Abstract
Objective
To evaluate non‐invasive vagus nerve stimulation (nVNS) as an acute cluster headache (CH) treatment.
Background
Many patients with CH experience excruciating attacks at a frequency that is not sufficiently addressed by current symptomatic treatments.
Methods
One hundred fifty subjects were enrolled and randomized (1:1) to receive nVNS or sham treatment for ≤1 month during a double‐blind phase; completers could enter a 3‐month nVNS open‐label phase. The primary end point was response rate, defined as the proportion of subjects who achieved pain relief (pain intensity of 0 or 1) at 15 minutes after treatment initiation for the first CH attack without rescue medication use through 60 minutes. Secondary end points included the sustained response rate (15‐60 minutes). Subanalyses of episodic cluster headache (eCH) and chronic cluster headache (cCH) cohorts were prespecified.
Results
The intent‐to‐treat population comprised 133 subjects: 60 nVNS‐treated (eCH, n = 38; cCH, n = 22) and 73 sham‐treated (eCH, n = 47; cCH, n = 26). A response was achieved in 26.7% of nVNS‐treated subjects and 15.1% of sham‐treated subjects (P = .1). Response rates were significantly higher with nVNS than with sham for the eCH cohort (nVNS, 34.2%; sham, 10.6%; P = .008) but not the cCH cohort (nVNS, 13.6%; sham, 23.1%; P = .48). Sustained response rates were significantly higher with nVNS for the eCH cohort (P = .008) and total population (P = .04). Adverse device effects (ADEs) were reported by 35/150 (nVNS, 11; sham, 24) subjects in the double‐blind phase and 18/128 subjects in the open‐label phase. No serious ADEs occurred.
Conclusions
In one of the largest randomized sham‐controlled studies for acute CH treatment, the response rate was not significantly different (vs sham) for the total population; nVNS provided significant, clinically meaningful, rapid, and sustained benefits for eCH but not for cCH, which affected results in the total population. This safe and well‐tolerated treatment represents a novel and promising option for eCH. ClinicalTrials.gov identifier: NCT01792817.
Keywords: episodic cluster headache, chronic cluster headache, non‐invasive vagus nerve stimulation, acute treatment, neuromodulation, randomized controlled trial
Abbreviations
- ACT1
Non‐invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache
- ADE
adverse device effect
- AE
adverse event
- cCH
chronic cluster headache
- CH
cluster headache
- CI
confidence interval
- DHE
dihydroergotamine
- DNIC
diffuse noxious inhibitory control
- eCH
episodic cluster headache
- FDA
U.S. Food and Drug Administration
- GM
gray matter
- ICHD
International Classification of Headache Disorders
- ITT
intent‐to‐treat
- nVNS
non‐invasive vagus nerve stimulation
- SADE
serious adverse device effect
- SD
standard deviation
- SoC
standard of care
- SPG
sphenopalatine ganglion
- VNS
vagus nerve stimulation
PLEASE SEE THE ACCOMPANYING EDITORIAL BY PROFESSOR MORRIS LEVIN IN THIS ISSUE OF THE JOURNAL
INTRODUCTION
Cluster headache (CH) is a primary headache disorder characterized by recurrent unilateral exacerbations of severe or very severe pain lasting ∼15 minutes to 3 hours and accompanied by transient symptoms such as rhinorrhea, lacrimation, miosis, ptosis, and periorbital edema.1 The condition is now classified as a trigeminal autonomic cephalalgia,1 with the attacks resulting from vascular changes in cranial circulation driven by trigeminal autonomic reflex activation.2 A worldwide lifetime CH prevalence of 0.12% has been reported;3 most patients have episodic cluster headache (eCH), and 10% to 15% have chronic cluster headache (cCH).1 The International Classification of Headache Disorders (ICHD) recognizes eCH and cCH as clinically distinct subtypes.1, 4 In eCH, attack periods may last from 7 days to 1 year and are separated by ≥1‐month pain‐free intervals.1 Chronic CH attack periods last for >1 year either without remission or with <1‐month remission periods.1 CH imposes substantial burdens on quality of life and health care resource utilization, worsens work absenteeism and social functioning, and is typically accompanied by clinically significant disability that can engender psychiatric comorbidities with possible suicidal tendencies.5, 6
According to evidence‐based recommendations, the primary symptomatic treatments for CH attacks are subcutaneous sumatriptan and inhaled oxygen.7, 8 Alternative acute CH treatments include intranasal triptans and intravenous dihydroergotamine (DHE).7, 8, 9 Only subcutaneous sumatriptan and intravenous DHE are approved in the United States for the acute pharmacologic treatment of CH.10, 11 Treatment with intravenous DHE is impractical,12 and the U.S. Food and Drug Administration (FDA)‐approved labeling for sumatriptan in CH indicates a maximum of two doses per day,11 which may be inadequate for many patients including those with frequent attacks (ie, 3 to 8 per day)13 and may lead to medication overuse headache with multiple daily dosing.1, 14 The limited therapeutic options for the acute treatment of CH reflect an unmet medical need.
Vagus nerve stimulation (VNS) is a neuromodulation technique, administered via an implantable or non‐invasive method, that affects several central pathways including those involved in CH.15 The hypocretin and orexin pathway has been suggested to have a role in the dorsal vagal complex and CH pathophysiology,16, 17 supporting the therapeutic potential of VNS in patients with the disorder. The investigational nVNS gammaCore® device (electroCore, LLC; Basking Ridge, NJ, USA) transfers electrical impulses transcutaneously to the cervical branch of the vagus nerve.15 Data from a 1‐year open‐label study (N = 19) suggested that nVNS is potentially efficacious for acute and prophylactic management of eCH and cCH.18 In the randomized controlled study of Non‐invasive Vagus Nerve Stimulation (nVNS) for PREVention and Acute Treatment of Chronic Cluster Headache, weekly attack frequency reductions were significantly more pronounced with daily prophylactic nVNS as an adjunct to standard of care (SoC) than with SoC alone (P = .02).19 On the basis of these previous findings, we hypothesized that acute nVNS therapy is effective and safe for the treatment of CH attacks. Here, we report results from the study of nVNS for the ACute Treatment of Cluster Headache (ACT1).
METHODS
ACT1 Study Design
This pivotal, randomized, double‐blind, sham‐controlled prospective study was conducted from February 2013 to October 2014 across 20 U.S. centers, including university‐based/academic medical centers and headache/pain/neurological clinics and institutes (ClinicalTrials.gov identifier: NCT01792817). The study was designed to assess the superiority of nVNS treatment in comparison with a sham device and comprised two phases: (1) a double‐blind phase in which subjects were randomized to receive nVNS or sham treatment for 1 month or until five CH attacks were treated and (2) an open‐label phase in which subjects who completed the double‐blind phase could subsequently receive 3 months of nVNS treatment. Investigators obtained institutional review board approval, and subjects provided written informed consent. Authors had full access to all study data.
