Abstract
Plasmodium falciparum malaria is a deadly infectious disease in which antibodies play a critical role in naturally acquired immunity. However, at present the specificity and nature of antibodies elicited in response to malaria is only partially understood. Autoreactivity and polyreactivity are common features of antibody responses in several infections and have been suggested to contribute to effective pathogen-specific antibody responses. Here we report on the regulation of B cells expressing the inherently autoreactive VH4-34 heavy chain (identified by the 9G4 monoclonal antibody) and 9G4+ plasma IgG in adults and children living in a P. falciparum malaria-endemic area in West Africa. The frequency of 9G4+ peripheral blood CD19+ B cells was similar in U.S. adults and African adults and children. However, more 9G4+ B cells appeared in classical and atypical memory B cell compartments in African children and adults as compared to U.S. adults. The levels of 9G4+ IgG increased following acute febrile malaria, but did not increase with age as humoral immunity is acquired or correlate with protection from acute disease. This was the case even though a portion of 9G4+ B cells acquired phenotypes of atypical and classical memory B cells and 9G4+ IgG contained equivalent numbers of somatic hypermutations as compared to all other VHs, a characteristic of secondary antibody repertoire diversification in response to antigen stimulation. Determining the origin and function of 9G4+ B cells and 9G4+ IgG in malaria may contribute to a better understanding of the varied roles of autoreactivity in infectious diseases.
Introduction
Plasmodium falciparum malaria is a deadly infectious disease that takes the lives of nearly 600,000 individuals each year, nearly all African children and pregnant women (1). At present we have no highly effective vaccine for malaria (2) and understanding the nature of naturally acquired malaria immunity would contribute to vaccine development efforts (3). Abs play a central role in naturally acquired immunity to malaria as demonstrated by the passive transfer of Abs from malaria resistant adults to children with clinical malaria that resulted in a reduction in the levels of parasitemia and fever in these children (4). Individuals living in malaria endemic areas acquire protective Abs, but the process is remarkably slow requiring many years of repeated P. falciparum infections (5). The inefficient acquisition of protective Abs has been attributed to both the extensive genetic diversity of P. falciparum parasites (6) and to infection-mediated dysregulation of B cell responses (5, 7, 8). Understanding the mechanisms at play in the development of malaria immunity is limited by our incomplete understanding of the nature and specificities of the Abs elicited in response to malaria.
A common feature of protective Abs elicited during several viral infections is their auto- and poly-reactivity. High levels of autoreactive Abs have been described in several viral infections in humans including HIV, Epstein-Barr virus, hepatitis viruses and chicken pox, measles and mumps viruses (9). The relationship between autoreactivity and polyreactivity is perhaps best studied in Ab responses to HIV. The HIV envelope is highly diverse and the vast majority of HIV-specific Abs elicited in infected individuals are incapable of neutralizing multiple HIV viral clades (10). Although rare, many broadly neutralizing (bN) HIV envelope-specific mAbs have been isolated (11–13) and a recent study showed that auto- and poly-reactivity are significantly more frequent in the bNAb as compared non-neutralizing HIV-specific mAbs (14). These data suggest that auto- and poly-reactivity of bNAbs reflect the nature of the specific neutralizing epitope and are not the result of the HIV infection per se. Moreover, the observation that HIV bNAbs are auto- and poly-reactive supports the hypothesis that conserved neutralizing epitopes of HIV mimic host proteins to exploit immunological tolerance mechanisms that would remove B cells expressing Abs specific for such epitopes from the B cell repertoire (15). Auto- and poly-reactivity of HIV bNAbs may be beneficial and contribute to enhanced virus neutralization by allowing bivalent heteroligation between a high affinity HIV- envelope-specific Ab binding site and a low affinity interaction with other ligands on the viral surface (16).
These results raise the question, are auto- or poly-reactive Abs a component of immune responses in malaria. A variety of auto-Abs have been described in the serum of malaria-exposed individuals (17) and conversely, in at least one case, sera from individuals with a systemic autoimmune disease namely, systemic lupus erythematosus (SLE)2 reacted against P. falciparum parasites (18). Individuals with SLE have elevated levels of Abs encoded by the VH4-34 immunoglobulin heavy chain gene detected by the 9G4 idiotype-specific mAb (19, 20). 9G4+ Igs have an intrinsic autoreactivity that is primarily determined by the VH4-34 heavy chain (21) and recognize N-acetyl-lactosamine (NAL) epitopes expressed on a variety of glycoproteins (22–25). Indeed, 9G4+ Abs from individuals with SLE bind to a variety of self antigens including the NAL on the Ii antigen expressed on the surface of red blood cells, the same glycan on the abundant B cell surface protein, B220, according to distinct structural features of the 9G4+ Abs (21). It was recently shown that HIV-infected individuals had elevated 9G4+ Abs and that these 9G4+ Abs were HIV-envelope- specific but lacked many of the key self-reactive properties of 9G4+ Abs in individuals with SLE (26).
Indeed, a link has been suggested between the control of autoimmunity in Africans and malaria exposure. Greenwood et al. (27) observed a low incidence of rheumatoid arthritis and other systemic autoimmune diseases in tropical Africa and suggested that the African environment plays a part in protection from autoimmune disease, particularly the multiple parasitic infections that individuals are exposed to from childhood, key among these malaria (27). Taken together with the frequent occurrences of SLE in African Americans as compared to Americans of European descent (28) this observation suggests that the African genome may encode autoimmune susceptibility genes that are suppressed by malaria infection. This hypothesis was tested by Greenwood et al. (29) in animal models of spontaneous autoimmune disease and showed that infections with the nonlethal rodent parasite P. chabaudi had protective effects against the development of autoimmune disease in autoimmune susceptible (New Zealand Black × New Zealand White) F1 mice. Conversely, we provided evidence that a genetic susceptibility to autoimmunity in mice due to a deficiency in FcγRIIB or overexpression of Toll-like receptor 7 protected against lethal cerebral malaria caused by infection with the rodent parasite P. berghei ANKA (30). Despite enormous advances in the field of malaria immunology in recent years (31–33), the cellular and molecular mechanisms underlying these observations remain incompletely understood.
To address the role of autoreactivity in malaria we undertook an analysis of the expression of 9G4+ B cells and 9G4+ Ig in a cohort of malaria-exposed Malian children and adults. Our results provide evidence that as compared to U.S. adults, more 9G4+ B cells acquire the phenotypes of classical and atypical memory B cells and that 9G4+ VH contain similar numbers of somatic hypermutations, characteristic of antigen-driven diversification, as compared to all other VH. Even though the levels of 9G4+ Ig increase following acute febrile malaria, levels of 9G4+ Ig did not increase with age or correlate with protection from acute disease. Determining the origins and function of 9G4+ B cells and 9G4+ Ig in malaria may further our understanding of the possible roles of autoreactivity in infectious diseases.
