Abstract
Aim: Sterol regulatory element-binding protein (SREBP)-1c is the dominant liver insulin-stimulated isoform and strongly correlates with diabetic dyslipidemia characterized by hyperinsulinemia [i.e., high-density lipoprotein cholesterol (HDL-C) levels and hypertriglyceridemia]. MicroRNA (miRNA) 33b is harbored in the intron of SREBP-1c and represses ATP-binding cassette, sub-family A, and member 1 (ABCA1) expression, essential for HDL formation. We measured plasma miRNA33b levels as possible biomarkers for diabetic dyslipidemia in patients with type 2 diabetes mellitus (T2DM) showing insulin resistance.
Methods: The participants included 50 patients with T2DM (M/F 31/19) enrolled in an educational program for controlling blood glucose levels at Hirosaki University Hospital. HbA1c, fasting plasma glucose, insulin, and lipid levels were determined. Plasma miRNA33b, miRNA33a and miRNA148a were quantified using a TaqMan® MicroRNA Assay, and values were corrected with reference to miRNA16.
Results: Mean BMI of participants were 28.2 ± 6.6 (kg/m2) and the Homeostasis Model Assessment of Insulin Resistance was 4.3 ± 2.7. Patients' laboratory findings indicated diabetic dyslipidemia with insulin resistance. Plasma miRNA33b/16 levels revealed a positive correlation with plasma insulin level (r = 0.326, P = 0.021), serum C-peptide (r = 0.280, P = 0.049), and triglyceride (r = 0.351, P = 0.012), but no association with HDL-C (r = −0.210, P = 0.143). The blood level of miRNA33a was approximately 1/150th of that of miRNA33b and was not correlated with the above parameters.
Conclusion: We postulated that plasma miRNA33b may be useful as a new metabolic biomarker of dyslipidemia in patients with T2DM as well as metabolic syndrome via an insulin/SREBP-1c/miRNA33b/ABCA1 pathway.
Keywords: MicroRNA, MicroRNA33b, SREBP-1c, Type 2 diabetes mellitus, Dyslipidemia, Insulin resistance
See editorial vol. 23: 1259–1260
Introduction
Diabetic dyslipidemia is characterized by increased plasma triglyceride (TG), decreased high-density lipoprotein (HDL)-cholesterol (C) and existence of small dense low-density lipoprotein (LDL)-C. HDL-C levels are negatively associated with plasma TG levels1, 2). The etiology of diabetic dyslipidemia is intertwined with insulin resistance as follows: reduced hormonesensitive lipase (HSL) activity retards the catabolism of TG-rich very low-density lipoprotein (VLDL)3). In turn, VLDL-TGs can be exchanged for HDL cholesterol by cholesteryl ester transfer protein (CETP). TG-rich HDL particles can undergo further modification, including hydrolysis of their TG, which leads to the dissociation of the structurally important protein apolipoprotein A-I (ApoA-I). HDL cholesterol is then reduced because ApoA-I is a major component of the HDL particle4).
To date, it has been suggested that extracellular microRNAs (miRNAs) have specific physiological functions, depending on their cellular origin, in regulating the immune response, development, stem cell differentiation and, most recently, lipid metabolism5–8). In particular, an association between miRNA33b with diabetic dyslipidemia and insulin resistance has been assumed from the following observations: increased insulin activates the transcription of the sterol regulatory element-binding protein (SREBP)-1c, while miRNA33b is encoded in the human SREBP-1c gene. SREBP-1c activates the genes necessary to produce fatty acids and, in turn, plasma TG levels rise8, 9).
Recent advances in research on the ATP-binding cassette, sub-family A, member 1 (ABCA1) protein suggests that the lipidation of ApoA-I via interaction with ABCA1 is an essential, initial step for the formation of HDL, and this ultimately determines plasma HDL-C levels10, 11). Increased production of miRNA33b in response to the insulin-stimulated SREBP-1c gene may cause a reduction in HDL levels by repressing the expression of ABC A1/G112).
A recent Genome Wide Association Study (GWAS) has demonstrated that several miRNAs (miR128-1, miR148a, miR130b, and miR301b) except for miRNA33b control the expression of key proteins involved in cholesterol-lipoprotein trafficking, such as ABCA1, cholesterol transporter13). Recently, miR148a in these miRNAs played an important role of controlling circulating lipoprotein levels via ABCA1 expression14).
In this study, we determined plasma levels of miRNA33b in patients with type 2 diabetic mellitus (T2DM) showing a dyslipidemia associated with hyper-insulinemia (insulin resistance). We postulate that circulating miRNAs may be excellent comprehensive biomarkers that provide an integrated view of the lipid profiles of type 2 diabetic patients with insulin resistance 15–18). Additionally, we measured miRNA148a and examine the relationship between this miRNA and clinical parameters.