Subjects
Subjects were recruited from investigator databases and via clinical practice Web sites and Web advertisement. All subjects were nonpregnant/nonlactating 18‐ to 75‐year‐old adults diagnosed with eCH or cCH according to ICHD, 2nd edition criteria.4 Key exclusion criteria were a history of aneurysm, intracranial hemorrhage, brain tumors, significant head trauma, prolonged QT interval, arrhythmia, ventricular tachycardia/fibrillation, syncope, or seizure; structural intracranial/cervical vascular lesions; another significant pain disorder; cardiovascular disease; uncontrolled hypertension; abnormal baseline electrocardiogram; botulinum toxin injections in the past 3 months; nerve blocks in the past 1 month; previous CH surgery, bilateral/right cervical vagotomy, carotid endarterectomy, or right vascular neck surgery; electrical device implantation; and current use of prophylactic medications for indications other than CH.
Interventions
The nVNS device (Fig. 1) produces a proprietary low‐voltage electrical signal comprising a 5‐kHz sine wave burst lasting for 1 millisecond (five sine waves, each lasting 200 microseconds), with such bursts repeated once every 40 milliseconds (25 Hz), generating a 24‐V peak voltage and 60‐mA peak output current; users could adjust the stimulation amplitude. The appearance, weight, visual and audible feedback, and user application were identical for the sham and nVNS devices. The sham device produces a low‐frequency (0.1 Hz) biphasic signal that does not stimulate the vagus nerve or generally cause muscle contraction. After applying conductive gel to the two stainless steel contact surfaces, subjects administered three consecutive 2‐minute stimulations (Fig. 2) to the right side of the neck at the onset of premonitory symptoms or pain. Subjects self‐treated up to five CH attacks in the double‐blind phase; only one attack could be treated during a 12‐hour period. There were no limitations on the number of attacks that could be treated in the open‐label phase. As‐needed use of abortive or pain‐relieving rescue medications was permitted as soon as 15 minutes after initiation of each nVNS treatment.
Figure 1.
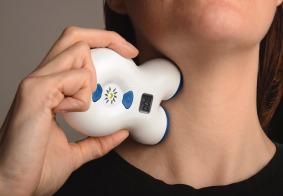
Non‐invasive vagus nerve stimulation device. Image provided courtesy of electroCore, LLC. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
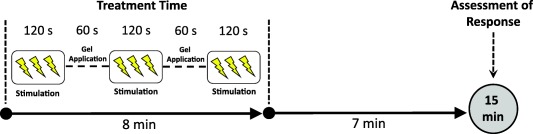
Time to first measurement of response used to define the primary end point. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Randomization and Blinding
Using independent statistician–generated randomization schedules, subjects were randomly assigned (1:1) to receive nVNS or sham treatment (variable block design, stratified by site). Devices labeled with a 3‐digit randomization number were not outwardly identified as active or sham and were allocated to the sites by a third‐party distributor according to the randomization scheme. Trained study site personnel (investigator or study coordinator) distributed devices to subjects in chronological order according to the randomization number. Investigators, subjects, and study coordinators were blinded to treatment assignments. After the first treatment and at the end of the double‐blind phase, subjects indicated the treatment they thought they had received (nVNS or sham) via blinding questionnaires.
Study End Points
The primary efficacy end point was response rate, assessed in the double‐blind phase and defined as the proportion of all subjects who achieved a pain intensity score of 0 or 1 on a 5‐point scale (0, no pain; 4, very severe pain) at 15 minutes after treatment initiation (Fig. 2) for the first CH attack; rescue medication use within 60 minutes was considered a treatment failure. The response rate was also evaluated at the end of the open‐label phase in a post hoc analysis. Secondary end points, assessed in the double‐blind phase, included sustained treatment response rate (defined as the proportion of subjects with a pain intensity score of 0 or 1 without rescue medication use at 15 through 60 minutes after treatment initiation for the first CH attack) and average of all subjects’ mean pain intensities at 15 minutes after treatment initiation for all attacks (up to five attacks per subject). To enhance understanding of the clinical potential of nVNS, percentages of patients who were responders (pain intensity score of 0 or 1) and of those who were pain‐free (pain intensity score of 0) at 15 minutes for ≥50% of treated attacks in the double‐blind phase were evaluated in post hoc analyses. Prespecified exploratory end points in the double‐blind phase included attack duration, rescue medication use, and perception of the device. All efficacy end points were evaluated in subgroup analyses of the eCH and cCH cohorts and in the total study population.
The primary safety end point was the occurrence of serious adverse device effects (SADEs) in the double‐blind phase, including those related to the sham device and/or CH events. Other safety outcomes included all adverse event (AE) occurrences in both study phases.
Sample Size Determination
A sample size of 120 subjects (60 per treatment arm) was determined to provide 82% power with respect to the primary end point, with a significance level of P ≤ .05 for a 2‐sided test. To allow for an approximate 20% attrition rate (eg, subject withdrawals), a total of up to 150 subjects was planned for enrollment and randomization. Assumed response rates were 25% for the sham group and 50% for the nVNS group. On 50% completion of enrollment, a review of prespecified interim analysis results by the data safety monitoring board revealed no safety concerns or other reasons for study discontinuation, and enrollment of the planned sample size was completed. No P value adjustments were required after completion of this interim data analysis.
Data Collection
After baseline information was collected during screening, subjects used diaries to record pain intensity (rated at 15 minutes, 30 minutes, 1 hour, and 2 hours after treatment), attack duration, rescue medication use, AEs, device perceptions, and blinding questionnaire responses for each attack.
Statistical Analyses
Statistical efficacy analyses were conducted on the intent‐to‐treat (ITT) population, defined as all randomly assigned subjects who treated ≥1 CH attack. Attack duration and device perception analyses were conducted on observed cases (ie, subjects from the ITT population who provided data for these end points), and attacks that lasted longer than 180 minutes were excluded according to ICHD criteria.4 Descriptive statistics were used for continuous variables. Categorical variables were summarized by frequency distribution and proportion; Clopper‐Pearson (exact) 95% confidence intervals (CIs) were calculated for response rates. Group differences for the primary end point and other categorical variable comparisons were performed using Fisher's exact test (if expected frequency ≤5 for ≥1 cell) or the chi‐square test. Linear mixed‐effect regression models were used to compare mean treatment group intensities to account for repeated measures per subject. Attack duration comparisons were performed using the t test. Comparisons of within‐subject response rates between the double‐blind and open‐label phases were performed using the McNemar test for paired proportions. Missing data were imputed as failures for response variables and using the last observation carried forward for attack intensity. For subjects who did not enter the open‐label phase, data for this phase were imputed to failure. For all response variables, subjects with missing data at any time point(s) for rescue medication use (ie, 15, 30, and/or 60 minutes) were considered non‐responders. Statistical significance was set at P < .05. Subgroup efficacy analyses of eCH and cCH cohorts were prespecified on the basis of distinct ICHD clinical definitions4 and the possibility that acute attacks of eCH and cCH might respond differently to treatment20 but were not independently powered to demonstrate statistical significance. P values are provided for these analyses without adjustment for multiple comparisons. Safety analyses were conducted on all treated subjects. All statistical analyses were performed independently by North American Science Associates Inc. (Minneapolis, MN, USA) using SAS® 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Subjects
A total of 150 subjects (Fig. 3) were enrolled in ACT1 and randomly assigned to receive nVNS (n = 73) or sham (n = 77) treatment, including 133 who met criteria for the ITT population (nVNS, n = 60; sham, n = 73). Of the 128 subjects who entered the open‐label phase, 100 completed the study. Reasons for discontinuation are shown in Figure 3. Demographic and baseline characteristics (Table 1) were similar between the nVNS and sham groups and were consistent with those of a typical CH population.4 Of all 150 subjects, most had eCH (67%) and the remaining 33% had cCH, and 102 (68%) subjects were receiving prophylactic therapy for CH at baseline.
