysaccharides released by Schizosaccharomyces strains after 14 days of alcoholic fermentation. Data are representative of two independent experiments. Error bars represent standard deviation of two independent experiments, each carried out in duplicate
contents of polysaccharides released by Schizosaccharomyces strains after 14 days of alcoholic fermentation. Data are representative of two independent experiments. Error bars represent standard deviation of two independent experiments, each carried out in duplicate
The percentage of glucose on the polysaccharides of all of the Schizosaccharomyces strains ranged from 9.4% and 15%, with exception of strain #7, which showed the highest percentage of glucose (18.4%). The percentage of glucosamine ranged from 0.26 % to 0.34 % for all Schizosaccharomyces strains, again with the exception of S. pombe strain # 7 (0.60%). Glucosamine likely derived from acid hydrolysis of chitin (Hardy et al., 1988), a long-chain polymer of N-Acetylglucosamine, which has been shown to be present, in small quantity (0.5%), in the S. pombe cell wall (Sietsma and Wessels, 1990).
The protein percentage of the polysaccharide was lower in all of the S. pombe strains (ranging from 0.7% to 1.21%), with the exception of the strain # 7 (2.28%) as compared to the three S. japonicus yeast strains (ranging from 1.57% to 1.82%). In any case the protein concentration of the glycoprotein component was lower than that of the strain EC1118 of S. cerevisiae (3.68%), as previously reported (Domizio et al., 2014). These results, besides confirming the difference between the two yeast species, highlight the different features of S. pombe strain #7 with respect to the other S. pombe strains evaluated. The higher percentage of glucose of this strain could be due to a higher release of a small part of its inner layer of the cell wall composed of β (1-3), β (1-6), and α (1-3) glucans (Pérez and Ribas, 2004), or to a different cell wall architecture.
The analysis of the released N-glycans (now oligosaccharides) by MALDI-TOF (Fig. 9) provided a snapshot of the most likely composition of the complex polysaccharides present. The S. pombe strains, showed a core of N-glycans with mass ranging from 9 up to 15 hexose residues. This is consistent with the reported structure of S. pombe galactomannoproteins, containing a heterogeneous small “core” oligosaccharide fraction linked to asparagine with sugar compositions that range from Man9(GlcNAc)2 to Gal4Man10(GlcNAc)2, (Gal: Galactose, Man: Mannose, GlcNAc: N-Acetylglucosamine) (Ballou et al., 1994). These compounds represent the smaller members of a series of a much larger N-linked glycans that have been shown to have an average composition of (Gal)52(Man)64(GlcNAc)2 (Gemmill and Trimble, 1996). The smallest glycan detected in the present study among the S. pombe strains, with the exception of the strain #7, was (Hex)9(GlcNAc)2. This is in agreement with previous work reporting that S. pombe, unlike S. cerevisiae, lacks an endoplasmic reticulum Man9-α1,2-mannosidase and therefore is not able to trim Man9GlcNAc2 to Man8GlcNAc2 prior to incorporation into hybrid and complex sugars (Gemmill and Trimble, 1996; Ziegler et al., 1994). Man9-α1,2-mannosidase is an important enzyme involved in glycoprotein quality control and is important for the targeting of misfolded glycoprotein for degradation. However, the presence of a smaller glycan (Hex)8(GlcNAc)2 found in S. pombe #7, is particularly interesting and could confirm what Movsichoff et al. (2005) reported that S. pombe yeast exhibits little α-mannosidase activity against misfolded glycoproteins.
Figure 9.
