Abstract
Memory for antigen is a defining feature of adaptive immunity. Antigen-specific lymphocyte populations show an increase in number and function after antigen encounter and more rapidly re-expand upon subsequent antigen exposure. Studies of immune memory have primarily focused on effector B cells and T cells with microbial specificity, using prime challenge models of infection. However, recent work has also identified persistently expanded populations of antigen-specific regulatory T cells that protect against aberrant immune responses. In this Review, we consider the parallels between memory effector T cells and memory regulatory T cells, along with the functional implications of regulatory memory in autoimmunity, antimicrobial host defence and maternal fetal tolerance. In addition, we discuss emerging evidence for regulatory T cell memory in humans and key unanswered questions in this rapidly evolving field.
In addition to the ability to respond to an almost infinite range of foreign antigens, the cells of the adaptive immune system can also ‘remember’ prior antigen encounters. Despite a fairly rudimentary knowledge of the mediators responsible for immunological memory, Edward Jenner first recognized this remarkable facet of adaptive immunity more than 200 years ago through experimental cowpox vaccination. More recently, we have come to understand that immunological memory is conferred by specialized adaptive immune cells that robustly expand upon primary antigen exposure and that retain the ability to respond with more accelerated kinetics upon subsequent encounter with the same antigen. To exploit immune memory against micro-organisms, vaccines are now being engineered to induce long-term persistence of protective pathogen-specific antibodies, along with antibody-producing B cells and effector T cells. However, these findings also raise exciting new questions about whether newly identified regulatory immune cell subsets can also remember previous antigenic exposures.
Memory T cells have an essential role in immunity against microbial pathogens. As our appreciation of the diversity of functional T cell lineages has increased, so has our recognition of the memory features that are shared among many T cell subsets. Immunological memory has been most extensively characterized for CD8+ T cells. Long-standing work in this field has established the existence of multiple subsets of memory CD8+ T cells, which differ in terms of their tissue distribution and their capacity to traffic between peripheral tissues and lymphoid organs. These memory CD8+ T cell subsets can be distinguished on the basis of their expression of cell surface markers and transcription factors, along with their distinct epigenetic landscapes and metabolic profiles (reviewed in REFS 1–3) (TABLES 1,2).
Table 1.
Markers for memory T cell subsets*
| Memory T cell subset | Mouse phenotype | Human phenotype | |
|---|---|---|---|
| Conventional T cells | Central memory | CCR7hi, CD44hi, CD127hi, L-selectinhi and KLRG1low | CD44hi, CD45ROhi, CD45RAlow, CD127hi and express high levels of IL-2 and intermediate levels of IFNγ and TNF |
| Effector memory | CCR7low, CD44hi, CD127hi, L-selectinlow and KLRG1hi | CD44hi, CD45ROhi, CD45RAlow, CD127hi, L-selectinlow, express high levels of IFNγ and TNF and express low levels of IL-2 | |
| Tissue-resident memory | CCR7low, CD69hi, CD103hi and KLRG1low | CD45ROhi, CD45RAlow, CD69hi; and CD103hi and CD103low subsets | |
| Stem cell memory | CD44low, L-selectinhi, CD95hi, CD122hi, SCA1hi, BCL-2hi and CD127hi | CD27hi, CD28hi, CD45ROlow, CD45RAhi, CD95hi, CD122hi, CD127hi, L-selectinhi, CCR7hi and express low levels of IFNγ and intermediate levels of IL-2 | |
| Memory regulatory T cells‡ | CD25hi, CD27hi, CD44hi, FOXP3hi, L-selectinlow, CTLA4hi and CD127hi§ | CD25hi, CD27hi, CD44hi, CD45ROhi, CD45RAlow, CCR7low, FOXP3hi, L-selectinlow, CTLA4hi, CD127low, ICOShi, BCL-2hi, Ki67low and HLA-DR expression not defined | |
BCL-2, B cell lymphoma 2; CCR7, CC-chemokine receptor 7; CTLA4, cytotoxic T lymphocyte antigen 4; FOXP3, forkhead box P3; ICOS, inducible T cell co-stimulator; IFNγ, interferon-γ; IL-2, interleukin-2; KLRG1, killer cell lectin-like receptor subfamily G member 1; SCA1, stem cell antigen 1; TNF, tumour necrosis factor.
It is currently unknown whether memory regulatory T cell subsets exist in mice or humans.
Shown only in mouse skin.
Table 2.
Selected markers for resting, effector and memory T cell subsets*
| Conventional T cells | Regulatory T cells | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Resting | Activated effector | Memory | Resting | Activated effector | Memory | |
| Selected phenotypic markers | CD25low | CD25hi | CD25low | CD25hi | CD25 expression variable | CD25hi |
| CD44low | CD44hi | CD44hi | CD44hi | CD44hi | ||
| CD45RAhi‡ | CD45RA expression variable‡ | CD45RAlow‡ | CD45RAhi‡ | CD44hi | CD45RAlow‡ | |
| CD45ROlow‡ | CD45ROhi‡ | CD45ROlow‡ | CD45RAlow‡ | CD45ROhi‡ | ||
| CD69low | CD45RO expression variable‡ | CD69 expression variable | CD69low | CD45ROhi‡ | CD69 expression unknown | |
| L-selectinhi | CD69hi | L-selectin expression variable | L-selectinhi | CD69hi | ||
| CD127high | L-selectinlow | CD127low | L-selectinlow | |||
| Ki67low | CD127low | CTLA4low | CD127low | L-selectinlow | ||
| BCL-2hi | Ki67hi | CD127hi | ICOSlow | CTLA4hi | CD127hi§ | |
| BCL-2low | CD27hi | HLA-DRlow‡ | ICOShi | CTLA-4hi | ||
| KLRG1hi | Ki67low | Ki67low | HLA-DRhi‡ | ICOShi | ||
| BCL-2hi | BCL-2hi | Ki67hi | HLA-DRexpression not defined | |||
| BCL-2low | ||||||
| KLRG1hi | CD27hi | |||||
| Ki67low | ||||||
| BCL-2hi | ||||||
| KLRG1 expression not defined | ||||||
|
| ||||||
| Chemokine receptors | CCR7hi | Several, including CCR3, CCR6, CCR8 and CXCR3 | Variable levels of CCR7 | CCR7hi | CCR7low | CCR7low |
|
| ||||||
| Transcription factors | FOXP3low KLF2 |
FOXP3 expression variable‡ Several, including T-bet (TH1 cell-associated), GATA3 (TH 2 cell-associated), RORγ (TH17 cell-associated) and BCL-6 (TFH cell-associated)|| |
FOXP3low | FOXP3hi | FOXP3high
Several others, including T-bet and IRF4 |
FOXP3hi |
BCL, B cell lymphoma; CCR CC-chemokine receptor; CTLA4, cytotoxic T lymphocyte antigen 4; CXCR, CXC-chemokine receptor; FOXP3, forkhead box P3; GATA3, GATA-binding factor 3; ICOS, inducible T cell co-stimulator; IRF4, IFN-regulatory factor 4; KLF2, Krueppel-like factor 2; KLRG1, killer cell lectin-like receptor subfamily G member 1; RORγ, retinoic acid receptor-related orphan receptor-γ; TFH, T follicular helper; TH, T helper.
Human only.
Shown only in mouse skin.
CD4+ T cells only.