Material and Methods
Study approval
The Ethics Committee of the Faculty of Medicine, Pharmacy, and Dentistry at the University of Sciences, Techniques, and Technologies of Bamako, and the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health approved this study. Written informed consent was received from participants prior to inclusion in the study. Written informed consent was obtained from parents or guardians of participating children prior to inclusion in the study.
Human samples
US adult PBMC and plasma samples were obtained from the NIH blood bank, where the medical history is unknown, but prior P. falciparum exposure is unlikely. The Malian samples were collected from children and adults enrolled in one of two multi-year longitudinal studies of the acquisition of immunity to malaria in two rural villages, Kambila (34) and Kalifabougou (35), Mali. For Fig. 1, adult peripheral blood samples were obtained from individuals enrolled in the Kambila study in 2009 and Malian children (6 year olds) peripheral blood samples were obtained from individuals enrolled in the Kalifabougou study in 2014. For Figs. 3B–E samples were from the Kambila study in 2009. Clinical malaria was defined as ≥ 2,500 asexual parasites/μL, an axillary temperature of ≥37.5°C or self-reported fever within 24 h, and no other cause of fever discernible by physical exam. For both the Kambila and Kalifabougou cohort studies, individuals with clinical signs and symptoms of malaria and parasitemia detected by microscopy were treated with anti-malarials according to the Malian National Malaria Control Program guidelines.
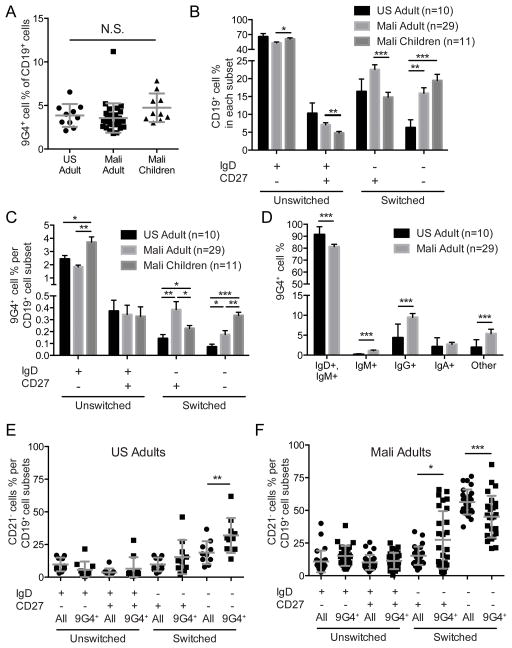
FIGURE 1.
Characterization of 9G4+ B cells in the peripheral blood of US adults and Malian adults and children. (A) Percent of CD19+ B cells that are 9G4+ in the PBMCs of U.S. adults and Malian adults and six year old children. Mean and standard deviation (SD) are shown. (B) Percent of all CD19+ B cells that are naïve (IgD+, CD27−), unswitched CD27+ (IgD+, CD27+), classical switched MBCs (IgD−, CD27+), or switched, CD27− B cells (IgD−, CD27−). Mean and standard error of the mean (SEM) are shown. (C) Percent of B cells in each subset shown in B that are9G4+. Mean and SEM are shown. (D) Percent of 9G4+ B cells that stained using anti-IgM, anti-IgD, anti-IgG or anti-IgA or were not stained by these reagents (Other). Mean and SEM are shown. (E–F) Percent of all CD19+ B cell in each subset (All) or percent of 9G4+ B cell in each subset (9G4+) that were CD21− for U.S. adults (E) and Malian adults (F). Mean and SD are shown. Statistical comparisons were by Student’s T-test (* = p-value < 0.05; ** = p-value < 0.005; *** = p-value < 0.0005).
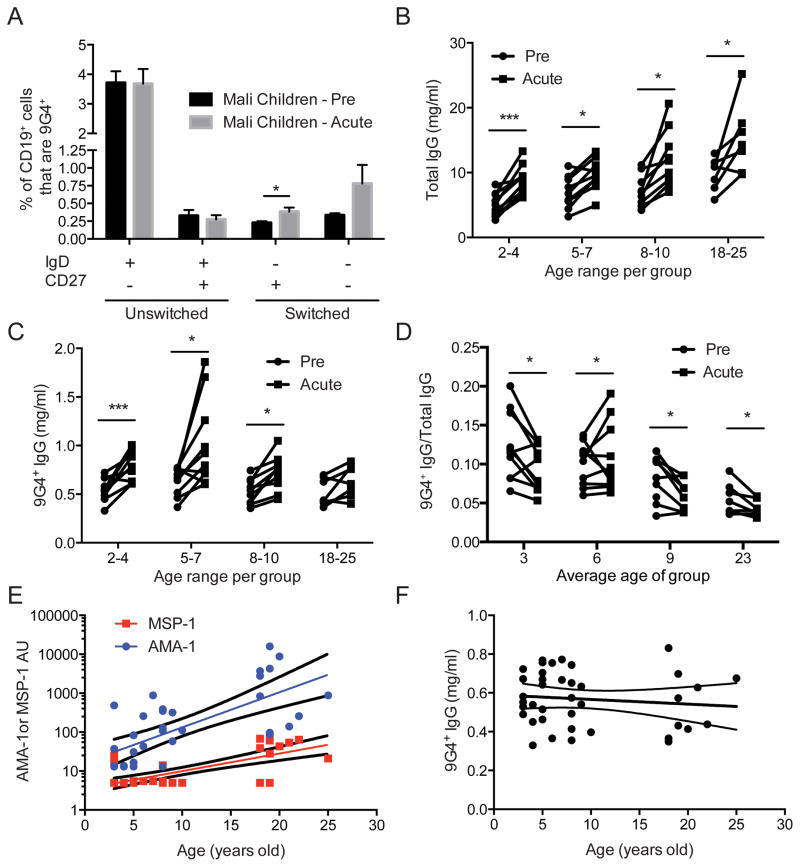
FIGURE 3.
Effect of acute febrile malaria on expression of 9G4+ B cells and 9G4+ IgG. (A) Percent of B cells that are 9G4+ in B cell subsets (as in Fig. 1C) in the PBMCs of 6 year old Malian children in May (Pre), just prior to the malaria transmission season and seven days following their first acute febrile malaria episodes (Acute). Mean and SEM are shown. Statistical comparisons were by Student’s T-test. (B) Total IgG in plasma from individuals prior to the malaria transmission season and 14 days after their first acute febrile malaria episode with subject data paired. (C) 9G4+-IgG levels in plasma samples from the individuals in B. Statistical comparisons were by Student’s T-test. (* = p-value < 0.05; ** = p-value < 0.005; *** = p-value < 0.0005) (D) The ratio of the levels of 9G4+ IgG (in C) to the levels of total IgG (in B). (E) Arbitrary ELISA units (AU) for MSP-142- and AMA1-specific IgG with age. Linear regressions and 95% confidence interval are shown. Both slopes are significantly greater than 0 (P value < 0.005). (F) 9G4+-IgG levels with age in the same subjects in D. Linear regression is shown with 95% confidence interval. The slope is not significantly different than 0.
