Abstract
In recent years, the impairment of RNA binding proteins that play key roles in the post-transcriptional regulation of gene expression has been linked to numerous neurological diseases. These RNA binding proteins perform critical mRNA processing steps in the nucleus, including splicing, polyadenylation, and export. In many cases, these RNA binding proteins are ubiquitously expressed raising key questions about why only brain function is impaired. Recently, mutations in the ZC3H14 gene, encoding an evolutionarily conserved, polyadenosine RNA binding protein, have been linked to a nonsyndromic form of autosomal recessive intellectual disability. Thus far, research on ZC3H14 and its Nab2 orthologs in budding yeast and Drosophila reveals that ZC3H14/Nab2 is important for mRNA processing and neuronal patterning. Two recent studies now provide evidence that ZC3H14/Nab2 may function in the quality control of mRNA splicing and export and could help to explain the molecular defects that cause neuronal dysfunction and lead to an inherited form of intellectual disability. These studies on ZC3H14/Nab2 reveal new clues to the puzzle of why loss of the ubiquitously expressed ZC3H14 protein specifically affects neurons.
Intellectual disability is a neurodevelopmental disorder characterized by reduced intellectual functioning (I.Q. ≤ 70) and deficits in adaptive behavior diagnosed by the age of 18 years [1-3]. Intellectual disability has been estimated to affect 1-3% of the population worldwide [4, 5]. Amongst the growing list of genes linked to intellectual disability (> 700) [2], many of these genes encode RNA binding proteins that play critical roles in post-transcriptional regulation of gene expression. Recently, mutations in the ZC3H14 gene, encoding an evolutionarily conserved, zinc finger polyadenosine RNA binding protein, have been linked to a severe nonsyndromic form of autosomal recessive intellectual disability (I.Q. ~30-50) [6]. Here, we highlight two recent studies [7, 8] on the role of the ZC3H14 protein and its budding yeast ortholog, Nab2, in the quality control of mRNA splicing and export that could provide insights into the function of ZC3H14 in the brain and how impairment of ZC3H14 could give rise to intellectual disability.
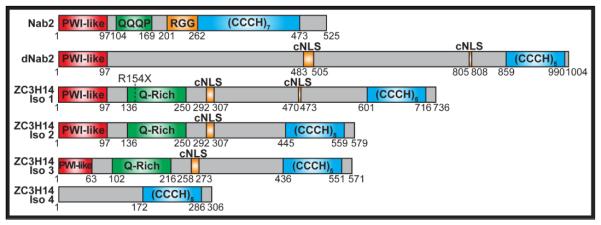
The human ZC3H14 (zinc finger CCCH domain-containing #14) protein, also known as MSUT2 [9], belongs to an evolutionarily conserved family of nuclear zinc finger polyadenosine RNA binding (Pab) proteins that include S. cerevisiae Nab2 and Drosophila dNab2 [6, 10, 11]. Splice variants of ZC3H14 give rise to at least four human ZC3H14 protein isoforms - Isoform 1-4; ZC3H14 Isoforms 1-3 are ubiquitously expressed in all tissues of the body while Isoform 4 is primarily expressed in the testes [12]. The best-characterized ZC3H14 mutation linked to intellectual disability creates a premature termination codon - R154X - that causes loss of expression of ZC3H14 Isoform 1-3 [6]. Human ZC3H14 Isoform 1-3, fly dNab2, and budding yeast Nab2 each contain an N-terminal PWI-like domain, a nuclear targeting signal (cNLS or RGG domain) and a C-terminal tandem CCCH zinc finger domain (Figure 1) [6, 10-14]. Studies on S. cerevisiae Nab2 have shown that Nab2 is an essential, nuclear protein that shuttles between the nucleus and cytoplasm and plays key roles in mRNA export, mRNA stability, and regulation of poly(A) tail length on bulk RNA [11, 13, 15-17]. The Nab2 PWI-like domain serves as a protein-protein interaction domain that binds nuclear pore-associated proteins, such as Mlp1, and other factors and is important for nuclear mRNA export [13, 14, 18, 19]. The Nab2 tandem zinc finger domain, containing seven CCCH zinc fingers, binds specifically to polyadenosine RNA with high affinity [10, 20]. Notably, nab2 N-terminal domain yeast mutants exhibit nuclear accumulation of poly(A) RNA and nab2 zinc finger mutants show extended bulk poly(A) tails [13, 15, 18, 20]. Like Nab2, the tandem zinc finger domains of ZC3H14 and dNab2, containing five zinc fingers, specifically bind to polyadenosine RNA [6, 10]. Importantly, cells depleted for ZC3H14 or dNab2 show extended bulk poly(A) tails, indicating that ZC3H14 and dNab2 also regulate poly(A) tail length [6, 21].