Compared with CD8+ T cells, memory within the CD4+ T cell compartment is less well understood. This probably stems from reduced proliferation kinetics and expansion potential that make enumerating CD4+ T cells with defined antigen-specificity technically more difficult. CD4+ T cells differentiate into functionally distinct effector subsets, including T helper 1 (TH1), TH2, TH17 and T follicular helper (TFH) cell subsets, each of which is responsible for activating specialized immunological pathways for optimal host defence against a range of microbial pathogens. This diversity makes it more challenging to quantify antigen-specific CD4+ memory T cells. In addition, each effector CD4+ T cell subset has inherent plasticity that further complicates tracking the persistence of functional memory CD4+ T cells. Another interesting distinction between memory CD4+ T cells compared with CD8+ T cells relates to durability. Although CD8+ T cells have consistently been shown to be maintained as a stable memory pool for extended time periods, antigen-specific memory CD4+ T cells decline in number over time4–6. Nevertheless, antigen-specific CD4+ T cells from each effector subset have been shown to persist long term after antigen elimination, as determined by unique expression patterns of transcription factors, cytokines, adhesion molecules and chemokine receptors7,8 (TABLES 1,2).
In contrast to effector CD4+ T cell subsets that promote pro-inflammatory responses, the forkhead box P3 (FOXP3)-expressing regulatory T (TReg) cell subset has potent immune suppressive properties9,10. Conceptually, the need for immunological memory within the effector T cell compartment is obvious — the ability to remember and to robustly respond to eradicate pathogenic micro-organisms more efficiently after secondary infection would enhance survival by augmenting immunity against recurrent infection. By contrast, the biological benefit of TReg cell memory is less apparent. It has been postulated that memory TReg cells mitigate tissue damage during the heightened responses of pro-inflammatory memory cells. In addition, memory TReg cells promote reproductive fitness by reinforcing fetal tolerance during pregnancy. The importance of regulatory memory is supported by several recent studies that identify long-term persistence of antigen-specific TReg cells with potent immunosuppressive properties despite the elimination of cognate antigen11–15. In this Review, we describe accumulating evidence for the existence of memory TReg cells and discuss the properties and the physiological functions of this newly identified cell population.
Regulatory memory
The concept and definition of memory TReg cells
During a primary immune response, antigen-presenting cells (APCs) activate T cells by presenting antigen and by providing additional co-stimulatory signals. This results in the expansion and functional differentiation of the T cells. Effector T cells that are generated migrate to the site of infection and eliminate the offending organism. Effector T cells are fairly short-lived cells; their life cycle is defined by a rapid expansion phase, a functional inflammatory or cytotoxic phase and a contraction phase during which they undergo apoptotic cell death. These cells are mainly present during active microbial infections. By contrast, during a primary response, a subset of T cells is generated that has potential for long-term survival — this subset is termed memory T cells. Memory T cells escape apoptosis during the contraction phase and persist in either secondary lymphoid organs — in the case of central memory T cells (TCM cells) — or in the recently infected peripheral tissue — in the case of effector memory T cells (TEM cells) and tissue-resident memory T cells16 (TRM cells) — for pro-longed periods of time after their cognate antigens have been cleared (FIG. 1). Upon re-exposure to antigen (that is, during the secondary immune response), memory T cells undergo rapid population expansion and mediate more robust effector functions compared with the primary immune response, which leads to rapid clearance of the infection. On the basis of this fundamental understanding of primary and secondary immune responses, several criteria have been suggested to distinguish memory T cells from effector T cells. It is generally accepted that essential features of memory T cells include first, evidence of prior expansion and/or activation, second, persistence in the absence of cognate antigen and third, enhanced functional activity upon antigen re-exposure.
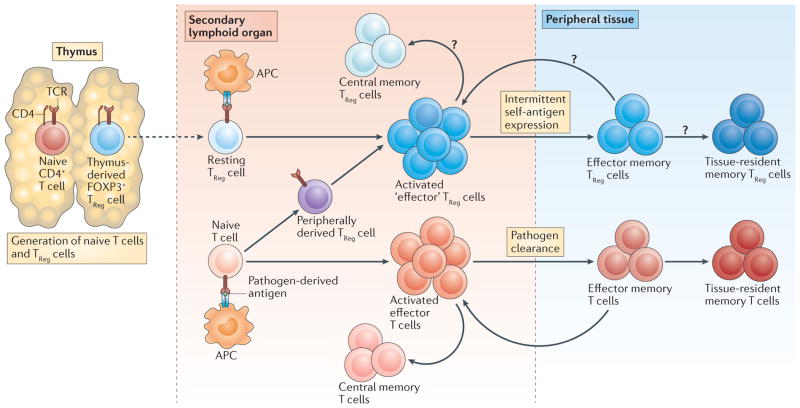
Figure 1. Life cycle of regulatory and conventional CD4+ T cells.
Naive conventional CD4+ T cells and regulatory T (TReg) cells are generated in the thymus. Upon antigen-specific activation in secondary lymphoid organs, these populations give rise to conventional effector T cells and ‘effector’ TReg cells. Effector TReg cells can arise from both thymus-derived and peripherally derived TReg cells. Central memory T cells are generated from a subset of activated conventional effector T cells and remain in secondary lymphoid organs. It is currently unknown whether central memory TReg cells are generated. Effector memory cells are generated from a subset of both conventional T cells and TReg cells. These cells migrate to antigen-expressing peripheral tissues where they stably reside (as tissue-resident memory T cells) or where they recirculate between blood and non-lymphoid tissues (as effector memory T cells). It is currently unknown whether the memory TReg cell populations that are found in peripheral tissues comprise tissue-resident memory TReg cells, effector memory TReg cells or both of these subsets. APC, antigen-presenting cell; FOXP3, forkhead box P3; TCR, T cell receptor.
Defining memory T cells by phenotypic markers
Classical definitions of immunological memory are based on our understanding of memory effector T cells. In the CD4+ T cell lineage, evidence of prior activation includes increased expression of CD44 and reduced expression of L-selectin (also known as CD62L), which enables migration to peripheral tissues by decreasing adhesion to high endothelial venules in secondary lymphoid organs17,18. As interleukin-7 (IL-7) signalling promotes long-term survival of T cells, expression of high levels of CD127 (also known as IL-7 receptor subunit-α) has been used as an additional marker of effector T cell memory19,20. In addition, CD47, the transcription factor T-bet, LY6G and specific epigenetic landscapes have all been used as evidence of prior activation and/or differentiation in mouse memory effector CD4+ T cells21–24.
Although a growing number of markers that reliably identify memory effector T cells have been identified (TABLES 1,2), similar indicators of functional memory for TReg cells are less clearly defined. This has been complicated by the fact that many of the markers used to identify memory effector T cells cannot be applied to TReg cells. Almost all TReg cells in secondary lymphoid organs and peripheral tissues express high levels of CD44 (REFS 25,26), which makes CD44 expression of little use in defining prior activation of TReg cells. In fact, there are few TReg cell-intrinsic molecules linked with immune suppression that have been shown to be expressed de novo on TReg cells upon activation. Instead, activated TReg cells generally increase their expression of molecules that they already express in the steady state. For example, upon encounter with antigen, TReg cells increase their expression of cytotoxic T lymphocyte antigen 4 (CTLA4), CD25 (also knwn as IL-2 receptor subunit-α), inducible T cell co-stimulator (ICOS), and glucocorticoid-induced TNFR-related protein (GITR; also known as TNFRSF18), all of which are expressed at fairly high levels on resting TReg cells27,28. Quantitative shifts in expression of these proteins are therefore the most useful markers of prior antigen encounter but are not definitive. Despite these caveats, some markers that are used to define TEM cells may be of value in defining memory TReg cells. It has been shown that a population of memory TReg cells in mouse skin can express high levels of CD127, which is expressed at low levels on TReg cells in secondary lymphoid organs29. However, this does not seem to be true for TReg cells with a memory phenotype found in human skin30, which suggests that this marker is not a robust indicator of memory TReg cells across species. In addition, expression of CD127 will probably vary with respect to the specific location at which memory TReg cells reside, as not all tissues express high levels of IL-7 (REF. 31).
