Abstract
Background
Rotavirus vaccination of all infants began in the United States in 2006. While the effect of vaccination on childhood hospitalizations for rotavirus have been well described, the effects of rotavirus vaccine on ED visits are less well documented.
Methods
Using the State Emergency Department Databases (SEDD) for 10 US states, we compared rates of gastroenteritis- and rotavirus-coded ED visits among children < 5 years of age in pre-vaccine (2003–2006) with post-vaccine (2008–2013) years; 2007 was excluded as a transition year. We analyzed ED visit rates by age group, sex, race, and rotavirus season.
Results
The pre-vaccine annual gastroenteritis-coded ED visit rate among children < 5 years of age of 426 per 10,000 (annual range, 396–477 per 10,000) declined to 382 per 10,000 in post-vaccine years, a 10.3% (±0.3%, p<.0001) rate reduction overall. Compared to pre-vaccine years, annual ED visit rates for gastroenteritis decreased by 6.5% (±0.6%) in 2008, 12.3% (±0.6%) in 2010, 14.8% (±0.5%) in 2011, 20.4% (±0.5%) in 2012 and 10.1% (±0.6%) in 2013; a small increase of 1.8% (±0.6%) was seen in 2009 (p<.0001 for all individual comparisons). Declines were similar by sex and race and were greater in children <2 years of age (range 14.1–20.6%, p<.0001) than older children (increase of 3.3% ± 0.6%, p<.0001). A decline of 21.2% (±0.4%, p<.0001) in ED visits was seen during the rotavirus season months from January through June versus an increase of 9.5% (±0.6%, p<.0001) during July to December. ED visits specifically coded for rotavirus showed more prominent declines than for all gastroenteritis.
Conclusions
ED visits for gastroenteritis in US children have declined since introduction of rotavirus vaccine.
Index Words: Rotavirus, Gastroenteritis, Diarrhea, Rotavirus Vaccines, Utilization
Background
Prior to the introduction of rotavirus vaccine for all infants in the US in 2006, rotavirus gastroenteritis caused an estimated 205,000 – 272,000 emergency department (ED) visits annually among children under 5 years of age [1, 2]. Current US guidelines recommend either 2 doses of a monovalent rotavirus vaccine (RV1; Rotarix, GSK Biologicals) or 3 doses of a pentavalent rotavirus vaccine (RV5; Rotateq, Merck) for routine immunization of all US infants before the age of 8 months [1].
Since rotavirus vaccine introduction, there has been a dramatic decline in the burden of severe rotavirus disease in US children [3–8]. Moreover, rotavirus vaccination in infants offers indirect benefits by reducing rotavirus infection in older children and adults [9]. While the effect of vaccination on childhood hospitalizations for rotavirus has been well described, the effects of rotavirus vaccine on ED visits are less well documented. While rotavirus vaccination was approved primarily to reduce the risk of severe diarrhea (as measured by hospitalizations and deaths), an added benefit of vaccination would be reducing ED congestion through less diarrhea visits. Active surveillance through the New Vaccine Surveillance Network (NVSN) showed a 76% reduction in laboratory-confirmed rotavirus infection in ED visits in the first two seasons after vaccine introduction, but these data were limited to hospitals in three sentinel US counties [10]. A prior analysis using insurance databases showed a decrease in ED visits for gastroenteritis during the first four years of the vaccination program from 2008–2011 [5], but these data cannot be generalized to the entire US population.
With maturation of the rotavirus vaccine program, immunization coverage in children less than 3 years of age has increased from 44% in 2009 to 73% in 2013 [11]. We aim to provide a comprehensive evaluation of ED utilization among children <5 years of age for acute gastroenteritis through 2013 using robust and more nationally representative discharge data. We estimated baseline pre-vaccine burden and monitored trends in gastroenteritis- and rotavirus-coded ED visits among US children younger than 5 years in 10 US states over an 11-year period to account for expected year-to-year variation in rotavirus trends [12].