Methods
Study Participants
We investigated 50 patients with T2DM (male/female 31/19) who were enrolled in an educational program related to the control of blood glucose levels at Hirosaki University Hospital. Their treatments included the following: diet (n = 21), oral hypoglycemic agents [n = 27, sulfonylurea/dipeptidyl peptidase (DPP4) inhibitor/biguanide/thiazolidinedione/alphaglucosidase inhibitors 16/18/9/3/2], and/or glucagonlike peptide (GLP)-1 receptor agonist (n = 4). Patients being treated with insulin were excluded.
Laboratory Measurements
Laboratory examinations of the following were performed; fasting plasma glucose (FPG), HbA1c, plasma insulin level (IRI), serum C-peptide (s-CPR) and urinary C-peptide (u-CPR; average of u-CPR levels for three consecutive days). To determine each patient's serum lipid profile, levels of total cholesterol (TC), TG, HDL-C, LDL-C, and ApoA-1, B, E, were measured. All blood measurements were performed in the clinical laboratory of Hirosaki University Hospital, using routine automated laboratory methods. HbA1c (%) was measured by high-performance liquid chromatography (HPLC) and expressed as a National Glycohemoglobin Standardization Program (NGSP) value. A Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)19, 20) was used, whereby values were calculated according to the following formula: FPG (mg/dL) × Fasting-IRI (µU/mL)/405.
Analysis of Plasma miRNAs
Measurement of plasma miRNAs (miRNA33b, miRNA33a, miRNA148a, and miRNA16) were performed as follows; circulating miRNAs were extracted using microRNA Extractor® SP kits (Wako Pure Chemical Industries Ltd., Osaka, Japan). This kit extracts circulating microRNA in 200 µL of serum or plasma. The extraction method of this kit is based on a procedure using a chaotropic reagent, which aims for a safe extraction without the use of hazardous phenol/chloroform and a spin column. Extracted total miRNA samples were stored at −80°C.
Human miRNA33a/b, miRNA148a, and miRNA16 reference genes were measured using TaqMan®Small RNA Assays (Applied Biosystems, Foster City, CA, USA). TaqMan®Small RNA Assays are preformulated primer and probe sets designed to detect and quantify mature microRNAs. Quantification using TaqMan Small RNA Assays is done using two-step reverse transcription (RT)-PCR. In the RT step, cDNA is reverse transcribed from total RNA in samples using a small RNA-specific, stem-loop RT primer from TaqMan Small RNA Assays and reagents from a TaqMan MicroRNA Reverse Transcription Kit. In the PCR step, PCR products were amplified from cDNA samples using TaqMan Small RNA Assays, together with TaqMan Universal PCR Master Mix II (ABI PRISM 7700 Sequence Detection System; Applied Biosystems, Yokohama, Japan).
We ordered predesigned and custom TaqMan Micro-RNA Assays for every miRNA type through the Applied Biosystems website: miRBase ID hsa-mir-33a-5p for miRNA33a, hsa-mir-33b-5p for miRNA33b, has-miR-148a-5p for miRNA148a, and hsa-mir-16-5p for miRNA16. Values were obtained using q-PCR, meaning that miRNAs were corrected with reference to total RNA. Determined plasma miRNA33a, 33b, and 148a levels were corrected with references to miRNA1621, 22) and expressed as miRNA33a/16, miRNA33b/16, and miRNA148a/16.
Each miRNA measurement was made in triplicate, and an average value was calculated. As to the extraction of miRNAs using an microRNA Extractor® SP kit, the intra-assay coefficient of variation (CV%) of quadruplicate measurements of five plasma samples was calculated as 4.2% ± 0.6%. The intra-assay CV values of RT-PCR measurements of miRNA33a, miRNA33b, and miRNA16 were calculated as 20.8% ± 7.8%, 22.7% ± 9.5%, 27.5% ± 7.0%, respectively, in a similar manner.
Statistics
Values are expressed as means ± standard deviations (SD). Differences between two groups were evaluated by a two-tailed, unpaired Student's t -test. Statistical significance was defined as P < 0.05.
Ethics
The Ethics Committee of the Hirosaki Graduate School of Medicine reviewed and approved the research protocol. All participants provided written, informed consent for blood sampling and measurments of miRNAs.
Results
Clinical Characteristics of Patients with T2DM
Mean BMI of participants was 28.2 ± 6.6 kg/m2) and BMI of 47 patients were over 25. Participants showed a mean HbA1c of 9.5 ± 1.8% (Table 1). Plasma insulin levels were high (IRI 10.0 ± 8.0 µU/mL; s-CPR 2.4 ± 0.9 ng/mL) and HOMA-IR was calculated as 4.3 ± 2.7, which signified insulin resistance (HOMA-IR >2.5 is usually judged to be insulinresistant). As for dyslipidemia, mean plasma levels of LDL-C, HDL-C and TG were 116.2 ± 36.4, 45.5 ± 12.5 and 160.4 ± 75.4 mg/dL, respectively. Seven patients were undergoing statin treatment.