Defining memory TReg cells by epigenetics
Shifts in the epigenetic landscape and in transcriptional signatures may represent complementary approaches for identifying memory TReg cells. Fully activated and lineage-committed TReg cells have defined epigenetic marks in FOXP3 (REF. 32). For example, demethylation of a conserved intronic regulatory element in FOXP3 (termed the conserved non-coding sequence 2 (CNS2) locus) is required for the maintenance of FOXP3 expression and for TReg cell stability upon exposure to inflammatory cytokines33. Furthermore, TReg cells activated in specific TH-skewing environments express transcription factors that are also expressed by the effector CD4+ T cell lineage they most potently suppress34–36. For example, TReg cells that preferentially suppress TH1 cell responses express T-bet, the canonical transcription factor that promotes TH1 cell differentiation35. Thus, it is conceivable that prior activation or differentiation in memory TReg cells can be identified by epigenetic markers that are indicative of stable and open FOXP3 expression in addition to transcriptional regulators that control effector CD4+ T cell differentiation.
Challenges in defining memory TReg cells
Perhaps the greatest challenge in defining memory TReg cells has been a lack of evidence that TReg cells can persist for prolonged periods of time in the absence of antigen. In the thymus, maturing thymocytes that express T cell receptors (TCRs) that have fairly high affinity for self peptide–MHC complexes differentiate into thymus-derived TReg cells37 (BOX 1). Thus, it is inferred that most, if not all, TReg cells have specificity for self antigen. In turn, given that most self antigens are constitutively expressed, a challenge in defining memory TReg cells is identifying cells of defined specificity that persist in the absence of cognate antigen. One approach to address this issue has relied on mouse models in which expression of surrogate self antigens in tissues can be precisely turned on and off, allowing identification of antigen-specific TReg cells that persist after antigen expression is extinguished11,13,29. In addition, there are TReg cells that recognize foreign microbial antigens expressed by pathogens that cause acute transient infection, facilitating the identification of pathogen-specific TReg cells that persist after the infection resolves14,15. These models have been instrumental in defining memory TReg cells and are discussed in detail below.
Box 1. Regulatory T cell subsets.
Forkhead box P3 (FOXP3)-expressing regulatory T (TReg) cells can be separated into several subsets on the basis of the sites in which they are generated, their relative differentiation state and the tissues in which they primarily reside. Cells derived in the thymus are often termed ‘natural’ TReg cells, and those derived outside of the thymus are often referred to as ‘induced’ or ‘adaptive’ TReg cells. There has been a consensus to rename these subsets ‘thymus-derived’ TReg cells (tTReg) and ‘peripherally-derived’ TReg cells (pTReg), respectively102. Naive or resting TReg cells are those that have yet to encounter their cognate antigen in the periphery or those that are constantly being exposed to antigen but the interactions are below the threshold for full activation. By contrast, effector TReg cells are cells that have received strong antigen stimulation outside of the thymus and have become fully activated, reflected by their proliferative index, changes in surface markers and enhanced suppressive function. Memory TReg cells have responded to antigen and are capable of surviving for fairly long periods of time even in the apparent absence of antigen (FIG. 1). TReg cells in tissues have different phenotypes and functional capacity compared with those found in secondary lymphoid organs and peripheral blood. Specialized populations of these cells have been identified in visceral adipose tissue, muscle, the gastrointestinal tract and skin (reviewed in REFS 38,40).
Intricately associated with the difficulty in testing whether TReg cells can persist in the absence of antigen stimulation are challenges in showing that these cells respond more robustly upon repeated antigen exposure. For memory effector T cells, this is measured by the kinetics and the magnitude of proliferation and effector cytokine production, as well as by the kinetics of pathogen clearance. However, these criteria cannot be used for memory TReg cells. TReg cells secrete a limited repertoire of cytokines, most of which are difficult to quantify on a per cell basis and may differ with respect to the tissues in which the cells reside38. The best criterion for functional changes associated with TReg cell memory is enhanced cell-intrinsic suppressive capacity and this is often difficult to measure with precision.
Early evidence for memory TReg cells
Despite inherent caveats in their phenotypic and functional characterization, there are several lines of evidence that support the existence of memory TReg cells. The first report of memory in a regulatory (also known as suppressor) T cell population was almost four decades ago39. Using an immunization approach with haptenated human IgG, Loblay and colleagues39 showed that suppressor cells were generated in the T cell compartment upon primary exposure to antigen. Using adoptive transfer experiments, they went on to show that these cells were long-lived (at least 9 months) and suppressed immune responses with accelerated kinetics upon secondary challenge. In addition, during the secondary response, far fewer of these suppressor cells (5–10-fold fewer) were required to achieve a level of suppression equivalent to that observed in primary responses. These authors postulated that memory suppressor cells could have an important role in maintaining long-lived tolerance to self antigens. Although this work introduced the concept of regulatory memory, experiments during this time were considerably hampered by the lack of markers to isolate and functionally characterize suppressor cell populations. However, the discovery of FOXP3 as a lineage-defining marker for TReg cells has enabled more precise analyses of regulatory cell populations9. A wealth of phenotypic and functional characterization of TReg cells has emerged, and the potential for memory in this compartment has been recently revisited using several experimental models (FIG. 2).
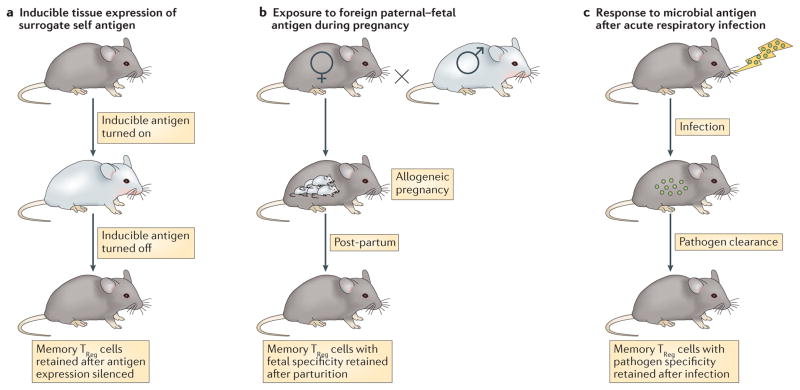
Figure 2. Mouse models for studying memory TReg cells.
Three primary mouse models have been used to identify and to characterize memory regulatory T (TReg) cells. a. In a tissue-specific inducible antigen model, expression of a pseudo-self antigen can be precisely turned on and off. This system facilitates the generation of memory TReg cells (by turning on the antigen) and their isolation and characterization in both secondary lymphoid organs and peripheral tissues after the antigen is turned off11,13. b. In an antigen-specific gestational model, maternal CD4+ T cells with surrogate fetal specificity can be precisely identified during primary pregnancy, post-partum and with fetal antigen restimulation in subsequent pregnancies12. c. In an acute infection model with influenza virus, the initial infection is rapidly cleared but virus-specific memory TReg cells are generated and maintained long term. These memory TReg cells mitigate the tissue damage that occurs upon reinfection with the virus14,15.
