Abstract
Summary: Gene therapeutic approaches to cure genetic diseases require tools to express the rescuing gene exclusively within the affected tissues. Viruses are often chosen as gene transfer vehicles but they have limited capacity for genetic information to be carried and transduced. In addition, to avoid off‐target effects the therapeutic gene should be driven by a tissue‐specific promoter in order to ensure expression in the target organs, tissues, or cell populations. The larger the promoter, the less space will be left for the respective gene. Thus, there is a need for small but tissue‐specific promoters. Here, we describe a compact unc45b promoter fragment of 195 bp that retains the ability to drive gene expression exclusively in skeletal and cardiac muscle in zebrafish and mouse. Remarkably, the described unc45b promoter fragment not only drives muscle‐specific expression but presents heat‐shock inducibility, allowing a temporal and spatial quantity control of (trans)gene expression. Here, we demonstrate that the transgenic expression of the smyd1b gene driven by the unc45b promoter fragment is able to rescue the embryonically lethal heart and skeletal muscle defects in smyd1b‐deficient flatline mutant zebrafish. Our findings demonstrate that the described muscle‐specific unc45b promoter fragment might be a valuable tool for the development of genetic therapies in patients suffering from myopathies. genesis 54:431–438, 2016. © 2016 The Authors. Genesis Published by Wiley Periodicals, Inc.
Keywords: unc45b, smyd1b, muscle, muscle diseases, zebrafish
Abbreviations
- AAV
adeno‐associated virus
- AP
alkaline phosphatase
- GFP
green fluorescent protein
- HCM
hypertrophic cardiomyopathies
- HMT
histone methyltransferase
- HRE
heat shock factor response elements
- HSF1
heat shock factor 1
- MCK
muscle creatine kinase
- RSV
Rous sarcoma virus
- SMYD1
SET and MYND domain containing protein 1
- TA
tibialis anterior.
In the context of heart and skeletal muscle disorders, the SET and MYND domain containing protein 1 (SMYD1) was identified as disease causing when mutated or defective (Abaci et al., 2010; Gottlieb et al., 2002; Just et al., 2011; Nagandla et al., 2016; Stewart et al., 2016). SMYD1 is exclusively expressed in cardiac and skeletal muscle cells from zebrafish and mice (Just et al., 2011; Nagandla et al., 2016) where it locates to the cell nucleus, as expected for a histone methyltransferase (HMT), and to the sarcomeric M‐band (Just et al., 2011). In zebrafish, Smyd1b was shown to be crucial to orchestrate thick filament assembly in cardiomyocytes and fast‐twitch skeletal muscle cells and when defective leads to severe cardiac and skeletal muscle dysfunction due to impaired myofibrillogenesis (Just et al., 2011; Li et al., 2013; Prill et al., 2015; Tan et al., 2006). Consistently, nullizygous SMYD1 mice die at embryonic day E10.5 due to severe heart malformations and cardiac dysfunction (Gottlieb et al., 2002). Conditional ablation of murine SMYD1 at the myoblast stage results in impaired myoblast differentiation accompanied with fewer myofibers and a reduction of the expression of muscle‐specific genes (Nagandla et al., 2016). By contrast, targeted SMYD1 elimination after myoblast differentiation leads to pronounced myopathy with severe myofibrillar disarray (Stewart et al., 2016). Interestingly, mutations in human SMYD1 were implicated to be involved in the development of hypertrophic cardiomyopathies (HCM) (Abaci et al., 2010).
To date, many genetic muscle diseases such as cardiomyopathies or muscular dystrophies, targeted gene therapy/transfer might be a promising strategy to attenuate pathological symptoms or even to cure the disease. Gene therapy often aims at reintroducing the wild‐type sequence of a deficient gene in the affected organs, tissues or cell populations. Therefore, the target sequence needs to be driven by effective tissue‐specific promoters, and packed into appropriate vectors such as viruses for their efficient delivery to and into the target cells (Kotterman and Schaffer, 2014).