Table 1. Clinical characteristics of 50 patients with T2DM.
| No (M/F) | 50 (31/19) | Treatment for DM | No (%) |
| Age (y.o.) | 52.7 ± 13.5 | Diet | 21 (42) |
| BMI (kg/m2) | 28.2 ± 6.6 | OHA | 27 (54) |
| FPG (mg/dL) | 186.5 ± 53.2 | SU | 16 |
| HbAlc (%) | 9.5 ± 1.8 | DPP4I | 18 |
| S-CPR (ng/mL) | 2.4 ± 0.9 | BG | 9 |
| U-CPR (mg/day) | 96.2 ± 44.0 | TZD | 3 |
| IRI (µU/mL) | 10.0 ± 8.0 | AGI | 2 |
| HOMA-IR | 4.3 ± 2.7 | GLP-1 | 4 (8) |
| TC (mg/dL) | 193.8 ± 40.0 | Others | |
| TG (mg/dL) | 160.4 ± 75.4 | statins | 7 (14) |
| HDL-C (mg/dL) | 45.5 ± 12.5 | ||
| LDL-C (mg/dL) | 116.2 ± 36.4 | ||
| Non-HDL-C (mg/dL) | 145.1 ± 46.1 |
Patients were obese and showed insulin resistance (HOMA-IR was calculated as 4.3 ± 2.7). Patients were treated with diet (n = 21), oral hypoglycemic agents (n = 27, sulfonylurea (SU)/ dipeptidyl peptidase (DPP4) inhibitor (DPP4I)/biguanide (BG)/thiazolidinedione (TZD)/alpha-glucosidase inhibitor (AGI) 16/18/9/3/2) or GLP-1 receptor agonist (n = 4). Patients who were being treated with insulin were excluded.
With regard to insulin levels, there was a significant positive correlation between plasma IRI and s-CPR (r = 0.758, P < 0.001), and between plasma and urinary CPR (r = 0.504, P < 0.001). Plasma TG levels showed a significant negative correlation with plasma HDL-C (r = −0.446, P = 0.001). Plasma HDL-C levels were significantly positively correlated with ApoA-I levels (r = 0.806, P < 0.001), because ApoA-I was a major constituent of the HDL particle (supplementary Fig. 1). All these findings were compatible with diabetic dyslipidemia and insulin resistance.
Supplementary Fig. 1.
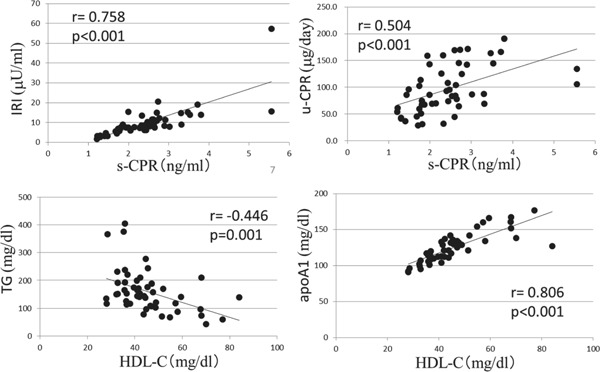
Clinical parameters and their relationship in 50 patients with T2DM
With regard to insulin levels, there was a significantly positive correlation between plasma IRI and s-CPR, and between plasma and urinary CPR. Plasma TG levels showed a significantly negative correlation with plasma HDL-C. Plasma HDL-C levels significantly correlated with ApoA-I levels.
Plasma miRNA33b/16 Levels and Clinical Parameters in Patients with T2DM
Plasma miRNA33b/16 levels were significantly positively correlated with IRI (r = 0.326, P = 0.021), and also with s-CPR levels (r = 0.280, P = 0.049; Table 2A and supplementary Fig. 2). Plasma miRNA33b/16 levels were not correlated with FBS or HbA1c levels. Plasma miRNA33b/16 levels also demonstrated a significant correlation with TG levels (r = 0.351, P = 0.012), and showed no correlation with HDL-C levels (r = −0.210, P = 0.143; Table 2A). Then, plasma miRNA33b/16 levels were not correlated with LDL-C/HDL-C ratio (r = 0.241, P = 0.091)
Table 2. Correlation of plasma miRNA33b/16 (A) or miRNA33b (B) levels and clinical parameters in patients with T2DM.
