Abstract
Aim: Both vascular function and structure are independent predictors of cardiovascular events. The purpose of this study was to evaluate vascular function and structure of a leg artery in patients with peripheral artery disease (PAD).
Methods: We measured flow-mediated vasodilatation (FMD) and nitroglycerine-induced vasodilation (NID) as indices of vascular function and intima-media thickness (IMT) as an index of vascular structure of the popliteal artery in 100 subjects, including 20 patients with Buerger disease and 30 patients with atherosclerotic PAD, 20 age- and sex-matched subjects without Buerger disease (control group) and 30 age- and sex-matched patients without atherosclerotic PAD (control group).
Results: IMT was significantly larger in the Buerger group than in the control group (Buerger, 0.63 ± 0.20 mm; control, 0.50 ± 0.07 mm; P = 0.01), whereas there were no significant differences in FMD and NID between the two groups. IMT was significantly larger in the atherosclerotic PAD group than in the control group (atherosclerotic PAD, 0.80 ± 0.22 mm; control, 0.65 ± 0.14 mm; P < 0.01), and FMD and NID were significantly smaller in the atherosclerotic PAD group than in the control group (FMD: atherosclerotic PAD, 3.9% ± 1.1%; control, 5.0% ± 1.8%; P < 0.01; and NID: atherosclerotic PAD, 6.1% ± 2.0%; control, 8.4% ± 2.1%; P < 0.01).
Conclusion: These findings suggest that vascular function is preserved in patients with Buerger disease and that both vascular function and vascular structure are impaired in patients with atherosclerotic PAD.
Keywords: Peripheral artery disease; Buerger disease; Flow-mediated vasodilatation; Nitroglycerine-induced vasodilation, Intima-media thickness
See editorial vol. 23: 1255–1256
Introduction
It is well known that patients with peripheral artery disease (PAD) have a markedly increased risk for cardiovascular events and advanced atherosclerotic disease1–3). However, mortality and morbidity rates of cardiovascular disease are not constant in PAD. Interestingly, the prevalence of cardiovascular events and any cause of death are significantly higher in patients with atherosclerotic PAD than in patients with Buerger disease, and the mortality rate in patients with Buerger disease is not higher than that in age-matched control subjects1, 4, 5).
Intima-media thickness (IMT) is an established index of structural change of an artery. IMT, particularly that of the carotid, is associated with the presence of cardiovascular risk factors6, 7) and is an independent predictor of cardiovascular events8–10). Recently, we have shown that IMT of the brachial artery also serves as a surrogate marker for progression of atherosclerosis11). However, there is little information on endothelial function and IMT of a leg artery in patients with PAD, including Buerger disease.
Endothelial function is initially impaired with progression of atherosclerosis, leading to cardiovascular events12, 13). Evaluation of flow-mediated vasodilatation (FMD) and nitroglycerine-induced vasodilation (NID) is useful for assessing vascular function14–20). Previous studies have shown that endothelial function assessed by FMD can serve as an independent predictor of cardiovascular events21–25). Several studies have demonstrated that not only FMD but also NID is impaired in subjects with cardiovascular risk factors, patients with coronary vascular disease, and patients with PAD19, 20, 26–28). Recently, we demonstrated that NID decreased in relation to cumulative cardiovascular risk factors and was therefore significantly correlated with cardiovascular risk factors as well as FMD and that impaired NID was an independent variable for critical limb ischemia19, 28). Vascular function assessed by NID per se also should predict cardiovascular outcomes.
Although some studies have shown interrelations between endothelial function assessed by FMD and that assessed by IMT in a general population, FMD and IMT were examined at different vasculatures, such as the brachial artery and common carotid artery in those studies29–31). There is no information on differences in IMT, FMD and NID of the peripheral artery between Buerger disease and atherosclerotic PAD. Therefore, in the present study, we evaluated FMD, NID, and IMT in the same leg artery in patients with Buerger disease and patients with atherosclerotic PAD.