Figure 3.
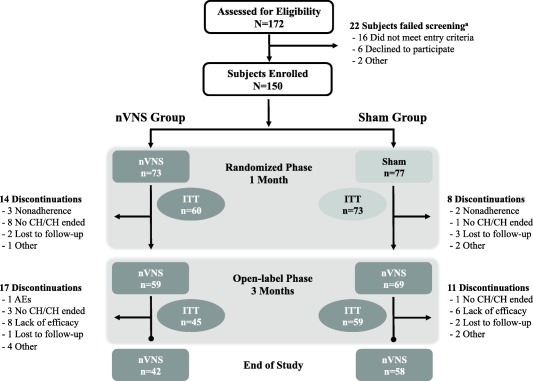
Subject disposition. AE, adverse event; CH, cluster headache; ITT, intent‐to‐treat; nVNS, non‐invasive vagus nerve stimulation. aSome subjects failed screening for >1 reason.
Table 1.
Demographic and Baseline Characteristics (All Treated Subjects)
| Characteristic | By Treatment Group (N = 150) |
By Cohort (N = 150) |
||
|---|---|---|---|---|
|
nVNS (n = 73) |
Sham (n = 77) |
eCH Cohort (n = 101) |
cCH Cohort (n = 49) |
|
| Age (y), mean ± SD | 47.1 ± 13.5 | 48.6 ± 11.7 | 48.4 ± 12.5 | 46.8 ± 13.0 |
| Male, No. (%) | 59 (80.8) | 67 (87.0) | 84 (83.2) | 42 (85.7) |
| Race, No. (%) | ||||
| Asian | 4 (5.5) | 1 (1.3) | 4 (4.0) | 1 (2.0) |
| Black | 5 (6.9) | 7 (9.1) | 9 (8.9) | 3 (6.1) |
| White | 63 (86.3) | 68 (88.3) | 87 (86.1) | 44 (89.8) |
| Missing | 1 (1.4) | 1 (1.3) | 1 (1.0) | 1 (2.0) |
| Duration of last CH attack (min), mean ± SD | 86 ± 119 | 64 ± 71 | 76.5 ± 104.4 | 68.9 ± 75.0 |
| CH Type, No. (%) | ||||
| eCH | 50 (68.5) | 51 (66.2) | 101 (100.0) | 0 |
| cCH | 23 (31.5) | 26 (33.8) | 0 | 49 (100.0) |
| Medications Used to Manage CH, No. (%) | ||||
| Triptans | 42 (57.5) | 54 (70.1) | 68 (67.3) | 28 (57.1) |
| Oxygen | 31 (42.5) | 29 (37.7) | 37 (36.6) | 23 (46.9) |
| Mild analgesics | 13 (17.8) | 16 (20.8) | 16 (15.8) | 13 (26.5) |
| Narcotics | 4 (5.5) | 4 (5.2) | 5 (5.0) | 3 (6.1) |
| Prophylactic medications | 42 (57.5) | 60 (77.9) | 65 (64.4) | 37 (75.5) |
| Verapamil | 11 (15.1) | 20 (26.0) | 25 (24.8) | 6 (12.2) |
| Lithium | 3 (4.1) | 3 (3.9) | 4 (4.0) | 2 (4.1) |
| Topiramate | 2 (2.7) | 7 (9.1) | 5 (5.0) | 4 (8.2) |
| Corticosteroids | 11 (15.1) | 8 (10.4) | 15 (14.9) | 4 (8.2) |
| Other | 21 (28.8) | 28 (36.4) | 28 (27.7) | 21 (42.9) |
| None | 4 (5.5) | 2 (2.6) | 5 (5.0) | 1 (2.0) |
cCH = chronic cluster headache; CH = cluster headache; eCH = episodic cluster headache; nVNS = non‐invasive vagus nerve stimulation; SD = standard deviation.
Response Rates (Double‐Blind Phase)
Response rates (Fig. 4) in the total population were 26.7% in the nVNS group and 15.1% in the sham group (P = .1). In subgroup analyses, a significantly higher response rate was demonstrated with nVNS (34.2%) than with sham treatment (10.6%) for the eCH cohort (P = .008) but not for the cCH cohort (nVNS, 13.6%; sham, 23.1%; P = .48).
Figure 4.
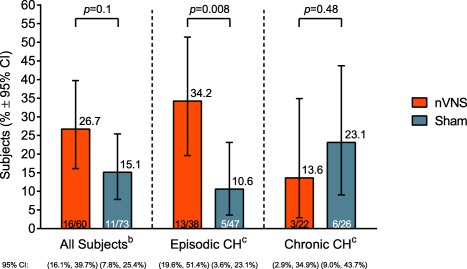
Response ratea (ITT Population). CH, cluster headache; CI, confidence interval; ITT, intent‐to‐treat; nVNS, non‐invasive vagus nerve stimulation. a Response rate was defined as the proportion of subjects who achieved a pain intensity score of 0 or 1 on a 5‐point scale (0, no pain; 4, very severe pain) at 15 minutes and had no rescue medication use through 60 minutes after treatment initiation for the first CH attack in the double‐blind phase. P values are from Fisher's exact test (if ≥1 cell had an expected frequency of ≤5) or the chi‐square test. bPrimary end point. cPrespecified subanalysis.
Sustained Treatment Response Rates and Pain Intensity (Double‐Blind Phase)
Sustained treatment response rates (Fig. 5) for the eCH cohort and total population were significantly higher with nVNS than with sham treatment (eCH: nVNS, 34.2%; sham, 10.6%; P = .008; total: nVNS, 26.7%; sham, 12.3%; P = .04). For the cCH cohort, sustained response rates were similar between groups (nVNS, 13.6%; sham, 15.4%; P = 1.0). For both cohorts and for the total population, the average of all subjects’ mean pain intensities at 15 minutes after treatment for all CH attacks was not significantly different between the nVNS and sham treatment groups (eCH: nVNS, 2.0 [95% CI: 1.8, 2.3]; sham, 2.0 [95% CI: 1.8, 2.3]; P = 1.0; cCH: nVNS, 2.3 [95% CI: 1.9, 2.6]; sham, 1.9 [95% CI: 1.6, 2.3]; P = .2; total: nVNS, 2.1 [95% CI: 1.9, 2.3]; sham, 2.0 [95% CI: 1.8, 2.2]; P = .4).
Figure 5.
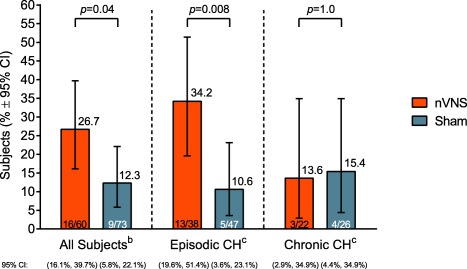
Sustained treatment response ratea (ITT Population). CH, cluster headache; CI, confidence interval; ITT, intent‐to‐treat; nVNS, non‐invasive vagus nerve stimulation. a Sustained treatment response rate was defined as the proportion of subjects with a pain intensity score of 0 or 1 without rescue medication use at 15 through 60 minutes after treatment initiation for the first CH attack in the double‐blind phase. P values are from Fisher's exact test (if ≥1 cell had an expected frequency of ≤5) or the chi‐square test. bSecondary end point. cPrespecified subanalysis.