| A. miRNA33b/16 | B. miRNA33b | ||||
|---|---|---|---|---|---|
| r | p value | r | p value | ||
| BMI | 0.194 | 0.177 | BMI | 0.112 | 0.441 |
| FBS | 0.059 | 0.682 | FBS | −0.133 | 0.356 |
| HbA1c | −0.032 | 0.828 | HbA1c | −0.095 | 0.51 |
| IRI | 0.326 | 0.021 | IRI | 0.263 | 0.065 |
| s-CPR | 0.28 | 0.049 | s-CPR | 0.237 | 0.098 |
| u-CPR | −0.049 | 0.733 | u-CPR | 0.098 | 0.499 |
| LDL-C | 0.163 | 0.259 | LDL-C | −0.09 | 0.533 |
| TG | 0.351 | 0.012 | TG | 0.086 | 0.545 |
| LDL-C/HDL-C | 0.241 | 0.091 | LDL-C/HDL-C | 0.059 | 0.687 |
| HDL-C | −0.21 | 0.143 | HDL-C | −0.197 | 0.171 |
| ApoA-I | −0.169 | 0.241 | ApoA-I | −0.245 | 0.086 |
Plasma miRNA33b/16 levels revealed a positive relationship with IRI or with s-CPR (A). Plasma miRNA33b/16 levels also demonstrated a positive correlation with TG and showed a negative tendency with HDL-C (A). Similar tendencies between miRNA33b levels (relative to total RNA) and the above-mentioned clinical parameters were revealed; however, statistically significant differences were not seen (B). Statistical significance was defined as P < 0.05.
Supplementary Fig. 2.
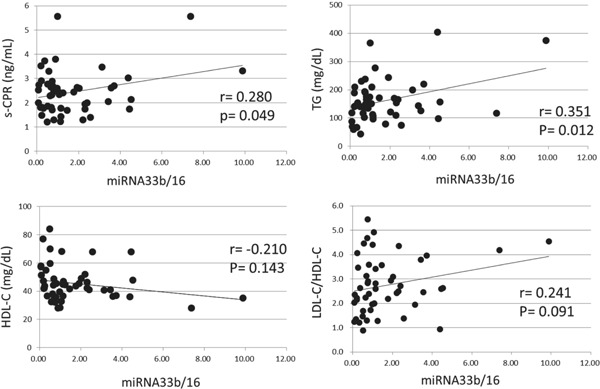
Correlation of plasma miRNA33b/16 with s-CPR, TG, HDL-C and LDL-C/HDL-C
Plasma miRNA33b/16 levels significantly correlated with IRI, and also with s-CPR levels. Plasma miRNA33b/16 levels also demonstrated a significant correlation with TG levels, and showed a negative tendency with HDL-C levels. Then, plasma miRNA33b/16 levels demonstrated positive tendency with LDL-C/HDL-C.
Similar trends between miRNA33b levels (relative to total RNA) and the above-mentioned clinical parameters were also found; however, a statistically significant difference was not seen with any parameter (Table 2B).
Plasma miRNA33a/16 Levels and Clinical Parameters in Patients with T2DM
The plasma level of miRNA33a/16 did not show any correlation with any of the clinical parameters described above. However, miRNA33a/16 showed a positive correlation with LDL-C (r = 0.304, P = 0.032) and tendency with LDL-C/HDL-C ratio (r = 0.269, P = 0.059) (Table 3). Of particular note, the blood level of miRNA33a was found to be approximately 1/150th the miRNA33b blood level; average plasma miRNA33a level was 0.083 ± 0.075 ng/uL and miRNA33b was 9.6 ± 8.7 ng/uL. Additionally, the plasma level of miRNA33a/16 showed no correlation with that of miRNA33b/16 (r = 0.107, P = 0.539).
Table 3. Correlations of plasma miRNA33a/16 levels and clinical parameters in patients with T2DM.
| miRNA33a/16 | ||
|---|---|---|
| r | p value | |
| BMI | −0.064 | 0.659 |
| FBS | 0.051 | 0.725 |
| HbA1c | −0.160 | 0.266 |
| IRI | −0.067 | 0.640 |
| s-CPR | −0.102 | 0.479 |
| u-CPR | −0.220 | 0.125 |
| LDL-C | 0.304 | 0.032 |
| TG | 0.066 | 0.650 |
| LDL-C/HDL-C | 0.269 | 0.059 |
| HDL-C | −0.099 | 0.494 |
| ApoA-I | −0.118 | 0.415 |
The plasma level of miRNA33a/16 did not show any correlation with any of the clinical parameters except with positive correlation with LDL-C and tendency with LDL-C/HDL-C ratio.