Methods
Subjects
A total of 100 subjects (mean age, 58.9 ± 14.1 years), including 20 patients with Buerger disease (18 men and two women, mean age: 48.9 ± 14.4 years), and 30 patients with atherosclerotic PAD (22 men and eight women, mean age: 69.5 ± 7.8 years), 20 age-and sex-matched subjects without Buerger disease (Buerger control group: 19 men and one woman, mean age: 43.0 ± 5.1 years), and 30 age- and sex-matched patients without atherosclerotic PAD (atherosclerotic PAD control group: 20 men and 10 women, mean age: 65.7 ± 7.5 years), were enrolled. Buerger disease was diagnosed by the previously reported criteria, including results of physical examinations, clinical symptoms, angiographic findings, and results of arterial duplex scanning32). To rule out other vasculitis and hypercoagulable states, rheumatoid factor, lupus anticoagulants, and serologic investigations were evaluated. Atherosclerotic PAD and Buerger disease were defined as current intermittent claudication with ankle-brachial index (ABI) < 0.9, or chronic ischemic rest pain, ischemic ulcers, or gangrene attributed to objectively proven arterial occlusive disease, or history of previous intervention including angioplasty, bypass graft, and limb amputation. Diagnosis of limb ischemia was confirmed by angiography. Control subjects were matched sex and age, and had no PAD. Hypertension was defined as treatment with oral antihypertensive agents or systolic blood pressure of more than 140 mmHg, or diastolic blood pressure of more than 90 mmHg, in a sitting position on at least three different occasions without medication33). Diabetes was defined according to the American Diabetes Association recommendation34). Dyslipidemia was defined according to the third report of the National Cholesterol Education Program35). We defined smokers as those who had ever smoked. Coronary heart disease included angina pectoris, myocardial infarction, and unstable angina. The vascular tests were performed without withholding medications. The ethical committees of our institutions approved the study protocol. Written informed consent for participation in the study was obtained from all subjects.
Study Protocol
We measured vascular responses to reactive hyperemia and sublingually administrated nitroglycerine in the popliteal artery without stenosis and in occluded arteries in subjects. Subjects fasted the previous night for at least 12 h. The study began at 8:30 AM. The subjects were kept in the prone position in a quiet, dark, air-conditioned room (constant temperature of 22°C–25°C) throughout the study. A 23-gauge polyethylene catheter was inserted into the left deep antecubital vein to obtain blood samples. Thirty minutes after maintaining the prone position, basal popliteal artery diameter and IMT were measured. Then, FMD was measured in the popliteal artery. After completion, we next measured NID with confirmation that the popliteal artery diameter had recovered to the baseline value. The observers were blind to the protocol of this study.
Measurement of Popliteal Artery IMT
The ultrasound unit Aloka-α7 (Aloka Co, Tokyo, Japan) equipped with a linear, phased-array high-frequency (13-MHz) transducer was used for scanning the popliteal artery. Ultrasound longitudinal images of the popliteal artery were acquired at the end of the diastole (defined as the R wave of an electrocardiogram), in which the far wall intima-media interface was clearly defined. The leading edge of the intima and the media-adventitia interface were traced as continuous lines. A total of 20 points over a 3-mm length of IMT in the 10-mm longitudinal image depicted in the analysis display were measured and the mean value per image was automatically calculated. IMT was measured at the same point in each different image. The average of mean values obtained from 10 cardiac cycles was defined as IMT of the popliteal artery. All of the duplex ultrasonography scans were performed by ultrasonograhic specialists.
The coefficient of variation for IMT was 4.9% in our laboratory.
Measurement of Vascular Function
A high-resolution linear artery transducer was coupled to computer-assisted analysis software (Aloka-α7, ALOKA Co., Tokyo, Japan) that used an automated edge detection system for measurement of artery diameter. A blood pressure cuff was placed around the thigh. The target artery was scanned longitudinally. When the clearest B-mode image of the intimal interfaces between the lumen and vessel wall was obtained, the transducer was held at the same point throughout the scan by a special probe holder (MP-PH0001, ALOKA Co.) to ensure consistency of the image. A baseline image was acquired and blood flow was estimated by time averaging the pulsed Doppler velocity signal obtained from a sample volume. Then the blood pressure cuff was inflated to 50 mm Hg above systolic pressure for 5 min. Depth and gain setting were set to optimize the images of the arterial lumen wall interface. When the tracking gate was placed on the intima, the artery diameter was automatically tracked and the waveform of diameter changes over the cardiac cycle was displayed in real time using the FMD mode of the tracking system. This allowed the ultrasound images to be optimized at the start of the scan and the transducer position to be adjusted immediately for optimal tracking performance throughout the scan. Pulsed Doppler flow was assessed at baseline and during peak hyperemic flow, which was confirmed to occur within 15 s after cuff deflation. Blood flow velocity was calculated from the color Doppler data and was displayed as a waveform in real time. The baseline longitudinal image of the artery was acquired for 30 s and then the blood pressure cuff was inflated to 50 mm Hg above systolic pressure for 5 min. The longitudinal image of the artery was recorded continuously until 5 min after cuff deflation. Pulsed Doppler velocity signals were obtained for 20 s at baseline and for 10 s immediately after cuff deflation. Changes in brachial artery diameter were immediately expressed as per cent change relative to the vessel diameter before cuff inflation. FMD was calculated as the per cent change in peak vessel diameter from the baseline value. %FMD [(peak diameter – baseline diameter)/baseline diameter] was used for analysis. Blood flow volume was calculated by multiplying the Doppler flow velocity (corrected for the angle) by heart rate and vessel cross-sectional area (-r2). Reactive hyperemia was calculated as the maximum percentage increase in flow after cuff deflation compared with baseline flow. After a 10-min period to allow baseline conditions of the artery to be reestablished, another baseline scan was performed.