PBMC and plasma processing
In Mali, citrate-containing cell preparation tubes (BD, Franklin Lakes, NJ) were used to collect venous blood from subjects. Samples were transported 45 km to the MRTC in Bamako, Mali where PBMCs and plasma were isolated per the manufacturer’s instructions. PBMCs were frozen in fetal bovine serum (FBS) (Gibco, Grand Island, NY) containing 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO), kept at −80°C for 24 h, and then stored in liquid nitrogen. Plasma was frozen in 1 ml aliquots at −80°C. These samples were then shipped to the US on dry ice for analysis. PBMCs were thawed and slowly diluted into complete media (RPMI + 10% FBS) by dropwise addition. Cells were counted using a hemacytometer and stained for flow cytometry analysis.
Antibodies and flow cytometry
For cell surface staining, PBMCs were washed in PBS and in PBS incubated with Live/dead fixable Aqua (Invitrogen, Carlsbad, CA) and fluorescently-labeled Ab as indicated (Supplementary Table 1). To identify 9G4+ B cells, B cells incubated with biotinylated 9G4 mAb, were washed in PBS, and the biotinylated 9G4 mAb was detected using Streptavidin-AF680. FACS analyses were performed on a BD LSR II flow cytometer (BD) and analyzed using FlowJo software (Tree Star, Inc, Ashland, OR).
ELISAs
9G4 ELISAs were carried out as described with some modifications (36). Briefly, Nunc Polysorb plates were coated with purified 9G4 mAb at 10ug/ml concentration overnight at 4°C. Plates were washed 3 times with PBS-Tween 0.1% and blocked with Super Blocking Solution (Thermoscientific, Waltham, MA) for 45 min at room temperature. Three serial 2-fold dilutions of samples in diluent (3 parts PBS, 1 part Super Block Solution, plus 0.1% Tween 20) starting at 1:4000 were incubated for 1 h at room temperature. Recombinant VH4-34 antibody (88F7) was used as a standard to allow quantification of 9G4+ Ig levels. After washing, plates were incubated with goat Abs specific for human IgG labeled with Alkaline Phosphatase (Sigma) at a dilution of 1:30,000 for 1 h at room temperature. Plates were washed and developing solution Blue Phospho substrate (KPL, Gaithersburg, MD) was added following manufacturer guidelines, and the optical density was measured at 650 nm using a microplate reader.
9G4 –IgM ELISAs were similar to 9G4-IgG ELISAs, except samples were at a starting concentration of 1:1000 and goat Abs specific for human IgM labeled with Alkaline Phosphatase (Sigma) were used at a dilution of 1:20,000. Results are presented as O.D. values were recorded. For total IgG and IgM ELISA, Nunc Polysorb plates were coated with either human anti-IgG (Jackson Immuno Research, West Grove, PA) or human anti-IgM (Jackson Immuno Research) in PBS at 2ug/ml and incubated overnight at 4 °C. Plates were washed and incubated with Superblock for 45 min at room temperature. Three serial 2-fold dilutions of samples in diluent starting at 1:64000 for IgG and 1:5000 for IgM were incubated for 1 h at room temperature. Eleven serial 2-fold dilutions of either IgG whole molecule (Jackson Immuno Research) or IgM whole molecule (Jackson Immuno Research) were used as standards, respectively. After washing, plates were incubated with either goat anti-human IgG antibody labeled with Alkaline Phosphatase or goat anti-human IgM antibody labeled with Alkaline Phosphatase for 1 h at room temperature. Plates washed and developing was performed as described above.
AMA-1 and MSP-142 ELISAs were performed as described previously (37). AMA1 was produced in Pichia pastoris and characterized as described (37). MSP-142 was produced in Escherichia coli, refolded and purified as described (38). Briefly, a mixture of FVO and 3D7 P. falciparum AMA1 and MSP-142 were used to coat the ELISA plates. The limit of detection for the ELISA was defined as the unit value at the lowest point on the standard curve multiplied by the dilution factor for plasma used. Eleven and 33 ELISA units were the minimal detection levels for MSP1 and AMA1 respectively. All data below the limit of detection were assigned 6 units for MSP1 and 17 units for AMA1 (approximately half the limit of detection).
IgH sequence analysis
Next generation sequencing of the IgH repertoires from three B cell populations, naïve, classical MBCs and atypical MBCs purified from the peripheral blood of four Malian adults was as previously described (39) (Sequencing Read Archive Accession No.: SRP087640, https://trace.ncbi.nlm.nih.gov/Traces/sra_sub/sub.cgi?acc=SRP087640&focus=SRP087640&from=list&action=show:STUDY). For the IgH sequence analysis of PBMCs of Malian infants and toddlers a molecular identifier, clustering-based immune repertoire sequencing (MIDCIRS) method was used to increase sequencing accuracy and minimize bias introduced during PCR amplification (Wendel et al. 2016 submitted for publication). The use of molecular identifiers to reduce the PCR and sequencing errors is similar to previously published methods (40). Consensus sequences were aligned to the IMGT antibody germline allele families, and possible novel germline alleles were detected via TIgGER (41). Sequences that mapped to possible novel germline alleles were excluded from these analyses. Mutations were counted as the Hamming Distance to the best aligned IMGT germline allele, excluding the CDR3 due to the difficulty determining the germline sequence. The average number of mutations was calculated weighted by the number of consensus sequences and reported for the V segment alone or for the V and J segments together (Sequencing Read Archive Accession No: phs001209, http://www.ncbi.nlm.nih.gov/gap).