Plasma miRNA148a/16 Levels and Clinical Parameters in Patients with T2DM
Plasma miRNA148a/16 levels demonstrated a negative tendency with HDL-C (r = −0.228, P = 0.056) and with ApoA-I levels (r = −0.141, P = 0.164; Table 4). However, miRNA148a/16 levels were not correlated with any parameter, such as FBS, HbA1c, TG, insulin level, LDL-C or LDL-C/HDL-C levels (Table 4). Plasma level of miRNA148a/16 showed no correlation with that of miRNA33b/16 (r = −0.006, P = 0.969).
Table 4. Correlations of plasma miRNA148a/16 levels and clinical parameters in patients with T2DM.
| miRNA148a/16 | ||
|---|---|---|
| r | p value | |
| BMI | 0.064 | 0.671 |
| FBS | 0.104 | 0.474 |
| HbA1c | 0.032 | 0.824 |
| IRI | 0.097 | 0.749 |
| s-CPR | 0.093 | 0.739 |
| u-CPR | 0.032 | 0.587 |
| LDL-C | 0.034 | 0.592 |
| TG | −0.020 | 0.445 |
| LDL-C/HDL-C | 0.152 | 0.855 |
| HDL-C | −0.228 | 0.056 |
| ApoA-I | −0.141 | 0.164 |
Plasma miRNA148a/16 levels demonstrated a negative tendency with HDL-C and with ApoA-I levels.
Discussion
The SREBP family consists of three isoforms: SREBP-1a and -1c and SREBP-223–25). SREBP-1c is the dominant insulin-stimulated isoform found in the liver responsible for inducing fatty acid synthesis and lipid accumulation, whereas SREBP-2 is induced by sterol deprivation and regulates genes involved in cholesterol synthesis and uptake26). Two miRNA33 genes with a two-nucleotide variation in their mature forms are present in human SREBP genes: miRNA33a, harbored in intron 16 of the SREBP-2 gene on chromosome 22, and miRNA33b, harbored in intron 17 of the SREBP-1 gene on chromosome 1727–31). One potentially important miRNA33 target exists in the 3′ untranslated region (3′ UTR) of ABCA1 messenger RNA (mRNA)32–34). The 3′ UTR of ABCA1 mRNA contains three highly conserved binding sites (as determined by sequence-specific hybridization to complementary target sites) for miRNA33a and/or miRNA33b. ABCA1 is responsible for the movement of free cholesterol out of the cell and for the generation of nascent HDL particles.
Although present in primates, miRNA33b is absent in rodents and has been less well studied than miRNA33a to date10, 35, 36). The expression of miRNA33 was found in various cells and tissues, including hepatic cells, macrophages, endothelial cells, brain, liver, colon, small intestine, and skeletal muscle12, 37).
Previous in vitro experiments showed that insulin increases the expression of SREBP-1c mRNA in parallel with other miRNAs in primary hepatocytes26, 38–41). Indeed, the expression of ABCA1 mRNA and protein is strongly repressed by miR-33 overexpression in a variety of cell types10–12, 32–34).
In addition to these, miRNA33a/33b, similarly, targets a variety of lipid metabolism-associated genes besides ABCA1, such as Crot, Cpt1a, Hadhb, and Ampkα5, 35, 37). Furthermore, miRNA33b also cooperates with SREBP1 in regulating glucose metabolism by targeting phosphoenolpyruvate carboxykinase (PCK1) and glucose-6-phosphatase (G6PC), key regulatory enzymes of hepatic gluconeogenesis42).
In this study, we clinically demonstrated a relationship between circulating plasma miRNA33b levels and metabolic parameters of 50 patients with T2DM who exhibited diabetic dyslipidemia with insulin resistance. We showed that plasma miRNA33b/16 was positively correlated with plasma insulin levels (IRI and CPR) and was negatively correlated with TG levels. Plasma miRNA33b/16 also showed a negative tendency with HDL-C. Although plasma HDL-C levels significantly correlated with ApoA-I levels (r = 0.806, P < 0.001; supplementary Fig. 1), ApoA-I showed no correlation with plasma miRNA33b/16 (r = −0.169, P = 0.241; Table 2A). These findings suggest that increased plasma insulin may activate SREBP-1c/miRNA33b in the liver; SREBP-1c may participates in the biosynthesis of TG and coordinately enhanced miRNA33b may restrict the transcription of ABCA1, which subsequent decreases the formation of HDL-C and ApoA-I (SREBP-1c/miRNA33b/ABCA1 axis; Fig. 1)43). We postulated that plasma miRNA33b itself may be an important biomarker which comprehensively express the physiological states of patients with T2DM; this probably also includes patients with metabolic syndrome37, 43, 44).
Fig. 1.