Other Efficacy Results (Double‐Blind Phase)
The proportion of subjects in the eCH cohort, but not in the cCH cohort or total population, who were responders at 15 minutes for ≥50% of treated attacks was significantly higher with nVNS (34.2%) than with sham treatment (14.9%; P = .04) (Table 2). Results were also significant in only the eCH cohort for the proportion of those who were pain‐free at 15 minutes for ≥50% of treated attacks (nVNS, 15.8%; sham, 2.1%; P = .04) (Table 2). Similarly, differences between groups that favored nVNS for mean duration of the first attack in the double‐blind phase were more pronounced in the eCH cohort (12.8 minutes; P = .21) than in the cCH cohort (3.1 minutes; P = .82) or in the total population (9.3 minutes; P = .25) (Table 2).
Table 2.
Exploratory Efficacy End Point Results
| End Point | All Subjects | eCH Cohort | cCH Cohort | |||
|---|---|---|---|---|---|---|
| nVNS | Sham | nVNS | Sham | nVNS | Sham | |
| Subjects who were responders at 15 minutes for ≥50% of their treated attacks in the double‐blind phase (%) † , ‡ | 26.7 | 20.6 | 34.2 | 14.9 | 13.6 | 30.8 |
| 95% CI | 16.1, 39.7 | 12.0, 31.6 | 19.6, 51.4 | 6.2, 28.3 | 2.9, 34.9 | 14.3, 51.8 |
| P value | .41 | .04 | .19 | |||
| Subjects who were pain‐free at 15 minutes for ≥50% of their treated attacks in the double‐blind phase (%) † , ‡ | 11.7 | 6.9 | 15.8 | 2.1 | 4.6 | 15.4 |
| 95% CI | 4.8, 22.6 | 2.3, 15.3 | 6.0, 31.3 | 0.1, 11.3 | 0.1, 22.8 | 4.4, 34.9 |
| P value | .33 | .04 | .36 | |||
| Duration of first CH attack in the double‐blind phase (min), § , ¶ mean ± SD | 50.6 ± 38.3 | 59.9 ± 47.5 | 48.4 ± 35.4 | 61.2 ± 49.5 | 54.5 ± 43.8 | 57.6 ± 44.8 |
| 95% CI | 40.0, 61.1 | 48.0, 71.7 | 36.0, 60.7 | 45.4, 77.1 | 33.4, 75.6 | 38.7, 76.5 |
| P value | .25 | .21 | .82 | |||
| Change in duration of attacks from baseline to the first attack in the double‐blind phase (min), §,††,‡‡ mean ± SD | −9.5 ± 51.8 | 12.8 ± 45.5 | −14.4 ± 59.5 | 16.3 ± 51.5 | 1.0 ± 28.6 | 5.4 ± 29.2 |
| 95% CI | −25.8, 6.9 | 0.2, 25.3 | −37.4, 8.7 | −1.1, 33.7 | −16.3, 18.3 | −9.7, 20.4 |
| P value | .03 | .03 | .69 | |||
| Rescue medication use in the first hour after treatment initiation (first attack) in the double‐blind phase (%) † | 38.3 | 50.7 | 42.1 | 48.9 | 31.8 | 53.9 |
| 95% CI | 26.1, 51.8 | 38.7, 62.6 | 26.3, 59.2 | 34.1, 63.9 | 13.9, 54.9 | 33.4, 73.4 |
| P value | .15 | .53 | .13 | |||
cCH = chronic cluster headache; CH = cluster headache; CI = confidence interval; eCH = episodic cluster headache; ITT = intent‐to‐treat; nVNS = non‐invasive vagus nerve stimulation; SD = standard deviation.
†ITT population; all subjects: nVNS, n = 60; sham, n = 73; eCH cohort: nVNS, n = 38; sham, n = 47; cCH cohort: nVNS, n = 22; sham, n = 26.
‡No rescue medication use through 60 minutes after treatment initiation; P values are from Fisher's exact test (if ≥1 cell had an expected frequency of ≤5) or the chi‐square test.
§Attacks with duration >180 minutes were excluded according to International Classification of Headache Disorders criteria; P values are from the t test.
¶Observed cases; all subjects: nVNS, n = 53; sham, n = 64; eCH cohort: nVNS, n = 34; sham, n = 40; cCH cohort: nVNS, n = 19; sham, n = 24.
††Change from the last attack before randomization (based on subject recollection) to the first attack in the double‐blind phase (based on objective recording).
‡‡Observed cases; all subjects: nVNS, n = 41; sham, n = 53; eCH cohort: nVNS, n = 28; sham, n = 36; cCH cohort: nVNS, n = 13; sham, n = 17.
Device Perceptions
At the end of the double‐blind phase, subjects rated treatment satisfaction (1, extremely satisfied; 5, not at all satisfied), their willingness to recommend the device to a friend or family member, and ease of device use (1, very easy; 4, very difficult). Proportions of subjects who were extremely satisfied, very satisfied, or satisfied with their treatment were 38.3% for nVNS and 31.9% for sham treatment. The percentage of subjects who indicated that they would recommend their study device to a friend or family member was similar between groups (∼55%). Of all subjects, ∼90% reported that their device was very easy or somewhat easy to use.
Blinding
In the nVNS group, the blinding estimate calculated using the Bang index21 (with values closer to 0 indicating better blinding effectiveness) was 0.20 (95% CI: 0.03, 0.37) after the first treatment, indicating that a considerable proportion of patients correctly guessed their treatment allocation beyond chance. However, successful blinding with nVNS was achieved at the end of the double‐blind phase, with a blinding estimate of 0.10 (95% CI: −0.08, 0.28). Blinding for the sham group also became successful during the study with negative estimates of −0.21 (95% CI: −0.37, −0.05) after the first treatment, which indicated an excess of incorrect guesses potentially related to incomplete blinding, and −0.11 (95% CI: −0.28, 0.06) at the end of the double‐blind phase.
Efficacy Results (Open‐Label Phase)
Rates of response to nVNS in the open‐label phase were similar for the eCH cohort (29.4%; 95% CI: 20.0%, 40.3%; n = 85) and cCH cohort (35.4%; 95% CI: 22.2%, 50.5%; n = 48; P = .47), whereas results seen in the double‐blind phase demonstrated numerically higher nVNS response rates in eCH (34.2%) than in cCH (13.6%; P = .08). In the total population, the response rate in patients who initially received nVNS in the double‐blind phase (26.7%) was maintained in the open‐label phase (30.0%; 95% CI: 18.8%, 43.2%; n = 60; P = .62). For those who initially received sham treatment, the response rate improved from the double‐blind phase (15.1%) to the open‐label phase (32.9%; 95% CI: 22.3%, 44.9%; n = 73; P = .007).
