Abstract
Background
The acute consumption of excessive quantities of alcohol causes well-recognized neurophysiological and cognitive alterations. As people reach advanced age, they are more prone to cognitive decline. To date, the interaction of current heavy alcohol (ETOH) consumption and aging remain unclear. The current paper tested the hypothesis that negative consequences of current heavy alcohol consumption on neurocognitive function are worse with advanced age. Further, we evaluated the relations between lifetime history of alcohol dependence and neurocognitive function
Methods
Sixty-six participants underwent a comprehensive neurocognitive battery. Current heavy ETOH drinkers were classified using NIAAA criteria (ETOH Heavy, n = 21) based on the Timeline follow-back and a structured clinical interview and compared to non-drinkers, and moderate drinkers (ETOH Low, n = 45). Fifty-three-point-three percent of the total population had a lifetime history of alcohol dependence. Neurocognitive data were grouped and analyzed relative to global and domain scores assessing: global cognitive function, attention/executive function, learning, memory, motor function, verbal function, and speed of processing.
Results
Heavy current ETOH consumption in older adults was associated with poorer global cognitive function, learning, memory, and motor function (p’s<.05). Furthermore, lifetime history of alcohol dependence was associated with poorer function in the same neurocognitive domains, in addition to the attention/executive domain, irrespective of age (p’s<.05).
Conclusions
These data suggest that while heavy current alcohol consumption is associated with significant impairment in a number of neurocognitive domains, history of alcohol dependence, even in the absence of heavy current alcohol use, is associated with lasting negative consequences for neurocognitive function.
Keywords: Alcohol Consumption, Alcohol Dependence, Cognitive Aging, ETOH, Cognitive Impairment
INTRODUCTION
The acute consumption of excessive quantities of alcohol causes well-recognized neurophysiological and cognitive alterations, including loss of consciousness, coma, or even death. Heavy alcohol consumption adversely affects the brain both directly and indirectly. Direct brain effects of alcohol include depression of central nervous system activity, alterations in cerebrovascular function, and neurotoxicity (Alexander et al., 2004, Haorah et al., 2005, Shih et al., 2001, Vinod and Hungund, 2005, Webb et al., 1997, Wilhelm et al., 2015). Indirect effects include neurotoxicity tied to hepatic, renal, and gastrointestinal dysfunction, as well as sleep disturbance, anoxia, head injury, and other disturbances that may occur with chronic alcohol intoxication (O’Dell et al., 2012, Marksteiner et al., 2002, Schuckit, 2009, Solomon et al., 1992, Spirduso et al., 1989, Wilde et al., 2004).
Despite a growing literature concerning the effects of acute and chronic heavy alcohol consumption, the neurocognitive manifestations of heavy alcohol consumption remain unresolved. Findings from past studies conducted to address this question have not been ubiquitous. While the neurocognitive effects of alcohol consumption appear to depend on the amount of alcohol consumed, the duration of use, and various other clinical factors, including age and comorbid neurological conditions, not all studies agree (Carey et al., 2004a, Carey et al., 2004b, Draper et al., 2011, Friend et al., 2005, Green et al., 2010, Houston et al., 2014, Marksteiner et al., 2002, Molina et al., 1994, O’Dell et al., 2012, Solomon et al., 1992, Squeglia et al., 2009, 2014, Sullivan et al., 2002, 2010).
For example, in the MATCH study of drinkers undergoing alcohol treatment for alcohol abuse-dependence, Friend, Malloy, and Sindelar found that while years of alcohol consumption was inversely associated with neuropsychological test scores, it did not account for much of the variance in these test scores (Friend et al., 2005). Yet, a recent study found in older adults that age of onset of alcohol dependence was not associated with greater cognitive deficits (Kist et al., 2014). However, this study also found that older adults had significantly poorer cognitive abilities when compared to non-alcohol dependent controls. A recent study found age effects in 51 adults with alcohol dependence diagnoses who were abstinent from alcohol for one month (Durazzo et al., 2013). Mild deficits of learning, memory, cognitive efficiency, executive functions, processing speed, and fine motor skills were associated with alcohol dependence, though these deficits were greatest among people who also smoked cigarettes. In addition, another study found deficits in executive function in 560 heavy drinking men and women (Houston et al 2014). In an earlier study Drake et al found that alcohol dependent adults who abstained from alcohol after a 28 day treatment program showed recovery of cognitive functions (Drake et al., 1995). Another study found that moderate alcohol consumption was not associated with either the occurrence or exacerbation of dementia (Panza et al., 2009), and there have been reports that drinking one glass of wine a day may actually be associated with reduced rates of Alzheimer’s Disease (Solfrizzi et al., 2007). Yet, a recent study of brain morphometry and cognition reported that late life consumption of alcohol is associated with episodic memory difficulties and also reduced hippocampal volume in the Framingham cohort (Downer et al., 2014). These contrasting results demonstrate the need for further study of the influence of advanced age on possible heavy alcohol consumption effects on neurocognitive function. Further still, even less is known about the impact of advanced age on possible alcohol related neurocognitive deficits.
In the current study, we sought to understand the relationship between age, heavy alcohol consumption, and neurocognitive function. As people reach advanced age and are more prone to cognitive decline (Woods et al., 2012, Woods et al., 2013), the adverse effects of heavy alcohol use may be exacerbated (Riege et al., 1981). In fact, dementia secondary to alcoholism is commonly diagnosed in elderly adults whose cognitive and functional decline is inconsistent with progressive neurodegenerative disorders like Alzheimer’s disease (AD), and whose clinical history indicates chronic heavy alcohol consumption (Tyas, 2001, Meyer et al., 1998). As people reach more advanced age they experience systemic physiological and neural alterations that may increase vulnerability to the effects of alcohol (Tyas, 2001, Meyer et al., 1998, Snow et al., 2009, Goldberg et al., 1994). Yet, relatively few studies have directly compared the neurocognitive performance of heavy drinkers with that of people who consume moderate quantities of alcohol or who are non-drinkers as a function of age. To address this question, the present study was conducted to examine the association of heavy alcohol consumption with neurocognitive function at different ages. We hypothesized that heavy alcohol consumption would be associated with significant cognitive impairments and that the adverse effects of heavy alcohol consumption would be greatest among older adults.