For the successful delivery of the gene product into post‐mitotic cells such as cardiomyocytes or skeletal muscle cells, adeno‐associated virus (AAV)‐based vectors have emerged as safe and effective technologies. In this context, serotype AAV9 shows high cardiac‐ and skeletal muscle tropism (Asokan et al., 2012; Katwal et al., 2013; Riaz et al., 2015), maximizing transgene expression. Nevertheless, since systemic application of AAV9 vectors also lead to the transduction of other organ systems, tissue‐specific promoters are essential to minimize off‐target effects or overall toxicity of the transgene (Wang et al., 2008). For instance, dogs injected with AAV‐derived vectors harboring a Rous sarcoma virus (RSV) promoter driving alkaline phosphatase (AP) reporter show widespread AP expression in every muscle, but also in many other organs, like kidney, pancreas, and peripheral nerves (Yue et al., 2015). Most muscle‐specific promoters such as that of the muscle creatine kinase (MCK) gene are very large (6.5 kb), and thus incompatible with AAVs having a 4.5 kb capacity (Wu et al., 2010). Efforts have been made to shorten the MCK promoter, giving rise to the dMTK (509 bp) or tMCK (720 bp) promoter that show skeletal muscle‐specific expression (Yue et al., 2015) and the MHCK7 (770 bp) promoter that drives cardiac and skeletal muscle expression (Salva et al., 2007).
Unc45b is a cardiac and skeletal muscle‐specific myosin chaperone shown to be essential for proper thick filament assembly in C. elegans, zebrafish, and mice (Chen et al., 2012; Etard et al., 2015, 2007; Hoppe et al., 2004). In 2013, a 503 bp fragment driving unc45b endogenous expression was described but no regulatory sequences were discovered (Berger and Currie, 2013). We recently identified the relevant regulatory sequences recapitulating its endogenous expression (Etard et al., 2015). By systematically shortening the promoter sequence, we found that a 195 bp unc45b promoter fragment in combination with a gata2 minimal promoter drives expression of a reporter in the same patterns as that of the endogenous unc45b gene.
Here, we show that the described 195 bp unc45b promoter fragment without the gata2 minimal promoter is able to direct highly specific reporter‐gene expression in skeletal and cardiac muscle in transgenic zebrafish. Furthermore, we find that the promoter fragment is heat‐shock inducible allowing quantity control of transgene expression in a temporal and spatial manner. Remarkably, unc45b promoter‐driven expression of wild‐type zebrafish smyd1b in cardiomyocytes and skeletal muscle cells was sufficient to reconstitute heart and skeletal muscle development and function in the smyd1b‐deficient zebrafish mutant flatline (fla). Furthermore, we find that the unc45b promoter fragment also effectively mediates reporter gene expression in transduced mouse muscle. In summary, our findings suggest that the described compact unc45b promoter might have a high potential in AVV‐mediated gene transfer approaches to treat monogenic muscle diseases.
RESULTS
Very recently, we identified the regulatory sequences of the unc45b gene promoter driving reporter gene expression specifically in cardiomyocytes and skeletal muscle under basal conditions and after further induction in response to misfolded myosin (Fig. 1A) (Etard et al., 2015). We found that a 195 bp fragment termed −505‐310(unc45b)gata2:gfp efficiently mediates green fluorescent protein (GFP) expression in skeletal and cardiac muscle when inserted in front of the heterologous gata2 minimal promoter in transient as well as in stable expression analyses (Fig. 1B,C) (Etard et al., 2015).
Figure 1.
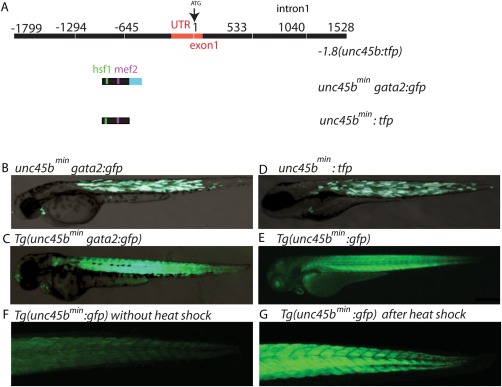
A small 195 bp unc45b promoter fragment drives cardiac and skeletal muscle expression. (A) Schematic representation of the full‐length unc45b promoter −1.8(unc45b:tfp), a 195 bp unc45b promoter fragment associated with gata2 (unc45bmingata2:gfp) and the 195 bp unc45b promoter fragment alone (unc45bmin:gfp). (B) Injection of unc45bmingata2:gfp into wild‐type embryos reveals GFP expression within cardiac and skeletal muscle (72 hpf). (C) Transgenic unc45bmingata2:gfp embryos Tg(unc45bmingata2:gfp) show a restricted expression in skeletal and cardiac muscles at 72 hpf. (D) Microinjection of unc45bmin:tfp into wild‐type embryos also reveals a restricted expression in skeletal and cardiac muscles (72 hpf). (E) Tg(unc45bmin:gfp) transgenic zebrafish embryos show heart‐ and skeletal muscle‐specific GFP expression (72 hpf). (F, G) Heat shock significantly increases GFP levels in Tg(unc45bmin:gfp) embryos (G) compared to untreated embryos (F) at 72 hpf.