Memory TReg cells with self antigen specificity
Many early studies of TReg cell biology focused on how these cells are generated in the thymus and the mechanisms by which they function in secondary lymphoid tissues. However, it has become increasingly appreciated that immune suppression by TReg cells is also required to regulate inflammation in non-lymphoid tissues. In turn, TReg cells recovered from different tissues seem to have distinct functional properties38,40. To investigate the nature of TReg cell responses in the skin, transgenic mice were generated in which a defined model antigen could be inducibly expressed in keratinocytes11. In this model, expression of the model antigen was constitutive in the thymus but tightly regulated in the skin, mimicking the expression pattern of tissue-restricted self antigens. Importantly, antigen expression in skin could also be silenced, allowing characterization of antigen-specific memory T cells that persist without ongoing exposure to cognate antigen. As expected, constitutive expression in the thymus resulted in the generation of a large population of antigen-specific TReg cells that seeded all secondary lymphoid organs. Upon antigen induction in the skin, these cells robustly proliferated, increased expression of TReg cell-intrinsic molecules that mediate immune suppression (such as CTLA4) and migrated to the skin to resolve the inflammatory response mediated by antigen-specific effector T cells11. Although few antigen-specific TReg cells were present before the induction of antigen expression, a distinct population that retained high levels of CTLA4 expression persisted in the skin long after antigen expression had been turned off. Upon re-expression of antigen (analogous to a secondary response), skin inflammation was attenuated and resolved with accelerated kinetics compared with the primary response. Depletion of TReg cells in the interval between initial and subsequent antigen expression ameliorated these beneficial effects against skin disease. This was the first evidence that antigen-specific FOXP3+ TReg cells could fulfil immunological criteria for memory and persist as bona fide memory cells.
In most TCR-transgenic systems, the α-chain of the expressed TCR can pair with endogenous TCR α-chains41. This results in more than one TCR specificity being expressed on a single transgenic T cell, giving the cell the potential to recognize multiple different antigens. This caveat is circumvented by breeding TCR-transgenic mice onto a recombination-activating gene (RAG)-deficient background. In this setting, endogenous TCR chains are not expressed and thus cannot combine with transgenic TCR chains, which results in the production of T cells that bear only the transgenic TCR. As experiments in the inducible skin antigen system described above used TCR-transgenic T cells on a RAG-sufficient background, it is conceivable that TReg cells that persisted after cessation of antigen expression were maintained in the tissue through continued recognition of self antigen by alternative TCRs. To circumvent this caveat and to definitively test whether TReg cells could persist in tissues in the absence of antigen, an adoptive transfer approach was used, in which TCR-transgenic T cells on a RAG-deficient background were transferred into recipient mice capable of induced antigen expression in skin13. Antigen was induced for only 7 days and then extinguished. Consistent with previous results, a subset of TReg cells persisted in the skin for at least 60 days after cessation of antigen expression and had a low basal rate of proliferation, properties that are characteristic of tissue memory cells42. Moreover, in this model, all TReg cells are generated peripherally (that is, outside of the thymus) as there are no thymus-derived TReg cells present in the initial inoculum, which suggests that memory TReg cells can be generated in vivo from a peripherally generated TReg cell population.
Memory TReg cells in antimicrobial host defence
The finding that high affinity for self antigen has a major role in generating TReg cells in the thymus suggests that most, if not all, thymus-derived TReg cells are specific for self. However, several studies have identified TReg cells that recognize pathogen-derived peptides and some of these TReg cells seem to be generated in the thymus43–45. The ability to identify and to track TReg cells that are specific for foreign microorganisms offers a more conventional setting in which memory TReg cells can be identified. In a model of acute lung infection with influenza, virus-specific TReg cells increased 50-fold during the primary infection15. Virus-specific TReg cells expressed low levels of L-selectin but did not differ from resting TReg cells with respect to CD44 expression (which, as discussed above, is constitutively expressed at high levels even on resting TReg cells). Similar to effector T cell populations, the majority of virus-specific TReg cell populations also contracted after resolution of the primary infection. However, a small fraction of antigen-specific TReg cells persisted for more than 50 days after infection, representing a surviving population of memory cells. Upon reinfection, the virus-specific memory TReg cell pool underwent a 10-fold expansion that closely mirrored the expansion of the memory effector T cell population in kinetics and magnitude. Moreover, memory TReg cells significantly suppressed effector T cell population expansion and cytokine production in both systemic and tissue-specific models of reinfection15. In addition, they mitigated tissue damage without compromising viral clearance. These results were essentially repeated by a different group using a very similar model of infection14. Taken together, this work supports the hypothesis that memory TReg cells are generated to regulate potent memory effector responses and to thwart collateral damage to tissues that occurs with robust immune stimulation during infection39. Nonetheless, how memory TReg cells attenuate tissue inflammation without compromising pathogen clearance remains to be determined.
Memory TReg cells in maternal–fetal tolerance
Among genetically distinct individuals in naturally occurring outbred populations, pregnancy requires maternal tolerance to genetically foreign paternal antigens expressed by the developing fetus. Multiple mechanisms have evolved to establish and to reinforce fetal tolerance to protect immunologically foreign fetal tissue from maternal rejection. One such mechanism is the systemic expansion of maternal TReg cell populations, which primarily comprise FOXP3+ cells with defined fetal specificity. Reciprocally, reduced expansion of these maternal TReg cell populations has been widely associated with human pregnancy complications including pre-elampsia, premature birth and spontaneous abortion46,47. However, the fate of these TReg cells after parturition is not clear. Are they lost shortly after birth and generated de novo with each pregnancy or are they maintained for long periods of time in the non-pregnant state? If they persist, would they confer a reproductive advantage by reinforcing fetal tolerance in subsequent pregnancies?
To address these questions, the differentiation of maternal CD4+ T cells with specificity for a defined surrogate fetal antigen was evaluated after primary pregnancy and in response to fetal antigen restimulation in subsequent pregnancies12. Fetus-specific maternal CD4+ T cells accumulated throughout pregnancy and persisted at increased levels for the first 100 days after parturition. However, the level of maternal TReg cells with fetal specificity also progressively diminished within this post-partum time frame, similarly to the reduction in numbers of antigen-specific effector CD4+ T cells with antigen elimination after acute infection. Re-exposure to the same fetal antigen in subsequent pregnancies primed previously generated memory TReg cell populations to re-expand with accelerated kinetics compared with the initial pregnancy. In turn, the highly enriched pool of fetus-specific TReg cells in secondary pregnancy compared with primary pregnancy conferred remarkable protective properties against disruptions in fetal tolerance12. These findings suggest that memory TReg cells in mothers reinforce fetal tolerance and establish an immunological basis for partner-specific protection against complications in secondary compared with primary human pregnancies48,49.