MATERIALS AND METHODS
Participants
Sixty-six participants in a NIAAA sponsored study of the effects of heavy alcohol use and aging on neurocognitive and brain functioning were assessed. The mean age of the sample was 38.5 ± 11.7 years (range = 21–69 years). Mean educational attainment was 13.7 ± 2.75 years. The racial composition of the overall sample was 30.3% African-American and 69.7% Caucasian. Thirty-five (53%) participants were women. The sample consisted of adults recruited from the Brown University Center for Aids Research (CFAR), who were at risk for HIV or HCV infection based on their association with HIV-infected friends or family, prior injection drug use, or sexual risk, but who were not infected with either HIV or HCV. Participants were recruited over 30 months using clinician referral, word of mouth, and flyers. All participants underwent a neurological examination and thorough medical history assessment. HIV infection was ruled out based on enzyme linked immunosorbent assay (ELISA) and confirmed by Western blot, while active HCV infection was ruled out by negative anti-HCV ELISA and negative qualitative HCV RNA by polymerase chain reaction. Participants were also excluded for history of (1) head injury with loss of consciousness > 10 minutes; (2) history of severe anxiety, depression or neurological disorders, including dementia, seizure disorder, stroke, and opportunistic brain infection; (3) severe psychiatric illness that might impact brain function (e.g., schizophrenia, bipolar illness; and (4) current (6-month) substance dependence or positive urine toxicology screen for cocaine, opiates, or illicit stimulants or sedatives. Inclusion/exclusion criteria were assessed using structured clinical interview by the study physician and self-reported medical history. The study was approved by the institutional review boards, and informed consent was obtained from each participant before enrollment.
Alcohol consumption
Participants were recruited with the goal of obtaining relatively equal samples of non-drinkers, people who drink moderate quantities of alcohol, and heavy alcohol users (ETOH none; ETOH moderate, ETOH High) based on current use. Participants were categorized into alcohol groupings based on NIAAA criteria (see Alcoholism NIoAA: http://rethinkingdrinking.niaaa.nih.gov/IsYourDrinkingPatternRisky/WhatsAtRiskOrHeavyDrinking.asp) derived from Timeline follow-back (TLFB, Fals-Steward et al., 2000) and a structured clinical interview by the study physician. The TLFB involves a self-report of drinking behavior over the past 90-days and was used to calculate the average number of drinks per week over the past 3 months. The ETOH-heavy group consisted of people who reported drinking 5 or more drinks in a single day for men (or average more than 14 per week), and 4 or more in a single day (or average more than 7 in a week) for women. The ETOH-moderate group consisted of people who reported consuming less than ETOH-heavy quantities, while ETOH-none reported no consumption of alcohol.
Given the study hypotheses of adverse neurocognitive effects among heavy drinkers, the ETOH none and ETOH moderate groups were pooled into a single group consisting of individuals who were currently drinking below the NIAAA threshold for “at-risk” alcohol consumption (ETOH Low). 31.8% (n = 21, 8 women) were heavy alcohol consumers compared to 68.2% (n=45, 27 women) who were not. There were no significant differences by ETOH level or age between ETOH None (n=11) versus moderate (n=34) participants on any cognitive domain examined (F’s(1, 45) < 1.4, p’s > .05). Age and years of education were not significantly different between the ETOH-heavy and ETOH-low-risk groups (p’s > .05). There was not a significant difference in racial composition between ETOH groups (p > .05). There were a greater percentage of women in the ETOH Low group (p>.05, addressed below in the statistical section). Demographic characteristics by ETOH grouping are presented in Table 1.
Table 1.
Sample Demographics by ETOH and Lifetime Alcohol Dependence History Groupings
| ETOH Group | Mean | Std. Dev. | Range | |
|---|---|---|---|---|
| Age | ETOH− (n=45) | 39.82 | 12.21 | 21–69 |
| ETOH+(n=21) | 35.38 | 10.20 | 22–54 | |
|
|
||||
| Dependence− (n=28) | 38.57 | 13.82 | 21–69 | |
| Dependence+ (n=32) | 38.84 | 9.87 | 22–56 | |
|
| ||||
| Education | ETOH− (w=27) | 13.84 | 2.80 | 8–20 |
| ETOH+ (w=8) | 13.05 | 2.94 | 7–18 | |
|
|
||||
| Dependence−* (w=18) | 14.61 | 2.47 | 11–20 | |
| Dependence+* (w=13) | 12.53 | 2.92 | 7–18 | |
ETOH+ = Heavy ETOH consumption, ETOH− = Non-Heavy ETOH Consumption, Dependence− = No history of alcohol dependence, Dependence+ = History of alcohol dependence, n = sample size, w = women,
p<.05
Drug and Alcohol Dependence
No participants were currently using cocaine or opiates based on self-report and urinalysis, and no participants met criteria for current cocaine or opiate dependence based on the Kreek-McHugh-Schluger-Kellogg scale (KMSK scale; Kellogg et al., 2003). KMSK scale was also used to assess lifetime history of alcohol dependence. The KMSK quantifies self-reported exposure to opiates, cocaine, alcohol, and/or tobacco. Each section of the KMSK scale assesses the frequency, amount, and duration of use of a substance during the person’s period of highest consumption. The scale also assesses the mode of use, whether the substance use is current or past, and whether each substance is the substance of choice. Six participants were excluded from Alcohol Dependence analyses because of incomplete KMSK scores. 53.3% (n = 32, 13 women) of the sample (sample n = 60) had a history of alcohol dependence, while 46.7% (n = 28, 18 women) did not have a lifetime history of alcohol dependence. 21.6% (n = 13) of participants with past history of alcohol dependence were currently alcohol dependent. Thus, 13 of 32 persons with current alcohol dependence overlapped with the total number of people with a lifetime history. Age was not significantly different between Lifetime Alcohol Dependence groups (p > .05), but education and sex was significantly different (p’s < .05; addressed below in statistical analyses). There was not a significant difference in racial composition between Dependence groups (p > .05). Demographic characteristics by Lifetime Alcohol Dependence grouping are presented in Table 1. It is important to note that Lifetime Alcohol Dependence was assessed based on positive or negative history of Alcohol Dependence, not a quantification of amount of alcohol consumed over the lifetime.