Results
Characterization of 9G4+ B cells in U.S. adults and Malian adults and children
We compared the percentage of CD19+ B cells that expressed B cell receptors (BCRs) containing the VH4-34 heavy chain recognized by the 9G4 mAb (9G4+ B cells) in peripheral blood of adults living in the U.S. and adults and 6 year old children living in P. falciparum malaria-endemic Mali. The Malian adults and children were enrolled in one of two multi-year longitudinal studies of the acquisition of immunity to malaria (34, 35). 9G4+ CD19+ B cells were detected by flow cytometry using the VH4-34 idiotype-specific mAb, 9G4, by the gating strategy shown in Fig. S1A. The percentage of 9G4+ CD19+ B cells was similar in peripheral blood from U.S. adults and Malian adults and children, approximately 4% (Fig. 1A). Because 9G4+ B cells have been shown to be restricted to naïve B cells in healthy U.S. individuals but increased in the MBC compartment in individuals with SLE (42, 43), we determined which B cell subsets contained 9G4+ B cells. To do so we first analyzed the distribution of all CD19+ B cells from U.S. adults and Malian adults and children in four B cell subsets: naïve B cells (IgD+ CD27−); unswitched CD27+ B cells (IgD+ CD27+); classical switched MBCs (IgD− CD27+) and switched, CD27− B cells (IgD− CD27−) using the gating strategy shown in Fig. S1A. The greatest difference between the U.S. and Malian B cells was in the percent of all CD19+ cells that were in the switched, CD27− B cell (IgD− CD27−) subset (Fig. 1B). In U.S. individuals the percent of cells in this subset was low, approximately 5%. In contrast, switched, CD27− B cells represented between 15–20% of the CD19+ B cells in the peripheral blood of Malian adults and children. We also observed a small but significant decrease in the percent CD19+ B cells that were naïve and an increase in the unswitched and switched, CD27+ B cells in Malian adults as compared to Malian children.
We then determined the percent of 9G4+ B cells in each subset. The majority of the 9G4+ B cells in both U.S. adults and Malian adults and children were naïve B cells (Fig. 1C). Malian children had a significantly larger proportion of 9G4+ naïve B cells as compared to U.S. and Malian adults. Of particular interest, both Malian adults and children had a larger percent of both switched, CD27+ and switched, CD27− B cells that were 9G4+ as compared to U.S. adults. As predicted from the B cell subset distribution the majority of 9G4+ B cells in U.S. and Malian adults were IgD+ and IgM+ and a greater number of 9G4+ B cells were isotype-switched to IgG in Malian adults as compared to U.S. adults (Fig. 1D).
We previously described a population of CD27−, CD21− B cells that was greatly expanded in the peripheral blood of Malian adults and children but in low frequency in U.S. adults, which we termed atypical MBCs (39, 44). Following the gating strategy in Fig. S1C, we determined the percent of all CD19+ that were atypical MBCs (CD19+, IgD−, CD27−, CD21−) and the percent of 9G4+ cells that were atypical MBCs (Fig. 1E, F). Among U.S. adults, only ~20% of IgD−, CD27− B cells were CD21− atypical MBCs and approximately 30% of 9G4+ B cells were atypical MBCs (9G4+, IgD−, CD27−, CD21−). In contrast, among Malian adults more than 50% of IgD−, CD27− B cells were CD21− atypical MBCs and approximately 45% of 9G4+ B cells were atypical MBCs (9G4+, IgD−, CD27−, CD21−). In addition, a greater percentage of 9G4+ IgD− CD27+ B cells were CD21− as compared to all of the IgD− CD27+ B cells (Fig. 1F).
Taken together these results indicate that although the percent of all CD19+ B cells that are 9G4+ is similar in individuals living in the U.S. and in malaria-endemic Mali, the frequency of 9G4+ B cells in both the classical and atypical MBC compartments is larger in malaria-exposed individuals as compared to individuals in the U.S.
Characterization of 9G4+-IgG in U.S. and Malian adult plasma
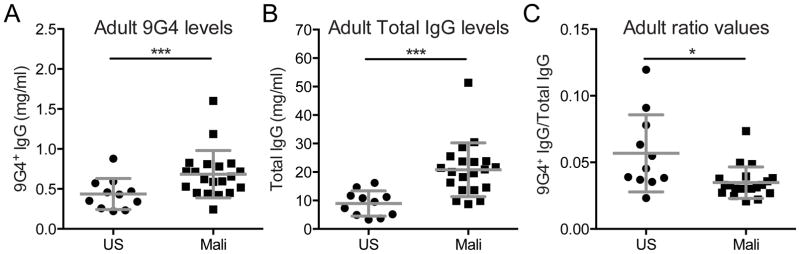
We quantified 9G4+-IgG in adult plasma samples and determined that Malian plasma contained significantly more 9G4+-IgG as compared to plasma from U.S. individuals (Fig. 2A). However, as the total amount of IgG was greater in Malian plasma (Fig. 2B) the mean proportion of plasma IgG that was 9G4+ was slightly less in plasma from adults in Mali as compared to plasma from adults in the U.S. (Fig. 2C). This result indicates that although the levels of 9G4+-IgG are elevated in Malians as compared to U.S. individuals, the 9G4+-IgG is not preferentially enriched in adult Malian plasma.
FIGURE 2.
9G4+ IgG and total IgG in US and Malian adult plasma. Quantification of IgG in the plasma of the same individuals in Fig. 1. (A) 9G4+ IgG (mg/ml) in plasma of US and Malian adults. (B) The total IgG (mg/ml) in US and Malian adult plasma. (C) The ratio of 9G4+ IgG and total IgG ([9G4+ IgG mg/ml]/[Total IgG mg/ml]) for each individual. Mean and SD are shown. Statistical comparisons were by Student’s T-test (** = p-value < 0.005; *** = p-value < 0.0005).
The effect of febrile malaria on 9G4+ B cells and 9G4+ IgG
We next determined the impact of acute febrile malaria on the subset distribution of 9G4+ B cells and on the levels of 9G4+-IgG. CD19+ 9G4+ B cells were quantified in the four B cell subsets in PBMCs collected from 6 year old children at the end of the six month dry season in Mali—during which there is negligible malaria transmission—and seven days after their first episode of acute febrile malaria during the ensuing transmissions season. We observed a small but statistically significant increase in the mean percentage of 9G4+ B cells in the switched, CD27+ MBC subset following acute malaria (Fig. 3A). The mean percentage of B cells in the switched, CD27− B cell subset (that contained approximately 60% atypical MBCs; Fig. 1F) also appeared to increase but this was not statistically significant.
We also determined the effect of acute malaria on the levels of 9G4+-IgG in the plasma in Malian children and adults (age 2–25 years). Our previous studies showed that Abs specific for a large number of P. falciparum antigens, as measured using protein microarrays, rise during the six month transmission season (45). However, in children, by the end of the subsequent dry season the Ab levels drop to levels just slightly above the previous dry season. This pattern continued year after year in children ultimately resulting in the acquisition of P. falciparum-specific Abs by early adolescence that were maintained through the dry season in adults. Assuming that the increase in stable antigen-specific Ab levels reflected increases in antigen-specific long-lived plasma cells, we concluded that most P. falciparum-specific Abs following acute malaria are produced by short-lived plasma cells but with age long-lived P. falciparum-specific plasma cells accumulate (5). In similar studies, we did not observe increases in the levels of IgG specific for an irrelevant antigen, namely tetanus toxoid, during the transmission season indicating a lack of obvious non-specific, polyclonal malaria-induced activation of B cell (46).