Safety and Tolerability
No SADEs occurred during the study. Of all subjects, 48% (72/150) had ≥1 AE during the study (double‐blind phase, 49/150 subjects [nVNS group, 18; sham group, 31]; open‐label phase, 42/128 subjects). ADEs occurred in 35 subjects during the double‐blind phase (nVNS group, 11; sham group, 24) and 18 subjects during the open‐label phase (Table 3). Application site reactions and nervous system AEs occurred more frequently with sham treatment than with nVNS in the double‐blind phase. Of 13 non–device‐related serious AEs in the nVNS group, CH pain and hospitalization was reported twice by one subject during the double‐blind phase and once by two subjects during the open‐label phase, including one subject who also reported multiple upper extremity deep vein thromboses, abdominal aortic aneurysm, pneumonia, anasarca, acute respiratory failure, and urethral trauma in the open‐label phase. Mesenteric ischemia, herniated disk, and ureteral calculus were reported once by one subject each in the open‐label phase. Only one subject discontinued from the study because of AEs, which were mild or moderate, were not device related, and occurred in the open‐label phase.
Table 3.
Incidence of Adverse Events and Adverse Device Effects (All Treated Subjects)
| AEs and ADEs | Double‐Blind Phase | Open‐Label Phase | |
|---|---|---|---|
| nVNS (n = 73) | Sham (n = 77) | nVNS (n = 128) | |
| Subjects with ≥1 AE, No. (%) | 18 (24.7) | 31 (40.3) | 42 (32.8) |
| Subjects with ≥1 serious AE, No. (%) | 1 (1.4) † , ‡ | 0 | 5 (3.9) ‡ , § |
| Subjects with ≥1 ADE, No. (%) | 11 (15.1) | 24 (31.2) | 18 (14.1) |
| ADEs Occurring in ≥5% of Subjects in Any Treatment Group, No. (%) | |||
| Application site reactions | |||
| Burning/tingling/soreness/stinging | 2 (2.7) | 7 (9.1) | 4 (3.1) |
| Skin irritation/redness/erythema | 0 | 9 (11.7) | 2 (1.6) |
| Musculoskeletal disorders | |||
| Lip or facial drooping/pulling/twitching | 8 (11.0) | 0 | 9 (7.0) |
| Nervous system disorders | |||
| Dysgeusia/metallic taste | 0 | 7 (9.1) | 2 (1.6) |
Abbreviations: ADE = adverse device effect; AE = adverse event; nVNS = non‐invasive vagus nerve stimulation.
†Serious AE of cluster headache (2 occurrences).
‡Serious AEs were not considered related to the study device.
§Serious AEs included cluster headache (1 occurrence; 1 subject); cluster headache as well as multiple left extremity deep vein thromboses, abdominal aortic aneurysm, pneumonia, anasarca, acute respiratory failure, and urethral trauma (1 occurrence each in the same subject); mesenteric ischemia (1 occurrence; 1 subject); herniated disk (1 occurrence; 1 subject); and ureteral calculus (1 occurrence; 1 subject).
DISCUSSION
The ACT1 study is one of the first and largest randomized, double‐blind, sham‐controlled trials to evaluate the effects of a non‐invasive neuromodulation device for the acute treatment of eCH and cCH. Significant effects of nVNS were not observed for the response rate in the total population but were seen in the eCH cohort across a broad range of end points including response rate, sustained treatment response rate, and percentages of patients who were responders, and of those who were pain‐free, for ≥50% of treated attacks (Table 4). Results in this cohort may suggest that an initial treatment response to nVNS is an indicator of subsequent consistency of response (ie, for ≥50% of attacks). Although significant differences were observed between the nVNS and sham groups among the eCH cohort, these differences were not observed among the cCH cohort, a finding that affected the consistency of significant results among the total population (Table 4). A difference of ∼10 minutes between the nVNS and sham groups in the total population for mean duration of the first attack during the double‐blind phase may be clinically meaningful (ie, provides practical advantages that address current therapeutic challenges22) in CH despite the lack of statistical powering. Acute nVNS therapy for CH attacks was well tolerated and achieved clinically meaningful efficacy results (vs sham).
Table 4.
Summary of Results From the Double‐Blind Phase
| End Point | All Subjects | eCH Cohort | cCH Cohort |
|---|---|---|---|
| Response rate |
NS (P = .1) |
SIG
(P = .008) |
NS (P = .48) |
| Sustained treatment response rate |
SIG
(P = .04) |
SIG
(P = .008) |
NS (P = 1.0) |
| Pain intensity |
NS (P = .4) |
NS (P = 1.0) |
NS (P = .2) |
| Responder for ≥50% of treated attacks |
NS (P = .41) |
SIG
(P = .04) |
NS (P = .19) |
| Pain‐free for ≥50% of treated attacks |
NS (P = .33) |
SIG
(P = .04) |
NS (P = .36) |
| Duration of first attack |
NS (P = .25) |
NS (P = .21) |
NS (P = .82) |
| Change in attack duration |
SIG
(P = .03) |
SIG
(P = .03) |
NS (P = .69) |
| Rescue medication use in the first hour after the first attack |
NS (P = .15) |
NS (P = .53) |
NS (P = .13) |
cCH = chronic cluster headache; eCH = episodic cluster headache; NS = not significant; SIG = significant.
Study limitations include the analysis of the cCH cohort as part of the primary end point, the need for careful interpretation of subanalyses results, challenges with blinding inherent in medical device studies, and the time to first measurement of response used to define the primary efficacy end point. Primary end point results were significant for the eCH cohort but were diminished overall by the cCH cohort results. When subanalyses results are interpreted, the lack of statistical powering and the potential for type 1 and type 2 errors (in the eCH and cCH cohorts, respectively) should be considered. The difference in AE descriptions provided by subjects treated with the nVNS (eg, drooping/pulling of the lip/face) and sham (eg, burning, soreness, stinging) devices may help to explain results of the blinding analyses, which are similar to those observed in previous sham‐controlled trials.23, 24 The burning sensation and other pain‐related AEs reported by the sham‐treated group in ACT1 may have led to a placebo effect based on impressions that the subjects were receiving active treatment. Sham device–associated pain may have also produced a diffuse noxious inhibitory control (DNIC) effect, a phenomenon in which the application of a noxious electrical stimulus to remote body regions inhibits dorsal horn activity and attenuates the original pain.25, 26 Potential placebo and DNIC effects in the sham group may have reduced the magnitude of the therapeutic benefit associated with nVNS treatment.
Another limitation was that the time point used to define the ACT1 primary end point was 15 minutes after treatment initiation, which has been used in other CH studies,27, 28, 29 rather than after treatment completion. In ACT1, this 15‐minute interval comprised an 8‐minute nVNS stimulation period followed by only a 7‐minute period that appeared to be sufficient for significant treatment effects to become evident in the eCH cohort but not in the cCH cohort or total population (Fig. 2). The 15‐minute assessment time point may have also contributed to the nonsignificant difference in average pain intensities between the nVNS and sham groups; other potential contributing factors include the combined statistical influence of the responders and nonresponders as well as the assessment after all attacks (rather than after the first attack). Therefore, methodological implications in ACT1 regarding distinct effects among the eCH and cCH cohorts, the painful sham stimulation, and the use of a longer time to first measurement of response such as 30 minutes, as used in CH studies of other therapies,20, 28, 30 should be considered for future randomized controlled trials.