Neurocognitive Assessment
All participants completed a battery of standardized neuropsychological tests widely used in past studies by our group and others to assess the following cognitive domains: Speed of information processing, attention//executive functioning, learning, recall memory, verbal fluency, and psychomotor speed. The battery was comprised of the following tests chosen for their sensitivity to HIV-associated neurocognitive deficit (HAND): Hopkins Verbal Learning Test – Revised (HVLT-R; verbal learning and memory; Benedict et al., 1998, Brandt and Benedict, 1991) Brief Visuospatial Memory Test – Revised (BVMT-R; visuospatial learning and memory; Benedict, 1997); Controlled Oral Word Association Test (COWAT–FAS; verbal fluency; Benton et al., 1994) category fluency (animals; categorical verbal fluency); Stroop Color and Word Test; (attention/executive function; (Golden, 1978) Trails Making Test, Parts A and B; (executive function; Reitan, 1992); Letter-Number Sequencing (working memory) from the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III; Wechsler, 1997); Grooved Pegboard Test; (fine motor speed; Kløve, 1963) and the Digit Symbol–Coding and Symbol Search (speed of processing measures) tests from the WAIS-III (Wechsler, 1997). T-scores from delayed recall on the HVLT-R and BVMT-R were averaged to calculate the delayed recall domain. Learning trial performance (T-scores) on these two tasks was averaged to create the learning domain. COWAT and animal naming T-scores were averaged for the verbal fluency domain. Stroop, Letter-Number Sequencing, and Trails A and B T-scores were averaged to compute the attention/working memory/executive functioning domain. Digit Symbol–Coding and Symbol Search were averaged to calculate the speed of processing domain. The Grooved Pegboard Test T-score was used for the psychomotor speed domain.
Demographically (age, education, gender, race) corrected t-scores were calculated using established norms. A global index of neurocognitive function was calculated by averaging all domain composite T-scores.
Statistical Analyses
Statistical analyses were performed using SPSS-22 software (IBM). Demographic and clinical characteristics of the overall sample were determined, and differences in these characteristics among the ETOH Low and ETOH High groups examined using independent t-tests and χ2. Differences in neurocognitive performance as a function of alcohol grouping or lifetime alcohol dependence groups, and age were examined using general linear modeling. The primary analyses consisted of two way ANOVAs (e.g., ETOH × age or Dependence × age), in which the dependent measure was each of the domain scores and the global index. Age was dichotomized based on the median of the sample (median = 39 years) such that adults 40 years or older were compared to adults younger than 40 years. As age was corrected for using T-scores in the dependent measures, age was included in the models to specifically assess for abnormal change in the normal trajectory of age-related neurocognitive decline. Thus, presence of an age effect denotes exacerbation of normal age-related decline in neurocognitive function. Interactions and simple effects were examined based on the results of the overall ANOVAs. Both ETOH groupings and Lifetime dependence groupings had significant differences in the distribution of sex. Analyses including sex as a factor in each of the two way ANOVAs failed to show any significant interactions or main effects of sex on cognitive measures (p’s>.05). Thus, sex was not included in the models presented below. Tables 2 and 3 provide mean values in T-scores used for analyses. Except for Figure 1, T-scores were transformed into z-score format for ease of interpretation. Figure 1 depicts performance per domain by t-scores.
Table 2.
Cognitive Performance by ETOH and Age Groups (T-Scores)
| Domain Score | ETOH Group | Age Group | Mean | Std. Error | 95% Confidence Interval
|
|
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Global Cognition* | ETOH− | Younger | 48.274 | 1.644 | 44.987 | 51.560 |
| Older | 51.616 | 1.681 | 48.255 | 54.976 | ||
|
|
||||||
| ETOH+ | Younger | 51.666 | 2.107 | 47.453 | 55.878 | |
| Older | 45.489 | 2.980 | 39.532 | 51.446 | ||
|
| ||||||
| Speed of Processing | ETOH− | Younger | 52.551 | 1.887 | 48.779 | 56.322 |
| Older | 54.227 | 1.929 | 50.371 | 58.083 | ||
|
|
||||||
| ETOH+ | Younger | 54.381 | 2.418 | 49.547 | 59.215 | |
| Older | 48.643 | 3.420 | 41.807 | 55.479 | ||
|
| ||||||
| Attention/Executive | ETOH− | Younger | 53.841 | 1.755 | 50.333 | 57.348 |
| Older | 54.227 | 1.794 | 50.641 | 57.814 | ||
|
|
||||||
| ETOH+ | Younger | 54.381 | 2.249 | 49.885 | 58.877 | |
| Older | 49.714 | 3.181 | 43.356 | 56.073 | ||
|
| ||||||
| Learning* | ETOH− | Younger | 39.030 | 2.456 | 34.120 | 43.940 |
| Older | 44.545 | 2.511 | 39.524 | 49.565 | ||
|
|
||||||
| ETOH+ | Younger | 45.417 | 3.148 | 39.123 | 51.710 | |
| Older | 34.662 | 4.452 | 25.762 | 43.562 | ||
|
| ||||||
| Memory* | ETOH− | Younger | 38.762 | 2.632 | 33.501 | 44.024 |
| Older | 47.720 | 2.691 | 42.341 | 53.100 | ||
|
|
||||||
| ETOH+ | Younger | 45.072 | 3.374 | 38.328 | 51.816 | |
| Older | 38.097 | 4.771 | 28.560 | 47.634 | ||
|
| ||||||
| Verbal | ETOH− | Younger | 52.391 | 2.087 | 48.220 | 56.563 |
| Older | 55.932 | 2.134 | 51.667 | 60.197 | ||
|
|
||||||
| ETOH+ | Younger | 54.571 | 2.675 | 49.225 | 59.918 | |
| Older | 55.500 | 3.783 | 47.938 | 63.062 | ||
|
| ||||||
| Motor* | ETOH− | Younger | 48.217 | 2.292 | 43.635 | 52.800 |
| Older | 50.432 | 2.344 | 45.747 | 55.117 | ||
|
|
||||||
| ETOH+ | Younger | 53.857 | 2.938 | 47.984 | 59.730 | |
| Older | 43.643 | 4.155 | 35.337 | 51.949 | ||
ETOH+ = Heavy ETOH consumption, ETOH− = Non-Heavy ETOH Consumption, Std. = Standard, Younger = years of age < 40 years, Older = years of age ≥ 40 years, Age range of sample = 21–69 years,
= Significant age × ETOH interaction at p<.05
Table 3.
