Abstract
Bacterial endospores are among the most resilient forms of life on earth and are intrinsically resistant to extreme environments and antimicrobial treatments. Their resilience is explained by unique cellular structures formed by a complex developmental process often initiated in response to nutrient deprivation. Although the macromolecular structures of spores from different bacterial species are similar, their resistance to environmental insults differs widely. It is not known which of the factors attributed to spore resistance confer very high-level heat resistance. Here, we provide conclusive evidence that in Bacillus subtilis, this is due to the presence of a mobile genetic element (Tn1546-like) carrying five predicted operons, one of which contains genes that encode homologs of SpoVAC, SpoVAD and SpoVAEb and four other genes encoding proteins with unknown functions. This operon, named spoVA2mob, confers high-level heat resistance to spores. Deletion of spoVA2mob in a B. subtilis strain carrying Tn1546 renders heat-sensitive spores while transfer of spoVA2mob into B. subtilis 168 yields highly heat-resistant spores. On the basis of the genetic conservation of different spoVA operons among spore-forming species of Bacillaceae, we propose an evolutionary scenario for the emergence of extremely heat-resistant spores in B. subtilis, B. licheniformis and B. amyloliquefaciens. This discovery opens up avenues for improved detection and control of spore-forming bacteria able to produce highly heat-resistant spores.
Introduction
The bacterial endospore is one of the most resistant life forms on earth with astounding longevity that may exceed thousands of years (Cano and Borucki, 1995; Vreeland et al., 2000). Endospores can survive not only exposure to extremes of temperature but also other stresses such as desiccation, radiation and disinfectants (Setlow, 2006). Spore-forming species belonging to the Firmicutes have broad biotechnological applications in fermentation processes, gut health promotion (probiotics), crop protection and increasing crop yields, as carriers for vaccine antigens, and in the production of a range of useful chemicals, enzymes and fuels (Duc et al., 2003; Cutting, 2011). However, some of the species are pathogenic and their spores have a pivotal role in the spread of infection (for example, Bacillus anthracis, Clostridium difficile, Clostridium perfringens, Clostridium tetani, Clostridium botulinum; Ivanova et al., 2003; Read et al., 2003; Rupnik et al., 2009; Peck et al., 2011). Pathogenic sporeformers are estimated to cause over one million cases of foodborne illness in the USA alone (for example, due to C. perfringens and B. cereus; Scallan et al., 2011). Furthermore, several non-pathogenic bacterial sporeformers are a cause of major financial losses to the food industry through the presence of heat-resistant spores that survive processes such as pasteurization or even sterilization, leading to reduced shelf life, food spoilage and subsequent food waste (Scheldeman et al., 2006; Scallan et al., 2011; Wells-Bennik et al., 2016).
The tremendous importance of spores has prompted considerable effort to understand the molecular mechanisms responsible for their resistance properties (Nicholson et al., 2000; Errington, 2003; Gould, 2006; Sunde et al., 2009). It is known that different features of the spore, including components in the core, cortex, coat and membranes, contribute to resistance properties (Setlow, 2006; Brul et al., 2011). However, it remains unknown why spores of some strains are able to withstand extreme heat treatments, whereas others succumb quickly (Oomes et al., 2007; Lima et al., 2011; Berendsen et al., 2015b).
In this study, we investigated determinants of high-level heat resistance of spores of B. subtilis strains that were isolated from diverse sources. Therefore, the genome sequences and the heat-resistance properties of spores of these strains were determined. A comparative genomics approach revealed the presence of a transposon only in strains producing high-level heat-resistant spores. The genes in the transposon were shown to contribute to high-level heat resistance of spores and their occurrence was assessed in other species belonging to the family of Bacillaceae.