Methods
Data Source
We analyzed data from the State Emergency Department Databases (SEDD) over an 11-year period (2003–2013). The SEDD is a component of the Healthcare Cost and Utilization Project (HCUP), produced by the Agency for Healthcare Research & Quality (AHRQ), and captures emergency visits at hospital-affiliated EDs [13]. SEDD files include all patients, regardless of payer. We restricted our analysis to ten states that continuously reported data to SEDD over the 11-year period: Connecticut, Georgia, Indiana, Maryland, Missouri, Nebraska, South Carolina, Tennessee and Vermont. In 2013, there were 3,225,505 children under 5 years of age in these ten states, equal to about 16% of the total U.S. population under 5 years of age. Seven states - Georgia, Indiana, Maryland, Missouri, South Carolina, Tennessee and Vermont – consistently reported race information, and were included in analysis by racial groups.
Definition of Gastroenteritis & Rotavirus-Coded ED Visit
Gastroenteritis ED visits were identified with the use of the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: diarrhea of determined etiology (bacterial [001–005 and 008.0–008.5, excluding 003.2]; parasitic [006–007, excluding 006.3–006.6]; and viral [008.6 and 008.8]), and diarrhea of undetermined etiology (presumed infectious [009.0–009.3] and presumed noninfectious [558.9 and 787.91]) [14]. Rotavirus-coded emergency department visits were a subset of gastroenteritis visits that included the specific ICD-9-CM code for rotavirus (008.61).
Demographic and Other Characteristics
Gastroenteritis- and rotavirus-coded ED visits among children < 5 years of age for years 2003–2013 were examined by sex, month and year of visit, age group by month (0–2, 3–5, 6–11, 12–23, and 24–59), and race. ED visits with missing information for a specific variable were excluded from analysis of that variable but were included for all other analyses.
Rate Calculations and Pre- and Post-vaccine Comparisons
ED visit rates were calculated by dividing the number of ED visits for gastroenteritis or rotavirus by the number of children < 5 years of age in participating states, according to the National Center for Health Statistics’ bridged-race post-censal population estimates [15]. ED visit rates were calculated by month of year, age group, sex, and race. ED visit rates were also characterized by rotavirus season, defined as the months from January-June of all years as has been done for other analyses using laboratory and hospital discharge data [6, 8, 16, 17].
To compare the relative difference in pre- and post-vaccine years, gastroenteritis- and rotavirus-coded ED visit rates for pre-vaccine years 2003–2006 were compared with the post-vaccine rates in every year from 2008–2013. Data from 2007 were excluded because rotavirus vaccine uptake was low and uneven during the first year after vaccine introduction. Rate ratios with 95% confidence intervals were calculated using Poisson regression analysis. Statistical significance was defined as a 2-sided P value of <0.05. SAS version 9.3 (SAS Institute Inc) was used for all analyses.
This analysis was conducted as a collaboration between CDC and AHRQ. As all analyses were completed with aggregated and de-identified ED discharge records and no human subjects were involved, informed consent and bioethical review were not required.
Results
A total of 1,450,474 ED visits coded for gastroenteritis and 13,350 ED visits coded for rotavirus occurred in children <5 years of age in the 10 states from 2003–2013. Overall gastroenteritis-coded ED visits decreased significantly by 10.3% (±0.3%, p<.0001) in the six post-vaccine years (2008–2013) compared with the pre-vaccine years (2003–2006). ED visits were significantly reduced in 5 of the 6 post-vaccine years compared to the pre-vaccine period, with the largest decline of 20.4% (±0.5%, p<.0001) seen in 2012 (Table). Declines were similar among male and female children. By age group, there was a greater reduction in ED visits in age groups less than 2 years old (from 14.1%–20.6%, p<.0001), while visits remained unchanged or increased for children 2–5 years old. Hispanic children experienced a larger reduction in ED visits (23.1% ±1.0%) than White (13.7% ±0.5%) or Black (13.1% ±0.6%) children (p<.0001 for all comparisons).