Cognitive Performance by ETOH and Age Groups (Raw-Scores)
| Test Name | ETOH Group | Age Group | Mean | Std. Error | Test Name | ETOH Group | Age Group | Mean | Std. Error |
|---|---|---|---|---|---|---|---|---|---|
| HVLT-R Learning | ETOH− | Younger | 23.65 | 1.23 | Trails A (sec) | ETOH− | Younger | 28.04 | 1.84 |
| Older | 23.91 | 0.93 | Older | 29.41 | 1.75 | ||||
|
|
|
||||||||
| ETOH+ | Younger | 25.50 | 1.48 | ETOH+ | Younger | 28.00 | 2.39 | ||
| Older | 18.86 | 1.72 | Older | 36.86 | 1.96 | ||||
|
| |||||||||
| HVLT-R Delayed | ETOH− | Younger | 7.91 | 0.59 | Trails B (sec) | ETOH− | Younger | 64.83 | 5.33 |
| Older | 9.00 | 0.44 | Older | 81.45 | 10.31 | ||||
|
|
|
||||||||
| ETOH+ | Younger | 8.93 | 0.69 | ETOH+ | Younger | 82.79 | 15.42 | ||
| Older | 7.00 | 0.78 | Older | 81.14 | 6.39 | ||||
|
| |||||||||
| BVMT-R Learning | ETOH− | Younger | 22.30 | 1.57 | Letter Number Sequencing | ETOH− | Younger | 10.74 | 0.74 |
| Older | 23.41 | 1.24 | Older | 10.23 | 0.71 | ||||
|
|
|
||||||||
| ETOH+ | Younger | 26.14 | 1.81 | ETOH+ | Younger | 11.64 | 0.85 | ||
| Older | 20.43 | 2.74 | Older | 9.43 | 1.08 | ||||
|
| |||||||||
| BVMT-R Delayed | ETOH− | Younger | 8.61 | 0.57 | Grooved Pegboard (D, sec) | ETOH− | Younger | 71.17 | 4.39 |
| Older | 9.27 | 0.51 | Older | 74.14 | 2.65 | ||||
|
|
|
||||||||
| ETOH+ | Younger | 9.86 | 0.74 | ETOH+ | Younger | 63.57 | 4.38 | ||
| Older | 7.71 | 1.12 | Older | 84.71 | 4.68 | ||||
|
| |||||||||
| COWAT | ETOH− | Younger | 37.43 | 2.55 | Grooved Pegboard (ND, sec) | ETOH− | Younger | 76.68 | 3.51 |
| Older | 40.86 | 2.98 | Older | 79.36 | 3.79 | ||||
|
|
|
||||||||
| ETOH+ | Younger | 40.86 | 3.14 | ETOH+ | Younger | 75.00 | 6.05 | ||
| Older | 37.29 | 4.05 | Older | 100.29 | 5.65 | ||||
|
| |||||||||
| Animal Naming | ETOH− | Younger | 21.96 | 1.51 | Digit Symbol Coding | ETOH− | Younger | 71.26 | 4.25 |
| Older | 21.00 | 0.95 | Older | 66.55 | 3.82 | ||||
|
|
|
||||||||
| ETOH+ | Younger | 22.69 | 1.72 | ETOH+ | Younger | 77.21 | 4.41 | ||
| Older | 19.71 | 1.97 | Older | 63.00 | 4.28 | ||||
|
| |||||||||
| Stroop Color and Word Test | ETOH− | Younger | 83.48 | 1.61 | Symbol Search | ETOH− | Younger | 38.87 | 2.02 |
| Older | 38.00 | 2.28 | Older | 32.55 | 1.57 | ||||
|
|
|
||||||||
| ETOH+ | Younger | 43.50 | 2.82 | ETOH+ | Younger | 39.36 | 3.20 | ||
| Older | 32.14 | 0.80 | Older | 28.71 | 1.88 | ||||
HVLT-R = Hopkins Verbal Learning Test – Revised, BVMT-R = Brief Visual Memory Test – Revised, COWAT = Controlled oral word association test, D = Dominant, ND = Non-Dominant, ETOH+ = Heavy ETOH consumption, ETOH− = Non-Heavy ETOH Consumption, Std. = Standard, Younger = years of age < 40 years, Older = years of age ≥ 40 years, Age range of sample = 21–69 years, sec = seconds
Figure 1.
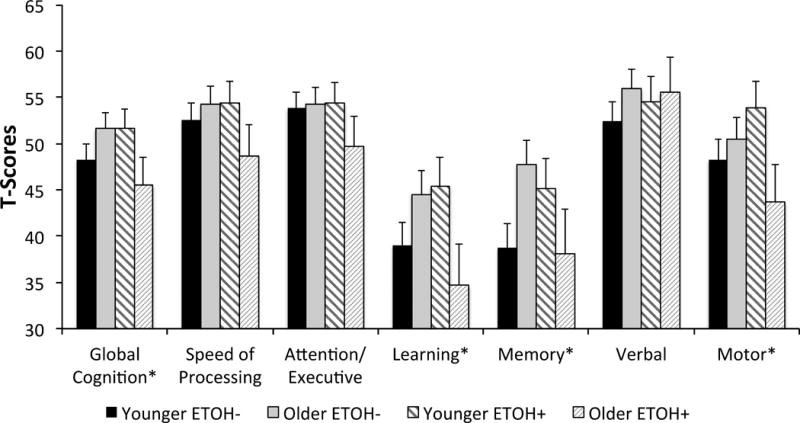
Cognitive performance by ETOH and age groups. T-score data are presented with standard error bars for each age and ETOH group. Although visually different, Younger ETOH− and Younger ETOH+ were not significantly different on Learning and Memory domains (F’s<2.1, p’s>.15).
RESULTS
Current alcohol consumption
Descriptive statistics for ETOH High and Low groups by age group are provided in Tables 2 (T-scores) and 3 (raw scores). The interactions of age by ETOH for global cognitive performance, learning, memory, and motor function are shown in Figures 2.
Figure 2.
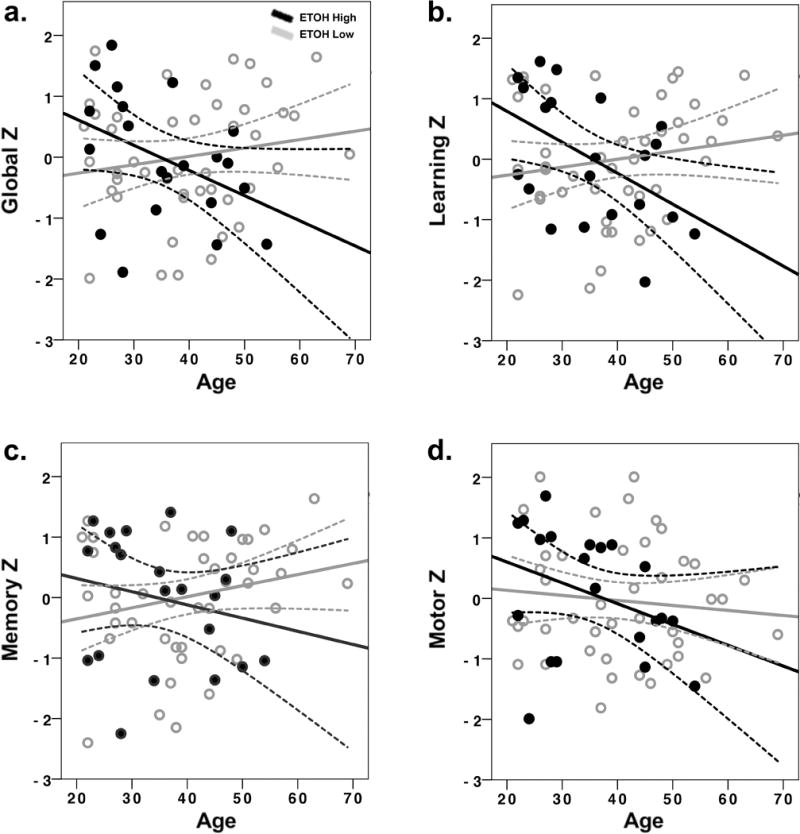
Effects of age and current alcohol consumption on neurocognitive domains. T-scores were converted to z-scores for ease of interpretation. Figure 2a: Global cognitive function, 1b: Learning, 1c: Memory, 1d: Motor. ETOH-High = Heavy alcohol consumption, ETOH-Low: None/ Moderate alcohol consumption. Dashed lines represent 95% confidence limits.