Figure 1. Domain structure of S. cerevisiae Nab2, Drosophila melanogaster dNab2, human ZC3H14 Isoform 1-4 polyadenosine RNA binding protein.
The ZC3H14/Nab2 proteins contain the following domains: an N-terminal Pro-Trp-Ile (PWI)-like fold domain (red), important for protein-protein interaction and mRNA export in Nab2 [13, 14, 18, 19, 31], a Glu (Q)-rich domain (green), an RGG motif/predicted classical nuclear localization signal (cNLS) (orange), important for localization to the nucleus in Nab2 [13], and a C-terminal tandem Cys3His (CCCH) zinc finger domain (blue), critical for specific binding to polyadenosine RNA [10, 11, 20]. The Arg154Stop (R154X) premature termination codon mutation in ZC3H14 linked to intellectual disability that eliminates ZC3H14 Isoform 1-3 [6] is depicted above ZC3H14 Isoform 1. Amino acid positions of domains are shown below each protein.
Studies on dNab2 using dNab2 mutant fly models have provided insight into the critical function of dNab2 in neurons. Mutant flies that lack dNab2 have reduced viability and locomotor activity, impaired short-term memory, and defects in the neuronal patterning in the learning and memory center (mushroom body) of the fly brain [6, 22]. Critically, expression of dNab2 only in the neurons of dNab2 zygotic mutant flies rescues the viability, locomotor activity and neuronal patterning in the flies, demonstrating that dNab2 is essential for proper neuronal function and also that expression of dNab2 only in neurons is sufficient to support proper neuronal function [6, 22]. In addition, neuronal expression of human ZC3H14 Isoform 1 in dNab2 mutant flies rescues function, indicating that ZC3H14 is a functional ortholog of dNab2 [21].
Work on ZC3H14 has shown that mouse ZC3H14 is enriched in murine hippocampal neurons that are critical for memory in the brain, supporting a role for ZC3H14 in neuronal function [6]. Moreover, mouse ZC3H14 colocalizes with poly(A) RNA in nuclear speckles, which are known centers of pre-mRNA processing, in rodent hippocampal neurons [6], suggesting ZC3H14 could function in neuronal RNA processing. Human ZC3H14 Isoform 1, but not Isoform 4, also localizes to nuclear speckles in mammalian cells [12]. Combined, these data suggest that ZC3H14 could regulate specific RNA processing steps to coordinate neuronal function and that ZC3H14 loss could lead to neuronal dysfunction and intellectual disability via dysregulation of neuronal RNA processing. Further studies on the molecular functions of ZC3H14 and its orthologs are therefore warranted to elucidate the critical role(s) of ZC3H14 in proper neuronal function.