The response to nitroglycerine was used for assessment of endothelium-independent vasodilation. NID was measured as described previously28). Briefly, after acquiring baseline rest images for 30 s, a sublingual tablet (75 µg nitroglycerine) was given, and images of the artery were recorded continuously until the dilation reached a plateau after administration of nitroglycerine. Subjects who had received nitrate treatment and subjects in whom the sublingually administered nitroglycerine tablet did not dissolved during the measurement, were excluded from this study. NID was automatically calculated as a per cent change in peak vessel diameter from the baseline value. Percentage of NID [(peak diameter – baseline diameter)/baseline diameter] was used for analysis.
The coefficient of variation for the baseline diameter was 2.9% in our laboratory.
Analytical Methods
Samples of venous blood were placed in tubes containing sodium ethylenediamine tetraacetic acid (1 mg/mL) and in polystyrene tubes. The ethylenediamine tetraacetic acid-containing tubes were chilled promptly in an ice bath. Plasma was immediately separated by centrifugation at 3100 g for 10 min at 4°C, and serum was separated by centrifugation at 1000 g for 10 min at room temperature. Samples were stored at −80°C until the time of assay. Serum concentrations of total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, glucose, and electrolytes were determined by routine chemical methods.
Statistical Analysis
Results are presented as the means ± standard deviation (SD) for continuous variables and as percentages for categorical variables. Statistical significance was set at a level of P < 0.05. Comparisons between groups were carried out using P for trend analysis. Unpaired Student's t-test was used for group comparisons. The data were processed using the software package Stata version 9 (Stata Co, College Station, TX, USA).
Results
Baseline Clinical Characteristics
The baseline clinical characteristics are summarized in Table 1. Of the 20 patients with Buerger disease, 18 (90.0%) were men and two (10.0%) were women; three (15.0%) had hypertension, four (20.0%) had dyslipidemia, one (5.0%) had diabetes mellitus, four (20.0%) were current smokers and none had previous coronary heart disease. Serum concentration of high-density lipoprotein cholesterol and ABI were significantly lower in the Buerger group than in the control group. There were no significant differences in other parameters between the two groups.
Table 1. Clinical Characteristics of The Subjects.
| Variable | Total (n = 100) | Buerger disease No (n = 20) | Buerger disease Yes (n = 20) | P value | Atherosclerotic PAD No (n = 30) | Atherosclerotic PAD Yes (n = 30) | P value |
|---|---|---|---|---|---|---|---|
| Age, yr | 58.9 ± 14.1 | 43.0 ± 5.1 | 48.9 ± 14.4 | 0.09 | 65.7 ± 7.5 | 69.5 ± 7.8 | 0.06 |
| Sex, men/women | 79/21 | 19/1 | 18/2 | 0.54 | 20/10 | 22/8 | 0.57 |
| Body mass index, kg/m2 | 22.7 ± 3.2 | 22.7 ± 3.0 | 22.4 ± 3.1 | 0.75 | 23.7 ± 3.1 | 21.9 ± 3.2 | 0.03 |