Here, we further characterized these unc45b regulatory sequences and assessed a potential use of the minimal unc45b promoter for gene transfer/therapy approaches to treat cardiac and skeletal muscle diseases. We first tested in transient expression analyses whether the 195 bp unc45b fragment can act as promoter by inserting it upstream of the teal fluorescent protein coding region ((−505‐310(unc45b):tfp) hereafter named unc45bmin:tfp). This construct lacks the gata2 minimal promoter. Injection of the unc45bmin:tfp containing vector construct into fertilized zebrafish oocytes at the one‐cell stage led to a specific and strong TFP expression in skeletal muscle cells and cardiomyocytes (Fig. 1D), demonstrating that the used 195 bp unc45bmin fragment can act as promoter fragment and is able to drive strong reporter gene expression in a pattern identical to the endogenous unc45b promoter. To further prove this finding, we next generated a transgenic zebrafish line using the pDest‐Tol2pA2 vector backbone including the unc45bmin:gfp construct (Tg(unc45bmin:gfp)). Consistently, Tg(unc45bmin:gfp) zebrafish embryos exhibit GFP expression specifically in skeletal muscle cells and cardiomyocytes at 48 (data not shown) and 72 h post fertilization (hpf) (Fig. 1E). Additionally, we evaluated whether reporter gene expression can be induced in Tg(unc45bmin:gfp) embryos as observed in Tg(unc45bmingata2:gfp) fish (Etard et al., 2015). Indeed, we found that incubation of 48 h old Tg(unc45bmin:gfp) embryos with pre‐heated E3 medium (39°C) for 1 h significantly increased GFP expression compared to untreated Tg(unc45bmin:gfp) embryos (Fig. 1F,G). These findings clearly demonstrate that the compact 195 bp unc45bmin promoter fragment is able to recapitulate spatial expression of the full‐length zebrafish unc45b promoter (Etard et al., 2015) and that transcriptional activity can be efficiently induced by heat shock. Very recently, we demonstrated that heat shock factor response elements (HRE) in the unc45b promoter are indispensable for transcriptional inductivity. Furthermore, we showed that misfolded myosin seems to be the major trigger for the induction of the unc45b promoter mediated by the activation and recruitment of heat shock factor 1 (HSF1) proteins to its binding sites within the unc45b promoter (Etard et al., 2015).
Next, to assess whether the identified minimal unc45b promoter is able to direct sufficient wild‐type gene expression to rescue muscle mutant embryos, we used the smyd1b‐deficient flatline mutants, which display severe cardiac and skeletal muscle dysfunction due to impaired myofibrillogenesis (Just et al., 2011). We first generated a transgenic zebrafish line expressing a Smyd1b‐GFP fusion protein under the control of the compact 195 bp unc45bmin promoter (Tg(unc45bmin:smyd1b‐gfp)). As shown in Figure 1E, we found that Smyd1b‐GFP is specifically expressed in cardiomyocytes and skeletal muscle cells (Fig. 2A,B) and that the transgene localizes to the sarcomere in an alternating pattern to α‐actinin, an established Z‐disk marker (Fig. 2C). This finding suggests that similar to the subcellular localization of endogenous Smyd1b, transgenic Smyd1b‐GFP