Materials and methods
Strains, sporulation and establishing spore heat resistance
For 18 strains of B. subtilis, 9 strains of B. amyloliquefaciens and 9 strains of B. licheniformis, the heat resistance of spores was characterized as described previously (Berendsen et al., 2015b; Supplementary Table 1). Detailed spore heat-inactivation kinetics were previously determined for 11 strains of B. subtilis and two strains of B. amyloliquefaciens (Berendsen et al., 2015b). For the other strains, spores were prepared and detailed inactivation kinetics were determined. The heat resistance of spores was visualized by plotting the calculated decimal reduction time (D-value) at a given temperature (for example, 100 °C or 112.5 °C) for spores of different strains (Berendsen et al., 2015b).
The genome sequences of 8 B. subtilis strains were publicly available, and the genomes of the other 10 B. subtilis strains were sequenced (Supplementary Table 2; Berendsen et al., 2016). In addition, the genomes of two strains of B. amyloliquefaciens and all nine strains of B. licheniformis were sequenced (Supplementary Table 2).
Phenotype-genotype matching and genome analysis
To compare the genome content of members of the Bacillaceae family, orthology matrices were constructed on the basis of the predicted protein content of the strains using Ortho-MCL (Li et al., 2003). Three different orthology matrices were constructed, namely, one for the 18 strains of B. subtilis (Supplementary Dataset 1), another for the strains of B. licheniformis, the B. amyloliquefaciens strains with available genome sequences, B. subtilis strain 168 and strain B4146 (Supplementary Dataset 2), and a third one for 103 spore-forming members of the Bacillaceae (Supplementary Dataset 3; Li et al., 2003). The latter orthology matrix contained 35 strains belonging to the B. subtilis group, 33 strains of B. cereus, 5 strains of Anoxybacillus flavithermus, 23 strains of Geobacillus spp., 2 strains of Caldibacillus debilis, 1 strain of B. sporothermodurans and 4 strains of B. thermoamylovorans (Supplementary Table 2).
Phenotype-genotype matching was performed for the 18 B. subtilis strains using Phenolink (Bayjanov et al., 2012), with low- or high-level heat resistance of spores as the phenotypic input, and the orthology matrix (Supplementary Dataset 1) as genotypic input. The genomic locations of target genes were visualized using Artemis (Carver et al., 2012) and Artemis Comparison Tool (Carver et al., 2005). For B. subtilis strains, detailed gene and operon predictions within the Tn1546 transposon were made using FGENESB (www.softberry.com) and predictions were manually inspected. Specific insertion locations of the transposon and the number of transposon elements present per strain was verified by PCR using primers as listed in Supplementary Table 3.
A maximum likelihood core genome phylogenetic tree was constructed on the basis of the predicted protein sequences of all genes that are conserved in a single copy in all 103 Bacillaceae strains that were selected. Protein alignments were made using MUSCLE (Edgar, 2004) and the phylogenetic trees were constructed using PHYML (Guindon and Gascuel, 2003). The number and organization of spoVA genes in the genomes was verified using the orthology matrix of the 103 strains. To find potential functional equivalents, a Hidden Markov Model was constructed per orthologous group, that was used to search against all genomes (Johnson et al., 2010). Protein sequences were extracted from the 103 genomes of spore-forming Bacillaceae for predicted SpoVAC and SpoVAD. Protein alignments and phylogenetic protein trees were prepared as described above, and manually inspected for evolutionary relatedness of the proteins. The operon structures were verified for all spoVA genes. In addition, it was determined whether the genomic location of the spoVA operon was on the chromosome or on a plasmid, and it was established whether the operon was part of transposable genetic elements.