| Systolic blood pressure, mmHg | 126.8 ± 19.8 | 118.1 ± 8.7 | 117.2 ± 16.7 | 0.84 | 130.9 ± 17.8 | 134.8 ± 24.1 | 0.49 |
| Diastolic blood pressure, mmHg | 74.5 ± 11.9 | 74.7 ± 7.4 | 70.2 ± 13.2 | 0.20 | 77.6 ± 11.5 | 74.3 ± 13.3 | 0.31 |
| Heart rate, bpm | 72.5 ± 12.1 | 71.3 ± 9.9 | 68.8 ± 11.9 | 0.48 | 69.3 ± 10.5 | 79.0 ± 13.0 | < 0.01 |
| Total cholesterol, mg/dL | 172.8 ± 31.4 | 178.4 ± 18.8 | 171.3 ± 34.8 | 0.57 | 175.0 ± 20.1 | 170.3 ± 39.3 | 0.61 |
| Triglycerides, mg/dL | 116.1 ± 58.5 | 81.4 ± 26.9 | 125.9 ± 67.1 | 0.07 | 127.7 ± 67.3 | 110.2 ± 47.7 | 0.28 |
| High-density lipoprotein cholesterol, mg/dL | 56.0 ± 17.2 | 63.1 ± 20.5 | 49.0 ± 11.3 | 0.03 | 57.9 ± 12.7 | 56.8 ± 21.8 | 0.83 |
| Low-density lipoprotein cholesterol, mg/dL | 98.0 ± 25.5 | 100.0 ± 20.4 | 102.4 ± 29.0 | 0.83 | 100.1 ± 19.6 | 92.2 ± 29.3 | 0.27 |
| Glucose, mg/dL | 116.4 ± 35.6 | 97.9 ± 16.6 | 101.1 ± 20.4 | 0.69 | 114.6 ± 21.8 | 133.9 ± 48.6 | 0.07 |
| Ankle-brachial index | 1.00 ± 0.24 | 1.20 ± 0.05 | 0.82 ± 0.25 | < 0.01 | 1.15 ± 0.07 | 0.83 ± 0.21 | < 0.01 |
| Rutherford class | |||||||
| 0, n (%) | 55 (55.0) | 20 (100.0) | 4 (20.0) | < 0.01 | 30 (100.0) | 1 (3.3) | < 0.01 |
| 1, n (%) | 5 (5.0) | 0 (0.0) | 4 (20.0) | 0.01 | 0 (0.0) | 1 (3.3) | 0.24 |
| 2, n (%) | 6 (6.0) | 0 (0.0) | 2 (10.0) | 0.09 | 0 (0.0) | 4 (13.3) | 0.02 |
| 3, n (%) | 7 (7.0) | 0 (0.0) | 1 (5.0) | 0.23 | 0 (0.0) | 6 (20.0) | < 0.01 |
| 4, n (%) | 5 (5.0) | 0 (0.0) | 1 (5.0) | 0.23 | 0 (0.0) | 4 (13.3) | 0.02 |
| 5, n (%) | 19 (19.0) | 0 (0.0) | 7 (35.0) | < 0.01 | 0 (0.0) | 12 (40.0) | < 0.01 |
| 6, n (%) | 3 (3.0) | 0 (0.0) | 1 (5.0) | 0.23 | 0 (0.0) | 2 (6.7) | 0.09 |
| Fontaine class | |||||||
| Stage I, n (%) | 55 (55.0) | 20 (100) | 4 (20.0) | < 0.01 | 3 (100) | 1 (3.3) | < 0.01 |
| Stage II, n (%) | 18 (18.0) | 0 (0) | 7 (35.0) | < 0.01 | 0 (0) | 11 (36.6) | < 0.01 |
| Stage III, n (%) | 5 (5.0) | 0 (0) | 1 (5.0) | 0.23 | 0 (0) | 4 (13.3) | 0.02 |
| Stage IV, n (%) | 22 (22.0) | 0 (0) | 8 (40.0) | < 0.01 | 0 (0) | 14 (46.7) | < 0.01 |
| Medical history, n (%) | |||||||
| Hypertension | 57 (57.0) | 1 (5.0) | 3 (15.0) | 0.28 | 25 (83.3) | 28 (93.3) | 0.22 |
| Dyslipidemia | 32 (32.0) | 2 (10.0) | 4 (20.0) | 0.37 | 15 (50.0) | 11 (36.7) | 0.30 |
| Diabetes mellitus | 38 (38.0) | 0 (0.0) | 1 (5.0) | 0.23 | 17 (56.7) | 20 (66.7) | 0.43 |
| Previous coronary heart disease | 22 (22.0) | 0 (0.0) | 0 (0.0) | ND | 7 (23.3) | 15 (50.0) | 0.03 |
| Smoker (current), % | 20 (20.0) | 4 (20.0) | 4 (20.0) | 1.0 | 8 (26.7) | 4 (13.3) | 0.19 |
| Smoker (past), % | 49 (49.0) | 8 (40.0) | 15 (75.0) | 0.02 | 10 (33.3) | 16 (53.3) | 0.12 |
| Medications, n (%) | |||||||
| Calcium-channel blockers | 24 (24.0) | 0 (0.0) | 1 (5.0) | 0.23 | 14 (46.7) | 9 (30.0) | 0.18 |
| Renin angiotensin system inhibitors | 34 (34.0) | 1 (5.0) | 1 (5.0) | 1.0 | 14 (46.7) | 18 (60.0) | 0.30 |
| Statins | 28 (28.0) | 2 (10.0) | 3 (15.0) | 0.63 | 15 (50.0) | 8 (26.7) | 0.06 |
| Medically treated diabetes | 29 (29.0) | 0 (0.0) | 0 (0.0) | ND | 14 (46.7) | 15 (50.0) | 0.80 |
PAD indicates peripheral arterial disease; ND, not detected.