Table.
Gastroenteritis-Coded Emergency Department Visits in 10 US States*, Before (2003–2006) and After (2008–2013) Rotavirus Vaccine Introduction
| RATE, ANNUAL EMERGENCY DEPARTMENT VISITS PER 10,000 CHILDREN RATE REDUCTION, % (95% CONFIDENCE INTERVAL) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 2003–2006 (Range) |
2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2008–2013 | |
| TOTAL | 426 (395–477) |
399 6.5 (±0.6) |
434 −1.8 (±0.6) |
374 12.3 (±0.6) |
363 14.8 (±0.5) |
339 20.4 (±0.5) |
383 10.1 (±0.6) |
382 10.3 (±0.3) |
| SEX | ||||||||
| Male | 454 (421–509) |
426 6.2 (±0.8) |
462 −1.8 (±0.7) |
397 12.6 (±0.7) |
388 14.5 (±0.8) |
360 20.7 (±0.7) |
407 10.3 (±0.7) |
407 10.4 (±0.4) |
| Female | 398 (369–443) |
371 6.9 (±0.8) |
405 −1.9 (±0.8) |
350 12.1 (±0.8) |
338 15.0 (±0.8) |
318 20.1 (±0.7) |
358 10 (±0.8) |
357 10.3 (±0.5) |
| AGE, mo | ||||||||
| 0–2 | 559 (543–581) |
533 4.6 (±2.2) |
536 4.1 (±2.2) |
375 32.9 (±1.8) |
449 19.7 (±2) |
428 23.3 (±2) |
423 24.4 (±2) |
458 18.0 (±1.1) |
| 3–5 | 621 (594–681) |
582 6.3 (±2.1) |
558 10.1 (±2) |
528 15.0 (±2) |
465 25.1 (±1.9) |
444 28.4 (±1.8) |
458 26.1 (±1.8) |
507 18.4 (±1.0) |
| 6–11 | 871 (794–1,022) |
787 9.7 (±1.2) |
793 9 (±1.2) |
715 17.9 (±1.2) |
608 30.2 (±1.0) |
602 30.9 (±1.0) |
639 26.7 (±1.1) |
692 20.6 (±0.6) |
| 12–23 | 654 (583–753) |
597 8.6 (±1.0) |
632 3.4 (±1.0) |
555 15.1 (±1.0) |
529 19.1 (±0.9) |
497 24.0 (±0.9) |
556 14.9 (±1.0) |
562 14.1 (±0.4) |
| 24–59 | 247 (234–265) |
236 4.5 (±1.0) |
290 −17.3 (±1.1) |
248 −0.3 (±1.0) |
255 −3.2 (±1.0) |
229 7.3 (±0.9) |
274 −10.8 (±1.0) |
255 −3.3 (±0.6) |
| RACE | ||||||||
| White | 372 (349–402) |
338 9.2 (±0.8) |
375 −0.7 (±0.9) |
308 17.3 (±0.8) |
313 15.9 (±0.8) |
278 25.3 (±0.8) |
313 15.9 (±0.8) |
321 13.7 (±0.5) |
| Black | 571 (530–653) |
531 7.0 (±1.1) |
572 −0.2 (±1.1) |