properly localizes to the sarcomeric M‐band (Just et al., 2011). Furthermore, similar to the situation in Tg(unc45bmin:gfp) zebrafish embryos, 1 h heat shock significantly increased GFP fluorescence intensity in Tg(unc45bmin:smyd1b‐gfp) embryos, indicating that the 195 bp unc45bmin promoter fragment can be utilized to titrate transgene expression levels in vivo (Fig. 2D,E) (Etard et al., 2015). Next, we crossed homozygous Tg(unc45bmin:smyd1b‐gfp) fish with heterozygous flatline (fla+/−) mutant fish and raised their offspring to adulthood. smyd1b‐deficient homozygous fla mutant embryos (fla−/−) are characterized by severe cardiac and skeletal muscle dysfunction caused by defective myofibrillogenesis in cardiomyocytes and skeletal muscle cells as depicted by an α‐actinin‐specific immunostaining and birefringence analyses (Fig. 3A–D) (Just et al., 2011). After genotyping and transgene detection, we incrossed Tg(unc45bmin:smyd1b‐gfp)/(fla+/−) fish and evaluated their offspring. Usually, homozygous flatline mutant embryos (fla−/−) can be detected already at 24 hpf by the lack of cardiac contractions and complete paralysis (Just et al., 2011). None of the investigated embryos exhibited the typical fla heart and skeletal muscle phenotype as demonstrated by measuring cardiac and skeletal muscle functionality (Fig. 3E–I, movie). Next, all individuals were first subjected to in vivo birefringence analysis, second to α‐actinin immunostainings to evaluate sarcomere organization and finally to a genotyping assay to identify homozygous flatline mutant embryos (fla−/−). Remarkably, we found that all fla−/− embryos carrying the smyd1b‐gfp transgene driven by the compact unc45bmin promoter show bright birefringence signals (Fig. 3G), suggesting that sarcomere organization is preserved in these individual fish. Indeed, α‐actinin immunostaining revealed regular sarcomerogenesis in fla−/− embryos (Fig. 3J). Furthermore, Smyd1b‐GFP regularly locates to the sarcomeric M‐band in the rescued fla−/− embryos (Fig. 3J). Together, these findings clearly demonstrate that unc45bmin promoter driven expression of smyd1b is able to structurally and functionally rescue the heart and skeletal muscle defects in smyd1b‐deficient fla mutant zebrafish embryos. Moreover, we found that Tg(unc45bmin:smyd1b‐gfp)/(fla−/−) fish can be raised to adulthood without showing an overt muscle phenotype (data not shown).
Figure 2.
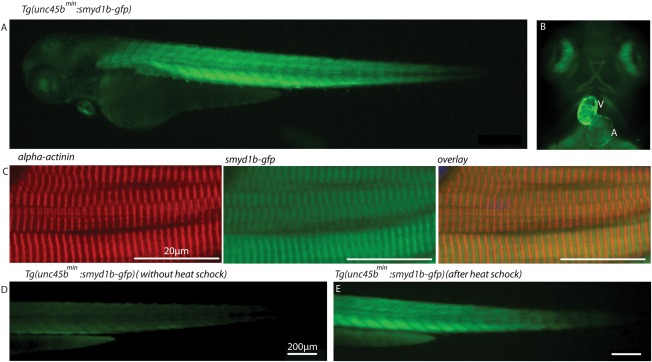
The transgenic zebrafish line Tg(unc45bmin:smyd1b‐gfp) exhibits Smyd1b expression specifically within cardiac and skeletal muscles. (A, B) At 72 hpf, Tg(unc45bmin:smyd1b‐gfp) transgenic embryos show strong and restricted expression of Smyd1b‐GFP fusion proteins within heart and skeletal muscles. A (Atrium), V (Ventricle). (C) α‐actinin‐specific immunostaining of Tg(Uncmin45b:smyd1b‐gfp) transgenic embryos reveals an alternating distribution of Smyd1b‐GFP and α‐actinin, suggesting Smyd1b localization at the sarcomeric M‐line (72 hpf). (D, E) smyd1b‐gfp expression was significantly enhanced by 1 h heat shock of the transgenic line Tg(unc45bmin:smyd1b‐gfp) (E) compared to untreated transgenic embryos (D) at 72 hpf.
Figure 3.