Findings from previous mechanistic/imaging and clinical studies may support the different effects seen between the two cohorts in ACT1 as well as the apparent treatment refractoriness seen in the cCH cohort during the study's double‐blind phase. In a voxel‐based morphometry study, patients experiencing eCH attacks had increases in gray matter (GM) (vs healthy controls) within most of the brain regions known to be associated with acute/transient pain, whereas patients with cCH had GM decreases within several regions known to be associated with pain processing and chronification, such as the posterior part of the anterior or cingulate cortex and amygdala.31 An observed negative correlation between GM volume and disease duration supports an impairment in recovery and the treatment resistance seen among the cCH cohort in ACT1.31 Similarly, clinical studies have shown that subjects with cCH had compromised responses to other acute therapies including sumatriptan,19, 32, 33 a primary symptomatic treatment.7, 8 Persistent interictal pain and allodynia, a known marker of treatment refractoriness in migraine,34, 35 may have affected responses in subjects with cCH,33 but the potential association between these features and treatment response was not examined in ACT1 and requires further evaluation in CH. These features, along with the ongoing attacks of cCH, may have cumulative effects on brain physiology31 and patients’ initial ability to respond to treatment. In contrast to results from the 1‐month double‐blind phase of ACT1, findings from the 3‐month open‐label phase showed that the response rate of the cCH cohort (35.4%) was similar to that of the eCH cohort (29.4%). These results suggest further benefit with continued acute nVNS use for patients with cCH, which is consistent with findings from prophylactic nVNS studies in cCH and chronic migraine as well as VNS studies in epilepsy and depression.19, 36, 37, 38
The ACT1 response definition, mild pain intensity or pain‐free at 15 minutes, was also used for primary analyses in a study of subcutaneous sumatriptan (N = 49) for a combined eCH and cCH population27 and a study of an implantable sphenopalatine ganglion (SPG) stimulation device (N = 32) for a cCH population.39 However, the ability to compare data from these three trials is limited. The subcutaneous sumatriptan and SPG stimulation studies involved relatively small sample sizes and did not report results specifically for subjects with eCH, whereas ACT1 demonstrated a significant benefit of nVNS in this subgroup. It should also be noted that SPG stimulation requires invasive surgical device implantation.39 Subcutaneous sumatriptan and inhaled oxygen are the most commonly used pharmacologic acute CH therapies.7, 8 In addition to the FDA‐approved maximum dosing of subcutaneous sumatriptan in CH (ie, 2 doses per day) being inadequate for patients with frequent attacks (ie, 3 to 8 attacks per day),13 this treatment is associated with injection site reactions (eg, pain, swelling) and neurologic symptoms (eg, dizziness, tiredness) and has cardiovascular contraindications.11, 27 Inhaled oxygen does not have a maximum frequency of use or associations with AEs, but its use may be limited because of the size and lack of portability of the tanks, coupled with the need for continuous access to the oxygen supply.12, 13 Considering the tolerability, dosing, and/or practicality issues associated with currently available symptomatic treatments, nVNS provides a safe, well‐tolerated, effective, and easy‐to‐use non‐invasive option for acute CH treatment that has produced significant and clinically meaningful responses within 15 minutes in patients with eCH. nVNS can be easily incorporated into the acute treatment paradigm for eCH. It may be particularly useful in clinical settings where the use of current acute treatment options is challenging due to the risk of medication overuse, a desire to minimize AEs, or an inability to treat in a timely manner.
CONCLUSIONS
This trial is among the largest randomized sham‐controlled studies of a therapeutic intervention for the acute treatment of CH. In the total population, a significant difference in response rates between the nVNS and sham groups was not observed. In subjects with eCH, nVNS therapy offered significant and clinically meaningful benefits over sham treatment, including rapid (within 15 minutes) and sustained (through 60 minutes) pain relief. Significant effects were not observed in subjects with cCH, a finding that affected results in the total population. The nVNS device was also safe and well tolerated and thus represents a novel acute treatment option with a positive risk‐benefit profile for patients with eCH. A report of a similar large, randomized, sham‐controlled trial completed in Europe is forthcoming and may validate the results seen in this study.
STATEMENT OF AUTHORSHIP
Category 1 (a) Conception and Design
Stephen D. Silberstein, Eric J. Liebler, Emily Rubenstein Engel, Stewart J. Tepper
(b) Acquisition of Data
Stephen D. Silberstein, Laszlo L. Mechtler, David B. Kudrow, Anne H. Calhoun, Joel R. Saper, Stewart J. Tepper
(c) Analysis and Interpretation of Data
Stephen D. Silberstein, Eric J. Liebler, Emily Rubenstein Engel, Candace McClure, Stewart J. Tepper
Category 2 (a) Drafting the Article
Stephen D. Silberstein, Eric J. Liebler, Stewart J. Tepper
(b) Revising It for Intellectual Content
Stephen D. Silberstein, Eric J. Liebler, Laszlo L. Mechtler, David B. Kudrow, Anne H. Calhoun, Candace McClure, Joel R. Saper, Emily Rubenstein Engel, Stewart J. Tepper
Category 3 (a) Final Approval of the Completed Article
Stephen D. Silberstein, Laszlo L. Mechtler, David B. Kudrow, Anne H. Calhoun, Candace McClure, Joel R. Saper, Eric J. Liebler, Emily Rubenstein Engel, Stewart J. Tepper
ACT1 Study Group