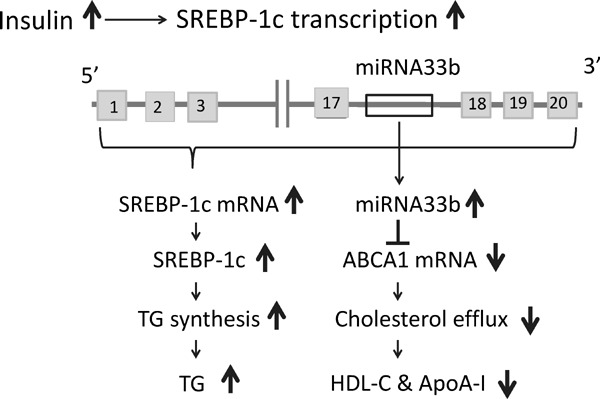
SREBP-1c/miRNA33b/ABCA1 axis
Increased plasma insulin may activate SREBP-1c in the liver, which participates in the biosynthesis of TG, and, coordinately enhanced miRNA33b may restrict the transcription of ABCA1, which subsequently decreases the formation of HDL-C and ApoA-I (the SREBP-1c/ miRNA33b/ ABCA1 axis). The authors have modified the figure from ref. 44.
It has been postulated that the relative amount of SREBF-2 mRNA in the liver is significantly less than that of SREBF1, and that miRNA33a would also be less abundant than miRNA33b2, 43). Our study confirmed that plasma miRNA33a levels were indeed over one hundred times lower compared with miRNA33b levels. This may presumably suggest that the origin of plasma miR33a and miR33b might quantitatively derive from the liver.
In the present study, miRNA33a/16 showed no correlation with parameters such as insulin, glucose, and lipid levels (Table 3). Indeed, a correlation was also not observed between miRNA33a/16 and 33b/16. With respect to the regulation of miRNA33a, sterol deprivation in the cell triggers SREBP-2 gene expression.
We could not explain the positive correlation between plasma levels of miRNA33a/16 and LDL-C (Table 3). For seven participants treated with statins, LDL-C levels were significantly lower than those for non-users (76.6 ± 29.8 vs. 122.6 ± 33.1 mg/dL); however, miRNA33a/16 levels as well as miRNA33b/16 or mi148a/16 did not differ between these two groups (supplementary Table 1).
Supplementary Table 1. Comparison of plasma levels of miRNA33a/16, 33b/16 and 148a/16 in patients with or without statin.
| no | 33a/16 | 33b/16 | 148a/16 | |
|---|---|---|---|---|
| Statin (+) | 7 | 0.034 ± 0.039 | 2.146 ± 2.506 | 4.149 ± 2.592 |
| Statin (−) | 43 | 0.036 ± 0.100 | 1.600 ± 1.752 | 3.460 ± 7.015 |
| P value | 0.927 | 0.621 | 0.652 |
For seven subjects treated with statins, LDL-C levels were significantly lower than for non-users; however, miRNA33a/16 levels, as well as miRNA33b/16 or mi148a/16 did not differ between these two groups.
Recently, plasma miR33a and miR33b had been reported to be significantly upregulated in familial hypercholesterolaemic children and both miRNAs had shown a positive correlation with LDL-C, LDL-C/HDL-C ratio, apolipoprotein B, CRP (C-reactive protein) and glycaemia45). Authors quoted the report which illustrated SREBP transcriptional increase in fibroblasts derived from patients with genetic FH46). They hypothesized that the cholesterol content of hepatocytes in genetic FH participants is low, which should stimulate SREBP gene expression and, in turn, the expression of miR33a and miR33b45).
Nowadays, high-throughput genome-wide screening assay to systematically identify miRNA148a as a negative regulator of LDL receptor expression and activity and defined a SREBP-1-mediated pathway through which miRNA148a regulates LDL-C uptake and ABCA1 expression to control circulating lipoprotein levels14). MiRNA148a/16 levels in our study showed no significant correlation between LDL-C, LDL-C/HDL/C, or insulin levels. The liver X receptor (LXR), a nuclear hormone receptor, has been known as a factor promoting the expression of SREBP-1 other than insulin. It is unknown how LXR affected SREBP-1 expression in our study2, 31).
The current study has several limitations that may impact on the interpretation of results. Firstly, with regard to the quantitative miRNA analysis of blood samples, whether blood miRNA levels parallel those of protein in the liver is uncertain. In this study, levels of miRNA33b, harbored within the SREBP-1c gene, were demonstrated to be upregulated in response to insulin stimulation37, 44). In other words, this has led to the concept that circulating miRNA33b could be useful as a biomarker for the detection of SREBP-1c expression in the liver45, 47). Second, selection of an optimal reference gene for the normalization of circulating miRNA levels is problematic; a universal reference gene has not yet been established. The selection of an optimal control gene should be made on the basis of indicating steady-state expression in screening experiments. We selected miRNA16 as a control gene based on previous reports21, 22) and for the following reasons. We found plasma levels of miRNA33b and miRNA16 were within similar ranges. We also found that more comprehensive correlations were obtained when making corrections with miRNA16 than with total RNA (Table 2A vs. 2B). Regarding other limitations of the study, the relatively small size of the patient cohort used here indicates that further validation is required in larger studies. We also found a considerable lack of uniformity in samples from patients who had been subjected to a variety of treatments for diabetes and dyslipidemia. To clarify our result, we need to evaluate plasma miRNA33b levels in patients with age-matched controls who did not have insulin resistance and compare miRNA33b levels before and after DM treatment. As a necessary consideration of the future, we should examine precise miR33b functional approach using in vitro study.