The immunological parameters controlling post-partum retention of maternal TReg cells with pre-existing fetal specificity remains to be determined. However, the ubiquitous engraftment of genetically distinct fetal cells in mothers after pregnancy opens up the intriguing possibility that microchimeric fetal cells may provide a source of cognate antigen required for sustaining the accumulation of maternal memory TReg cells50,51. In other words, pregnancy-induced maternal TReg cells may not represent bona fide memory cell but may instead be maintained by antigenic stimulation from fetal cells that establish microchimerism in mothers after pregnancy52. This idea is consistent with recent findings that micro-chimeric maternal cells retained in offspring prime the sustained increase in TReg cells with non-inherited maternal antigen specificity53. Compulsory early developmental exposure to genetically foreign maternal antigens primes in offspring sustained tolerance to non-inherited maternal antigens and persistently increased TReg cells with specificity to these genetically foreign maternal antigens54–56. Conversely, targeted depletion of micro-chimeric maternal cells in offspring causes a rapid and precipitous decline in the increased accumulation of TReg cells with non-inherited maternal antigen specificity53. Whether these TReg cells are phenotypically more consistent with effector or memory TReg cells remains to be determined. Nevertheless, these results imply that some TReg cell subsets may require persistent cognate antigen stimulation for long-term numerical persistence, which is analogous to the necessity of antigen exposure reminders for numerical and functional maintenance of effector CD4+ T cells with microbial specificity57–59.
Evidence for memory TReg cells in humans
The mouse models described above define memory TReg cells phenotypically and functionally in various biological settings. In all cases, the approach used relied on inducing and analysing cells specific for defined antigens in a highly controlled in vivo environment. The inherent complexity of carrying out clinical experiments and the limited availability of tools for tracking TReg cells of defined antigen specificity precludes these types of studies in humans. Instead, studies of human memory TReg cells have mostly relied on phenotypic characterization and in vitro assays. Human T cells express the RO iso-form of CD45 in the thymus and convert to CD45RA upon emigration to peripheral tissues60,61. Upon antigen recognition in the periphery, these cells switch back to CD45RO. Almost all CD45RA-expressing CD4+ T cells in vitro lose CD45RA expression and transition to CD45RO+ cells after 4 days of TCR stimulation62. Thus, circulating human T cells are often termed ‘memory’ cells if they express the CD45RO isoform. Although this marker is commonly used to distinguish naive T cells from memory T cells, it should be noted that expression of CD45RO alone does not define a T cell as being a bona fide memory cell. This marker does not distinguish between cells that persist in the absence of antigen and those that are continually being exposed to antigen. In addition, isoform switching from CD45RO back to CD45RA in the periphery has been reported63. Nevertheless, expression of CD45 isoforms together with chemokine receptors and selectins is now widely used to distinguish naive and memory T cells in humans64.
Using a combination of CD25, CD45RA and FOXP3 expression, Miyara and colleagues65 showed that peripheral blood of healthy humans contains two phenotypically and functionally distinct subsets of TReg cells: CD45RA+FOXP3low and CD45RA−FOXP3hi cells, termed ‘resting’ and ‘activated’ TReg cells, respectively65. Both populations were stable, highly suppressive TReg cell subsets that lacked effector cytokine production. However, CD45RA−FOXP3hi TReg cells expressed higher levels of cell-intrinsic activation markers such as CTLA4, ICOS and HLA-DR. Resting TReg cells were highly prevalent in cord blood and expressed higher levels of CD31, which suggests recent emigration from the thymus, whereas activated TReg cells were reduced in cord blood and increased with age. In addition, resting TReg cells readily proliferated and converted to activated TReg cells upon stimulation in vitro and in vivo. Taken together, these results suggest that, in humans, naive or resting TReg cells emigrate from the thymus in early life and, upon encounter with antigen in the periphery, these cells proliferate and differentiate into ‘activated’ effector TReg cells. As the antigen specificity of these subsets was not determined, it is not known whether activated TReg cells were constantly being exposed to their cognate antigen or whether these cells persist at expanded levels in the absence of cognate antigen stimulation. However, given the fact that these results correlate well with the persistence of TReg cells with specificity for tissue-restricted self antigens that have been defined in mouse models, it is conceivable that a subset of activated TReg cells in human peripheral blood mononuclear cells are memory TReg cells that persist and remain activated in the absence of ongoing antigen stimulation.
Several reports show that CD45RA+ TReg cells progressively decline in peripheral blood with age, accompanied by a reciprocal increase in CD45RO+ TReg cells62,66,67. Human umbilical cord blood contains the highest percentage of CD45RA+ TReg cells66,68. These results are consistent with the idea that CD45RA+ TReg cells represent a resting population that convert to CD45RO+ activated or memory TReg cells upon antigen exposure in peripheral tissues. Interestingly, antigen-experienced CD45RA− TReg cells can be further subdivided on the basis of HLA-DR expression68. Distinct populations of CD45RA−HLA-DR− and CD45RA−HLA-DR+ TReg cells are present in human peripheral blood, thymus and umbilical cord blood. Phenotypic and functional analysis of these populations revealed that they express a common ‘core’ TReg cell gene signature and are both highly suppressive. However, they differ with respect to their activation state, suppressive capacity and cytokine secretion. Compared with HLA-DR− TReg cells, HLA-DR+ TReg cells expressed higher levels of TReg cell-associated activation markers, were more suppressive in vitro and produced lower levels of effector cytokines. Given the more differentiated phenotype observed in the HLA-DR+ fraction, it is interesting to speculate that these cells might represent bona fide memory TReg cells. By contrast, HLA-DR− cells may be recently activated but not fully differentiated TReg cells. However, because HLA-DR has been shown to be expressed on recently activated conventional T cells in humans69, it is possible that CD45RA−HLA-DR+ TReg cells represent recently activated ‘effector’ TReg cells and not memory TReg cells. Important next steps will be to establish the antigen specificity of these phenotypically distinct human TReg cell populations.
As a result of the technical limitations of analysing and isolating cells from non-haematopoietic tissues, the study of memory TReg cells in humans has mainly focused on peripheral blood cells. However, it is well known that a large fraction of memory cells reside within peripheral tissues and that blood may simply be a conduit for memory cells that are actively trafficking between tissues or between tissues and secondary lymphoid organs16. Thus, it is important to study human memory TReg cells in tissues in addition to blood. To this end, TReg cells have recently been isolated from human skin and phenotypically and functionally characterized30. It was found that almost all TReg cells in adult skin express CD45RO, whereas a considerable fraction of TReg cells in fetal skin lacked CD45RO expression and instead were CD45RA+. In addition, TReg cells in adult skin express high levels of other markers associated with T cell memory, including CD27 and B cell lymphoma 2 (BCL-2). Compared with cutaneous memory effector T cells, memory TReg cells expressed unique TCR sequences, did not express CC-chemokine receptor 7 (CCR7) and failed to migrate out of skin in vivo. These results suggest that human skin contains TReg cells with an ‘effector memory’ phenotype that recognize unique antigens and that stably reside in this tissue. This population may be most similar to tissue-resident memory cells16. The factors required for maintaining these activated memory TReg cells in skin and the specific antigens that they recognize remain to be elucidated.
Generation and maintenance of memory TReg cells
How memory TReg cells are generated and maintained is a fundamentally important question and an area of active investigation. Several factors are likely to be involved — we discuss these below, and a summary of the ontogeny of memory TReg cells as well as salient features of resting, effector and memory TReg cell subsets is suggested in FIG. 3.