Global cognitive function
A significant age by ETOH interaction was found for global cognitive function (F(1, 62) = 4.80, p < .05, partial eta squared = .07). Overall cognitive performance varied as a function of level of alcohol consumption and age. Tests of simple effects revealed a significant effect of age on cognitive performance for the ETOH High group (p < .05). Heavy drinkers 40 years and older had lower global cognitive scores than younger heavy drinkers (Figure 2). There was not an age effect on cognitive performance for the ETOH Low group. Tests of simple effects conducted to examine ETOH High and ETOH Low groups further demonstrates this relationship between age and alcohol use. Cognitive performance did not vary as a function of level of alcohol consumption for participants under the age of 40 years. In contrast, cognitive performance differed between the ETOH High and ETOH Low groups for participants 40 years and older, with heavy drinkers showing lower cognitive scores (p <.05; Figure 2a).
Learning and Memory
A significant age by ETOH interaction was found for composite learning performance. (F(1, 62) = 6.30, p < .05, partial eta squared = .09, Figure 2b). Tests of simple effects revealed that among people in the ETOH High group, a significant age effect existed (p < .05). Heavy drinkers 40 years and older had lower learning scores than younger heavy drinkers. Age group effects were not evident for the ETOH Low group. Tests of simple effects conducted to compare ETOH High and ETOH Low separately for the young and older age groups indicated similar effects. Among young drinkers, ETOH High and ETOH Low did not differ significantly, whereas a significant ETOH effect existed among the older drinkers, with lower learning scores for the ETOH High group (p <.05).
A significant age by ETOH interaction was also found for composite memory performance (F(1, 62) = 5.25, p < .05, partial eta squared = .08). Heavy drinkers 40 years and older had lower memory recall score than younger heavy drinkers. An age effect was not evident for the ETOH Low group. Tests of simple effects conducted to compare ETOH High and ETOH Low separately for the young and older age groups indicated similar effects. Among young drinkers, ETOH High and ETOH Low did not differ significantly, whereas a significant ETOH effect existed among the older drinkers with lower memory scores for ETOH High group (p <.05). The interaction of age by ETOH for learning and memory is shown in Figures 2b and 1c.
Motor function
A significant age by ETOH interaction was also found for motor function (F(1, 62) = 4.2, p < .05, partial eta squared = .06, Figure 2d). Tests of simple effects comparing ETOH High and ETOH Low separately indicated that differences between the age groups existed for the ETOH High group (p <.05), but not the ETOH Low group. For the ETOH High group, older heavy drinkers had poorer fine motor function than younger heavy drinkers, whereas young and older adults in the ETOH Low group did not differ in their motor function.
Other cognitive functions
There were not interactions of age by ETOH for the verbal, speed of processing, or attention-executive domains (F’s(1, 62) < 2.2, p’s > .05). Accordingly for these cognitive domains, performance did not differ among young and older participates based on their level of current alcohol consumption. There were also not significant main effects for age or ETOH with respect for these cognitive domains (F’s(1, 62) < 0.8, p’s > .05).
Alcohol dependence
In subsequent analyses, the influence of lifetime alcohol dependence history was analyzed to determine whether dependence was also associated with reduced cognitive performance. Unlike current heavy alcohol use, lifetime history of alcohol dependence did not interact with age to adversely affect cognitive performance (F’s(1, 60) < 1.5, p’s >.22). There was also not a main effect of age (F’s(1, 60) < 1.5, p’s >.22). In contrast, the main effect of alcohol dependence was significant for global cognitive function (F(1, 60) = 7.35, p = .001, partial eta squared = .017; Figure 3a), learning (F(1, 60) = 7.35, p = .001, partial eta squared = .22; Figure 3b), memory (F(1, 60) = 7.35, p = .001, partial eta squared = .32; Figure 3c), motor function (F(1, 60) = 7.35, p = .001, partial eta squared = .12; Figure 3d), and attention/executive function (F(1, 60) = 7.35, p = .001, partial eta squared = .08; Figure 3e), with cognitive performance lower in people with a history of lifetime alcohol dependence (p’s < .05; Table 4). Lifetime alcohol dependence did not significantly affect verbal or speed of processing (p’s > .05).
Figure 3.