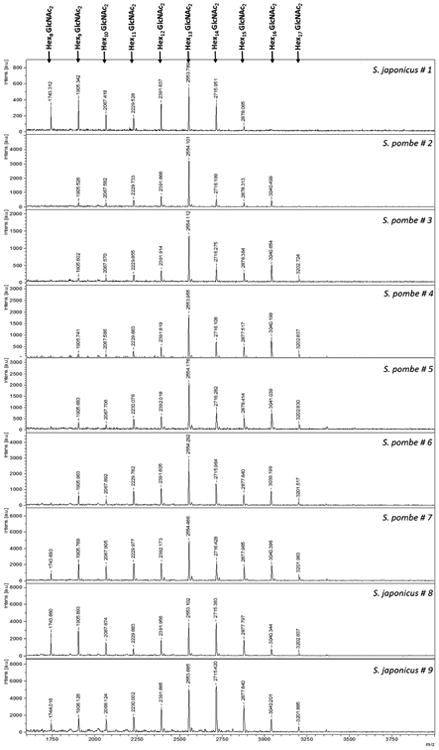
MALDI-TOF-MS profiles of N-Glycans prepared by PNGase F digestion. Digestion was conducted on the proteins released by each yeast strain at the end of the alcoholic fermentation. The chromatograms are representative of the analyses performed on each sample in triplicate from biological duplicate extractions. GlcNAc: N-Acetylglucosamine
In contrast to S. pombe, but in common with the budding yeasts such as S. cerevisiae, a smaller glycan unit (Hex)8(GlcNAc)2 was present in the polysaccharides of the three strains of S. japonicus. This is a unique feature among the eukaryotes (Gemmill and Trimble, 1996) and is consistent with our previous results (Domizio et al., 2014) where all the non-Saccharomyces yeasts displayed glycan (Hex)8(GlcNAc)2 as the smallest glycan unit.
The structure of the glycoproteins released by Schizosaccharomyces yeasts in the media are expected to be similar to that of the yeast cell wall, as has been observed for glycoproteins released into the wine for S. cerevisiae (Llaubères et al., 1987). An intraspecific similarity of the glycans profile, with comparable intensity for some of the glycans within each yeast species, was seen (Fig. 9). This observation suggests that rapid glycan fingerprint by MALDI-TOF could potentially be used as a taxonomic tool for these yeasts. Different N-glycan chains profiles were obtained from eight non-Saccharomyces yeasts, each belonging to different genera (Domizio et al., 2014). A different cell wall composition at the genera, species, and strain level has been reported (Ballou, 1976).
4. Conclusion
Given the ability of Schizosaccharomyces yeasts to release polysaccharides during the alcoholic fermentation, further research on strain diversity among Schizosaccharomyces yeasts is necessary. Indeed, Jeffares et al. (2015) showed that strains of S. pombe harbor genomes with an average nucleotide diversity on the same order of magnitude as that previously determined for S. cerevisiae (Schacherer et al. 2009). Particular attention should be given to the species S. japonicus. To our knowledge, use of S. japonicus in winemaking has not previously been proposed. S. japonicus was isolated and identified as a new species of fission yeast by Yukawa and Maki (1931). Since then, differences of some physiological features with respect to other fission yeasts have been reported (Hironori, 2014). In particular, S. japonicus yeasts present a larger cell size, different chromosomal behavior during cell division and hyphal growth. Our results highlight differences between S. pombe and S. japonicus. The main differences found in the present study are the fermentation performances, the quantity of polysaccharide released and, particularly interesting, the different ability to trim Man9GlcNAc2 to Man8GlcNAc2 prior to incorporation into hybrid and complex sugars. However, further studies are necessary in order to confirm this ER-specific mannosidase activity, here observed for all the three strains of S. japonicus.
Importantly, the release of high quantity polysaccharides in the media, in particular galactomannoproteins, renders these yeasts particularly interesting for the industrial production of exogenous polysaccharide preparations that could then be purified and used not only for winemaking purposes but also as novel functional foods.
To date the galactomannans polysaccharides commercially available are obtained from the endosperm of plant seeds, and have found many applications as in the pharmaceutical, biomedical, cosmetics, and food industries (Silveira and Bresolin, 2011; Srivastava and Kapoor, 2005). Microbial galactomannans represent a small fraction, mainly because their higher production cost if compared to the inexpensive plant based-products. However, fission yeasts, besides being non-pathogenic, could represent an interesting alternative to plant based products because the protein glycosylation occurs through a mechanism more related to that found in animal cells, generating hybrid-type oligosaccharides in glycoproteins, compared to that found in S. cerevisiae, which generates glycoproteins with high-mannose-type oligosaccharides (Kornfeld and Kornfeld, 1985; Ziegler, et al., 1994). In addition, the possibility to recover these compounds directly from the media could make these yeasts even more convenient from a production perspective for potential use as prebiotics as well. Many processes for the isolation and purification of polysaccharide have been developed. Some of them use enzymatic treatments in order to release the polysaccharide from the cell wall. Others use acids, hot alkali or a combination of both, which solubilize proteins and other polysaccharides. In this case, the acidic or alkaline conditions could lead to a more or less strong degradation of the sugar chains. Because the bioactivities of glycoprotein are significantly affected by structural features and molecular weight (Young et al., 1998) and, in turn, the structural features are affected by the extraction method, the possibility to keep the native structure of the glucan, avoiding drastic, glucan-destroying conditions, eliminating the necessity for cell wall enzymatic treatment, and maintaining at the same time an efficient yield of glycoproteins, could represent distinct advantages.