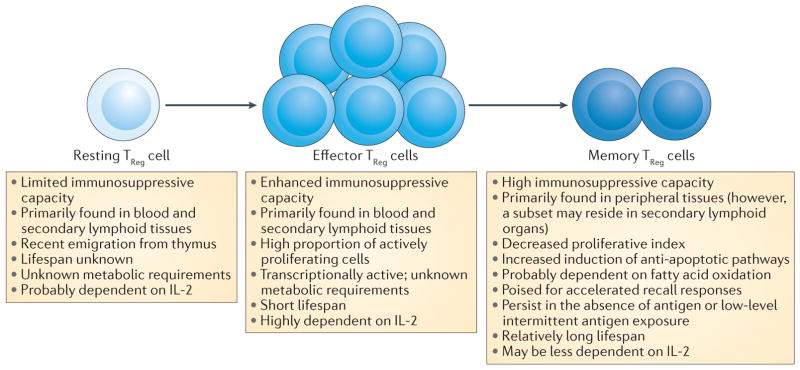
Figure 3. Predicted model for the relationship between resting, effector and memory TReg cells.
Regulatory T (TReg) cells that are derived in the thymus are maintained in the periphery in a resting or naive state. They are intermittently exposed to their cognate antigens but these T cell receptor MHC interactions are below the threshold for full activation. Upon strong stimulation with antigen in secondary lymphoid organs, resting T Reg cells are activated, proliferate and differentiate into effector TReg cells. A subset of effector TReg cells is capable of differentiating into long-lived memory TReg cells. Memory TReg cells can reside in non-lymphoid tissues and are capable of surviving in the absence of antigen or low levels of intermittent antigen exposure. The figure highlights key features of each population11,15,29,30,53,65,74,84. IL-2, interleukin-2.
Cytokines
Specific cytokine growth factors probably have a role in the generation and the maintenance of memory TReg cells. Both IL-2 and IL-7 have been implicated in the generation and the maintenance of memory CD4+ T cell subsets70. After acute infection with the intracellular bacterial pathogen Listeria monocytogenes, increased expression of CD25 (the high-affinity IL-2 receptor α-chain) was preferentially seen on bacteria-specific effector memory T cells, and cell-intrinsic genetic deficiency of CD25 significantly compromised the ability to generate this population71. In a separate model using TCR-transgenic cells, IL-2 signalling early after vaccination was required for IL-7 receptor (IL-7R) expression and for the generation of long-lived memory cells72. Given that IL-2-mediated signalling is important for the generation of effector memory cells and because IL-2 has a major role in TReg cell generation and maintenance73, it follows that this pathway might also be important in the generation of memory TReg cells.
The α-chain of the receptor for IL-7, CD127, is expressed at high levels on CD4+ effector memory T cells and has a major role in their maintenance in peripheral tissues19. The majority of TReg cells found in secondary lymphoid organs express low levels of IL-7R; however, memory TReg cells in skin have increased expression of IL-7R, which suggests that this pathway may be involved in maintaining these cells in tissues29. To dissect the relative contribution of the IL-2 and the IL-7 pathways in the generation and the maintenance of memory TReg cells, both genetic deletion and anti-body-mediated neutralization approaches have been used in the aforementioned model of inducible antigen expression in skin29. Results from these studies showed that IL-2 was necessary for the generation of memory TReg cells, whereas IL-7 but not IL-2 was required for their maintenance in skin. Consistent with these results, Smigiel and colleagues74 found that a subset of CD44hiCD62LlowCCR7low TReg cells have reduced levels of IL-2 receptor signalling and that IL-2 was not required to maintain these cells in vivo74.
Transcription factors
For CD8+ T cells, several studies have identified transcripts that are uniquely expressed in memory cells75–78; however, lineage-defining genes that drive differentiation of long-lived memory subsets have not been defined. Thus, the prevailing view is that complex networks coordinately drive memory cell generation and stability. Relative levels of specific transcription factors and specific epigenetic landscapes seem to be major determinants. For example, high levels of the transcription factor T-bet with concomitant low levels of the transcription factor eomesodermin (EOMES) promote the differentiation of naive CD8+ cells into short-lived effector cells, whereas low levels of T-bet and high levels of EOMES drive their development into memory cells79,80. The same paradigm seems to hold true for CD4+ T cells. The transcription factors B lymphocyte-induced maturation protein 1 (BLIMP1; also known as PRDM1) and BCL-6 have been shown to coordinately influence the development of effector and memory CD4+ T cell fates81,82. Interestingly, high levels of BLIMP1 are expressed in a subset of TReg cells with an ‘effector’ phenotype and in follicular TReg cells, which also express BCL-6 (REFS 83,84). Thus, it is interesting to speculate that gradients of specific transcription factors drive effector and memory TReg cell fates, similarly to what is seen in other CD4+ T cell subsets.
Epigenetic modifications
Although differential transcription factor expression will probably have a major role in promoting memory TReg cell development, binding of these factors to the appropriate DNA elements will be crucial. Chromatin accessibility and specific epigenetic modifications are proving to be major determinants of T cell memory fate24,85,86. How chromatin is assembled and modified early after TReg cell activation will probably differ between cells that are destined to be short-lived effectors and long-lived memory TReg cells. Elucidating these differences and the mechanisms by which they are established is central to our understanding of how memory TReg cells are generated and maintained. An assay for transposase-accessible chromatin using sequencing (ATAC-seq) is currently being used to map chromatin accessibility across the entire genome with great resolution using very small numbers of cells87. This technology may be well suited to discover how epigenetic landscapes differ between naive and memory TReg cell subsets and has the potential to reveal which transcription factors bind at specific enhancer regions in these cells.
Metabolic pathways
Different metabolic pathways are used during different stages of T cell activation and differentiation. It is now well understood that the metabolic demands of resting T cells are quite different from cells that are actively proliferating and mediating effector functions in the face of an ongoing immune response. Memory CD8+ T cells use unique metabolic pathways compared with both naive and short-lived effector cells. Whereas proliferating effector cells rely more on aerobic glycolysis, memory cells are dependent on fatty acid oxidation88,89. Sustained glycolytic activity inhibits the formation of memory, whereas inhibiting glycolysis promotes the development of memory cells90,91. Consistent with this, inhibition of mammalian target of rapamycin (mTOR) promotes fatty acid oxidation and increases the formation of memory cells92. It is hypothesized that memory T cells rely on fatty acid oxidation because it affords a greater capacity to generate energy under stress and enables a more rapid response upon reinfection93. Interestingly, the same metabolic pathways that promote T cell memory also promote the development of TReg cells. Compared with effector T cells, TReg cells express lower levels of glucose transporter 1 (GLUT1; also known as SLC2A1) and have higher basal lipid oxidation rates, which suggests that they primarily rely on fatty acid oxidation for their energy requirements94. Consistent with this, blocking either glycolysis or mTOR signalling promotes TReg cell development95–97. Given the metabolic similarities between memory effector cells and TReg cells, it has been suggested that activated naive cells differentiate into effector TReg cells if mTOR signalling is high and into long-lived memory TReg cells in the presence of low levels of mTOR activating signals98,99. Local levels of transforming growth factor-β (TGFβ) are thought to have a role in this process98. Taken together, these studies support the idea that the metabolic requirements for TReg cells differ from those of other CD4+ T cell populations. However, it is currently unknown how metabolism differs between specific TReg cell subsets. Whether memory TReg cells can be distinguished from naive and effector TReg cells on the basis of different metabolic requirements (that is, glycolysis versus fatty acid oxidation) remains to be determined.