527 7.6 (±1.1) |
468 18.0 (±1.0) |
406 28.9 (±1.0) |
473 17.2 (±1.0) |
496 13.1 (±0.6) |
| Hispanic | 435 (417–465) |
320 26.6 (±1.5) |
359 17.5 (±1.7) |
358 17.8 (±1.8) |
331 24.0 (±1.6) |
294 32.4 (±1.6) |
347 20.2 (±1.7) |
335 23.1 (±1.0) |
| SEASON | ||||||||
| Jan–Jun | 552 (500–603) |
419 24.0 (±0.6) |
535 3.1 (±0.7) |
406 26.4 (±0.6) |
422 23.5 (±0.6) |
344 37.7 (±0.6) |
482 12.6 (±0.6) |
435 21.2 (±0.4) |
| Jul–Dec | 301 (276–351) |
378 −25.6 (±1.1) |
334 −10.8 (±1.0) |
342 −13.4 (±1.0) |
305 −1.2 (±1.0) |
335 −11.3 (±1.1) |
284 5.7 (±0.9) |
330 −9.5 (±0.6) |
Connecticut, Georgia, Indiana, Maryland, Minnesota, Missouri, Nebraska, South Carolina, Tennessee and Vermont State Emergency Department Databases (SEDD), Healthcare Cost and Utilization Project (HCUP). Negative rate reductions indicate increased rate
The declines in ED visits were restricted to the months of the year from January to June during which rotavirus circulates in US Children, with a 21.2% (±0.4%, p<.0001) reduction in ED visits during these months overall in children <5 years of age in the post-vaccine period compared with pre-vaccine years. Similar to the annual reductions, the greatest reduction of 37.7% (±0.6%, p<.0001) during the rotavirus season was also seen in 2012. In contrast to the decline in the rotavirus-season months, a 9.5% (±0.6%, p<.0001) increase in ED visits was seen during the non-rotavirus season months from July to December.
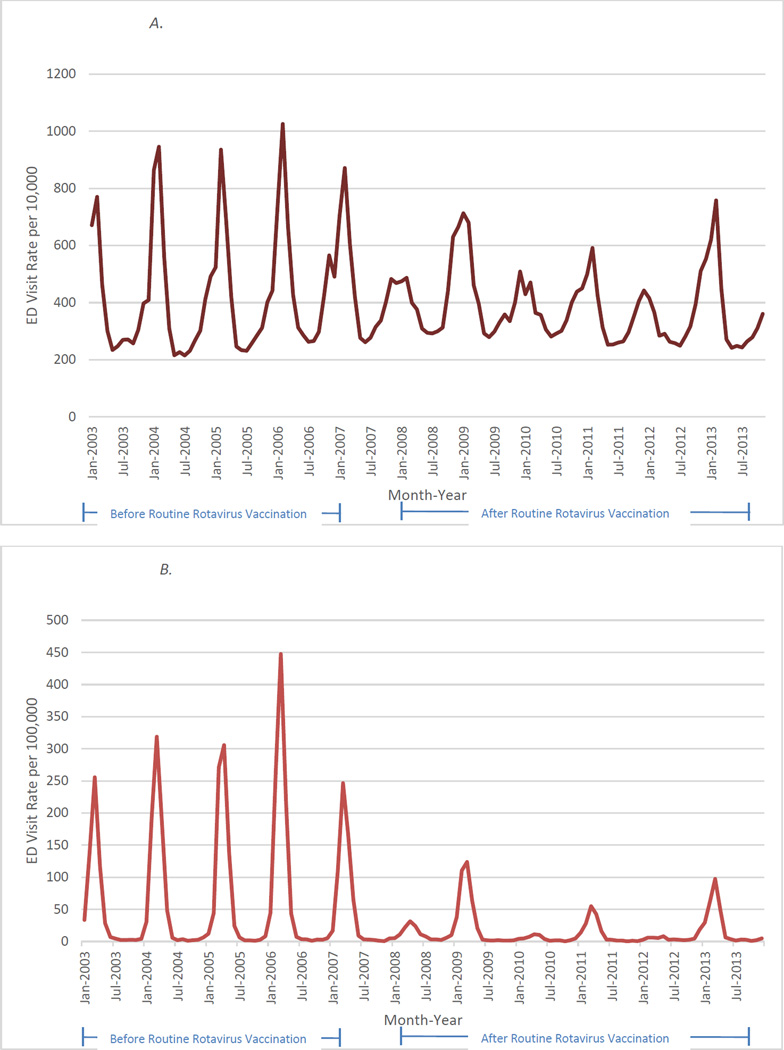
Monthly gastroenteritis-coded ED visits (Figure 1A) decreased notably during rotavirus seasons in post-vaccine years relative to pre-vaccine years. The reductions in all-cause gastroenteritis ED visits mirrored the decline in ED visits assigned the rotavirus-specific code (Figure 1B), and, as expected, more prominent declines were seen in rotavirus-specific ED visits.