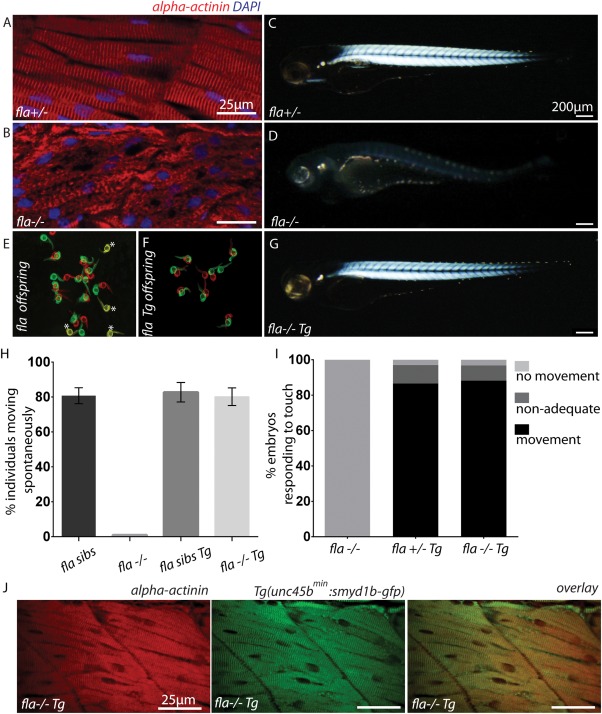
Functional and structural rescue of fla mutant embryos by the transgenic reintroduction of Smyd1b. (A, B) Immunostaining of fla siblings (A) and mutants (B) with α‐actinin‐specific antibodies (red) and DAPI (blue). Normal sarcomeric striation was visible in siblings, whereas complete lack of sarcomeric organization was found in homozygous fla mutant embryos. (C, D) fla siblings show strong birefringence signals at 72 hpf (C) whereas fla mutants lack proper sarcomeric organization and thereby birefringence signal (D). (E, F) Representative overview of spontaneous movement assays (at 24 hpf) with false‐colored and superimposed pictures of fla offspring derived from intercrossing heterozygous fla fish. Genotyping of these embryos revealed that all non‐moving (yellow*) embryos are homozygous fla mutants (E). By contrast, all homozygous fla mutant embryos carrying the transgene (Tg(unc45bmin:smyd1b‐gfp)) are able to move (F) (red to green shift). Red pictures 0 s, green pictures 10 s. (G) Transgenic reintroduction of Smyd1b (Tg(unc45bmin:smyd1b‐gfp)) into homozygous fla mutant embryos leads to normal birefringence signal. (H) Statistical analysis of the spontaneous movement assay. 80 ± 3.6% of genotyped fla siblings (fla+/+ and fla+/−) showed normal motility after 24 hpf (n = 111; three independent experiments). By contrast, homozygous fla mutant embryos are completely paralyzed (n = 35; three independent experiments). In comparison, 82.7 ± 5.6% of fla sibs (fla+/+ and fla+/−) (n = 98; three independent experiments) and 80.7 ± 4.6% of homozygous fla mutants carrying the transgene that expresses smyd1b showed normal spontaneous movements (n = 32; three independent experiments). Error bars indicate sd. (I) Quantification of the touch‐evoked flight response at 72 hpf. Homozygous fla mutants are completely paralyzed and do not flight upon tactile stimulation (n = 47; three independent experiments). 86.6% of fla siblings (fla+/+ and fla+/−) carrying the transgene (n = 61; three independent experiments) show regular motility at 72 hpf. Similarly, 88.2% of homozygous fla mutant embryos carrying the transgene (n = 26; three independent experiments) are able to respond regularly upon tactile stimulation. (J) α‐actinin‐specific immunostainings of skeletal muscles reveal normal sarcomeres in fla mutants carrying Tg(unc45bmin:smyd1b‐gfp), indicating a complete structural rescue by the transgenic reintroduction of Smyd1b in homozygous fla mutants.
Finally, to evaluate whether the zebrafish unc45bmin promoter is also capable to drive gene expression in mammalian myocytes, we transfected mouse hind limb tibialis anterior (TA) muscles with cDNA constructs encoding TFP under the control of different unc45b promoter fragments such as the full‐length unc45b promoter (−1.8(unc45b:tfp)), the compact 195 bp fragment (unc45bmin:tfp) and a 570 bp unc45b (−369 + 195(unc45b):tfp) fragment (Fig. 4A) (Etard et al., 2015). Ten days after electroporation, the mouse was anaesthetized, TA muscle was exposed and then examined by in vivo confocal fluorescence microscopy as described previously (Rudolf et al., 2012). Corroborating the observations in zebrafish, the truncated 195 bp fragment drove GFP expression with similar efficiency to that of the full‐length unc45b promoter (Fig. 4B), while the control promoter −369 + 195(unc45b):tfp (lacking the 195 bp fragment) did not mediate detectable GFP expression (Fig. 4B) (Etard et al., 2015). These findings demonstrate that the zebrafish 195 bp unc45bmin promoter fragment is able to drive reporter gene expression in mammalian muscle cells thereby suggesting a high potential of this compact muscle‐specific promoter for therapeutic gene transfer approaches in diseased muscles.