Carry over of transposon
Natural transfer of the Tn1546 transposon was achieved by generalized transduction from strain B4067, which produces high-level heat-resistant spores, to recipient strain 168-spR which produces low-level heat-resistant spores (Supplementary Figure 1). Details of this procedure are given below. Strain B4067 carries a prophage in the genome (locus tags B4067_4636 to B4067_4698, comprising 39 kb) that was induced with mitomycin C (1 μg ml−1, Sigma, Zwijndrecht, The Netherlands) to produce phages as described previously (Moineau et al., 1994). The phages were isolated for DNA sequencing as follows. Briefly, the lysed culture was centrifuged (10 min, 6000 g) and the supernatant was filter-sterilized using a filter with a pore size of 0.22 μm (Merck Millipore, Amsterdam, The Netherlands). The filtered supernatant was incubated with RNAse (10 μg ml−1, Sigma) and DNAse (1 μg ml−1, Sigma) at 37 °C for 1 h. Subsequently, NaCl (1M) and polyethylene glycol (10%) were added to the phages, followed by incubation for 18 h at 4 °C. The phages were centrifuged (10 min, 6000 g) and re-suspended in phage buffer (100 mM NaCl, 5 mM CalCl2, 1 mM MgSO4, 0.01% gelatin, pH 7.5). DNA was isolated from the phages followed by DNA sequencing as described previously (Krawczyk et al., 2015). The sequences of the DNA isolated from the phages and the coverage were visualized on the B. subtilis B4067 genome using Artemis (Carver et al., 2012) and found to contain the whole genome sequence of the strain.
Transfer of the Tn1546 transposon element from strain B4067 to strain 168-spR was achieved as follows. Upon induction of the prophage in strain B4067 by adding mitomycin C (1 μg ml−1), a phage lysate was obtained. This lysate was mixed with cells of recipient strain B. subtilis 168-spR and incubated for 1 h at 37 °C on a nitrocellulose filter (0.2 μm pore size, Nalgene, Rochester, NY, USA) that was placed on a Luria Broth plate (as described by Auchtung et al., 2005). The recipient cells (with some assumed to have received the Tn1546 transposon element) were transferred to sporulation plates and spores were prepared as described above. The resulting spores were subjected to a heat treatment of 100 °C for 60 min. This high heat treatment allowed for the survival of spores that were produced by cells of strain 168-spR that had incorporated a DNA element encompassing the Tn1546 transposon in the genome, while spores of cells that received DNA elements unrelated to high-level spore heat resistance were fully inactivated and not recovered. In addition to selection on the basis of heat resistance of spores, resulting strains were also selected on the basis of antibiotic resistance (due to the spec marker, 100 μg ml−1) and tryptophan deficiency. The donor strain B4067 could not grow in the presence of spectinomycin in the concentrations used. The presence of the Tn1546 transposon in the resulting colonies of 168-spR was verified by PCR (primers are listed in Supplementary Table 3). One of the colonies containing the Tn1546 transposon was selected (designated 168HR, NIZO culture collection strain B4417) and the genome sequence was determined as described previously (Krawczyk et al., 2015).
Gene deletion and cloning
Specific deletion mutants (Supplementary Table 1) were constructed in strain 168HR using the cre/lox system, as previously described, with slight alterations (Lambert et al., 2007; Yan et al., 2008). The PCR fragment lox66-P32-cat-lox71 cassette from pNZ5319 was fused by overhang PCR with the flanking regions of the genes to be deleted (primers are listed in Supplementary Table 3). The fused fragments were cloned into pNZ5319 using the SwaI/Ecl136II restriction sites (Lambert et al., 2007). Following this strategy, the gene yitF was deleted from B. subtilis 168, and in strain 168HR, deletion of the entire Tn1546 transposon and predicted operons and genes in Tn1546 was achieved (Supplementary Table 1). Deletions of target genes or operons by replacement with the lox66-P32-cat-lox71 cassette in mutants were verified by PCR. The spoVA2mob operon from B. subtilis B4067 was cloned into pDG1730 (Guerout-Fleury et al., 1996), for ectopic expression from the amyE locus in B. subtilis 168. The construct was integrated into the amyE locus of B. subtilis 168, yielding strain 168 amyE::spoVA2mob. Heat resistance of spores for the constructed strains was assessed by exposure to 100 °C for 1 h as described above.