Unanswered questions and future directions
Functional studies in mice and complementary studies with human cells and tissues have identified the existence of FOXP3-expressing memory TReg cells. However, our current understanding of memory TReg cell biology is rudimentary compared with that of memory effector T cells. This primarily stems from the very recent identification of memory TReg cells and the lack of memory-specific phenotypic markers for identifying these cells. Fundamental questions remain to be answered, with perhaps the first being, are there specific markers that can reliably separate memory TReg cells from short-lived ‘effector’ TReg cells and resting TReg cells? Comprehensive gene-expression profiling combined with flow cytometric characterization of memory TReg cells in mouse models in which these cells can be reliably generated and purified will be required to elucidate a ‘core’ memory TReg cell signature. Contrasting this profile with that of purified resting and recently activated effector TReg cells will probably establish a set of parameters that can more reliably identify memory TReg cells in both mice and humans. As heterogeneity will probably exist in seemingly pure TReg cell populations, single cell expression analysis may be required to more precisely define these subsets. To determine whether memory TReg cells consist of central, effector and tissue-resident subsets, this analysis will need to be carried out on cells isolated from both secondary lymphoid organs and peripheral tissues. Once candidate markers are identified, crucial next steps include investigating which represent true lineage-defining indicators of cell ‘fate’ versus those that reflect a transient cell ‘state’ influenced by local inflammatory signals — a task currently plaguing the much more mature memory CD8+ T cell field1. In addition, it will be important to discern whether memory exists in other regulatory immune populations, such as type 1 regulatory T (TR1) cells 100 and regulatory B cells101. It is conceivable that these cells work together with FOXP3-expressing memory TReg cells to promote self tolerance and to maintain immune homeostasis.
The identification of TReg cells as a dedicated immune suppressive CD4+ T cell lineage and the essential role that these cells have in maintaining immune homeo-stasis has raised many exciting new questions regarding the fundamental biology of these cells. These efforts are beginning to bear fruit, as novel treatment strategies aiming to either augment or to inhibit TReg cells are beginning to enter the clinic to treat human disease. We now appreciate that TReg cells are heterogeneous, comprised of multiple subsets that differ depending on the tissues in which they reside and on their differentiation state. Comprehensively defining these subsets, both phenotypically and functionally, may result in new and more targeted therapeutic strategies. We are in the early stages of dissecting the biology of memory TReg cells and the potential role that they have in health and disease. However, the highly suppressive nature of these cells and the exciting potential for their persistence as memory cells make them promising candidates for therapeutic manipulation in a range of clinical settings (for example, transplantation, autoimmunity, microbial immunity and maternal–fetal medicine) in which the balance between immune stimulation and suppression requires more stringent regulation.
Acknowledgments
S.S.W. is supported by the NIH through awards R01AI100934, R01AI120202 and R21AI112186, the March of Dimes Foundation and the Investigator in the Pathogenesis of Infectious Disease program from the Burroughs Wellcome Fund. M.D.R. is supported by the NIH through awards DP2AR068130, K08AR062064, R21AR066821 and UM1AI110498, by the Burroughs Wellcome Fund Career Award for Medical Scientists, the Scleroderma Research Foundation, the National Psoriasis Foundation and the Dermatology Foundation Stiefel Scholar Award in Autoimmune &/or Connective Tissue Diseases.
Glossary
- Memory TReg cells
Previously activated regulatory T (TReg) cells that persist in the absence of antigen expression or in the presence of intermittent low-level antigen expression. It is currently unknown whether central memory T cell, effector memory T cell or tissue-resident memory T cell subsets of memory TReg cells exist.
- Central memory T cells (TCM cells)
Generated in secondary lymphoid tissues and reside in secondary lymphoid tissues in the absence of antigen.
- Effector memory T cells (TEM cells)
Generated in secondary lymphoid tissues and recirculate between blood and non-lymphoid tissues in the absence of antigen.
- Tissue-resident memory T cells (TRM cells)
Generated in non-lymphoid tissues and stably reside in these tissues in the absence of antigen.
- Tissue-restricted self antigens
Self antigens that are expressed in specific tissues during defined periods of time. Hair follicle-associated antigens are an example of tissue-restricted self antigens in skin.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 3.Wakim LM, Bevan MJ. From the thymus to longevity in the periphery. Curr Opin Immunol. 2010;22:274–278. doi: 10.1016/j.coi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 5.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 6.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 7.Gasper DJ, Tejera MM, Suresh M. CD4 T-cell memory generation and maintenance. Crit Rev Immunol. 2014;34:121–146. doi: 10.1615/critrevimmunol.2014010373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepper M, Jenkins MK. Origins of CD4+ effector and central memory T cells. Nat Immunol. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FOXP3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. This is a landmark paper that shows that FOXP3 is the master transcription factor driving development of TReg cells. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by FOXP3+ regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Rosenblum MD, et al. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. This work phenotypically and functionally defines memory TReg cells in a mouse model of autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. This work phenotypically and functionally defines memory TReg cells in a mouse model of fetal–maternal tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gratz IK, et al. Cutting edge: self-antigen controls the balance between effector and regulatory T cells in peripheral tissues. J Immunol. 2014;192:1351–1355. doi: 10.4049/jimmunol.1301777. This paper shows that persistent self antigen expression in tissues leads to the preferential accumulation of TReg cells instead of effector T cells. It also shows that memory TReg cells can be generated from peripherally derived TReg cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brincks EL, et al. Antigen-specific memory regulatory CD4+FOXP3+ T cells control memory responses to influenza virus infection. J Immunol. 2013;190:3438–3446. doi: 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez AM, Zhu J, Huang X, Yang Y. The development and function of memory regulatory T cells after acute viral infections. J Immunol Baltim Md. 2012;189:2805–2814. doi: 10.4049/jimmunol.1200645. 1950. References 14 and 15 phenotypically and functionally define memory TReg cells in a mouse model of infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerottini JC, MacDonald HR. The cellular basis of T-cell memory. Annu Rev Immunol. 1989;7:77–89. doi: 10.1146/annurev.iy.07.040189.000453. [DOI] [PubMed] [Google Scholar]
- 18.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197:751–762. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondrack RM, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenz DC, et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc Natl Acad Sci USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van VQ, et al. CD47low status on CD4 effectors is necessary for the contraction/resolution of the immune response in humans and mice. PLoS ONE. 2012;7:e41972. doi: 10.1371/journal.pone.0041972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall HD, et al. Differential expression of LY6C and T-bet distinguish effector and memory TH1 CD4+ cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youngblood B, Hale JS, Ahmed R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology. 2013;139:277–284. doi: 10.1111/imm.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Soong L, Liu G, König R, Chopra AK. CD44 expression positively correlates with FOXP3 expression and suppressive function of CD4+ TReg cells. Biol Direct. 2009;4:40. doi: 10.1186/1745-6150-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firan M, Dhillon S, Estess P, Siegelman MH. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood. 2006;107:619–627. doi: 10.1182/blood-2005-06-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Oppenheim JJ. Resolving the identity myth: key markers of functional CD4+FOXP3+ regulatory T cells. Int Immunopharmacol. 2011;11:1489–1496. doi: 10.1016/j.intimp.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 2012;26:2253–2276. doi: 10.1096/fj.11-193672. [DOI] [PubMed] [Google Scholar]
