Abstract
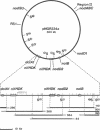
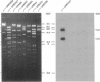
Many of the bacterial genes involved in nodulation (nod) and nitrogen fixation (nif) are dispersed over the 500-kilobase plasmid pNGR234a of the broad host-range Rhizobium species NGR234. As a first step toward generating a complete physical and genetic map of the plasmid, a full overlapping collection of cosmids was derived from a total genomic library. Clones were aligned by combining fingerprinting, hybridization, and pulsed-field gel electrophoresis data. Symbiotic loci were localized by probing a representative set of cosmids with both homologous and heterologous genes. nodABC, nodD1, nodD2, nodSU, nolB, and region II are widely dispersed over pNGR234a, while the two functional copies of nifKDH are separated by only 28 kilobases. Interestingly, sequences homologous to nodE, nodG, nodP, and nodQ have been assigned to another autonomously replicating element in Rhizobium species NGR234. Similarly one copy of the structural dctA gene is located on the symbiotic plasmid (dctA1) while the other is on what we assume to be the chromosome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Badenoch-Jones J., Holton T. A., Morrison C. M., Scott K. F., Shine J. Structural and functional analysis of nitrogenase genes from the broad-host-range Rhizobium strain ANU240. Gene. 1989 Apr 15;77(1):141–153. doi: 10.1016/0378-1119(89)90368-5. [DOI] [PubMed] [Google Scholar]
- Bassam B. J., Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Identification of a nodD-dependent locus in the Rhizobium strain NGR234 activated by phenolic factors secreted by soybeans and other legumes. Mol Plant Microbe Interact. 1988 Apr;1(4):161–168. doi: 10.1094/mpmi-1-161. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Brenner S., Livak K. J. DNA fingerprinting by sampled sequencing. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8902–8906. doi: 10.1073/pnas.86.22.8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton W. J., Dilworth M. J., Passmore I. K. Base ratio determination using unpurified DNA. Anal Biochem. 1972 Mar;46(1):164–172. doi: 10.1016/0003-2697(72)90408-3. [DOI] [PubMed] [Google Scholar]
- Broughton W. J., Heycke N., Z A H. M., Pankhurst C. E. Plasmid-linked nif and "nod" genes in fast-growing rhizobia that nodulate Glycine max, Psophocarpus tetragonolobus, and Vigna unguiculata. Proc Natl Acad Sci U S A. 1984 May;81(10):3093–3097. doi: 10.1073/pnas.81.10.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton W. J., Wong C. H., Lewin A., Samrey U., Myint H., Meyer H., Dowling D. N., Simon R. Identification of Rhizobium plasmid sequences involved in recognition of Psophocarpus, Vigna, and other legumes. J Cell Biol. 1986 Apr;102(4):1173–1182. doi: 10.1083/jcb.102.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cami B., Kourilsky P. Screening of cloned recombinant DNA in bacteria by in situ colony hybridization. Nucleic Acids Res. 1978 Jul;5(7):2381–2390. doi: 10.1093/nar/5.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon F. C., Riedel G. E., Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterized. Mol Gen Genet. 1979 Jul 2;174(1):59–66. doi: 10.1007/BF00433306. [DOI] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debellé F., Sharma S. B. Nucleotide sequence of Rhizobium meliloti RCR2011 genes involved in host specificity of nodulation. Nucleic Acids Res. 1986 Sep 25;14(18):7453–7472. doi: 10.1093/nar/14.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux C., Troiano R., Zito G., Devereux R. B., Kopecky K. J., Friedman R., Dowling P. C., Hafstein M. P., Rohowsky-Kochan C., Cook S. D. Effect of total lymphoid irradiation on functional status in chronic multiple sclerosis: importance of lymphopenia early after treatment--the pros. Neurology. 1988 Jul;38(7 Suppl 2):32–37. [PubMed] [Google Scholar]
- Edlund T., Normark S. Recombination between short DNA homologies causes tandem duplication. Nature. 1981 Jul 16;292(5820):269–271. doi: 10.1038/292269a0. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Fisher R. F., Jacobs T. W., Mulligan J. T., Long S. R. Nucleotide sequence of Rhizobium meliloti 1021 nodulation genes: nodD is read divergently from nodABC. DNA. 1985 Jun;4(3):241–248. doi: 10.1089/dna.1985.4.241. [DOI] [PubMed] [Google Scholar]
- Flores M., González V., Pardo M. A., Leija A., Martínez E., Romero D., Piñero D., Dávila G., Palacios R. Genomic instability in Rhizobium phaseoli. J Bacteriol. 1988 Mar;170(3):1191–1196. doi: 10.1128/jb.170.3.1191-1196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. J., Coulson A. R., Sulston J. E., Little P. F. Lorist2, a cosmid with transcriptional terminators insulating vector genes from interference by promoters within the insert: effect on DNA yield and cloned insert frequency. Gene. 1987;53(2-3):275–281. doi: 10.1016/0378-1119(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Harnish B. W. Transposition of a chromosomal segment bounded by redundant rRNA genes into other rRNA genes in Escherichia coli. J Bacteriol. 1982 Feb;149(2):449–457. doi: 10.1128/jb.149.2.449-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Bachem C. W., Schell J., Kondorosi A. Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 1987 Apr;6(4):841–848. doi: 10.1002/j.1460-2075.1987.tb04829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin A., Cervantes E., Chee-Hoong W., Broughton W. J. nodSU, two new nod genes of the broad host range Rhizobium strain NGR234 encode host-specific nodulation of the tropical tree Leucaena leucocephala. Mol Plant Microbe Interact. 1990 Sep-Oct;3(5):317–326. doi: 10.1094/mpmi-3-317. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morrison N. A., Hau C. Y., Trinick M. J., Shine J., Rolfe B. G. Heat curing of a sym plasmid in a fast-growing Rhizobium sp. that is able to nodulate legumes and the nonlegume Parasponia sp. J Bacteriol. 1983 Jan;153(1):527–531. doi: 10.1128/jb.153.1.527-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedock J., Long S. R. Nucleotide sequence and protein products of two new nodulation genes of Rhizobium meliloti, nodP and nodQ. Mol Plant Microbe Interact. 1989 Jul-Aug;2(4):181–194. doi: 10.1094/mpmi-2-181. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Sulston J., Mallett F., Staden R., Durbin R., Horsnell T., Coulson A. Software for genome mapping by fingerprinting techniques. Comput Appl Biosci. 1988 Mar;4(1):125–132. doi: 10.1093/bioinformatics/4.1.125. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Fountain J. W., Brereton A. M., Collins F. S. Direct construction of a chromosome-specific NotI linking library from flow-sorted chromosomes. Nucleic Acids Res. 1989 Feb 25;17(4):1665–1677. doi: 10.1093/nar/17.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]