Figure 4.
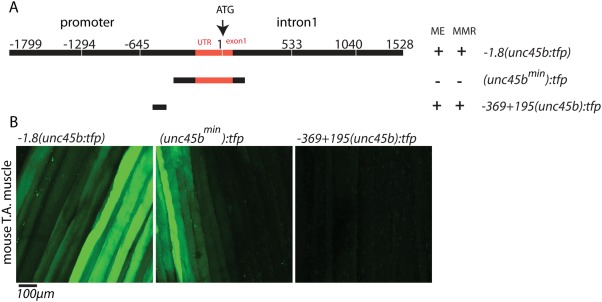
−505‐310(unc45b)tfp drive TFP expression within mouse muscle. (A) Scheme showing different unc45b promoter constructs used for electroporation into mouse muscles. (B) Electroporation of full‐length −1.8(unc45:tfp) and (unc45bmin):tfp constructs show expression of GFP in skeletal muscle fibers of mouse tibialis anterior (T.A) muscle, whereas −369 + 195(unc45b):tfp is not able to drive GFP expression.
MATERIAL AND METHODS
Fish Stock
Fish were bred and raised as previously described (Westerfield, 1993). The following mutant alleles were used: smyd1bzf340/zf340 (Just et al., 2011).
Microinjection
Microinjection was carried out as described (Müller et al., 1999), briefly, 1.5–2 nl of injection solution containing 20 ng/µl reporter plasmid DNA and 15 ng/µl Tol2 transposase mRNA, supplemented with 0.1% phenol red (injection marker), was injected into zebrafish eggs using a FemtoJet microinjector (Eppendorf).
Cloning
For −1.8(unc45b:tfp), −510‐310(unc45b)tfp, and −369 + 195(unc45b)tfp construct, see Etard et al. (2015).
The −505‐310(unc45b) promoter construct was amplified out of genomic DNA and cloned into the gateway 5′ entry p5E‐MCS. A full‐length cDNA encoding zebrafish smyd1b was amplified and cloned into pDONRzeo (Invitrogen). For transgenesis pDestTol2pA2 was used as backbone for multisite reaction with p3E‐EGFPpA as 3′ entry (Kwan et al., 2007). For each construct, at least six transgenic lines were identified and used for further analysis.
Histology, Immunohistochemistry, and Microscopy
The non‐invasive birefringence and touch‐evoke escape response analysis were carried out as described (Buhrdel et al., 2015). False‐colored and superimposed overviews of 24 hpf embryos were analyzed for spontaneous movement assays. Whole‐mount fluorescent immunostaining were carried out as described in (Inoue and Wittbrodt, 2011), embedded in JB‐4 (Polysciences) and 5 µm sections were cut. As primary antibodies we used: polyclonal rabbit anti‐GFP (1:200, Thermo Fisher Scientific, #A11122) and monoclonal mouse anti‐α‐actinin (1:10, Sigma Aldrich, #A7811). As secondary antibodies we used: goat anti‐rabbit IgG Alexa Fluor 488 and goat anti‐mouse IgG Alexa Fluor 555 (1:200, Thermo Fisher Scientific).
Heat shocks were performed for 1 h at 39°C with 48 hpf embryos. Heat shock images were taken at the Keyence BZ‐9000E, whole mount stainings at the Zeiss LSM710.
Mouse Skeletal Muscle Transfection and In Vivo Microscopy
All animals were kept and treated according to EU directives and national law. Adult male and female C57BL/10J were used for experiments. For cDNA electroporation into tibialis anterior muscle, the animals were anaesthetized under Isofluorane and the muscle was exposed for injection of a cDNA solution containing 10 µg of cDNA encoding TFP under the full‐length unc45b promoter (−1.8(unc45b:tfp)), short 195 bp fragment (unc45bmin:tfp), and 570 bp unc45b (−369 + 195(unc45b:tfp)) (Fig. 4A) (Etard et al., 2015). Post‐cDNA solution injection, electrodes connected to an electric pulse generator were placed under and above the T.A muscle and the muscles were subjected to five electric shocks, each of 20 V intensity, 20 ms duration, and 200 ms apart (Dona et al., 2003). Next the surgical opening of skin was sutured and 10 days post‐transfection, for in vivo microscopy anesthesia and preparation of mice were performed as described (Choi et al., 2012).