To this end, recent studies by Soucek et al. on budding yeast Nab2 function [8] and Wigington et al. on human ZC3H14 function [7] suggest that ZC3H14/Nab2 plays a role in the quality control of mRNA splicing and export. Soucek et al. set out to determine whether Nab2 affects the splicing of mRNA transcripts in yeast cells and found that nab2 zinc finger mutant cells exhibit increased levels of unspliced intron-containing pre-mRNAs, but do not show a strong effect on splicing in vitro [8]. In addition, Soucek et al. identified physical and genetic interactions between Nab2 and splicing factors in yeast cells [8], most notably the Mud2 and Msl5 proteins - the budding yeast orthologs of human U2 snRNA auxiliary factor 2 (U2AF2)/U2AF65 and branchpoint binding protein (BBP)/splicing factor 1 (SF1) - that recognize the branchpoint sequence in introns [23-26]. Importantly, Soucek et al. also identified physical interactions between mouse ZC3H14 and splicing factors in mouse brain, including U2AF2, supporting a conserved link between ZC3H14 and splicing in neuronal cells [8]. Finally, Soucek et al. observed that the function and pre-mRNA splicing defects of nab2 mutant cells are rescued by inactivation of the Rrp6 riboexonuclease subunit of the nuclear RNA exosome [8] - a conserved ribonuclease complex that is critical for RNA processing/degradation of non-coding RNA and pre-mRNA [27-30].
Combined, these results from Soucek et al. suggest a model (Figure 2) for ZC3H14/Nab2 quality control of mRNA splicing and export where ZC3H14/Nab2 binds the poly(A) tail of transcripts and detects improperly spliced and unspliced pre-mRNA via interaction with early splicing factors, such as branchpoint recognition factors, U2AF2/Mud2 and BBP/Msl5. ZC3H14/Nab2 binding to the splicing factors on the pre-mRNA could mark the transcript as unspliced, cause the retention of the transcript, and trigger the recruitment of the RNA exosome to the transcript for degradation. If ZC3H14/Nab2 does not bind to the splicing factors, the transcript could be marked as spliced and ZC3H14/Nab2 and other export factors could stabilize and remodel the transcript and target the transcript to the nuclear pore complex for export to the cytoplasm. Reduced RNA binding by ZC3H14/Nab2 due to disruption of Nab2 zinc fingers or depletion of ZC3H14 would be predicted to impair detection of unspliced pre-mRNA in the nucleus, leading to pre-mRNA escape from degradation, pre-mRNA accumulation and disruption of cellular function.
Figure 2. Model for ZC3H14/Nab2 function in quality control of mRNA splicing and export.
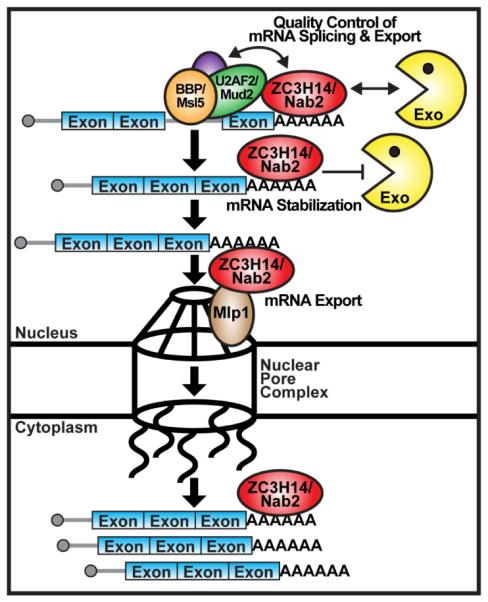
ZC3H14/Nab2 binds to the poly(A) tail of a transcript and checks for interaction with early splicing factors, such as branchpoint recognition factors, U2AF2/Mud2 and BBP/Msl5, to detect improperly spliced and unspliced pre-mRNA. If ZC3H14/Nab2 binds to splicing factors on the pre-mRNA, ZC3H14/Nab2 could mark the transcript as unspliced, retain the transcript in the nucleus, and trigger recruitment of the RNA exosome (Exo) to the transcript for degradation. If ZC3H14/Nab2 does not bind to splicing factors, the mRNA transcript could be marked as spliced and ZC3H14/Nab2, together with other export factors, could stabilize and remodel the transcript and target the transcript to the nuclear pore complex via interaction with nuclear pore-associated proteins (Mlp1) for export to the cytoplasm.