Figure 1.
A. Monthly Acute Gastroenteritis-Coded Emergency Department Visits Among Children Younger than 5 Years in 10 States During January 2003 Through December 2013
B. Monthly Rotavirus-Coded Emergency Department Visits Among Children Younger than 5 Years in 10 States During January 2003 Through December 2013
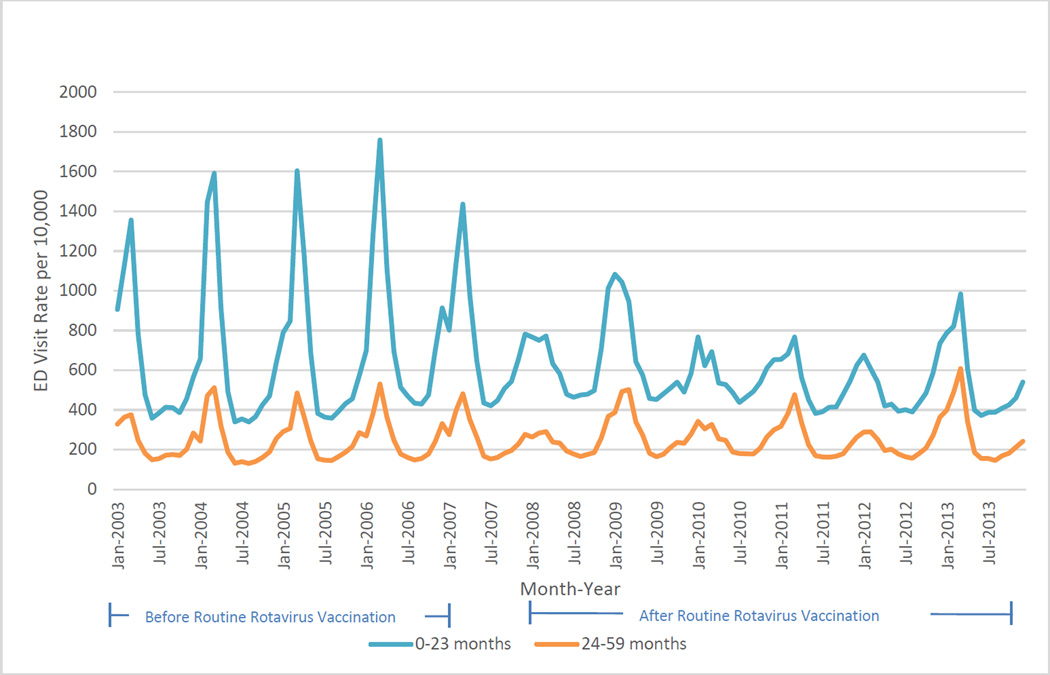
The largest and most consistent reductions in ED visits for gastroenteritis were in children <23 months of age (Figure 2).
Figure 2.
Monthly Acute Gastroenteritis-Coded Emergency Department Visits for Children < 23 months and 24–59 months of age in 10 States During January 2003 Through December 2013
Conclusions
Since the introduction of rotavirus vaccination in 2006, emergency department visits for all-cause acute gastroenteritis in children <5 years of age have declined through December 2013. The reduction in ED utilization has occurred primarily from January-June, the months when rotavirus infection is most common, and in children <2 years of age, the age group that is most likely to experience rotavirus infection [2]. These findings suggest strongly that the reduction in ED visits for gastroenteritis is likely attributable to rotavirus vaccination, as opposed to unmeasured contemporaneous factors or changes in coding practices.