All results are presented as means ± SD.
Of the 30 subjects with atherosclerotic PAD, 22 (73.3%) were men and eight (26.7%) were women; 28 (93.3%) had hypertension, 11 (36.7%) had dyslipidemia, 20 (66.7%) had diabetes mellitus, four (13.3%) were current smokers, and 15 (50.0%) had previous coronary heart disease. Heart rate, serum concentration of glucose, and ratio of previous coronary heart disease were significantly higher in the atherosclerotic PAD group than in the atherosclerotic PAD control group. Body mass index and ABI were significantly lower in the atherosclerotic PAD group than in the atherosclerotic PAD control group. There were no significant differences in other parameters between the two groups.
Vascular Function and Structure in Patients with Buerger Disease and Patients with Atherosclerotic PAD
There were no significant differences in FMD and NID between the Buerger group and control group (FMD: Buerger group, 5.7% ± 1.8%; control group, 6.1% ± 0.9%; P = 0.40; and NID: Buerger group, 8.6% ± 3.6%; control group, 9.8% ± 1.7%; P = 0.19, Fig. 1). IMT was significantly larger in the Buerger group than in the control group (Buerger group, 0.63 ± 0.20 mm; control group, 0.50 ± 0.07 mm; P = 0.01, Fig. 2).
Fig. 1.
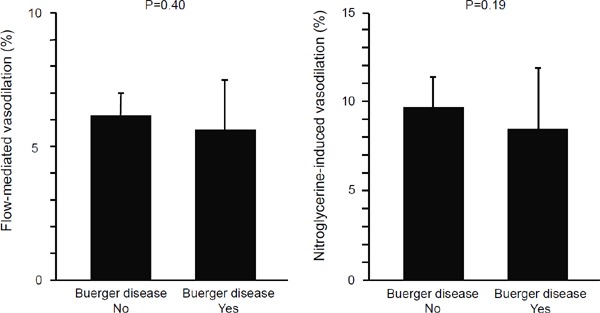
Bar graphs show flow-mediated vasodilation and nitroglycerine-induced vasodilation in patients with and without Buerger disease.
Fig. 2.
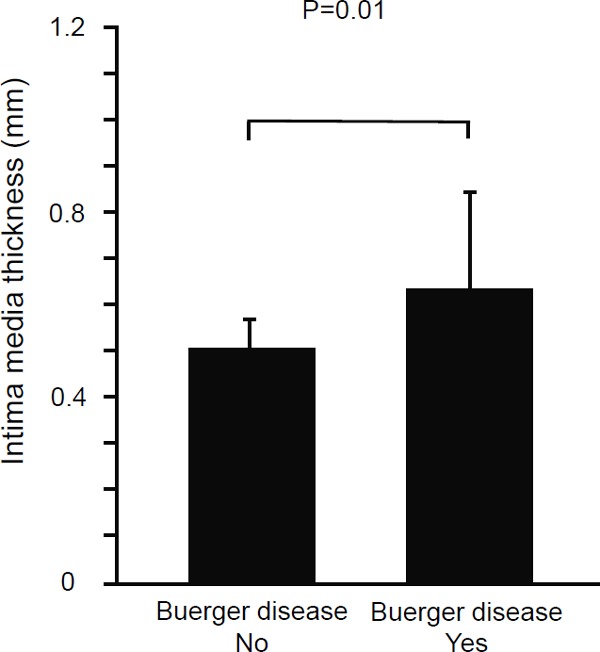
Bar graph shows the brachial intima-media thickness in patients with and without Buerger disease.
FMD and NID were significantly smaller in the atherosclerotic PAD group than in the control group (FMD: atherosclerotic PAD group, 3.9% ± 1.1%; control group, 5.0% ± 1.8%; P < 0.01 and NID: atherosclerotic PAD group, 6.1% ± 2.0%; control group, 8.4% ± 2.1%; P < 0.01, Fig. 3). IMT was significantly larger in the atherosclerotic PAD group than in the control group (atherosclerotic PAD group, 0.80 ± 0.22 mm; control group, 0.65 ± 0.14 mm; P < 0.01, Fig. 4).