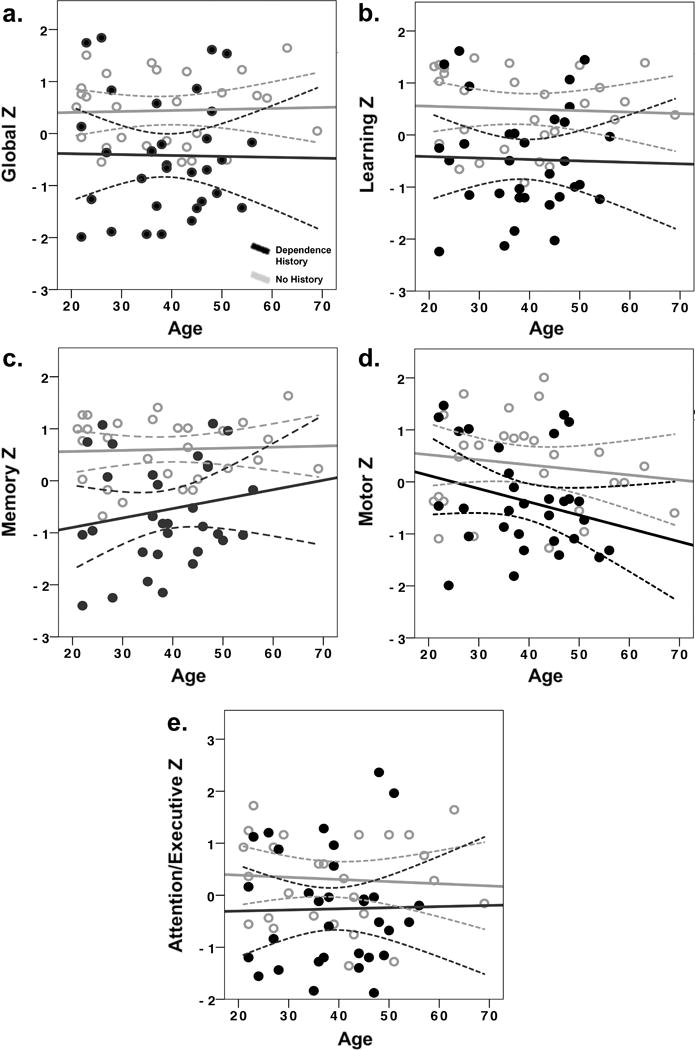
Effects of lifetime history of alcohol dependence on neurocognitive domains. T-scores were converted to z-scores for ease of interpretation. Figure 3a: Global cognitive function, 2b: Learning, 2c: Memory, 2d: Motor, 2e: Attention/Executive Function. Dependence History = lifetime alcohol dependence history. Dashed lines represent 95% confidence limits.
Table 4.
Cognitive Performance by Lifetime Alcohol Dependence History (T-Scores)
| Composite Cognitive Measure | ETOH DH | Mean | Std. Error | 95% Confidence Interval
|
|
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Global Cognition* | None | 53.313 | 1.463 | 50.382 | 56.243 |
| DH | 46.522 | 1.376 | 43.766 | 49.278 | |
|
| |||||
| Speed of Processing | None | 54.244 | 1.799 | 50.640 | 57.847 |
| DH | 51.990 | 1.692 | 48.601 | 55.379 | |
|
| |||||
| Attention/Executive* | None | 56.163 | 1.597 | 52.964 | 59.362 |
| DH | 51.390 | 1.502 | 48.382 | 54.399 | |
|
| |||||
| Learning* | None | 47.753 | 2.131 | 43.484 | 52.022 |
| DH | 36.216 | 2.004 | 32.202 | 40.231 | |
|
| |||||
| Memory* | None | 50.849 | 2.103 | 46.636 | 55.063 |
| DH | 36.226 | 1.978 | 32.263 | 40.188 | |
|
| |||||
| Verbal | None | 55.754 | 1.929 | 51.889 | 59.619 |
| DH | 52.855 | 1.814 | 49.221 | 56.490 | |
|
| |||||
| Motor* | None | 53.359 | 2.016 | 49.319 | 57.398 |
| DH | 45.629 | 1.896 | 41.830 | 49.428 | |
ETOH DH = Alcohol Dependence History, DH = Lifetime Alcohol Dependence History, None = No Dependence History, Std. = Standard,
= main effect of lifetime alcohol dependence history at p<.05
DISCUSSION
The results of this study indicate an interaction between quantity of current alcohol consumption and age with respect to global cognitive performance, as well as performance in the cognitive domains of learning, memory, and motor function. Current heavy drinkers who, by definition, consumed more alcohol on a weekly basis than the NIAAA threshold for “high-risk” drinking (ETOH High), exhibited greater cognitive deficits as a function of age compared to younger current heavy drinkers, and compared to adults who were current non-heavy drinkers or abstainers (ETOH Low). There was not an age association with cognitive performance for the ETOH Low group. Adults who were not currently heavy drinkers tended to have average cognitive performance, relative to demographically corrected normative values. The fact that people who did not drink alcohol at all did not differ significantly from people who consumed minimal to moderate quantities on any cognitive domain supports our original hypothesis that adverse cognitive effects would primarily be observed among current heavy drinkers.
That neurocognitive performance did not vary as a function of age in the ETOH Low group is perhaps not surprising given that the mean age of the study cohort was only 39 years, with no participants over the age of 65. Age-associated cognitive decrements are not expected among healthy adults during mid-life and are usually minimal until the seventh decade of life. The absence of aging associations in the ETOH Low group, after normative correction for age, education, and socioeconomic status, demonstrates that neither abstaining from alcohol nor non-heavy drinking altered the normal trajectory of cognitive aging. The observed age findings among heavy drinkers are more the anomaly, suggesting that people who consume large quantities of alcohol may be prone to premature cognitive aging.
Neurocognitive deficits in older current heavy drinking were not universal. Specifically, older current heavy drinkers had significantly lower performance on tasks related to learning, memory, and motor function. In contrast, attention/executive functions, verbal fluency, and speed of processing did not differ as a function of age and current alcohol consumption. In terms of motor function, the measure used in the current study was designed to assess psychomotor speed in a fine motor control task. Learning and memory composite scores were calculated using both visual and verbal learning and memory indices. These functionally specific results may provide insight into candidate neural structures for future investigations into the neural correlates of our findings, such as hippocampus, cerebellum, and primary and supplementary motor association cortices. As the functions of these brain regions are impacted acutely during heavy alcohol consumption (e.g., black-outs, loss of coordination, etc.), our data may suggest that these acute effects are more lasting in consequence.
In contrast to findings for current heavy alcohol consumption, lifetime history of alcohol dependence did not interact with age. Rather, neurocognitive deficits were evident in persons with a history of alcohol dependence irrespective of age. Global cognitive performance with specific deficits in learning, memory, motor function, and attention/executive function were associated with lifetime history of alcohol dependence. While neurocognitive effects of current heavy alcohol consumption appear to be exacerbated by age, long-term decline in cognitive function from lifetime history of alcohol dependence does not. Regardless, the same functions affected by current heavy consumption of alcohol were also affected in those with a lifetime history of alcohol consumption, in addition to attention/executive function. As with current heavy alcohol consumption, neurocognitive effects were not universal, with no evidence of change in speed of processing or verbal fluency. The consistency between current and lifetime history, as well as anecdotal reports of acute effects of heavy alcohol consumption, suggest that these patterns represent a consistent cascade of short and long term consequences from heavy alcohol consumption.