For the microscopic analysis of the TFP expression in T.A muscles, we used a DMRE TCS SP2 confocal microscope equipped with a KrAr laser (488 nm, 514 nm) and a 20×/0.7 N. A. HC PL APO CS IMM/CORR UV (immersion medium, Visc‐Ophtal gel; Dr. Winzer Pharma).
ACKNOWLEDGMENTS
We thank the Core Facility Confocal and Multiphoton Microscopy Ulm for their support. This research work is part of the project “Molecular Interaction Engineering: From Nature's Toolbox to Hybrid Technical Systems,” which is funded by the German Federal Ministry of Education and Research (BMBF), funding code 031A095 C.
LITERATURE CITED
- Abaci N, Gulec C, Bayrak F, Komurcu Bayrak E, Kahveci G, Erginel Unaltuna N. 2010. The variations of BOP gene in hypertrophic cardiomyopathy. Anatol J Cardiol 10:303–309. [DOI] [PubMed] [Google Scholar]
- Asokan A, Schaffer DV, Samulski RJ. 2012. The AAV vector toolkit: Poised at the clinical crossroads. Mol Ther 20:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Currie PD. 2013. 503unc, a small and muscle‐specific zebrafish promoter. Genesis 51:443–447. [DOI] [PubMed] [Google Scholar]
- Buhrdel JB, Hirth S, Kessler M, Westphal S, Forster M, Manta L, Wiche G, Schoser B, Schessl J, Schroder R, Clemen CS, Eichinger L, Furst DO, van der Ven PF, Rottbauer W, Just S. 2015. In vivo characterization of human myofibrillar myopathy genes in zebrafish. Biochem Biophys Res Commun 461:217–223. [DOI] [PubMed] [Google Scholar]
- Chen D, Li S, Singh R, Spinette S, Sedlmeier R, Epstein HF. 2012. Dual function of the UNC‐45b chaperone with myosin and GATA4 in cardiac development. J Cell Sci 125:3893–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KR, Berrera M, Reischl M, Strack S, Albrizio M, Roder IV, Wagner A, Petersen Y, Hafner M, Zaccolo M, Rudolf R. 2012. Rapsyn mediates subsynaptic anchoring of PKA type I and stabilisation of acetylcholine receptor in vivo. J Cell Sci 125:714–723. [DOI] [PubMed] [Google Scholar]
- Dona M, Sandri M, Rossini K, Dell'Aica I, Podhorska‐Okolow M, Carraro U. 2003. Functional in vivo gene transfer into the myofibers of adult skeletal muscle. Biochem Biophys Res Commun 312:1132–1138. [DOI] [PubMed] [Google Scholar]
- Etard C, Armant O, Roostalu U, Gourain V, Ferg M, Strahle U. 2015. Loss of function of myosin chaperones triggers Hsf1‐mediated transcriptional response in skeletal muscle cells. Genome Biol 16:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard C, Behra M, Fischer N, Hutcheson D, Geisler R, Strahle U. 2007. The UCS factor Steif/Unc‐45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev Biol 308:133–143. [DOI] [PubMed] [Google Scholar]
- Gottlieb PD, Pierce SA, Sims RJ, Yamagishi H, Weihe EK, Harriss JV, Maika SD, Kuziel WA, King HL, Olson EN, Nakagawa O, Srivastava D. 2002. Bop encodes a muscle‐restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nat Genet 31:25–32. [DOI] [PubMed] [Google Scholar]
- Hoppe T, Cassata G, Barral JM, Springer W, Hutagalung AH, Epstein HF, Baumeister R. 2004. Regulation of the myosin‐directed chaperone UNC‐45 by a novel E3/E4‐multiubiquitylation complex in C. elegans. Cell 118:337–349. [DOI] [PubMed] [Google Scholar]
- Inoue D, Wittbrodt J. 2011. One for all—A highly efficient and versatile method for fluorescent immunostaining in fish embryos. PloS One 6:e19713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just S, Meder B, Berger IM, Etard C, Trano N, Patzel E, Hassel D, Marquart S, Dahme T, Vogel B, Fishman MC, Katus HA, Strahle U, Rottbauer W. 2011. The myosin‐interacting protein SMYD1 is essential for sarcomere organization. J Cell Sci 124:3127–3136. [DOI] [PubMed] [Google Scholar]