Fig. 3.
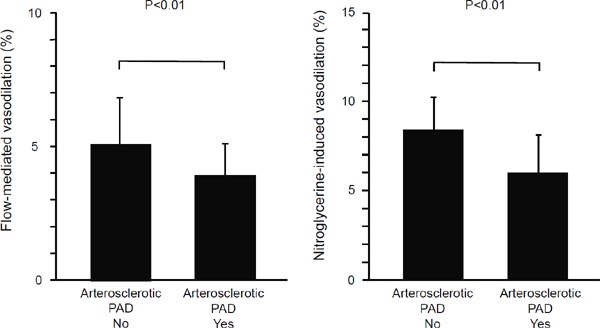
Bar graphs show flow-mediated vasodilation and nitroglycerine-induced vasodilation in patients with and without atherosclerotic peripheral arterial disease.
Fig. 4.
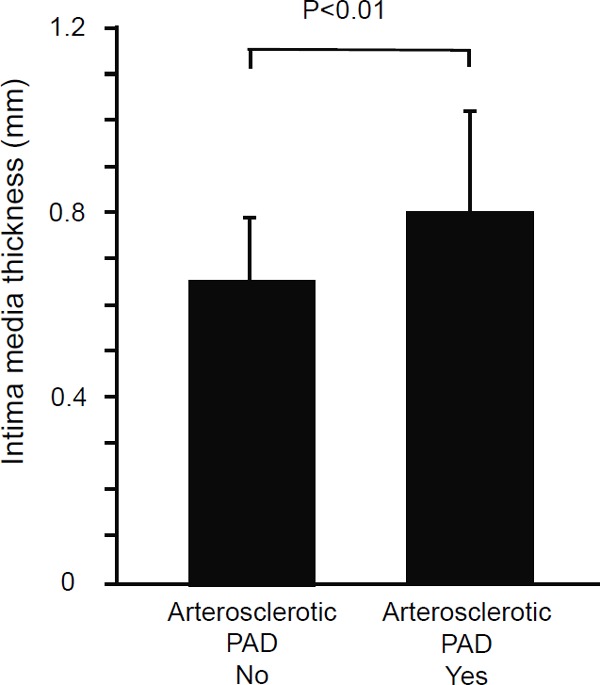
Bar graph shows the brachial intima-media thickness in patients with and without atherosclerotic peripheral arterial disease.
Discussion
In the present study, we demonstrated that IMT of the popliteal artery was increased both in patients with Buerger disease and patients with atherosclerotic PAD compared with those in control groups. FMD and NID of the popliteal artery were impaired in patients with atherosclerotic PAD but not in patients with Buerger disease. These findings suggest that vascular function of a leg artery is preserved in patients with Buerger disease and that both vascular function and vascular structure of a leg artery are impaired in patients with atherosclerotic PAD.
It is well known that patients with PAD, particularly atherosclerotic PAD, have a high prevalence of cardiovascular morbidity and mortality1, 2). Atherosclerosis progressively develops with aging in patients with atherosclerotic PAD. Endothelial dysfunction is the initial step in the pathogenesis of atherosclerosis, resulting in cardiovascular complications12, 13). Recently, we reported that both FMD and NID were impaired in patients with PAD19). In addition, brachial IMT was correlated with NID, suggesting that vascular smooth muscle function is also impaired in relation to increased brachial IMT11). In the present study, in the popliteal artery also, IMT was increased and FMD and NID were impaired in patients with atherosclerotic PAD, suggesting that atherosclerotic PAD has an advanced vascular failure. Severe vascular failure may contribute to the high cardiovascular morbidity and mortality rates in atherosclerotic PAD.
On the other hand, it has been reported that there is no significant difference in the rate of mortality between patients with Buerger disease and normal populations4, 5). Some investigators have reported that the survival rate of patients with Buerger disease is significantly lower than that in the general population4, 36). Cumulative survival rate was significantly higher in patients with Buerger disease than in patients with atherosclerotic PAD1, 5, 37). In the present study, vascular function, including endothelial function and vascular smooth muscle function, were not impaired in patients with Buerger disease. In addition, in a previous study, we have shown that confounding factors for endothelial function, such as oxidative stress markers, number of endothelial progenitor cells, and cell migration response to vascular endothelial growth factor, other than an inflammation marker, are similar in patients with Buerger disease and healthy controls38). Interestingly, IMT in the popliteal artery was larger in the Buerger group than in the control group, while IMT in the popliteal artery was smaller in the Buerger group than in the atherosclerotic PAD group. There has been no information on IMT in leg arteries of patients with Buerger disease. The precise reason for the increase in IMT in the popliteal artery in Buerger disease remains unclear.