Complementary to this work, Wigington et al. sought to identify target mRNA transcripts regulated by human ZC3H14 in human cells and discovered that ZC3H14 affects the steady-state level of only a small number of transcripts [7]. For further analysis, Wigington et al. selected the ATP5G1 transcript, encoding a critical subunit of the mitochondrial ATPase synthase F0 subunit, and found that depletion of ZC3H14 reduces the stability of ATP5G1 mRNA [7]. Importantly, Wigington et al. showed that ZC3H14 binds to ATP5G1 mRNA in the nucleus and preferentially binds to unspliced ATP5G1 pre-mRNA [7]. In addition, Wigington et al. found that depletion of ZC3H14 increases the level of unspliced ATP5G1 pre-mRNA in the cytoplasm [7]. Together, these results suggest that ZC3H14 can detect the difference between unspliced pre-mRNA and mature mRNA, facilitate retention of pre-mRNA in the nucleus and protect mature mRNA from degradation. Notably, ZC3H14 interactions with splicing factors, like U2AF2, would allow ZC3H14 to recognize the unspliced pre-mRNA. The data on ZC3H14 from Wigington et al. are consistent with the Nab2 data from Soucek et al. and previous work on Nab2 and support a role for ZC3H14 in quality control of mRNA splicing and export (Figure 2).
These studies begin to suggest that ZC3H14/Nab2 plays a critical role in ensuring that pre-mRNAs are properly processed in the nucleus before the transcripts are exported to the cytoplasm. This model fits well with previous data showing that Nab2 is important for poly(A) RNA export in yeast cells and suggesting that Nab2 facilitates concentration of properly processed mRNA at the nuclear pore for export [16, 18, 19, 31]. ZC3H14/Nab2 interaction with splicing factors and pre-mRNA and ZC3H14 localization to pre-mRNA processing nuclear speckles [6-8, 12] supports a splicing-associated function for ZC3H14/Nab2 that could involve quality control. Genetic interactions between Nab2 and the Rrp6 riboexonuclease of the RNA exosome [8] and evidence that Nab2 can physically interact with Rrp6 [32, 33] also suggest that Nab2 could affect pre-mRNA degradation via recruitment or regulation of Rrp6. Alternatively, Nab2 interactions with splicing factors could cause a conformational switch in Nab2 that alters the accessibility of the 3’-end of the pre-mRNA to Rrp6 degradation. The kinetics of ZC3H14/Nab2 association with splicing factors could also contribute to the signal of whether to protect or destroy the transcript.
To further understand the molecular functions of ZC3H14/Nab2 in pre-mRNA processing and export, a number of challenges remain. The splicing proteins and other factors that directly bind to ZC3H14/Nab2 need to be defined to gain insight into how ZC3H14/Nab2 recognizes and discerns the difference between unprocessed pre-mRNA and mature mRNA. In addition, the RNA elements/structures in the mRNA targets bound by ZC3H14/Nab2 need to be examined to determine if they contribute to ZC3H14/Nab2-mediated recognition/regulation of the mRNA. Notably, RNA secondary structures, such as stem-loops, have been identified in several yeast introns that impact splicing efficiency [34-37]. The mechanism by which loss or impairment of ZC3H14/Nab2 leads to extended poly(A) tails on bulk RNA also requires deeper analysis. Whether ZC3H14/Nab2 directly interacts with the polyadenylation machinery or associates with splicing factors or other proteins to regulate polyadenylation needs to be examined. On this note, splicing and polyadenylation steps in pre-mRNA processing are known to be tightly coupled and splicing factors, such as U2AF2/U2AF65, which interact with ZC3H14, have been shown to stimulate cleavage and polyadenylation [38-40].