In summary, our study postulates that circulating miRNA33b could act as a new marker to detect varying physiological states of dyslipidemia in patients with T2DM as well as in those with metabolic syndrome, who show SREBP-1c overexpression in response to hyperinsulinemia through the activities of insulin/SREBP-1c/miRNA33b/ABCA148, 49). These findings highlight the clinical potential of the antisense targeting of miRNA33b as a therapy to raise HDL levels in the treatment of cardiovascular disease during metabolic syndrome and T2DM35, 36, 42).
Conflict of Interest
The authors wish to declare that they have no conflicts of interest.
Reference
- 1). Taskinen MR: Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003. 46: 733-749 [DOI] [PubMed] [Google Scholar]
- 2). Mooradian AD: Dyslipidemia in type 2 diabetes mellitus. Nature Clinical Practice Endocrinology & Metabolism 2009, 5: 150-159 [DOI] [PubMed] [Google Scholar]
- 3). Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY: Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005, 437: 569-573 [DOI] [PubMed] [Google Scholar]
- 4). Lamarche B, Uffelman KD, Carpentier A, Cohn JS, Steiner G, Barrett PH, Lewis GF: Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J Clin Invest 1999, 103: 1191-1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 2010,101: 2087-2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Zhu H, Fan GC. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis 2011, 1: 138-149 [PMC free article] [PubMed] [Google Scholar]
- 7). Zhang E, Wu Y. MicroRNAs: important modulators of oxLDL-mediated signaling in atherosclerosis. J Atheroscler Thromb. 2013; 20: 215-227 [DOI] [PubMed] [Google Scholar]
- 8). Horie T, Baba O, Kuwabara Y, Yokode M, Kita T, Kimura T, Ono K. MicroRNAs and Lipoprotein Metabolism. J Atheroscler Thromb. 2014; 21: 17-22 [DOI] [PubMed] [Google Scholar]
- 9). Soufi Muhidien, Ruppert Volker, Kurt Bilgen, Schaefer Juergen R. The impact of severe LDL receptor mutations on SREBP-pathway regulation in homozygous familial hypercholesterolemia (FH). Gene 2012; 499: 218-222 [DOI] [PubMed] [Google Scholar]
- 10). Yokoyama S: ABCA1 and biogenesis of HDL. J Atheroscler Thromb 2006, 13: 1-15 [DOI] [PubMed] [Google Scholar]
- 11). Fitzgerald ML, Mujawar Z, Tamehiro N: ABC transporters, atherosclerosis and inflammation. Atherosclerosis. 2010, 211: 361-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, Hasegawa K, Yokode M, Kimura T, Kita T: MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci USA. 2010, 107: 17321-17326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Wagschal A, Najafi-Shoushtari SH, Wang L, Goedeke L, Sinha S, deLemos AS, Black JC1, Ramírez CM, Li Y, Tewhey R, Hatoum I, Shah N, Lu Y, Kristo F, Psychogios N, Vrbanac V, Lu YC, Hla T, de Cabo R, Tsang JS, Schadt E, Sabeti PC, Kathiresan S, Cohen DE, Whetstine J, Chung RT, Fernández-Hernando C, Kaplan LM, Bernards A, Gerszten RE, Näär AM. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat Med. 2015; 21: 1290-1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Goedeke L, Rotllan N, Canfrán-Duque A, Aranda JF, Ramírez CM, Araldi E, Lin CS, Anderson NN, Wagschal A, de Cabo R, Horton JD, Lasunción MA, Näär AM, Suárez Y, Fernández-Hernando C. MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat Med. 2015; 21: 1280-1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Guay C, Roggli E, Nesca V, Jacovetti C, Regazzi R. Diabetes mellitus, a microRNArelated disease? Transl Res 2011, 157: 253-264 [DOI] [PubMed] [Google Scholar]
- 16). Zampetaki A, Willeit P, Drozdov I, Kiechl S, Mayr M. Profiling of circulating microRNAs: from single biomarkers to re-wired networks. Cardiovasc Res 2012, 93: 555-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 2010, 107: 810-817 [DOI] [PubMed] [Google Scholar]
- 18). Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol 2013, 9: 513-521 [DOI] [PubMed] [Google Scholar]
- 19). Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Diabetologia. 1985, 28: 412-419 [DOI] [PubMed] [Google Scholar]
- 20). Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004, 27: 1487-1495 [DOI] [PubMed] [Google Scholar]
- 21). Brase JC, Wuttig D, Kuner R, Sültmann H. Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer. 2010, 9: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges Clin Chem. 2011, 57: 833-840 [DOI] [PubMed] [Google Scholar]