Our current findings provide evidence that the adverse effects of alcohol use on neurocognitive function may interact with both age and quantity of alcohol consumed. Heavy alcohol consumption appears to have adverse cognitive effects, whereas drinking minimal to moderate amounts of alcohol does not produce these associations, even in older adults. The fact that heavy alcohol effects on cognition were associated with age in a cohort that was less than 70 years of age suggests that very advanced age is not a prerequisite for these adverse effects, and that susceptibility may increase dramatically during mid-life. Evidence for greater compromise of neurocognitive function in older adults with current heavy alcohol consumption may have significant implications for personal and public health, as these individuals are likely more susceptible to decline in driving performance, increased rates of injury, hospitalization and dependence on assisted living, poorer medical outcomes, increased mortality rates, and other factors commonly associated with cognitive decline in older adults (Woods et al., 2013, Woods et al., 2011). Evidence for long-term consequences of alcohol dependence are also potentially important. These data suggest that those with a lifetime history of alcohol dependence may suffer deleterious effects that compromise neurocognitive function throughout life, not merely during acute periods of heavy alcohol consumption. However, the alternative is also possible and cannot be discounted in the current study. That is to say, premorbid deficits in neurocognitive function may predispose people toward alcohol abuse and dependence. It is also important to note that these findings are specifically relevant to the presence or absence of lifetime history of alcohol dependence, not a direct quantification of the amount of alcohol consumed over the lifetime. Such data may be important for further exploring these effects.
Furthermore, prior studies found mixed results when investigating the consequences of heavy alcohol consumption on neurocognitive function. Our results provide evidence supporting recent studies on the interaction of age and heavy alcohol consumption and extend our understanding of their neurocognitive consequences. This study provides strong evidence that heavy alcohol consumption has both short and long-terms consequence for neurocognitive function, and that these consequences increase with advancing age. Furthermore, our data suggest that heavy alcohol consumption is associated with accelerated cognitive aging.
Limitations and Future Directions
The population of non-infected but at-risk persons recruited from a larger ETOH focused study on HIV may represent a significant sampling bias that could exaggerate the impact of ETOH on cognitive function. However, these data also represent realistic insight into a population with high rates of ETOH abuse and thus are, at the very least, representative of similar populations. Use of normative data between groups to assess age effects over different test measures might be viewed as a limitation versus a matched sample control across groups. Future study of persons not at-risk for contracting HIV and HCV will help to support the applicability of these data to the population at large. In addition, longitudinal studies would allow for better understanding of the long-term consequences of these effects. Use of self-report measures of alcohol consumption and lifetime history of dependence may have introduced an extra degree of variability over objective measurement. However, such objective measures are often impossible, especially when assessing past alcohol consumption. These self-report measures may actually underestimate the level of current consumption and presence of past dependence. While this study demonstrates the neurocognitive consequences of heavy alcohol consumption, the structural, metabolic, and functional brain changes underlying long-term consequences of heavy alcohol consumption remains unclear. Furthermore, the causal direction of the relationship between neurocognitive function and alcohol abuse-dependence requires further study. As such, future studies are needed to characterize the relationship between alcohol-associated cognitive impairments versus cognitive deficit-associated increase in alcohol consumption, metabolic and functional brain abnormalities that can be assessed using neuroimaging and other methods, and the amount of recovery of function versus persistent brain dysfunction that is likely to occur with reduced alcohol consumption as people age.
Acknowledgments
Funding: The research was supported in part by the NIAAA P01AA019072, P30 AG028740, KL2TR001429, the McKnight Brain Research Foundation, and the UF Center for Cognitive Aging and Memory.
References
- Alcoholism NIoAAa. What’s at risk or “heavy drinking”. Available at: http://rethinkingdrinking.niaaa.nih.gov/IsYourDrinkingPatternRisky/WhatsAtRiskOrHeavyDrinking.asp.
- Alexander S, Kerr ME, Yonas H, Marion DW. The effects of admission alcohol level on cerebral blood flow and outcomes after severe traumatic brain injury. Journal of Neurotrauma. 2004;21:575–583. doi: 10.1089/089771504774129900. [DOI] [PubMed] [Google Scholar]
- Benedict RHB. Brief Visuospatial Memory Test-Revised. Psychological Assessment Resources; Odessa, FL: 1997. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. AJA Associates; Iowa City: 1994. [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised (HVLT-R) Psychological Assessment Resources, Inc; Lutz, FL: 1991. [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK, Group H. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004a;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, Grant I, Heaton RK, Group H. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol. 2004b;18:234–248. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- Devlin KN, Gongvatana A, Clark US, Chasman JD, Westbrook ML, Tashima KT, Navia B, Cohen RA. Neurocognitive effects of HIV, hepatitis C, and substance use history. J Int Neuropsychol Soc. 2012;18:68–78. doi: 10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer B, Jiang Y, Zanjani F, Fardo D. Effects of Alcohol Consumption on Cognition and Regional Brain Volumes Among Older Adults. Am J Alzheimers Dis Other Demen. 2014 doi: 10.1177/1533317514549411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AI, Butters N, Shear PK, Smith TL, Bondi M, Irwin M, Schuckit MA. Cognitive recovery with abstinence and its relationship to family history for alcoholism. J Stud Alcohol. 1995;56:104–109. doi: 10.15288/jsa.1995.56.104. [DOI] [PubMed] [Google Scholar]
- Draper B, Karmel R, Gibson D, Peut A, Anderson P. Alcohol-related cognitive impairment in New South Wales hospital patients aged 50 years and over. The Australian and New Zealand Journal of Psychiatry. 2011;45:985–992. doi: 10.3109/00048674.2011.610297. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Pennington DL, Schmidt TP, Mon A, Abe C, Meyerhoff DJ. Neurocognition in 1-month-abstinent treatment-seeking alcohol-dependent individuals: interactive effects of age and chronic cigarette smoking. Alcoholism, Clinical and Experimental Research. 2013;37:1794–1803. doi: 10.1111/acer.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The Timeline Follow-back reports of psychoactive substance use by drug-abusing patients: Psychometric properties. J Consult Clin Psych. 2000;68:134–44. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Friend KB, Malloy PF, Sindelar HA. The effects of chronic nicotine and alcohol use on neurocognitive function. Addict Behav. 2005;30:193–202. doi: 10.1016/j.addbeh.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Burchfiel CM, Reed DM, Wergowske G, Chiu D. A prospective study of the health effects of alcohol consumption in middle-aged and elderly men. The Honolulu Heart Program. Circulation. 1994;89:651–659. doi: 10.1161/01.cir.89.2.651. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop color and word test. Stoelting; Chicago: 1978. [Google Scholar]