As neurons appear uniquely sensitive to changes in RNA binding proteins and post-transcriptional processing of RNA [41], dysregulation of pre-mRNA levels and/or pre-mRNA escape to the cytoplasm due to loss of ZC3H14 could specifically disturb the function of neuronal cells, leading to intellectual disability in patients. Alternatively, the changes to transcripts that occur when ZC3H14 is lost, such as lengthening of poly(A) tails, could impact the fate or function of the transcript in the cytoplasm. Unraveling the functions of ZC3H14/Nab2 in the future will outline new details on the mRNA processing landscape and shed light on the causes of neuronal dysfunction in intellectual disability.
Acknowledgements
We thank members of the Corbett lab, in particular Dr. Sara W. Leung, for helpful discussions and comments. This work was supported by NIH R01 grant (GM058728) to AHC.
Footnotes
Conflicting interests
The authors have declared that no conflict of interests exist.
References
- 1.Kaufman L, Ayub M, Vincent JB. The genetic basis of non-syndromic intellectual disability: a review. J Neurodev Disord. 2010;2:182–209. doi: 10.1007/s11689-010-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vissers LE, Gilissen C, Veltman JA. Genetic studies in intellectual disability and related disorders. Nat Rev Genet. 2016;17:9–18. doi: 10.1038/nrg3999. [DOI] [PubMed] [Google Scholar]
- 3.Ellison JW, Rosenfeld JA, Shaffer LG. Genetic basis of intellectual disability. Annu Rev Med. 2013;64:441–450. doi: 10.1146/annurev-med-042711-140053. [DOI] [PubMed] [Google Scholar]
- 4.Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. 2011;32:419–436. doi: 10.1016/j.ridd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Leonard H, Wen X. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev. 2002;8:117–134. doi: 10.1002/mrdd.10031. [DOI] [PubMed] [Google Scholar]
- 6.Pak C, Garshasbi M, Kahrizi K, Gross C, Apponi LH, Noto JJ, et al. Mutation of the conserved polyadenosine RNA binding protein, ZC3H14/dNab2, impairs neural function in Drosophila and humans. Proc Natl Acad Sci U S A. 2011;108:12390–12395. doi: 10.1073/pnas.1107103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wigington CP, Morris KJ, Newman LE, Corbett AH. The Polyadenosine RNA Binding Protein, Zinc Finger Cys3His Protein #14 (ZC3H14), Regulates the pre-mRNA Processing of a Key ATP Synthase Subunit mRNA. J Biol Chem. 2016 doi: 10.1074/jbc.M116.754069. doi: 10.1074/jbc.M116.754069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soucek S, Zeng Y, Bellur DL, Bergkessel M, Morris KJ, Deng Q, et al. The Evolutionarily-conserved Polyadenosine RNA Binding Protein, Nab2, Cooperates with Splicing Machinery to Regulate the Fate of pre-mRNA. Mol Cell Biol. 2016 doi: 10.1128/MCB.00402-16. doi: 10.1128/MCB.00402-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guthrie CR, Greenup L, Leverenz JB, Kraemer BC. MSUT2 is a determinant of susceptibility to tau neurotoxicity. Hum Mol Genet. 2011;20:1989–1999. doi: 10.1093/hmg/ddr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly SM, Pabit SA, Kitchen CM, Guo P, Marfatia KA, Murphy TJ, et al. Recognition of polyadenosine RNA by zinc finger proteins. Proc Natl Acad Sci U S A. 2007;104:12306–12311. doi: 10.1073/pnas.0701244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JT, Wilson SM, Datar KV, Swanson MS. NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol. 1993;13:2730–2741. doi: 10.1128/mcb.13.5.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung SW, Apponi LH, Cornejo OE, Kitchen CM, Valentini SR, Pavlath GK, et al. Splice variants of the human ZC3H14 gene generate multiple isoforms of a zinc finger polyadenosine RNA binding protein. Gene. 2009;439:71–78. doi: 10.1016/j.gene.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marfatia KA, Crafton EB, Green DM, Corbett AH. Domain analysis of the Saccharomyces cerevisiae heterogeneous nuclear ribonucleoprotein, Nab2p. Dissecting the requirements for Nab2p-facilitated poly(A) RNA export. J Biol Chem. 2003;278:6731–6740. doi: 10.1074/jbc.M207571200. [DOI] [PubMed] [Google Scholar]