Consistent with these published data we observed that the levels of total IgG in plasma significantly increased in individuals following acute febrile malaria as compared to IgG levels in the same individuals at baseline before the malaria transmission season (Fig. 3B). In addition, the levels of total IgG at baseline increased with age as shown by a linear regression of the baseline total IgG levels with age (P value< 0.0001). Similarly the amount of 9G4+-IgG increased following acute febrile malaria in children three to 10 years of age but not significantly in adults (Fig. 3C). However, in contrast to the increase in total IgG with age, the amount of 9G4+-IgG in plasma at baseline before malaria did not increase with age (Fig. 3C). The ratios of 9G4+ IgG to the total IgG indicate that for most individuals the 9G4+ IgG increased less relative to the levels of total IgG following acute malaria (Fig. 3D). Comparing the levels of Abs specific for the P. falciparum antigens MSP-1 and AMA-1 to that of 9G4+-IgG in the same children we observed that the levels of both MSP-1- and AMA-1-specific Abs rose with age, as previously observed (46) (Fig. 3E). In contrast, the levels of 9G4+-IgG remained constant with increasing age (Fig. 3F).
Taken together these results suggest that the production of 9G4+-IgG following acute malaria is not selectively induced as many P. falciparum-specific Abs also increase following acute malaria as do the levels of total IgG. However, the stable levels of 9G4+-IgG appear to be differentially regulated. If the increase of P. falciparum-specific Ab levels with time is due to the accumulation of long-lived plasma cells as we have hypothesized (5) then the failure of 9G4+-IgG levels to increase with age suggests that 9G4-expressing plasma cells are not expanding and not entering into the long-lived plasma cell pool in malaria-exposed individuals.
The rate of somatic hypermutation (SHM) in 9G4+ Ig in Malian children and adults
SHM is an important characteristic of Ab repertoire secondary diversification due to antigen stimulation (47). To determine if 9G4+ Igs accumulated similar numbers of SHM as compared to all other VH we analyzed two data sets of high coverage IgVH repertoire sequences. The first was an analysis of SHM in B cells obtained from PBMCs of Malian infants (< 12 months old) and toddlers (13–42 months old) before and seven days after their first episode of acute febrile malaria [Wendel et al. 2016, submitted for publication]. The second was an analysis of SHM in three subpopulations of B cells in the PBMCs of four Malian adults, namely naïve B cells, conventional MBCs and atypical MBCs (39). For both data sets we determined the number of SHMs in the VH4-34 sequences and compared these to the number of SHMs in all other VH sequences (Fig. 4A,B).
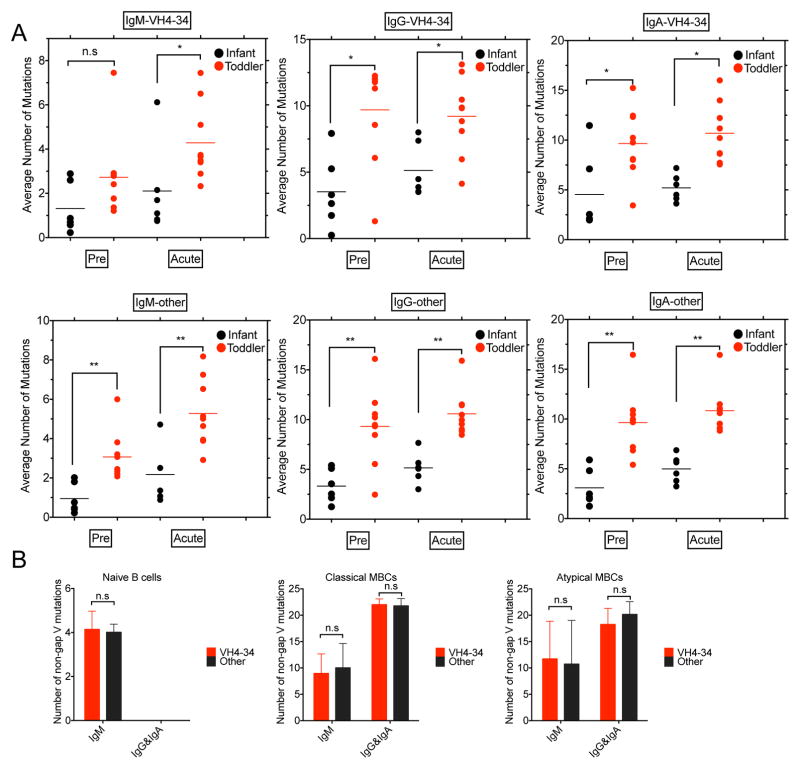
FIGURE 4.
Somatic hypermutation analysis of Malian young children and adult VH. (A) IgH sequence analysis for PBMCs acquired from 6 infants (3–11 months) and 9 toddlers (13–42 months) prior to the malaria transmission season (Pre) and 17 days following acute febrile malaria (Acute). Top panel. Average number of mutations in IgM-, IgG- and IgA-VH4-34. Bottom panel. Average number of mutations in IgM, IgG and IgA for all other VH genes. Statistical comparisons were by Mann Whitney U Test (ns = p value > 0.05; * = p value ≤ 0.05; ** = p value ≤ 0.01; *** = p value ≤ 0.001; **** = p value ≤ 0.0001). (B) Number of non-gap VH mutations for IgM and both IgG and IgA for naïve B cells, classical MBCs and atypical MBCs purified from the PBMCs acquired from four Malian adults for VH4-34 and all other VH.
The average number of SHMs in VH4-34 for both infants and toddlers were similar to that for all other VH for all isotypes with the IgMs expressing fewer SHMs than IgG and IgA for both VH4-34 and all other VH (Fig. 4A). For VH4-34 and for all other VH the number of SHMs were higher in toddlers as compared to infants before the malaria transmission season and after acute febrile malaria. The only exception was observed for VH4-34 IgM prior to the malaria transmission season in which case the number of SHMs were not significantly different in infants and toddlers. Given that the number of IgM SHMs in toddlers was higher than in infants following acute febrile malaria these data suggest that toddler VH4-34-IgM-expressing B cells that acquired SHMs following acute malaria did not detectably persist through the six month dry season in contrast to IgG and IgA VH4-34 and IgM, IgG and IgA of all other VH.
The number of SHMs in VH4-34 expressed by B cells in adults were similar to the number of SHMs in adult B cells expressing all other VH for each of the three B cell subpopulations analyzed, namely naïve, classical MBCs and atypical B cells (Fig. 4B). The number of SHMs in atypical and classical MBCs were greater than those in naïve B cells. Thus, even though the SHMs acquired in the VH4-34 IgM following acute malaria in young children appeared short lived as compared to the SHMs in VH4-34 IgG and IgA and in all other VH following acute malaria, B cells expressing VH4-34 IgM with acquired SHMs appear to eventually enter the long lived B cell pool.