Highlights.
We evaluated the ability of nine Schizosaccharomyces yeast strains to release polysaccharides during the alcoholic fermentation
Schizosaccharomyces yeasts showed higher capacity to release polysaccharides as compared to S. cerevisiae
S. japonicus strains released higher quantity of polysaccharide with respect to S. pombe strains
Polysaccharides analysis showed divergence between S. pombe and S. japonicus
An intraspecific similarity of the glycans profile was observed
Rapid glycan fingerprint by MALDI TOF could be used as a taxonomic tool for these yeasts
Acknowledgments
This work was supported in part by the National Institutes of Health (NIH) award R01 AT008759-03
The authors acknowledge technical support from C.M. Lucy Joseph, Carolyn Doyle, Dr. Richard Jeannotte and Dr. Annabelle LeParc Le Bouedec.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14:93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Ballou CE, Ballou L, Ball G. Schizosaccharomyces pombe glycosylation mutant with altered cell surface properties. Proc Natl Acad Sci USA. 1994;91:9327–31. doi: 10.1073/pnas.91.20.9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito S, Palomero F, Morata A, Calderón F, Suárez-Lepe JA. New applications for Schizosaccharomyces pombe in the alcoholic fermentation of red wines. Int J Food Sci Tech. 2012;47:2101–2108. doi: 10.1111/j.1365-2621.2012.03076. [DOI] [Google Scholar]
- Benito S, Palomero F, Calderón F, Palmero D, Suárez-Lepe JA. Selection of appropriate Schizosaccharomyces strains for winemaking. Food Microbiol. 2014;42:218–24. doi: 10.1016/j.fm.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Benito Á, Calderón F, Palomero F, Benito S. Combine Use of Selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecule. 2015;20(6):9510–23. doi: 10.3390/molecules20069510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito Á, Jeffares D, Palomero F, Calderón F, Bai F, Bähler J, Benito S. Selected Schizosaccharomyces pombe strains have characteristics that are beneficial for winemaking. PLoS ONE. 2016;11(3):e0151102. doi: 10.1371/journal.pone.0151102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordiga M, Travaglia F, Meyrand M, German JB, Lebrilla CB, Coisson JD, Arlorio M, Barile D. Identification and Characterization of Complex Bioactive Oligosaccharides in White and Red Wine by a Combination of Mass Spectrometry and Gas Chromatography. J Agric Food Chem. 2012;60:3700–3707. doi: 10.1021/jf204885s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown SL, Stockdale VJ, Pettolino F, Pocock KF, de Barros Lopes M, Williams PJ, Bacic A, Fincher GB, Høj PB, Waters EJ. Reducing haziness in white wine by overexpression of Saccharomyces cerevisiae genes YOL155c and YDR055w. Appl Microbiol Biotechnol. 2007;73:1363–76. doi: 10.1007/s00253-006-0606-0. [DOI] [PubMed] [Google Scholar]
- Calleja GB, Johnson BF. Flocculation in a fission yeast: an initial step in the conjugation process. Can J Microbiol. 1971;17:1175–1177. doi: 10.1139/m71-187. [DOI] [PubMed] [Google Scholar]
- Calleja GB, Yoo BY, Johnson BF. Fusion and erosion of cell walls during confugation in the fussion yeast (Schizosaccharomyces pombe) J Cell Sci. 1977;25:139–155. doi: 10.1242/jcs.25.1.139. [DOI] [PubMed] [Google Scholar]
- Chalier P, Angot B, Delteil D, Doco T, Gunata Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007;100:22–30. doi: 10.1016/j.foodchem.2005.09.004. [DOI] [Google Scholar]
- Ciani M. Continuous deacidification of wine by immobilized Schizosaccharomyces pombe cells: evaluation of malic acid degradation rate and analytical profiles. J App Bacteriol. 1995;79:631–634. [Google Scholar]
- Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Cortés JCG, Sato M, Muñoz J, Belén Moreno M, Clemente-Ramos JA, Ramos M, Okada H, Osumi M, Durán A, Ribas JC. Fission yeast Ags1 confers the essential septum strength needed for safe gradual cell abscission. J Cell Biol. 2012;198:637–656. doi: 10.1083/jcb.201202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Dedo JE, Duenñas E, Arnáiz Y, Del Rey F, De Aldana CRV. β-glucanase Eng2 is required for ascus wall endolysis after sporulation in the fission yeast Schizosaccharomyces pombe. Eukaryot Cell. 2009;8:1278–1286. doi: 10.1128/EC.00148-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker N, van Rijssel J, Distel B, Hochstenbach F. Role of the alpha-glucanase Agn2p in ascus-wall endolysis following sporulation in fission yeast. Yeast (Chichester, England) 2007;24:279–88. doi: 10.1002/yea.1464. [DOI] [PubMed] [Google Scholar]
- Dekker N, Speijer D, Grün CH, van den Berg M, de Haan A, Hochstenbach F. Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol Biol Cell. 2004;15:3903–14. doi: 10.1091/mbc.E04-04-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmadhikari MR, Wilker KL. Deacidification of high malate must with Schizosaccharomyces pombe. Am J Enol Vitic. 1998;49:408–412. [Google Scholar]
- Domizio P, Liu Y, Bisson LF, Barile D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014;43:5–15. doi: 10.1016/j.fm.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Domizio P, Romani C, Comitini F, Gobbi M, Lencioni L, Mannazzu I, Ciani M. Potential spoilage non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Ann Microbiol. 2011a;61:137–144. doi: 10.1007/s13213-010-0125-1. [DOI] [Google Scholar]
- Domizio P, Romani C, Lencioni L, Comitini F, Gobbi M, Mannazzu I, Ciani M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int J Food Microbiol. 2011b;147:170–180. doi: 10.1016/j.ijfoodmicro.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Dupin IVS, McKinnon BM, Ryan C, Boulay M, Markides aJ, Jones GP, Williams PJ, Waters EJ. Saccharomyces cerevisiae mannoproteins that protect wine from protein haze: Their release during fermentation and lees contact and a proposal for their mechanism of action. J Agric Food Chem. 2000;48:3098–3105. doi: 10.1021/jf0002443. [DOI] [PubMed] [Google Scholar]
- Escot S, Feuillat M, Dulau L, Charpentier C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust J Grape Wine Res. 2001;7:153–159. doi: 10.1111/j.1755-0238.2001.tb00204. [DOI] [Google Scholar]
- Fleet GH. Cell wall. In: Rose AH, Harrison JS, editors. The Yeasts;Yeast Organelles. Vol. 4. Academic Press; London: 1991. pp. 199–277. [Google Scholar]
- François JM. A simple method for quantitative determination of polysaccharides in fungal cell walls. Nat Protoc. 2006;1:2995–3000. doi: 10.1038/nprot.2006.457. [DOI] [PubMed] [Google Scholar]
- Fuster A, Escot S. Élevage des vins rouges sur lies fines: Choix de la levure fermentaire et ses conséquences sur les interactions polysaccharydes pariétaux/polyphénols. Rev Des Oenol. 2002;104:20–22. [Google Scholar]
- Gao C, Fleet GHH. Degradation of Malic and Tartaric-Acids by High-Density Cell-Suspensions of Wine Yeasts. Food Microbiol. 1995;12:65–71. doi: 10.1016/S0740-0020(95)80080-8. [DOI] [Google Scholar]
- García I, Jiménez D, Martín V, Durán A, Sánchez Y. The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol Cell. 2005;97:569–76. doi: 10.1042/BC20040096. [DOI] [PubMed] [Google Scholar]
- Gemmill TR, Trimble RB. Schizosaccharomyces pombe produces novel pyruvate-containing N-Linked oligosaccharides. J Biol Chem. 1996;271:25945–25949. doi: 10.1074/jbc.271.42.25945. [DOI] [PubMed] [Google Scholar]
- Gerbaud V, Gabas N, Bloiun J, Pellerin P, Moutounet M. Influence of wine polysaccharides and polyphenols on the crystallisation of potassium hydrogen tartrate. J Inter Sci Vigne Vin. 1997;31:65–83. [Google Scholar]