- Katwal AB, Konkalmatt PR, Piras BA, Hazarika S, Li SS, John Lye R, Sanders JM, Ferrante EA, Yan Z, Annex BH, French BA. 2013. Adeno‐associated virus serotype 9 efficiently targets ischemic skeletal muscle following systemic delivery. Gene Ther 20:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotterman MA, Schaffer DV. 2014. Engineering adeno‐associated viruses for clinical gene therapy. Nat Rev Genet 15:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. 2007. The Tol2kit: A multisite gateway‐based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236:3088–3099. [DOI] [PubMed] [Google Scholar]
- Li H, Zhong Y, Wang Z, Gao J, Xu J, Chu W, Zhang J, Fang S, Du SJ. 2013. Smyd1b is required for skeletal and cardiac muscle function in zebrafish. Mol Biol Cell 24:3511–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Chang B, Albert S, Fischer N, Tora L, Strahle U. 1999. Intronic enhancers control expression of zebrafish sonic hedgehog in floor plate and notochord. Development 126:2103–2116. [DOI] [PubMed] [Google Scholar]
- Nagandla H, Lopez S, Yu W, Rasmussen TL, Tucker HO, Schwartz RJ, Stewart MD. 2016. Defective myogenesis in the absence of the muscle‐specific lysine methyltransferase SMYD1. Dev Biol 410:86–97. [DOI] [PubMed] [Google Scholar]
- Prill K, Windsor Reid P, Wohlgemuth SL, Pilgrim DB. 2015. Still heart encodes a structural HMT, SMYD1b, with chaperone‐like function during fast muscle sarcomere assembly. PloS One 10:e0142528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz M, Raz Y, Moloney EB, van Putten M, Krom YD, van der Maarel SM, Verhaagen J, Raz V. 2015. Differential myofiber‐type transduction preference of adeno‐associated virus serotypes 6 and 9. Skeletal Muscle 5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Hafner M, Mongillo M. 2012. Investigating second messenger signaling in vivo. Methods Enzymol 505:363–382. [DOI] [PubMed] [Google Scholar]
- Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM, Finn EE, Nguyen QG, Blankinship MJ, Meuse L, Chamberlain JS, Hauschka SD. 2007. Design of tissue‐specific regulatory cassettes for high‐level rAAV‐mediated expression in skeletal and cardiac muscle. Mol Ther 15:320–329. [DOI] [PubMed] [Google Scholar]
- Stewart MD, Lopez S, Nagandla H, Soibam B, Benham A, Nguyen J, Valenzuela N, Wu HJ, Burns AR, Rasmussen TL, Tucker HO, Schwartz RJ. 2016. Mouse myofibers lacking the SMYD1 methyltransferase are susceptible to atrophy, internalization of nuclei and myofibrillar disarray. Dis Models Mech 9:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Rotllant J, Li H, De Deyne P, Du SJ. 2006. SmyD1, a histone methyltransferase, is required for myofibril organization and muscle contraction in zebrafish embryos. Proc Natl Acad Sci USA 103:2713–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li J, Fu FH, Chen C, Zhu X, Zhou L, Jiang X, Xiao X. 2008. Construction and analysis of compact muscle‐specific promoters for AAV vectors. Gene Therpy 15:1489–1499. [DOI] [PubMed] [Google Scholar]
- Westerfield M. 1993. The zebra fish book. Eugene: University of Oregon Press. [Google Scholar]
- Wu Z, Yang H, Colosi P. 2010. Effect of genome size on AAV vector packaging. Mol Ther 18:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Pan X, Hakim CH, Kodippili K, Zhang K, Shin JH, Yang HT, McDonald T, Duan D. 2015. Safe and bodywide muscle transduction in young adult Duchenne muscular dystrophy dogs with adeno‐associated virus. Human Mol Genet 24:5880–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]