- 14.Grant RP, Marshall NJ, Yang JC, Fasken MB, Kelly SM, Harreman MT, et al. Structure of the N-Terminal Mlp1-Binding Domain of the Saccharomyces cerevisiae mRNA-Binding Protein, Nab2. J Mol Biol. 2008;376:1048–1059. doi: 10.1016/j.jmb.2007.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hector RE, Nykamp KR, Dheur S, Anderson JT, Non PJ, Urbinati CR, et al. Dual requirement for yeast hnRNP Nab2p in mRNA poly(A) tail length control and nuclear export. Embo J. 2002;21:1800–1810. doi: 10.1093/emboj/21.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DM, Marfatia KA, Crafton EB, Zhang X, Cheng X, Corbett AH. Nab2p Is Required for Poly(A) RNA Export in Saccharomyces cerevisiae and Is Regulated by Arginine Methylation via Hmt1p. J Biol Chem. 2002;277:7752–7760. doi: 10.1074/jbc.M110053200. [DOI] [PubMed] [Google Scholar]
- 17.Schmid M, Olszewski P, Pelechano V, Gupta I, Steinmetz LM, Jensen TH. The Nuclear PolyA-Binding Protein Nab2p Is Essential for mRNA Production. Cell Rep. 2015;12:128–139. doi: 10.1016/j.celrep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Fasken MB, Stewart M, Corbett AH. Functional significance of the interaction between the mRNA-binding protein, Nab2, and the nuclear pore-associated protein, Mlp1, in mRNA export. J Biol Chem. 2008;283:27130–27143. doi: 10.1074/jbc.M803649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saroufim MA, Bensidoun P, Raymond P, Rahman S, Krause MR, Oeffinger M, et al. The nuclear basket mediates perinuclear mRNA scanning in budding yeast. J Cell Biol. 2015;211:1131–1140. doi: 10.1083/jcb.201503070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly SM, Leung SW, Apponi LH, Bramley AM, Tran EJ, Chekanova JA, et al. Recognition of polyadenosine RNA by the zinc finger domain of nuclear poly(A) RNA-binding protein 2 (Nab2) is required for correct mRNA 3'-end formation. J Biol Chem. 2010;285:26022–26032. doi: 10.1074/jbc.M110.141127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly SM, Leung SW, Pak C, Banerjee A, Moberg KH, Corbett AH. A conserved role for the zinc finger polyadenosine RNA binding protein, ZC3H14, in control of poly(A) tail length. RNA. 2014;20:681–688. doi: 10.1261/rna.043984.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly SM, Bienkowski R, Banerjee A, Melicharek DJ, Brewer ZA, Marenda DR, et al. The Drosophila ortholog of the Zc3h14 RNA binding protein acts within neurons to pattern axon projection in the developing brain. Dev Neurobiol. 2016;76:93–106. doi: 10.1002/dneu.22301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abovich N, Liao XC, Rosbash M. The yeast MUD2 protein: an interaction with PRP11 defines a bridge between commitment complexes and U2 snRNP addition. Genes Dev. 1994;8:843–854. doi: 10.1101/gad.8.7.843. [DOI] [PubMed] [Google Scholar]