Investigators are listed by study site at the time of the trial. 1. Associated Neurologists of Southern Connecticut, Fairfield, CT – Peter J. McAllister, MD (principal investigator), Thomas B. Toothaker, MD (principal investigator), Dario M. Zagar, MD (sub‐investigator), Jeffrey L. Gross, MD (sub‐investigator), Srinath Kadimi, MD (sub‐investigator), Anthony Quan Hong, MD (sub‐investigator), Nicholas A. Blondin, MD (sub‐investigator), Bozena Czapka, MHS, PA‐C (sub‐investigator), and Karen Brown, MMS, PA‐C (sub‐investigator); 2. California Medical Clinic for Headache, Santa Monica, CA – David B. Kudrow, MD (principal investigator); 3. Carolina Headache Institute, Durham, NC – Anne Calhoun, MD (principal investigator) and Keven A. Kahn, MD (sub‐investigator); 4. Cleveland Clinic Foundation, Cleveland, OH – Stewart Tepper, MD (principal investigator), Cynthia Bamford, MD (sub‐investigator), Emad Estemalik, MD (sub‐investigator), Mark Stillman, MD (sub‐investigator), Deborah Tepper, MD (sub‐investigator), Mary Ann Mays, MD (sub‐investigator), and Jennifer Kriegler, MD (sub‐investigator); 5. Clinvest Headache Care Center, Springfield, MO – Roger Cady, MD (principal investigator) and John Dexter, MD (sub‐investigator); 6. Colorado Neurological Institute, Englewood, CO – Cori Millen‐Schnurr, DO (principal investigator); 7. Dent Neurologic Institute, Amherst, NY – Laszlo Mechtler, MD (principal investigator), Mary K. Betz, RPA‐C (sub‐investigator), Karly A. Benamati, PA (sub‐investigator), Elizabeth D. Smith, NP (sub‐investigator), Jennifer W. McVige, MD (sub‐investigator), Nicolas P. Saikali, MD (sub‐investigator), Kathleen M. Mogensen, NP (sub‐investigator), Minsoo Kang, MD (sub‐investigator), and Maria T. Rizzo, PA (sub‐investigator); 8. Diamond Headache Clinic, Chicago, IL – Alexander Feoktistov, MD, PhD (principal investigator) and George Urban, MD (sub‐investigator); 9. Jefferson Headache Center, Philadelphia, PA – Stephen Silberstein, MD (principal investigator), William Young, MD (sub‐investigator), Michael Marmura, MD (sub‐investigator), and Stephanie Nahas‐Geiger, MD (sub‐investigator); 10. Michigan Head Pain & Neurological Institute, Ann Arbor, MI – Joel Saper, MD (principal investigator), Arnaldo Da Silva, MD (sub‐investigator), James Weintraub, MD (sub‐investigator), and Alicia Prestegaard, MD (sub‐investigator); 11. Mid‐Atlantic Headache Institute, Pikesville, MD – Marcia C. Ribeiro, MD (principal investigator); 12. Montefiore Headache Center, Bronx, NY – Brian Grosberg, MD (principal investigator), Sarah Vollbracht, MD (sub‐investigator), Richard Lipton, MD (sub‐investigator), Jelena Pavlovic, MD (sub‐investigator), and Matthew Robbins, MD (sub‐investigator); 13. New England Regional Headache Center, Worchester, MA – Herbert G. Markley, MD (principal investigator), Shivang Joshi, MD (sub‐investigator), and Carolyn Benson, NP (sub‐investigator); 14. Norton Neuroscience Institute Headache and Concussion Center, Louisville, KY – Brian M. Plato, DO (principal investigator) and Robert S. Tillett, MD (sub‐investigator); 15. Stanford University Medical Center, Department of Neurology, Stanford, CA – Sheena K. Aurora, MD, FAHS (principal investigator), Nada Hindiyeh, MD (sub‐investigator), Robert Cowan, MD (sub‐investigator), and Meredith Barad, MD (sub‐investigator); 16. Tampa General Hospital Headache and Pain Center, Tampa, FL – Maria‐Carmen Wilson, MD (principal investigator) and Cristina Cabret‐Aymat, MD (sub‐investigator); 17. The Center for Headache Care and Research/Island Neurological Associates, PC, Plainview, NY – Ira Turner, MD (principal investigator) and Jennifer Ahmed, MD (sub‐investigator); 18. University of Iowa Hospital and Clinics, Iowa City, IA – Connie Pieper, MD (principal investigator) and Carolyn Johnson, PA (sub‐investigator); 19. UT Southwestern Medical Center, Department of Neurology and Neurotherapeutics, Dallas, TX – Deborah L. Friedman, MD, MPH (principal investigator) and Priyanka Chaudhry, MD (sub‐investigator); 20. West Virginia University Hospitals, Department of Neurology, Morgantown, WV – David B. Watson, MD (principal investigator), Christopher S. Nance, MD (sub‐investigator), and Tiffany Lannan, FNP‐BC (sub‐investigator).
Acknowledgments
Authors would like to acknowledge Lia Spitzer of electroCore, LLC, for her contributions to study conception and design and the analysis and interpretation of data. Professional writing and editorial support were provided by Stefanie Dorlas, BMath, BEd, of MedLogix Communications, LLC, under the direction of authors.
Conflict of Interest: Stephen D. Silberstein, MD, has received consultancy and advisory board fees from Alder Biopharmaceuticals Inc., Allergan, Inc., Amgen, Inc., Avanir Pharmaceuticals, Inc., Depomed, Inc., Dr. Reddy's Laboratories Ltd., electroCore, LLC, eNeura Inc., Ipsen Biopharmaceuticals Inc., Medscape, LLC, Medtronic, Inc., Mitsubishi Tanabe Pharma America, Inc., National Institute of Neurological Disorders and Stroke, St. Jude Medical, Inc., Supernus Pharmaceuticals, Inc., Teva Pharmaceutical Industries Ltd., and Trigemina, Inc.; Laszlo L. Mechtler, MD, has received speaker fees from Allergan, Inc., Depomed, Inc., and Supernus Pharmaceuticals, Inc., and research support from Celldex Therapeutics, Cincinnati Children's Hospital Medical Center, GlaxoSmithKline, PharmaNet Group Ltd., and Questcor Pharmaceuticals, Inc.; David B. Kudrow, MD, has received speaker fees from Teva Pharmaceutical Industries Ltd. and grant funding from Depomed, Inc.; Anne H. Calhoun, MD, has received advisory board fees from Allergan, Inc., Depomed, Inc., Eli Lilly and Company, and Teva Pharmaceutical Industries Ltd., and speaker fees from Depomed, Inc., Merck & Co., Inc., and Teva Pharmaceutical Industries Ltd. Dr. Calhoun has also received research support from Autonomic Technologies, Inc., electroCore, LLC, and Scion NeuroStim, LLC; Candace McClure, PhD, is an employee of NAMSA; Joel R. Saper, MD, has received consultancy fees from Alder Biopharmaceuticals Inc., Allergan, Inc., Johnson & Johnson (Ethicon, Inc.), Migraine Research Foundation, NuPathe Inc., Purdue Pharma L.P., Supernus Pharmaceuticals, Inc., Teva Pharmaceutical Industries Ltd., and Tian Pharmaceutical Co. Ltd. Dr. Saper has also received research grants from Achelios Therapeutics, Inc., Alder Biopharmaceuticals Inc., Allergan, Inc., Amgen, Inc., Astellas Pharma Inc., Autonomic Technologies, Inc., Cerephex Corporation, Daiichi‐Sankyo Co. Ltd., Dr. Reddy's Laboratories Ltd., Eli Lilly and Company, GlaxoSmithKline, Labrys Biologics, Inc., Merck & Co., Inc., Pfizer, Inc., Scion NeuroStim, LLC, Vanda Pharmaceuticals, and Winston Laboratories, Inc.; Eric J. Liebler is an employee of electroCore, LLC, and receives stock ownership; Emily Rubenstein Engel, MD, has received consultancy, speaker, and advisory board fees from Allergan, Inc., Depomed, Inc., and electroCore, LLC; and Stewart J. Tepper, MD, has received consultancy fees from Acorda Therapeutics, Inc., Allergan, Inc., Amgen, Inc., Autonomic Technologies, Inc. (ATI), Avanir Pharmaceuticals, Inc., Depomed, Inc., Dr. Reddy's Laboratories Ltd., electroCore, LLC, Impax Pharmaceuticals, Inc., Pfizer, Inc., Scion NeuroStim, LLC, Teva Pharmaceutical Industries Ltd., Theorem Clinical Research, Zogenix, Inc., and Zosano Pharma Corporation. Dr. Tepper has also received research grants/support from Allergan, Inc., Amgen, Inc., Autonomic Technologies, Inc. (ATI), Avanir Pharmaceuticals, Inc., electroCore, LLC, eNeura Inc., GlaxoSmithKline, Merck & Co., Inc., Pfizer, Inc., Optinose US Inc./Avanir Pharmaceuticals, Inc./Otsuka Pharmaceutical Co., Ltd., Teva Pharmaceutical Industries Ltd., and Zogenix, Inc. He has also received stock options from Autonomic Technologies, Inc. (ATI) and royalties from the University Press of Mississippi and Springer.