- 23). Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89: 331-340 [DOI] [PubMed] [Google Scholar]
- 24). Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002, 109: 1125-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Osborne TF. Sterol regulatory element-binding proteins (SREBPs): key regulators of nutritional homeostasis and insulin action. J. Biol. Chem. 2000, 275: 32379-32382 [DOI] [PubMed] [Google Scholar]
- 26). Foretz M, Guichard C, Ferre P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl. Acad. Sci. USA. 1999, 96: 12737-12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, Esplugues E, Fisher EA, Penalva LO, Moore KJ, Suarez Y, Lai EC, Fernandez-Hernando C. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA. 2011, 108: 9232-9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, MacDougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J. Biol. Chem. 2010, 285: 33652-33661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. USA. 2010, 107: 12228-12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010, 328: 1566-1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. miR-33 contributes to the regulation of cholesterol homeostasis. Science 2010, 328: 1570-1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Ambros V. The functions of animal microRNAs. Nature. 2004, 431: 350-355 [DOI] [PubMed] [Google Scholar]
- 33). Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009, 136: 215-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008, 9: 102-114 [DOI] [PubMed] [Google Scholar]
- 35). Moore KJ, Rayner KJ, Suárez Y, Fernández-Hernando C. The role of microRNAs in cholesterol efflux and hepatic lipid metabolism. Annu Rev Nutr. 2011, 31: 49-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Rottiers V, Najafi-Shoushtari SH, Kristo F, Gurumurthy S, Zhong L, Li Y, Cohen DE, Gerszten RE, Bardeesy N, Mostoslavsky R, Näär AM. MicroRNAs in metabolism and metabolic diseases. Cold Spring Harb Symp Quant Biol. 2011, 76: 225-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol. 2012, 13: 239-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Foretz M, Pacot C, Dugail I, Lemarchand P, Guichard C, Le Liepvre X, Berthelier-Lubrano C, Spiegelman B, Kim JB, Ferre P, Foufelle F. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glu cose. Mol. Cell. Biol. 1999, 19: 3760-3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Owen JL, Zhang Y, Bae SH, Farooqi MS, Liang G, Hammer RE, Goldstein JL, Brown MS. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc. Natl. Acad. Sci. USA. 2012, 109: 16184-16189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999, 96: 13656-13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 2000, 6: 77-86 [PubMed] [Google Scholar]
- 42). Ramírez CM1, Goedeke L, Rotllan N, Yoon JH, Cirera-Salinas D, Mattison JA, Suárez Y, de Cabo R, Gorospe M, Fernández-Hernando C. MicroRNA 33 regulates glucose metabolism. Mol Cell Biol. 2013, 33: 2891-2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Rayner KJ, Fernandez-Hernando C, Moore KJ. MicroRNAs regulating lipid metabolism in atherogenesis. Thromb Haemost. 2012, 107: 642-647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Brown MS, Ye J, Goldstein JL. Medicine. HDL miR-ed down by SREBP introns. Science 2010, 328, 1495-1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Martino F, Carlomosti F, Avitabile D, Persico L, Picozza M, Barillà F, Arca M, Montali A, Martino E, Zanoni C, Parrotto S, Magenta A. Circulating miR-33a and miR-33b are up-regulated in familial hypercholesterolaemia in paediatric age. Clin Sci 2015, 129; 963-972 [DOI] [PubMed] [Google Scholar]
- 46). Mayr M, Zampetaki A, Willeit P, Willeit J, Kiechl S. MicroRNAs within the continuum of postgenomics biomarker discovery. Arterioscler Thromb Vasc Biol 2013, 33: 206-214 [DOI] [PubMed] [Google Scholar]
- 47). Fernández-Hernando C, Suárez Y, Rayner KJ, Moore KJ. MicroRNAs in lipid metabolism. Curr Opin Lipidol. 2011. April; 22: 86-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Joyce CE, Zhou X, Xia J, et al. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum Mol Genet 2011, 20: 4025-4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Ortega FJ, Mercader JM, Moreno-Navarrete JM, Rovira O, Guerra E, Esteve E, Xifra G, Martínez C, Ricart W, Rieusset J, Rome S, Karczewska-Kupczewska M, Straczkowski M, Fernández-Real JM. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care. 2014, 37: 1375-1383 [DOI] [PubMed] [Google Scholar]