- 24.Berglund JA, Chua K, Abovich N, Reed R, Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89:781–787. doi: 10.1016/s0092-8674(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 25.Rain JC, Legrain P. In vivo commitment to splicing in yeast involves the nucleotide upstream from the branch site conserved sequence and the Mud2 protein. Embo J. 1997;16:1759–71. doi: 10.1093/emboj/16.7.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rain JC, Rafi Z, Rhani Z, Legrain P, Kramer A. Conservation of functional domains involved in RNA binding and protein-protein interactions in human and Saccharomyces cerevisiae pre-mRNA splicing factor SF1. RNA. 1998;4:551–565. doi: 10.1017/s1355838298980335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3'-->5' exoribonucleases. Cell. 1997;91:457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 28.Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, Tollervey D. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 1999;18:5399–410. doi: 10.1093/emboj/18.19.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousquet-Antonelli C, Presutti C, Tollervey D. Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell. 2000;102:765–775. doi: 10.1016/s0092-8674(00)00065-9. [DOI] [PubMed] [Google Scholar]
- 30.Schneider C, Tollervey D. Threading the barrel of the RNA exosome. Trends Biochem Sci. 2013;38:485–493. doi: 10.1016/j.tibs.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green DM, Johnson CP, Hagan H, Corbett AH. The C-terminal domain of myosin-like protein 1 (Mlp1p) is a docking site for heterogeneous nuclear ribonucleoproteins that are required for mRNA export. Proc Natl Acad Sci U S A. 2003;100:1010–1015. doi: 10.1073/pnas.0336594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid M, Poulsen MB, Olszewski P, Pelechano V, Saguez C, Gupta I, et al. Rrp6p controls mRNA poly(A) tail length and its decoration with poly(A) binding proteins. Mol Cell. 2012;47:267–280. doi: 10.1016/j.molcel.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klass DM, Scheibe M, Butter F, Hogan GJ, Mann M, Brown PO. Quantitative proteomic analysis reveals concurrent RNA-protein interactions and identifies new RNA-binding proteins in Saccharomyces cerevisiae. Genome Res. 2013;23:1028–1038. doi: 10.1101/gr.153031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AbuQattam A, Gallego J, Rodriguez-Navarro S. An intronic RNA structure modulates expression of the mRNA biogenesis factor Sus1. RNA. 2016;22:75–86. doi: 10.1261/rna.054049.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabunilas J, Chanfreau G. Splicing-Mediated Autoregulation Modulates Rpl22p Expression in Saccharomyces cerevisiae. PLoS Genet. 2016;12:e1005999. doi: 10.1371/journal.pgen.1005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooks KB, Naseeb S, Parker S, Griffiths-Jones S, Delneri D. Novel Intronic RNA Structures Contribute to Maintenance of Phenotype in Saccharomyces cerevisiae. Genetics. 2016;203:1469–1481. doi: 10.1534/genetics.115.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gahura O, Hammann C, Valentova A, Puta F, Folk P. Secondary structure is required for 3' splice site recognition in yeast. Nucleic Acids Res. 2011;39:9759–9767. doi: 10.1093/nar/gkr662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyburz A, Friedlein A, Langen H, Keller W. Direct interactions between subunits of CPSF and the U2 snRNP contribute to the coupling of pre-mRNA 3' end processing and splicing. Mol Cell. 2006;23:195–205. doi: 10.1016/j.molcel.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 39.Millevoi S, Loulergue C, Dettwiler S, Karaa SZ, Keller W, Antoniou M, et al. An interaction between U2AF 65 and CF I(m) links the splicing and 3' end processing machineries. EMBO J. 2006;25:4854–4864. doi: 10.1038/sj.emboj.7601331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaida D. The reciprocal regulation between splicing and 3'-end processing. Wiley Interdiscip Rev RNA. 2016;7:499–511. doi: 10.1002/wrna.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cookson MR. RNA-binding proteins implicated in neurodegenerative diseases. Wiley Interdiscip Rev RNA. 2016 doi: 10.1002/wrna.1397. doi: 10.1002/wrna.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]