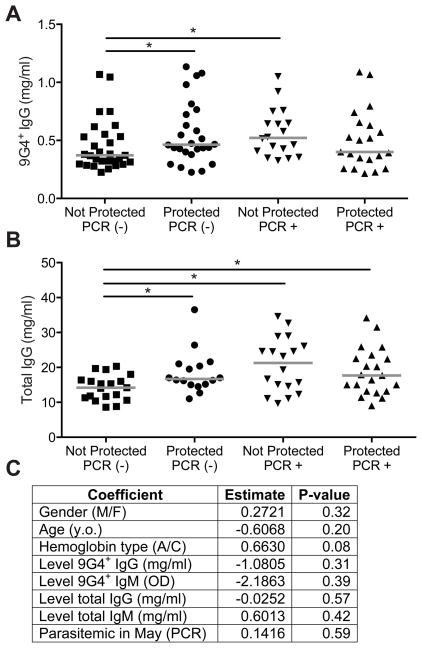
The levels of 9G4+ IgG in children resistant to or susceptible to febrile malaria
We determined the levels of 9G4+-IgG in children who were protected or not from febrile malaria. We focused on seven-nine year old children because children in this age group in our cohorts undergo a transition from malaria susceptible to malaria resistant (45). We analyzed the 9G4+-IgG in plasma samples taken at the end of the dry season before the malaria transmission season. Because we previously showed that P. falciparum PCR-positivity in asymptomatic children at the end of the dry season correlated with a decreased risk of febrile malaria during the ensuing transmission season (34), we separately analyzed asymptomatic children who were P. falciparum-infected and uninfected determined by PCR. The results showed that levels of 9G4+-IgG at the end of the dry season were slightly higher in children protected from acute febrile malaria during the ensuing transmission season as compared to susceptible children (Fig. 5A). This was only the case in children who were uninfected at the end of the dry season. However, the total IgG levels were also higher in protected PCR− children as compared to children who were not protected from febrile malaria (Fig. 5B). We then used logistic regression to test whether levels of 9G4+-IgG at the end of the dry season predicted whether or not a child would be resistant to acute febrile malaria in the ensuing malaria transmission season (Fig. 5C). First, 9G4+-IgG and -IgM and total IgG and IgM levels were tested individually in four simple logistic regressions, but none of the four models was significant. A multiple logistic regression model was built using stepwise model selection to determine whether any of the 9G4+-IgG or -IgM or total IgG and IgM levels were significant after adjustments for patient age, gender, Hb type and asymptomatic infections (PCR+) at the end of the dry season. The stepwise procedure did not identify any model with a significant relationship between malaria resistance and Ab levels. Model coefficients and their p-values are reported for the best fitting model (Fig. 5C), even though none of its coefficients were significant and its total R2 was low (R2 = 0.0855).
FIGURE 5.
Correlation of 9G4+ IgG levels and protection from acute febrile malaria. Seventy-four seven to nine year old children (nine seven year olds; 51 eight year olds; 14 nine year olds) were grouped as protected or not from acute malaria and either PCR+ for malaria parasites or not before the malaria season. (A). 9G4+-IgG levels in May at the end of the dry season prior to the malaria transmission season. Medians are shown. Statistical comparisons were by Mann-Whitney test. (* = p < 0.05) (B) Total IgG levels in May at the end of the dry season prior to the malaria transmission season. Medians are shown. Statistical comparisons were by Mann-Whitney test. (* = p < 0.05) (C) Multiple logistic regression testing whether 9G4+ IgG or 9G4+ IgM levels at the end of the dry season predicted resistance to acute febrile malaria during the ensuing malaria transmission season. Logistic regression was fit using JMP11.
Taken together with the observation that 9G4+-IgG levels did not increase with age as resistance to acute febrile malaria was acquired suggests that preexisting levels of 9G4+-IgG are not protective in malaria.
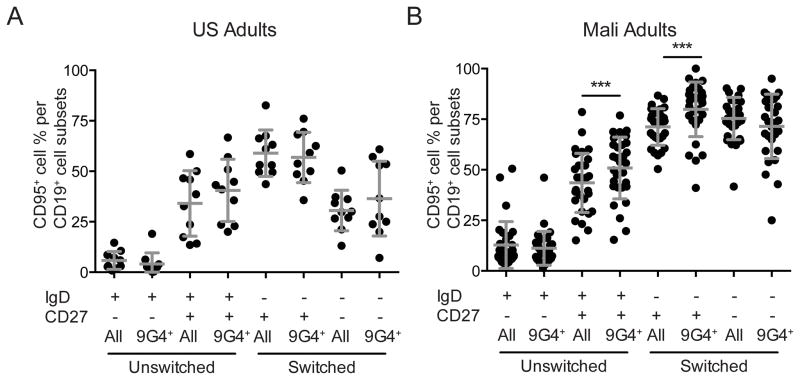
The expression of the apoptosis-inducing receptor CD95 on B cells
Having observed the failure of 9G4+-IgG to increase with age even though more 9G4+-B cells appeared to have differentiated into switched CD27+ classical and CD27− atypical MBCs, we analyzed B cells for the expression of CD95, the apoptosis-inducing receptor for Fas ligand (Fig. 6). We observed in Malian adults that the percent of B cells that express CD95 is significantly higher in 9G4+, IgD+ CD27+ B cells and in 9G4+ IgD−, CD27+ MBCs as compared to the same subsets of all CD19+ B cells. We did not observe any differences in CD95 expression in the same subpopulations in U.S. adults. This result suggests that 9G4+-B cells may be more sensitive to apoptosis and may as a consequence be short-lived.
FIGURE 6. The expression of CD95 in B cell subsets in US and Malian adults.
Percent of total (All) CD19+ B cells in each subset or percent of 9G4+ B cells in each subset (9G4+) that were CD95+ in the peripheral blood of A. US adults and B. Malian adults for individuals shown in Fig. 1A. The mean and SD are shown. Statistical analyses was by Student’s T-test. (* = p-value < 0.05 ** = p-value < 0.005, *** = p-value < 0.0005).