Financial Support: This study was sponsored by electroCore, LLC. Professional writing and editorial support from MedLogix Communications, LLC, funded by electroCore, LLC, were under the direction of authors throughout draft development and revisions in accordance with International Committee of Medical Journal Editors (ICMJE) criteria for authorship. Data analysis support from NAMSA was funded by electroCore, LLC. The authors are guarantors of this document, which expresses the opinions and conclusions of the authors and not those of their corresponding affiliations.
REFERENCES
- 1. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 2. Goadsby PJ. Pathophysiology of cluster headache: A trigeminal autonomic cephalgia. Lancet Neurol. 2002;1:251‐257. [DOI] [PubMed] [Google Scholar]
- 3. Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: A meta‐analysis of population‐based studies. Cephalalgia. 2008;28:614‐618. [DOI] [PubMed] [Google Scholar]
- 4. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24:9‐160. [DOI] [PubMed] [Google Scholar]
- 5. Jensen RM, Lyngberg A, Jensen RH. Burden of cluster headache. Cephalalgia. 2007;27:535‐541. [DOI] [PubMed] [Google Scholar]
- 6. Jürgens TP, Gaul C, Lindwurm A, et al. Impairment in episodic and chronic cluster headache. Cephalalgia. 2011;31:671‐682. [DOI] [PubMed] [Google Scholar]
- 7. Francis GJ, Becker WJ, Pringsheim TM. Acute and preventive pharmacologic treatment of cluster headache. Neurology. 2010;75:463‐473. [DOI] [PubMed] [Google Scholar]
- 8. May A, Leone M, Afra J, et al. EFNS Task Force. EFNS guidelines on the treatment of cluster headache and other trigeminal‐autonomic cephalalgias. Eur J Neurol. 2006;13:1066‐1077. [DOI] [PubMed] [Google Scholar]
- 9. Becker WJ. Cluster headache: Conventional pharmacological management. Headache. 2013;53:1191‐1196. [DOI] [PubMed] [Google Scholar]
- 10. Dihydroergotamine Mesylate [Package Insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2002. [Google Scholar]
- 11. IMITREX [Package Insert]. Research Triangle Park, NC: GlaxoSmithKline; 2015. [Google Scholar]
- 12. Ashkenazi A, Schwedt T. Cluster headache–acute and prophylactic therapy. Headache. 2011;51:272‐286. [DOI] [PubMed] [Google Scholar]
- 13. Ekbom K, Hardebo JE. Cluster headache: Aetiology, diagnosis and management. Drugs. 2002;62:61‐69. [DOI] [PubMed] [Google Scholar]
- 14. Braunstein D, Donnet A, Pradel V, et al. Triptans use and overuse: A pharmacoepidemiology study from the French health insurance system database covering 4.1 million people. Cephalalgia. 2015;35:1172‐1180. [DOI] [PubMed] [Google Scholar]
- 15. Yuan H, Silberstein SD. Vagus nerve stimulation and headache. Headache. 2015 Oct 16. doi: 10.1111/head.12721. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Ferguson AV, Samson WK. The orexin/hypocretin system: A critical regulator of neuroendocrine and autonomic function. Front Neuroendocrinol. 2003;24:141‐150. [DOI] [PubMed] [Google Scholar]
- 17. Martelletti P, Mitsikostas DD. Cluster headache: A quasi‐rare disorder needing a reappraisal. J Headache Pain. 2015;16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nesbitt AD, Marin JCA, Tompkins E, Ruttledge MH, Goadsby PJ. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249‐1253. [DOI] [PubMed] [Google Scholar]
- 19. Gaul C, Diener HC, Silver N, et al. Non‐invasive vagus nerve stimulation for prevention and acute treatment of chronic cluster headache (PREVA): A randomised controlled study. Cephalalgia. 2016;36:534‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lipton RB, Micieli G, Russell D, Solomon S, Tfelt‐Hansen P, Waldenlind E. Guidelines for controlled trials of drugs in cluster headache. Cephalalgia. 1995;15:452‐462. [PubMed] [Google Scholar]
- 21. Bang H, Flaherty SP, Kolahi J, Park J. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin Res Regul Aff. 2010;27:42‐51. [Google Scholar]
- 22. Kazdin AE. The meanings and measurement of clinical significance. J Consult Clin Psychol. 1999;67:332‐339. [DOI] [PubMed] [Google Scholar]
- 23. Broadbent HJ, van den Eynde F, Guillaume S, et al. Blinding success of rTMS applied to the dorsolateral prefrontal cortex in randomised sham‐controlled trials: A systematic review. World J Biol Psychiatry. 2011;12:240‐248. [DOI] [PubMed] [Google Scholar]
- 24. Rakel B, Cooper N, Adams HJ, et al. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11:230‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non‐convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979;6:305‐327. [DOI] [PubMed] [Google Scholar]
- 26. Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6:283‐304. [DOI] [PubMed] [Google Scholar]
- 27. The Sumatriptan Cluster Headache Study Group. Treatment of acute cluster headache with sumatriptan. N Engl J Med. 1991;325:322‐326. [DOI] [PubMed] [Google Scholar]
- 28. Cohen AS, Burns B, Goadsby PJ. High‐flow oxygen for treatment of cluster headache: A randomized trial. JAMA. 2009;302:2451‐2457. [DOI] [PubMed] [Google Scholar]
- 29. Schuh‐Hofer S, Reuter U, Kinze S, Einhäupl KM, Arnold G. Treatment of acute cluster headache with 20 mg sumatriptan nasal spray–an open pilot study. J Neurol. 2002;249:94‐99. [DOI] [PubMed] [Google Scholar]
- 30. Law S, Derry S, Moore RA. Triptans for acute cluster headache. Cochrane Database Syst Rev. 2013;7:CD008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naegel S, Holle D, Desmarattes N, et al. Cortical plasticity in episodic and chronic cluster headache. Neuroimage Clin. 2014;6:415‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bahra A, Gawel MJ, Hardebo JE, Millson D, Breen SA, Goadsby PJ. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology. 2000;54:1832‐1839. [DOI] [PubMed] [Google Scholar]
- 33. Marmura MJ, Pello SJ, Young WB. Interictal pain in cluster headache. Cephalalgia. 2010;30:1531‐1534. [DOI] [PubMed] [Google Scholar]
- 34. Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: A race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19‐26. [DOI] [PubMed] [Google Scholar]
- 35. Landy S, Rice K, Lobo B. Central sensitisation and cutaneous allodynia in migraine: Implications for treatment. CNS Drugs. 2004;18:337‐342. [DOI] [PubMed] [Google Scholar]
- 36. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment‐resistant depression: Acute and chronic effects. Brain Stimul. 2013;6:631‐640. [DOI] [PubMed] [Google Scholar]
- 37. Murphy JV. Left vagal nerve stimulation in children with medically refractory epilepsy. The Pediatric VNS Study Group. J Pediatr. 1999;134:563‐566. [DOI] [PubMed] [Google Scholar]
- 38. Silberstein SD, Calhoun AH, Lipton RB, et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Headache. 2016 Jul 13. pii: 10.1212/WNL.0000000000002918. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schoenen J, Jensen RH, Lantéri‐Minet M, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH‐1: A randomized, sham‐controlled study. Cephalalgia. 2013;33:816‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]