Our results are consistent with and expand on results from prior studies showing a reduction in rotavirus detection, hospitalization, and resource utilization since vaccine introduction [3–6, 10, 17–20]. The most direct comparison can be made with an analysis of ED utilization using the 2001–2011 Truven Health MarketScan Commercial Claims and Encounters Database, an insurance claims database that is largely from large, self-insured companies, and excludes Medicaid recipients and the uninsured [5]. This analysis showed reductions in ED visits for gastroenteritis from −2 to 31% in the first five years following rotavirus vaccine introduction from 2007–2011, and a smaller decrease in -1 to 20% in outpatient (non-ED) visits for gastroenteritis in the same time period.
The change in ED visits for gastroenteritis (ranging from an increase of 1.8% to a decrease of 20.4% in individual years from 2008–2013) is not as dramatic as the change in hospitalizations (decreases from 31–55% in individual years from 2008–2012), using similar methods and a similar dataset [4]. There are at least two possible explanations for this finding. First, childhood gastroenteritis caused by rotavirus infection is more severe than that from other causes [21–23]; thus, the proportion of hospitalized diarrhea cases that are caused by rotavirus and thus are potentially vaccine preventable is greater than the proportion of diarrhea cases seen in ED settings. Second, the efficacy of rotavirus vaccines is greater against more severe rotavirus gastroenteritis [24–28], and thus vaccines would avert a greater proportion of rotavirus hospitalizations than ED visits.
Because laboratory testing for rotavirus is infrequently conducted among diarrhea patients seen in ED settings, our analysis focused on the less specific disease outcome of all-cause acute gastroenteritis. Despite the under-reporting for rotavirus in ED settings, it is reassuring that ED visits for rotavirus and all-cause gastroenteritis show matching reductions since vaccine introduction and matching post-vaccination biennial peaks, likely due to incomplete vaccine coverage.
Given the ecologic design our study, our examination of disease trends is potentially susceptible to bias from secular changes in the number of diarrhea-associated ED visits caused by pathogens other than rotavirus. In particular, secular trends associated with the emergence of new viral strains are well recognized for norovirus [29], which has emerged to become the leading cause of diarrhea hospitalization in US children following the decline in rotavirus after vaccine implementation [30]. In this regard, it is worth noting that the increase in ED visits seen in 2012–2013 temporally correspond to the emergence of the norovirus outbreak strain GII.4 Sydney [29].
In summary, our results contribute to the growing evidence of the impact of rotavirus vaccination in reducing the burden and severity of gastroenteritis and reducing utilization at multiple levels of the healthcare system in the United States. While rotavirus vaccination reduced ED visits more modestly than has been cited for hospitalizations, it still offers a valuable tool in reducing ED congestion, particularly in winter months. Increasing rotavirus vaccine coverage in eligible children remains a public health priority to sustain the declines in the burden of severe gastroenteritis in US children.
Acknowledgments
Support: None
We would like to thank Rick Jordan for his assistance in abstracting data from the State Emergency Department Databases.
Footnotes
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the Agency for Healthcare Research and Quality (AHRQ).