Discussion
To better understand the regulation of autoimmune Abs in individuals living in malaria endemic areas in Africa we followed the expression of the inherently autoreactive VH4-34, recognized by the mAb, 9G4, in adults and children enrolled in two different longitudinal studies of the acquisition of immunity to P. falciparum malaria. The regulation of 9G4+ B cells and 9G4+ Ig is of particular interest as 9G4+ auto-Abs expand in SLE patients in a disease specific fashion and recognize a variety of self antigens (21). The expansion of 9G4+ Abs in SLE appears to be due to a defect in the exclusion of autoreactive B cells from germinal centers (42, 43). In healthy individuals 9G4+ B cells are excluded from germinal center reactions at an early stage, in contrast to SLE patients in whom 9G4+ B cells successfully participate in germinal center reactions and expand in IgG MBC and plasma cell compartments. In individuals living with HIV, 9G4+ Abs are also prevalent and encode HIV-specific neutralizing Abs, however, these 9G4+ Abs appear to be distinct from those in SLE and their expansion in HIV-infected individuals does not appear to be due to a general breakdown in tolerance (26).
Here we report that although the frequency of 9G4+ CD19+ B cells in the peripheral blood of adults and children living in malaria endemic Mali are similar to those in healthy individuals living in the U.S., in malaria-exposed individuals 9G4+ B cells expand into what appear to be classical MBC and atypical MBC compartments. We would like to understand what factors drive the differentiation of 9G4+ atypical and classical MBCs and what function they might provide. The observation that VH4-34 accumulated SHMs in classical and atypical MBCs in adults to similar extents as all other VHs suggest that 9G4+ atypical and classical MBCs arise in response to antigenic stimulation. Although whether these cells respond to self-antigens or are cross-reactive with malarial antigens is not known. Atypical MBCs have been described in several chronic infections including HIV (48, 49) and TB (50) and in autoimmune diseases (51), common features of which are chronic exposure to antigen and inflammation. Clearly inflammation could play a role in driving 9G4+ atypical MBCs in malaria. Concerning function, our recently published results indicate that atypical MBCs are not able to be activated to produce Abs or cytokines and thus are predicted not to contribute to sustained Ab levels (39). Although we observed increased numbers of 9G4+ B cells with phenotypic markers of classical MBCs in Malian adults and children as compared to U.S. adults, the failure to develop stable levels of 9G4+-IgG suggests that the 9G4+-B cells may, in fact, not be expanding into functional long-lived MBCs or LLPCs. Further analysis of the 9G4+ IgD− CD27+ MBCs will be required to determine if these do indeed function as long-lived MBCs. Consistent with the possibility that these 9G4+-MBCs are not long-lived is the observation that they express higher levels of CD95 suggesting these cells may be more susceptible to apoptosis.
We also observed higher levels of 9G4+ IgG in Malian adults as compared to U.S. adults. However, the 9G4+ IgG did not appear to be selectively increased as the levels of total IgG were also increased. Importantly, the levels of 9G4+ IgG increased following acute febrile malaria. However, the levels of 9G4+-IgG did not increase with age as protection is acquired and the levels of 9G4+-IgG at the end of the dry season did not predict resistance to acute febrile malaria in the ensuing malaria transmission season. Thus, 9G4+-IgGs do not appear to play a role in establishing resistance to clinical malaria. The failure to increase with age is an interesting property of the 9G4+ IgG and suggests that the production of 9G4+ IgG may be differentially regulated to prevent the detrimental effects of the accumulation of high levels of auto-reactive IgG. Clearly, determining the origins and function of the 9G4+ B cells and 9G4+ Ig in malaria may contribute to a better understanding of autoreactive Abs in infectious diseases.
Supplementary Material
Acknowledgments
We thank the residents of Kambila and Kalifabougou, Mali for participating in this study.
Abbreviations used in this article
- SLE
systemic lupus erythematosus
- bN
broadly neutralizing
- MBC
memory B cell
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health and by grants 5R37AI049660 and U19 AI110483 Autoimmunity Center of Excellence awarded by the National Institutes of Health to I.S.
References
- 1.WHO. World malaria report 2014. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 2.Tran TM, Portugal S, Draper SJ, Crompton PD. Malaria Vaccines: Moving Forward After Encouraging First Steps. Current tropical medicine reports. 2015;2:1–3. doi: 10.1007/s40475-015-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol. 2014;32:157–187. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 5.Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol. 2013;190:3039–3046. doi: 10.4049/jimmunol.1203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol. 2009;31:560–573. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholzen A, Sauerwein RW. How malaria modulates memory: activation and dysregulation of B cells in Plasmodium infection. Trends Parasitol. 2013;29:252–262. doi: 10.1016/j.pt.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Hviid L, Barfod L, Fowkes FJ. Trying to remember: immunological B cell memory to malaria. Trends Parasitol. 2015;31:89–94. doi: 10.1016/j.pt.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Mouquet H, Nussenzweig MC. Polyreactive antibodies in adaptive immune responses to viruses. Cellular and molecular life sciences: CMLS. 2012;69:1435–1445. doi: 10.1007/s00018-011-0872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol GPI, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu M, Yang G, Wiehe K, Nicely NI, Vandergrift NA, Rountree W, Bonsignori M, Alam SM, Gao J, Haynes BF, Kelsoe G. Polyreactivity and autoreactivity among HIV-1 antibodies. J Virol. 2015;89:784–798. doi: 10.1128/JVI.02378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 16.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, Walker BD, Ho DD, Wilson PC, Seaman MS, Eisen HN, Chakraborty AK, Hope TJ, Ravetch JV, Wardemann H, Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel-Ribeiro CT, Zanini G. Autoimmunity and malaria: what are they doing together? Acta Trop. 2000;76:205–221. doi: 10.1016/s0001-706x(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 18.Zanini GM, De Moura Carvalho LJ, Brahimi K, De Souza-Passos LF, Guimaraes SJ, Da Silva Machado E, Bianco C, Junior, Riccio EK, De Sousa MA, Alecrim MD, Leite N, Druilhe P, Daniel-Ribeiro CT. Sera of patients with systemic lupus erythematosus react with plasmodial antigens and can inhibit the in vitro growth of Plasmodium falciparum. Autoimmunity. 2009;42:545–552. doi: 10.1080/08916930903039810. [DOI] [PubMed] [Google Scholar]