In conclusion, patients with Buerger disease had normal vascular function but large IMT compared with that in age- and sex-matched controls, and patients with atherosclerotic PAD had abnormal vascular function and large IMT compared with that in age- and sex-matched controls. Our results may partially explain why there are differences in the rates of morbidity and mortality of cardiovascular diseases between patients with Buerger disease and patients with atherosclerotic PAD. Further studies are needed to evaluate vascular function and structure, and outcomes during a long-term follow-up period in patients with Buerger disease and patients with atherosclerotic PAD.
Acknowledgments
We thank Megumi Wakisaka, Ki-ichiro Kawano and Satoko Michiyama for their excellent secretarial assistance.
Sources of Founding
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (1859081500 and 21590898) and a Grant in Aid of Japanese Arteriosclerosis Prevention Fund.
Disclosures
None.
References
- 1). Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992; 326: 381-386 [DOI] [PubMed] [Google Scholar]
- 2). Steg PG, Bhatt DL, Wilson PW, D'Agostino R, Sr, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S. REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297: 1197-1206 [DOI] [PubMed] [Google Scholar]
- 3). Kojima I, Ninomiya T, Hata J, Fukuhara M, Hirakawa Y, Mukai N, Yoshida D, Kitazono T, Kiyohara Y. A low ankle brachial index is associated with an increased risk of cardiovascular disease: the Hisayama study. J Atheroscler Thromb. 2014; 21: 966-973 [DOI] [PubMed] [Google Scholar]
- 4). Ohta T, Shionoya S. Fate of the ischaemic limb in Buerger's disease. Br J Surg. 1988; 75: 259-262 [DOI] [PubMed] [Google Scholar]
- 5). Mills JL, Porter JM. Buerger's disease: a review and update. Semin Vasc Surg. 1993; 6: 14-23 [PubMed] [Google Scholar]
- 6). O'Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, Tracy R, Gardin JM, Price TR, Furberg CD. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke 1996; 27: 224-231 [DOI] [PubMed] [Google Scholar]
- 7). Lind L. Flow-mediated vasodilation was found to be an independent predictor of changes in the carotid plaque status during a 5-year follow-up. J Atheroscler Thromb. 2014; 21: 161-168 [DOI] [PubMed] [Google Scholar]
- 8). O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340: 14-22 [DOI] [PubMed] [Google Scholar]
- 9). Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2000; 151: 478-487 [DOI] [PubMed] [Google Scholar]
- 10). Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997; 96: 1432-1437 [DOI] [PubMed] [Google Scholar]
- 11). Iwamoto Y, Maruhashi T, Fujii Y, Idei N, Fujimura N, Mikami S, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, Noma K, Nakashima A, Higashi Y. Intima-media thickness of brachial artery, vascular function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2012; 32: 2295-2303 [DOI] [PubMed] [Google Scholar]
- 12). Ross R. Atherosclerosis: An inflammatory disease. N Engl J Med 1999; 340: 115-126 [DOI] [PubMed] [Google Scholar]
- 13). Higashi Y, Noma K, Yoshizumi M, Kihara Y. Oxidative stress and endothelial function in cardiovascular diseases (Review). Circ J 2009; 73: 411-418 [DOI] [PubMed] [Google Scholar]
- 14). Celermajer DS, Sorensen KE, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340: 1111-1115 [DOI] [PubMed] [Google Scholar]
- 15). Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: The Framingham Heart Study. Circulation 2004; 109: 613-619 [DOI] [PubMed] [Google Scholar]
- 16). Soga J, Hata T, Hidaka T, Fujii Y, Idei N, Fujimura N, Mikami S, Maruhashi T, Kihara Y, Chayama K, Kato H, Noma K, Liao JK, Higashi Y. for ROCK investigator group. Rho-associated kinase activity, endothelial function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2011; 31: 2353-2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Fujimura N, Hata T, Soga J, Hidaka T, Idei N, Fujii Y, Mikami S, Maruhashi T, Iwamoto Y, Kihara Y, Chayama K, Kato H, Noma K, Liao JK, Higashi Y. for ROCK investigator group. Mineralocorticoid receptor blocker eplerenone improves endothelial function and inhibits Rho-associated kinase activity in patients with hypertension. Clin Pharmacol Ther 2012; 91: 289-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Maruhashi T, Soga J, Idei N, Fujimura N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, Noma K, Nakashima A, Tomiyama H, Takase B, Yamashina A, Higashi Y. Hyperbilirubinemia, augmentation of endothelial function and decrease in oxidative stress in Gilbert syndrome. Circulation. 2012; 126: 598-603 [DOI] [PubMed] [Google Scholar]