- Giovani G, Rosi I, Bertuccioli M. Quantification and characterization of cell wall polysaccharides released by non-Saccharomyces yeast strains during alcoholic fermentation. Int J Food Microbiol. 2012;160:113–118. doi: 10.1016/j.ijfoodmicro.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Gobbi M, Comitini F, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013;33:271–281. doi: 10.1016/j.fm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ramos D, Cebollero E, Gonzalez R. A recombinant Saccharomyces cerevisiae strain overproducing mannoproteins stabilizes wine against protein haze. App Environ Microb. 2008;74:5533–5540. doi: 10.1128/AEM.00302-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe Z, Ayestarán B. Polysaccharide profile and content during the vinification and aging of tempranillo red wines. J Agric Food Chem. 2007;55:10720–10728. doi: 10.1021/jf0716782. [DOI] [PubMed] [Google Scholar]
- Guadalupe Z, Ayestarán B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and color composition in red wines. J Agric Food Chem. 2008;56:9022–9. doi: 10.1021/jf801535k. [DOI] [PubMed] [Google Scholar]
- Guadalupe Z, Martínez L, Ayestarán B. Yeast mannoproteins in red winemaking: Effect on polysaccharide, polyphenolic, and color composition. Am J Enol Vitic. 2010;61:191–200. [Google Scholar]
- Hardy MR, Townsend RR, Lee YC. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988;170:54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- Herrero E, Sanz P, Sentandreu R. Cell wall proteins liberated by Zymolyase from several ascomycetous and imperfect yeasts. J Gen Microbiol. 1987;133:2895–2903. [Google Scholar]
- Hironori N. Schizosaccharomyces japonicus: the fission yeast is a fusion of yeast and hyphae. Yeast. 2014;31:83–90. doi: 10.1002/yea.2996. [DOI] [PubMed] [Google Scholar]
- Horisberger M, Rouvet-Vauthey M. Cell wall architecture of the fission yeast Schizosaccharomyces pombe. Experientia. 1985;41:748–750. [Google Scholar]
- Jeffares DC, et al. The genomic and phenotypic diversity of Schizosaccharomyces pombe. Nat Genet. 2015;47:235–241. doi: 10.1038/ng.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BF, Yoo BY, Calleja GB. Cell division in yeasts: movement of organelles associated with cell plate growth of Schizosaccharomyces pombe. J Bacteriol. 1973;115:358–366. doi: 10.1128/jb.115.1.358-366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJ. Schizosaccharomyces japonicus yeast poised to become a favorite experimental organism for eukaryotic research. G3. 2013;3:1869–1873. doi: 10.1534/g3.113.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecká M, Fleet GH, Phaff HJ. Ultrastructure of the cell wall of Schizosaccharomyces pombe following treatment with various glucanases. J Struct Biol. 1995;114(2):140–52. doi: 10.1006/jsbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu rev biochem. 1985;54:631–64. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Llaubères RM, Dubourdieu D, Villetaz JC. Exocellular polysaccharides from Saccharomyces in wine. J Sci Food Agr. 1987;41:277–280. [Google Scholar]
- Lubbers S, Charpentier C, Feuillat M, Voilley A. Influence of yeast walls on the behavior of aroma compounds in a model wine. Am J Enol Vitic. 1994;45:29–33. [Google Scholar]
- Magyar I, Panyik I. Biological deacidification of wine with Schizosaccharomyces pombe entrapped in Ca-alginate gel. Am J Enol Vitic. 1989;40:233–240. [Google Scholar]
- Manners DJ, Meyer MT. The molecular structure of some glucans from the cell walls of Schizosaccharomyces pombe. Carbohyd Res. 1977;57:189–203. 51. [Google Scholar]
- Moine-Ledoux V, Dubourdieu D. An invertase fragment responsible for improving the protein stability of dry white wines. J Sci Food Agr. 1999;79:537–543. [Google Scholar]