- 19.Isenberg D, Spellerberg M, Williams W, Griffiths M, Stevenson F. Identification of the 9G4 idiotope in systemic lupus erythematosus. British journal of rheumatology. 1993;32:876–882. doi: 10.1093/rheumatology/32.10.876. [DOI] [PubMed] [Google Scholar]
- 20.Isenberg DA, McClure C, Farewell V, Spellerberg M, Williams W, Cambridge G, Stevenson F. Correlation of 9G4 idiotope with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 1998;57:566–570. doi: 10.1136/ard.57.9.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson C, Chida AS, Adlowitz D, Silver L, Fox E, Jenks SA, Palmer E, Wang Y, Heimburg-Molinaro J, Li QZ, Mohan C, Cummings R, Tipton C, Sanz I. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J Immunol. 2013;191:4926–4939. doi: 10.4049/jimmunol.1202263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecomte J, Feizi T. A common idiotype on human macroglobulins with anti-I and anti-i specificity. Clin Exp Immunol. 1975;20:287–302. [PMC free article] [PubMed] [Google Scholar]
- 23.Silberstein LE, Jefferies LC, Goldman J, Friedman D, Moore JS, Nowell PC, Roelcke D, Pruzanski W, Roudier J, Silverman GJ. Variable region gene analysis of pathologic human autoantibodies to the related i and I red blood cell antigens. Blood. 1991;78:2372–2386. [PubMed] [Google Scholar]
- 24.Chapman CJ, Spellerberg MB, Smith GA, Carter SJ, Hamblin TJ, Stevenson FK. Autoanti-red cell antibodies synthesized by patients with infectious mononucleosis utilize the VH4-21 gene segment. J Immunol. 1993;151:1051–1061. [PubMed] [Google Scholar]
- 25.Parr TB, Johnson TA, Silberstein LE, Kipps TJ. Anti-B cell autoantibodies encoded by VH 4-21 genes in human fetal spleen do not require in vivo somatic selection. Eur J Immunol. 1994;24:2941–2949. doi: 10.1002/eji.1830241204. [DOI] [PubMed] [Google Scholar]
- 26.Alcena DC, Kobie JJ, Kaminski DA, Rosenberg AF, Mattiacio JL, Brewer M, Dewhurst S, Dykes C, Jin X, Keefer MC, Sanz I. 9G4+ antibodies isolated from HIV-infected patients neutralize HIV-1 and have distinct autoreactivity profiles. PLoS One. 2013;8:e85098. doi: 10.1371/journal.pone.0085098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwood BM, Herrick EM, Voller A. Can parasitic infection suppress autoimmune disease? Proc R Soc Med. 1970;63:19–20. doi: 10.1177/003591577006300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molokhia M, McKeigue P. Systemic lupus erythematosus: genes versus environment in high risk populations. Lupus. 2006;15:827–832. doi: 10.1177/0961203306070007. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood BM, Herrick EM, Voller A. Suppression of autoimmune disease in NZB and (NZB x NZW) F1 hybrid mice by infection with malaria. Nature. 1970;226:266–267. doi: 10.1038/226266a0. [DOI] [PubMed] [Google Scholar]
- 30.Waisberg M, Tarasenko T, Vickers BK, Scott BL, Willcocks LC, Molina-Cruz A, Pierce MA, Huang CY, Torres-Velez FJ, Smith KG, Barillas-Mury C, Miller LH, Pierce SK, Bolland S. Genetic susceptibility to systemic lupus erythematosus protects against cerebral malaria in mice. Proc Natl Acad Sci U S A. 2011;108:1122–1127. doi: 10.1073/pnas.1017996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley EM, V, Stewart A. Immune mechanisms in malaria: new insights in vaccine development. Nat Med. 2013;19:168–178. doi: 10.1038/nm.3083. [DOI] [PubMed] [Google Scholar]
- 32.Spence PJ, Langhorne J. T cell control of malaria pathogenesis. Curr Opin Immunol. 2012;24:444–448. doi: 10.1016/j.coi.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Cockburn IA, Zavala F. Dendritic cell function and antigen presentation in malaria. Curr Opin Immunol. 2016;40:1–6. doi: 10.1016/j.coi.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Crompton PD, Traore B, Kayentao K, Doumbo S, Ongoiba A, Diakite SA, Krause MA, Doumtabe D, Kone Y, Weiss G, Huang CY, Doumbia S, Guindo A, Fairhurst RM, Miller LH, Pierce SK, Doumbo OK. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, Niangaly M, Dara C, Kayentao K, Ongoiba A, Doumbo OK, Traore B, Crompton PD. An Intensive Longitudinal Cohort Study of Malian Children and Adults Reveals No Evidence of Acquired Immunity to Plasmodium falciparum Infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57:40–47. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wardemann H, Hammersen J, Nussenzweig MC. Human autoantibody silencing by immunoglobulin light chains. J Exp Med. 2004;200:191–199. doi: 10.1084/jem.20040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malkin E, Long CA, Stowers AW, Zou L, Singh S, MacDonald NJ, Narum DL, Miles AP, Orcutt AC, Muratova O, Moretz SE, Zhou H, Diouf A, Fay M, Tierney E, Leese P, Mahanty S, Miller LH, Saul A, Martin LB. Phase 1 study of two merozoite surface protein 1 (MSP1(42)) vaccines for Plasmodium falciparum malaria. PLoS clinical trials. 2007;2:e12. doi: 10.1371/journal.pctr.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Portugal S, Tipton CM, Sohn H, Kone Y, Wang J, Li S, Skinner J, Virtaneva K, Sturdevant DE, Porcella SF, Doumbo OK, Doumbo S, Kayentao K, Ongoiba A, Traore B, Sanz I, Pierce SK, Crompton PD. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife. 2015;4 doi: 10.7554/eLife.07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vollmers C, Sit RV, Weinstein JA, Dekker CL, Quake SR. Genetic measurement of memory B-cell recall using antibody repertoire sequencing. Proc Natl Acad Sci U S A. 2013;110:13463–13468. doi: 10.1073/pnas.1312146110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gadala-Maria D, Yaari G, Uduman M, Kleinstein SH. Automated analysis of high-throughput B-cell sequencing data reveals a high frequency of novel immunoglobulin V gene segment alleles. Proc Natl Acad Sci U S A. 2015;112:E862–870. doi: 10.1073/pnas.1417683112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–2182. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107:6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss GE, Traore B, Kayentao K, Ongoiba A, Doumbo S, Doumtabe D, Kone Y, Dia S, Guindo A, Traore A, Huang CY, Miura K, Mircetic M, Li S, Baughman A, Narum DL, Miller LH, Doumbo OK, Pierce SK, Crompton PD. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 2010;6:e1000912. doi: 10.1371/journal.ppat.1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 48.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kardava L, Moir S, Shah N, Wang W, Wilson R, Buckner CM, Santich BH, Kim LJ, Spurlin EE, Nelson AK, Wheatley AK, Harvey CJ, McDermott AB, Wucherpfennig KW, Chun TW, Tsang JS, Li Y, Fauci AS. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J Clin Invest. 2014;124:3252–3262. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joosten SA, van Meijgaarden KE, Del Nonno F, Baiocchini A, Petrone L, Vanini V, Smits HH, Palmieri F, Goletti D, Ottenhoff TH. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes following Successful Treatment. PLoS Pathog. 2016;12:e1005687. doi: 10.1371/journal.ppat.1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, Meffre E. Complement receptor 2/CD21− human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.