- Green A, Garrick T, Sheedy D, Blake H, Shores EA, Harper C. The effect of moderate to heavy alcohol consumption on neurpopscyhological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcoholism, Clinical and Experimental Research. 2010;34:442–450. doi: 10.1111/j.1530-0277.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. Journal of Leukocyte Biology. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500–neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society: JINS. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Houston RJ, Derrick J, Leonard K, Testa M, Quigley B, Kubiak A. Effects of heavy drinking on executive cognitive functioning in a community sample. Addiction Behavior. 2014;39:345–349. doi: 10.1016/j.addbeh.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kist N, Sandjojo J, Kok RM, van den Berg JF. Cognitive functionaing in older adults with early, late, and very late onset alcohol dependence. Int Psychogeriatr. 2014;26:1863–1869. doi: 10.1017/S1041610214000878. [DOI] [PubMed] [Google Scholar]
- Kløve H. Grooved Pegboard. Lafeyette Instruments; Lafeyette, IN: 1963. [Google Scholar]
- Marksteiner J, Bodner T, Gurka P. Alcohol-induced cognitive disorder: alcohol dementia. Wiener medizinische Wochenschrift. 2002;152:98–101. doi: 10.1046/j.1563-258x.2002.01132.x. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Terayama Y, Konno S, Akiyama H, Margishvili GM, Mortel KF. Risk factors for cerebral degenerative changes and dementia. European Neurology. 1998;39(Suppl 1):7–16. doi: 10.1159/000052064. [DOI] [PubMed] [Google Scholar]
- Molina JA, Bermejo F, del Ser T, Jimenez-Jimenez FJ, Herranz A, Fernandez-Calle P, Ortuno B, Villanueva C, Sainz MJ. Alcoholic cognitive deterioration and nutritional deficiencies. Acta neurologica Scandinavica. 1994;89:384–390. doi: 10.1111/j.1600-0404.1994.tb02651.x. [DOI] [PubMed] [Google Scholar]
- O’Dell KM, Hannay HJ, Biney FO, Robertson CS, Tian TS. The effect of blood alcohol level and preinjury chronic alcohol use on outcome from severe traumatic brain injury in Hispanics, anglo-Caucasians, and African-americans. The Journal of Head Trauma rehabilitation. 2012;27:361–369. doi: 10.1097/HTR.0b013e318266735c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Capurso C, D’Introno A, Colacicco AM, Frisardi V, Lorusso M, Santamato A, Seripa D, Pilotto A, Scafato E, Vendemiale G, Capurso A, Solfrizzi V. Alcohol drinking, cognitive functions in older age, predementia, and dementia syndromes. J Alzheimers Dis. 2009;17:7–31. doi: 10.3233/JAD-2009-1009. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test. Reitan Neuropsychology Laboratory; Tucson, AZ: 1992. [Google Scholar]
- Riege WH, Holloway JA, Kaplan DW. Specific memory deficits associated with prolonged alcohol abuse. Alcoholism, Clinical and Experimental Research. 1981;5:378–385. doi: 10.1111/j.1530-0277.1981.tb04920.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Shih CL, Chi SI, Chiu TH, Sun GY, Lin TN. Ethanol effects on nitric oxide production in cerebral pial cultures. Alcoholism, Clinical and Experimental Research. 2001;25:612–618. [PubMed] [Google Scholar]
- Snow WM, Murray R, Ekuma O, Tyas SL, Barnes GE. Alcohol use and cardiovascular health outcomes: a comparison across age and gender in the Winnipeg Health and Drinking Survey Cohort. Age and Ageing. 2009;38:206–212. doi: 10.1093/ageing/afn284. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Baldassarre G, Scapicchio P, Scafato E, Amodio M, Capurso A, Panza F. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790–1799. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Malloy PF. Alcohol, head injury, and neuropsychological function. Neuropsychol Rev. 1992;3:249–280. doi: 10.1007/BF01109050. [DOI] [PubMed] [Google Scholar]
- Spirduso WW, Mayfield D, Grant M, Schallert T. Effects of route of administration of ethanol on high-speed reaction time in young and old rats. Psychopharmacology. 1989;97:413–417. doi: 10.1007/BF00439461. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Boissoneault J, Van Skike CE, Nixon SJ, Matthews DB. Age-related effects of alcohol from adolescent, adult, and aged populations using human and animal models. Alcoholism, Clinical and Experimental Research. 2014;38:2509–2516. doi: 10.1111/acer.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts change in neuropsychological functioning for adolescent rils and boys. Psychology of Addictive Behavior. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harris RA, Pfefferbaum A. Alcohol’s effects on brain and behavior. Alcohol Research and Health. 2010;33:127–43. [PMC free article] [PubMed] [Google Scholar]
- Tyas SL. Alcohol use and the risk of developing Alzheimer’s disease. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2001;25:299–306. [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Hungund BL. Endocannabinoid lipids and mediated system: implications for alcoholism and neuropsychiatric disorders. Life Sciences. 2005;77:1569–1583. doi: 10.1016/j.lfs.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Webb B, Heaton MB, Walker DW. Ethanol effects on cultured embryonic hippocampal neuronal calcium homeostasis are altered by nerve growth factor. Alcoholism, Clinical and Experimental Research. 1997;21:1643–1652. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III(WAIS-III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wilde EA, Bigler ED, Gandhi PV, Lowry CM, Blatter DD, Brooks J, Ryser DK. Alcohol abuse and traumatic brain injury: quantitative magnetic resonance imaging and neuropsychological outcome. Journal of Neurotrauma. 2004;21:137–147. doi: 10.1089/089771504322778604. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Beard DK, Wiren KM. Females uniquely vulnerable to alcohol-induced neurotoxicity show altered glucocorticoid signaling. Brain Res. 2015 doi: 10.1016/j.brainres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Cohen RA, Pahor M. Cognitive frailty: frontiers and challenges. The Journal of Nutrition, Health & Aging. 2013;17:741–743. doi: 10.1007/s12603-013-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Mark VW, Pitts AC, Mennemeier M. Pervasive cognitive impairment in acute rehabilitation inpatients without brain injury. PM & R: the journal of injury, function, and rehabilitation. 2011;3:426–432. doi: 10.1016/j.pmrj.2011.02.018. quiz 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Mennemeier M, Garcia-Rill E, Huitt T, Chelette KC, McCullough G, Munn T, Brown G, Kiser TS. Improvement in arousal, visual neglect, and perception of stimulus intensity following cold pressor stimulation. Neurocase. 2012;18:115–122. doi: 10.1080/13554794.2011.568498. [DOI] [PMC free article] [PubMed] [Google Scholar]