- Morata A, Gómez-Cordovés MC, Colomo B, Suárez JA. Pyruvic Acid and Acetaldehyde Production by Different Strains of Saccharomyces cerevisiae: Relationship with Vitisin A and B Formation in Red Wines. J Agric Food Chem. 2003;51:7402–7409. doi: 10.1021/jf0304167. [DOI] [PubMed] [Google Scholar]
- Moreno S, Sanchez Y, Rodriguez L. Purification and characterization of the invertase from Schizosaccharomyces pombe. A comparative analysis with the invertase from Saccharomyces cerevisiae. Biochem J. 1990;267:697–702. doi: 10.1042/bj2670697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsichoff F, Castro OA, Parodi AJ. Characterization of Schizosaccharomyces pombe ER alpha-mannosidase: a reevaluation of the role of the enzyme on ER-associated degradation. Mol Biol Cell. 2005;16:4714–24. doi: 10.1091/mbc.E05-03-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon JR, Nagel CW. Comparison of methods of deacidification of musts and wines. Am J Enol Vitic. 1977;28:79–87. [Google Scholar]
- Palomero F, Morata A, Benito S, Calderón F, Suárez-Lepe JA. New genera of yeasts for over-lees aging of red wine. Food Chem. 2009;112:432–441. doi: 10.1016/j.foodchem.2008.05.098. [DOI] [Google Scholar]
- Peinado RA, Moreno JJ, Maestre O, Ortega JM, Medina M, Mauricio JC. Gluconic acid consumption in wines by Schizosaccharomyces pombe and its effect on the concentrations of major volatile compounds and polyols. J Agric Food Chem. 2004;52(3):493–497. doi: 10.1021/jf035030a. [DOI] [PubMed] [Google Scholar]
- Peinado RA, Moreno JJ, Maestre O, Mauricio JC. Removing gluconic acid by using different treatments with a Schizosaccharomyces pombe mutant: Effect on fermentation byproducts. Food Chem. 2007;104:457–465. [Google Scholar]
- Peinado RA, Maestre O, Mauricio JC, Moreno JC. Use of Schizosaccharomyces pombe mutant to reduce the content in gluconic acid of must obtained from rotten grapes. J Agric Food Chem. 2009;57:2368–2377. doi: 10.1021/jf803479r. [DOI] [PubMed] [Google Scholar]
- Pérez P, Ribas JC. Cell wall analysis. Methods. 2004;33:245–251. doi: 10.1016/j.ymeth.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Peyron D, Boukharta M, Cuby A, Feuillat M. Dosage des polysaccharides dans les vins rouges: intéractions avec les composés phénoliques. Sci Aliment. 1993;13:761–767. [Google Scholar]
- Poncet-Legrand C, Doco T, Williams P, Vernhet A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. Am J Enol Vitic. 2007;58(1):87–91. [Google Scholar]
- Pozo-Bayón MA, Andújar-Ortiz I, Moreno-Arribas MV. Scientific evidences beyond the application of inactive dry yeast preparations in winemaking. Food Res Int. 2009;42:754–761. doi: 10.1016/j.foodres.2009.03.004. [DOI] [Google Scholar]
- Quijada-Morín N, Williams P, Rivas-Gonzalo JC, Doco T, Escribano-Bailón MT. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chem. 2014;154:44–51. doi: 10.1016/j.foodchem.2013.12.101. [DOI] [PubMed] [Google Scholar]
- Rankine BC. Decomposition ofL-malic acid by wine yeasts. J Sci Food Agr. 1966;17:312–316. doi: 10.1002/jsfa.2740170707. [DOI] [PubMed] [Google Scholar]
- Riou V, Vernhet A, Doco T, Moutounet M. Aggregation of grape seed tannins in model wine –effect of wine polysaccharides. Food Hydrocolloids. 2002;16(1):17–23. [Google Scholar]
- Romani C, Domizio P, Lencioni L, Gobbi M, Comitini F, Ciani M, Mannazzu I. Polysaccharides and glycerol production by non-Saccharomyces wine yeasts in mixed fermentation. Quad Vitic Enol Univ Torino. 2010;31:185e189. 2009-2010. [Google Scholar]
- Rosi I, Gheri A, Domizio P, Fia G. Production de macromolecules pariétales de Saccharomyces cerevisiae au cours de la fermentation et leur influence sur la fermentation malolactique. Rev Des Oenol. 2000;94:18–20. [Google Scholar]
- Rosi I, Gheri A, Ferrari S. Effets des levures produisant des polysaccharides pariétaux sur certain caractéristiques des vins rouges pendant la fermentation. Rev Des Oenol. 1998;172:24–26. [Google Scholar]