- 19). Maruhashi T, Nakashima A, Matsumoto T, Oda N, Iwamoto Y, Iwamoto A, Kajikawa M, Kihara Y, Chayama K, Goto C, Noma K, Higashi Y. Relationship between nitroglycerine-induced vasodilation and clinical, severity of peripheral artery disease. Atherosclerosis 2014; 235: 65-70 [DOI] [PubMed] [Google Scholar]
- 20). Raitakari OT, Seale JP, Celermajer DS. Impaired vascular responses to nitroglycerin in subjects with coronary atherosclerosis. Am J Cardiol 2001; 87: 217-219 [DOI] [PubMed] [Google Scholar]
- 21). Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 2002; 40: 505-510 [DOI] [PubMed] [Google Scholar]
- 22). Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation 2002; 105: 1567-1572 [DOI] [PubMed] [Google Scholar]
- 23). Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: Additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 2003; 108: 2093-2098 [DOI] [PubMed] [Google Scholar]
- 24). Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation 2005; 111: 363-368 [DOI] [PubMed] [Google Scholar]
- 25). Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multiethnic study of atherosclerosis. Circulation 2009; 120: 502-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Adams MR, Robinson J, McCredie R, Seale JP, Sorensen KE, Deanfield JE, Celermajer DS. Smooth muscle dysfunction occurs independently of impaired endothelium-dependent dilation in adults at risk of atherosclerosis. J Am Coll Cardiol. 1998; 32: 123-127 [DOI] [PubMed] [Google Scholar]
- 27). Jarvisalo MJ, Lehtimaki T, Raitakari OT. Determinants of arterial nitrate-mediated dilatation in children: role of oxidized low-density lipoprotein, endothelial function, and carotid intima-media thickness. Circulation. 2004; 109: 2885-2889 [DOI] [PubMed] [Google Scholar]
- 28). Maruhashi T, Soga J, Idei N, Fujimura N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Higashi Y. Nitroglycerine-induced Vasodilation for Assessment of Vascular Function: A Comparison with Flow-mediated Vasodilation. Arterioscler Thromb Vasc Biol. 2013; 33: 1401-1408 [DOI] [PubMed] [Google Scholar]
- 29). Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Ronnemaa T, Raitakari OT. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation 2004; 110: 2918-2923 [DOI] [PubMed] [Google Scholar]
- 30). Yan RT, Anderson TJ, Charbonneau F, Title L, Verma S, Lonn E. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol 2005; 45: 1980-1986 [DOI] [PubMed] [Google Scholar]
- 31). Yeboah J, Burke GL, Crouse JR, Herrington DM. Relationship between brachial flow-mediated dilation and carotid intima-media thickness in an elderly cohort: the Cardiovascular Health Study. Atherosclerosis 2008; 197: 840-845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Shionoya S. Diagnostic criteria of Buerger's disease. Int J Cardiol 1998; 66 (Suppl 1): S243-245 [DOI] [PubMed] [Google Scholar]
- 33). Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Jr, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560-2572 [DOI] [PubMed] [Google Scholar]
- 34). American Diabetes Association: clinical practice recommendations 1999. Diabetes Care 1999; 22 (Suppl. 1): S1-114 [PubMed] [Google Scholar]
- 35). Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486-2497 [DOI] [PubMed] [Google Scholar]
- 36). Szuba A, Cooke JP. Thromboangiitis obliterans. An update on Buerger's disease. West J Med. 1998; 168: 255-260 [PMC free article] [PubMed] [Google Scholar]
- 37). Cooper LT, Tse TS, Mikhail MA, McBane RD, Stanson AW, Ballman KV. Long-term survival and amputation risk in thromboangiitis obliterans (Buerger's disease). J Am Coll Cardiol. 2004; 44: 2410-2411 [DOI] [PubMed] [Google Scholar]
- 38). Idei N, Nishioka K, Soga J, Hidaka T, Hata T, Fujii Y, Fujimura N, Maruhashi T, Mikami S, Teragawa H, Kihara Y, Noma K, Chayama K, Higashi Y. Vascular function and circulating progenitor cells in thromboangitis obliterans (Buerger's disease) and atherosclerosis obliterans. Hypertension 2011; 57: 70-78 [DOI] [PubMed] [Google Scholar]