References
- 1.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58(Rr-2):1–25. [PubMed] [Google Scholar]
- 2.Payne DC, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008;122(6):1235–1243. doi: 10.1542/peds.2007-3378. [DOI] [PubMed] [Google Scholar]
- 3.Aliabadi N, et al. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination-United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2015;64(13):337–342. [PMC free article] [PubMed] [Google Scholar]
- 4.Leshem E, et al. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. Jama. 2015;313(22):2282–2284. doi: 10.1001/jama.2015.5571. [DOI] [PubMed] [Google Scholar]
- 5.Leshem E, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007–2011) Pediatrics. 2014;134(1):15–23. doi: 10.1542/peds.2013-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tate JE, et al. Trends in national rotavirus activity before and after introduction of rotavirus vaccine into the national immunization program in the United States, 2000 to 2012. Pediatr Infect Dis J. 2013;32(7):741–744. doi: 10.1097/INF.0b013e31828d639c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reduction in rotavirus after vaccine introduction--United States, 2000–2009. MMWR Morb Mortal Wkly Rep. 2009;58(41):1146–1149. [PubMed] [Google Scholar]
- 8.Delayed onset and diminished magnitude of rotavirus activity--United States, November 2007–May 2008. MMWR Morb Mortal Wkly Rep. 2008;57(25):697–700. [PubMed] [Google Scholar]
- 9.Gastanaduy PA, et al. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. Jama. 2013;310(8):851–853. doi: 10.1001/jama.2013.170800. [DOI] [PubMed] [Google Scholar]
- 10.Kilgore A, et al. Rotavirus-associated hospitalization and emergency department costs and rotavirus vaccine program impact. Vaccine. 2013;31(38):4164–4171. doi: 10.1016/j.vaccine.2013.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elam-Evans LD, et al. National, state, and selected local area vaccination coverage among children aged 19–35 months - United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(34):741–748. [PMC free article] [PubMed] [Google Scholar]
- 12.Payne DC, et al. Secular Variation in United States Rotavirus Disease Rates and Serotypes: Implications for Assessing the Rotavirus Vaccination Program. The Pediatric Infectious Disease Journal. 2009;28(11):948–953. doi: 10.1097/INF.0b013e3181a6ad6e. [DOI] [PubMed] [Google Scholar]
- 13.HCUP Databases. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2015. Sep, www.hcup-us.ahrq.gov/seddoverview.jsp. [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. Washington, DC: US Department of Health and Human Services; International Classification of Diseases, Ninth Revision, Clinical Modification. [Google Scholar]
- 15.National Center for Health Statistics. Washington, DC: US Department of Health and Human Services; 2015. Vintage 2014 postcensal estimates of the resident population of the United States (April 1, 2010, July 1, 2012–July 1, 2014), by year, county, single-year of age (0,1,2,…,85 years and over), bridged race, Hispanic origin, and sex. [Google Scholar]
- 16.Tate JE, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009;124(2):465–471. doi: 10.1542/peds.2008-3528. [DOI] [PubMed] [Google Scholar]
- 17.Tate JE, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30(1 Suppl):S30–S34. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 18.Rha B, et al. Effectiveness and impact of rotavirus vaccines in the United States - 2006–2012. Expert Rev Vaccines. 2014;13(3):365–376. doi: 10.1586/14760584.2014.877846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne DC, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age: 2009–2011. Clin Infect Dis. 2013;57(1):13–20. doi: 10.1093/cid/cit164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai R, et al. All-cause gastroenteritis and rotavirus-coded hospitalizations among US children, 2000–2009. Clin Infect Dis. 2012;55(4):e28–e34. doi: 10.1093/cid/cis443. [DOI] [PubMed] [Google Scholar]
- 21.Koopman JS, et al. Patterns and etiology of diarrhea in three clinical settings. Am J Epidemiol. 1984;119(1):114–123. doi: 10.1093/oxfordjournals.aje.a113712. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs A, et al. Rotavirus gastroenteritis. Clinical and laboratory features and use of the Rotazyme test. Am J Dis Child. 1987;141(2):161–166. doi: 10.1001/archpedi.1987.04460020051025. [DOI] [PubMed] [Google Scholar]
- 23.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22(3):259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 24.Armah GE, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 25.Madhi SA, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Palacios GM, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 27.Vesikari T, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 28.Zaman K, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 29.Vega E, et al. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol. 2014;52(1):147–155. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne DC, et al. Norovirus and medically attended gastroenteritis in US children. N Engl J Med. 2013;368(12):1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]