- Schacherer J, Shapiro JA, Ruderfer DM, Kruglyak L. Comprehensive polymorphism survey elucidates population structure of Saccharomyces cerevisiae. Nature. 2009;458(7236):342–345. doi: 10.1038/nature07670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sietsma JH, Wessels JGH. The occurrence of glucosamino-glycan in the wall of Schizosaccharomyces pombe. J Gen Microbiol. 1990;136:2261–2265. doi: 10.1099/00221287-136-11-2261. [DOI] [PubMed] [Google Scholar]
- Silva S, Ramón-Portugal F, Andrade P, Texera M, Strehaino P. Malic acid consumption by dry inmobilized cells of Schizosaccharomyces pombe. Am J Enol Vitic. 2003;54:50–55. [Google Scholar]
- Silveira J, Bresolin T. Pharmaceutical use of galactomannans. Química Nova. 2011;34:292–299. doi: 10.1590/S0100-40422011000200023. [DOI] [Google Scholar]
- Sipiczki M. Splitting of the fission yeast septum. FEMS Yeast Res. 2007;7:761–70. doi: 10.1111/j.1567-1364.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- Snow PG, Gallander JF. Deacidification of white table wines through partial fermentation with Schizosaccharomyces pombe. Am J Enol Vitic. 1979;30:45–48. [Google Scholar]
- Spiropoulos A, Tanaka J, Flerianos I, Bisson LF. Characterization of hydrogen sulfide formation in commercial and natural wine isolates of Saccharomyces. Am J Enol Vitic. 2000;51:233–248. [Google Scholar]
- Srivastava M, Kapoor VP. Seed galactomannans: an overview. Chem Biodivers. 2005;2:295–317. doi: 10.1002/cbdv.200590013. [DOI] [PubMed] [Google Scholar]
- Taillandier P, Gilis M, Strehaino P. Deacidification by Schizosaccharomyces: interactions with Saccharomyces. J Biotechnol. 1995;40:199–205. [Google Scholar]
- Tanaka K, Hirata A. Ascospore development in the fission yeasts Schizosaccharomyces pombe and S. japonicus. J Cell Sci. 1982;56:263–279. doi: 10.1242/jcs.56.1.263. [DOI] [PubMed] [Google Scholar]
- Thornton RJ, Rodriguez SB. Deacidification of red and white wines by a mutant of Schizosaccharomyces malidevorans under commercial winemaking conditions. Food Microbiol. 1996;13:475–482. [Google Scholar]
- Tsai CS, Ye HG, Shi JL. Carbon-13 NMR studies and purification of gluconate pathway enzymes from Schizosaccharomyces pombe. Arch Biochem Biophys. 1995;316:155–162. doi: 10.1006/abbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- Vanrell G, Canals R, Esteruelas M, Fort F, Canals JM, Zamora F. Influence of the use of bentonite as a riddling agent on foam quality and proteinfraction of sparkling wines (Cava) Food Chem. 2007;104:148–155. [Google Scholar]
- Vidal S, Francis L, Williams P, Kwiatkowski M, Gawel R, Cheynier V, Waters EJ. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004;85(4):519–525. [Google Scholar]
- Waters EJ, Pellerin P, Brillouet JMA. Saccharomyces mannoprotein that protects wine from protein haze. Carbohyd Polym. 1994;23:185–191. [Google Scholar]
- Yokotsuka K, Otaky A, Naitoh A, Tanaka H. Controlled simultaneous deacidification and alcohol fermentation of high-acid grape must using two immobilized yeasts, Schizosaccharomyces pombe and Saccharomyces cerevisiae. Am J Enol Vitic. 1993;44:371–377. [Google Scholar]
- Young M, Davies MJ, Bailey D, Gradwell MJ, Smestad-Paulsen B, Wold JK, Barnes RM, Hounsell EF. Characterization of oligosaccharides from an antigenic mannan of Saccharomyces cerevisiae. Glycoconjugate J. 1998;15:815–22. doi: 10.1023/a:1006968117252. [DOI] [PubMed] [Google Scholar]
- Yukawa M, Maki T. Schizosaccharomyces japonicus nov. sp La Bul Sci Fakultat Terkultura Kjusu Imp Univ Fukuoka Japan. 1931;4:218–226. [Google Scholar]
- Ziegler FD, Gemmill TR, Trimble RB. Glycoprotein synthesis in yeast. Early events in N-linked oligosaccharide processing in Schizosaccharomyces pombe. J Biol Chem. 1994;269:12527–12535. [PubMed] [Google Scholar]