- 29.Gratz IK, et al. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol. 2013;190:4483–4487. doi: 10.4049/jimmunol.1300212. This work shows that memory TReg cells in mouse skin are dependent on IL-7 and not on IL-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez Rodriguez R, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124:1027–1036. doi: 10.1172/JCI72932. The paper phenotypically characterizes memory TReg cells in normal human skin and in the skin of patients with psoriasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang HY, Luther SA. Expression and function of interleukin-7 in secondary and tertiary lymphoid organs. Semin Immunol. 2012;24:175–189. doi: 10.1016/j.smim.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Morikawa H, Sakaguchi S. Genetic and epigenetic basis of TReg cell development and function: from a FOXP3-centered view to an epigenome-defined view of natural TReg cells. Immunol Rev. 2014;259:192–205. doi: 10.1111/imr.12174. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y, et al. Control of the inheritance of regulatory T cell identity by a cis element in the FOXP3 locus. Cell. 2014;158:749–763. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a STAT3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weissler KA, Caton AJ. The role of T-cell receptor recognition of peptide:MHC complexes in the formation and activity of FOXP3+ regulatory T cells. Immunol Rev. 2014;259:11–22. doi: 10.1111/imr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loblay RH, Pritchand-Briscoe H, Basten A. Suppressor T-cell memory. Nature. 1978;272:620–622. doi: 10.1038/272620a0. This is the first paper to define the phenomenon of regulatory memory. [DOI] [PubMed] [Google Scholar]
- 40.Gratz IK, Campbell DJ. Organ-specific and memory TReg cells: specificity, development, function, and maintenance. Front Immunol. 2014;5:333. doi: 10.3389/fimmu.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci USA. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacLeod MK, Kappler JW, Marrack P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology. 2010;130:10–15. doi: 10.1111/j.1365-2567.2010.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, et al. IFN-γ- and IL-10-expressing virus epitope-specific FOXP3+ TReg cells in the central nervous system during encephalomyelitis. J Exp Med. 2011;208:1571–1577. doi: 10.1084/jem.20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafiani S, et al. Pathogen-specific TReg cells expand early during mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity. 2013;38:1261–1270. doi: 10.1016/j.immuni.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 2010;6:e1001043. doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang TT, et al. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol. 2014;192:4949–4956. doi: 10.4049/jimmunol.1400498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 48.Campbell DM, MacGillivray I, Carr-Hill R. Pre-eclampsia in second pregnancy. Br J Obstet Gynaecol. 1985;92:131–140. doi: 10.1111/j.1471-0528.1985.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 49.Trupin LS, Simon LP, Eskenazi B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiol Camb Mass. 1996;7:240–244. doi: 10.1097/00001648-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson JL. The otherness of self: microchimerism in health and disease. Trends Immunol. 2012;33:421–427. doi: 10.1016/j.it.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinder JM, et al. Pregnancy-induced maternal regulatory T cells, bona fide memory or maintenance by antigenic reminder from fetal cell microchimerism? Chimerism. 2014;5:16–19. doi: 10.4161/chim.28241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinder JM, et al. Cross-generational reproductive fitness enforced by microchimeric maternal cells. Cell. 2015;162:505–515. doi: 10.1016/j.cell.2015.07.006. This works shows that microchimeric maternal cells provide a source of cognate antigen required for sustaining the postnatal accumulation of memory TReg cells with specificity for non-inherited maternal antigens in the offspring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dutta P, et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. 2009;114:3578–3587. doi: 10.1182/blood-2009-03-213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutta P, Burlingham WJ. Tolerance to noninherited maternal antigens in mice and humans. Curr Opin Organ Transplant. 2009;14:439–447. doi: 10.1097/MOT.0b013e32832d6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uzonna JE, Wei G, Yurkowski D, Bretscher P. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J Immunol. 2001;167:6967–6974. doi: 10.4049/jimmunol.167.12.6967. [DOI] [PubMed] [Google Scholar]
- 58.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 59.Nelson RW, McLachlan JB, Kurtz JR, Jenkins MK. CD4+ T cell persistence and function after infection are maintained by low-level peptide:MHC class II presentation. J Immunol. 2013;190:2828–2834. doi: 10.4049/jimmunol.1202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 61.Trowbridge IS, Thomas ML. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 62.Booth NJ, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 63.Henson SM, Riddell NE, Akbar AN. Properties of end-stage human T cells defined by CD45RA re-expression. Curr Opin Immunol. 2012;24:476–481. doi: 10.1016/j.coi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 65.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FOXP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. This paper phenotypically and functionally defines resting and effector TReg cells in human blood. [DOI] [PubMed] [Google Scholar]
- 66.Seddiki N, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 67.van der Geest KSM, et al. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp Gerontol. 2014;60:190–196. doi: 10.1016/j.exger.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Dong S, et al. Multiparameter single-cell profiling of human CD4+FOXP3+ regulatory T-cell populations in homeostatic conditions and during graft-versus-host disease. Blood. 2013;122:1802–1812. doi: 10.1182/blood-2013-02-482539. [DOI] [PubMed] [Google Scholar]
- 69.Moriya N, Sanjoh K, Yokoyama S, Hayashi T. Mechanisms of HLA-DR antigen expression in phytohemagglutinin-activated T cells in man. Requirement of T cell recognition of self HLA-DR antigen expressed on the surface of monocytes. J Immunol. 1987;139:3281–3286. [PubMed] [Google Scholar]
- 70.Katzman SD, et al. Opposing functions of IL-2 and IL-7 in the regulation of immune responses. Cytokine. 2011;56:116–121. doi: 10.1016/j.cyto.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pepper M, Pagán AJ, Igyártó BZ, Taylor JJ, Jenkins MK. Opposing signals from the BCL-6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7Rα-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin-2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smigiel KS, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. This work phenotypically and functionally defines resting and effector TReg cell subsets in mice. The authors show that resting TReg cells are highly dependent on IL-2 for survival, whereas effector TReg cells are dependent on signalling through ICOS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Obar JJ, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Best JA, et al. Transcriptional insights into the CD8+ T cell response to infection and memory T cell formation. Nat Immunol. 2013;14:404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Oestreich KJ, et al. BCL-6 directly represses the gene program of the glycolysis pathway. Nat Immunol. 2014;15:957–964. doi: 10.1038/ni.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banerjee A, et al. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 81.Kallies A, Xin A, Belz GT, Nutt SL. BLIMP1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 82.Rutishauser RL, et al. Transcriptional repressor BLIMP1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Linterman MA, et al. FOXP3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cretney E, et al. The transcription factors BLIMP1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 85.Vahedi G, et al. STATs shape the active enhancer landscape of T cell populations. Cell. 2012;151:981–993. doi: 10.1016/j.cell.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zediak VP, Johnnidis JB, Wherry EJ, Berger SL. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J Immunol. 2011;186:2705–2709. doi: 10.4049/jimmunol.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Windt GJW, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gubser PM, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 92.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Sullivan D, et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi LZ, et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and TReg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FOXP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 97.Zeng H, et al. mTORC1 couples immune signals and metabolic programming to establish TReg cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coe DJ, Kishore M, Marelli-Berg F. Metabolic regulation of regulatory T cell development and function. Front Immunol. 2014;5:590. doi: 10.3389/fimmu.2014.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Powell JD, Heikamp EB, Pollizzi KN, Waickman AT. A modified model of T-cell differentiation based on mTOR activity and metabolism. Cold Spring Harb Symp Quant Biol. 2013;78:125–130. doi: 10.1101/sqb.2013.78.020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 101.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 102.Abbas AK, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 103.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft- versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 105.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]