Spore characterization
The dipicolinic acid (DPA) contents of spores were determined for B. subtilis strain 168 and 168HR, as described previously (Kort et al., 2005). For analysis of proteins of spores of strain 168HR, total protein was extracted by bead beating (4 rounds, 40 s, 5 m s−1) of 0.5 ml of spore suspension (1 × 1010 colony forming units (CFU) ml−1), followed by addition of 1 ml Urea (8M) Tris (10 mM) at pH 8 and incubation at room temperature for 1 h. From the total protein extract, 10 μg was digested in-solution with trypsin upon reduction and alkylation. The resulting peptide fragments were purified and concentrated, and the peptide mixture was analyzed by nanoflow C18 reversed phase liquid chromatography (Bruker Daltonics, Breda, The Netherlands). For B. subtilis 168 and 168HR, the dimensions of the spore core and cortex were measured by imaging of cross sections of spores using transmission electron microscopy, as described previously (Lima, 2012). Measurement of the dimensions of sporoplast and core volume were performed using ImageJ (Schneider et al., 2012). The spore dimensions of the cortex and core were determined for 308 individual spores for B. subtilis 168 and 254 individual spores for B. subtilis 168HR.
Results and discussion
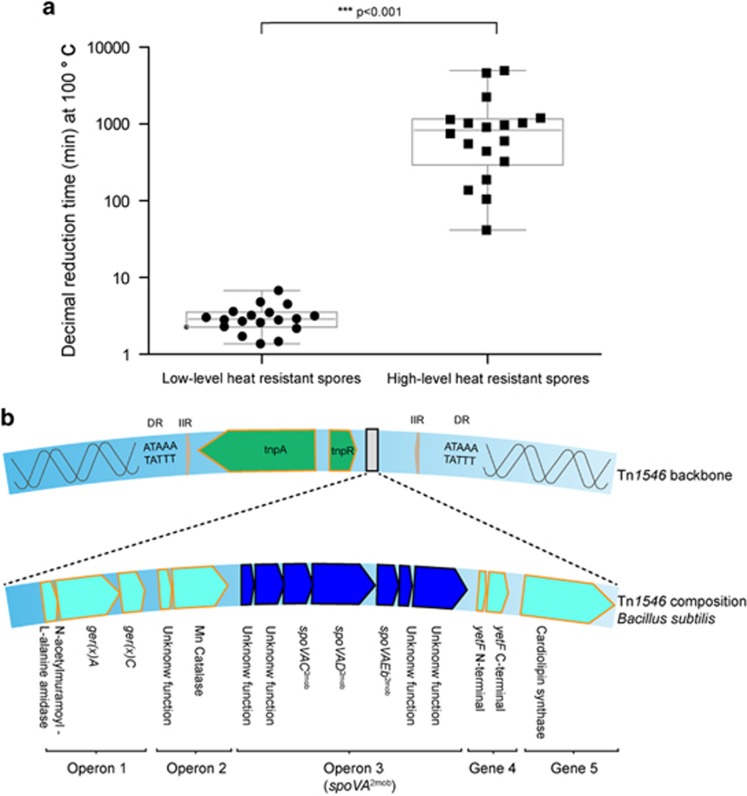
To find the cause of extreme heat resistance in spores, the genomes of 18 strains of B. subtilis with either high or low spore heat-resistance properties were analyzed. Nine of these strains were isolated from diverse food products and produced spores that easily survived prolonged periods of boiling (10.5 h at 100 °C needed for one decimal reduction), while the other nine, including laboratory strain 168, produced spores that were much more readily heat-inactivated (only 2.9 min at 100 °C led to one decimal reduction) (Figure 1a). The analysis of the genomes of all 18 strains (Supplementary Table 4 and Dataset 1) revealed that only the highly heat-resistant strains contained a unique transposon Tn1546, related to the class II cointegrative Tn3-type transposon first described in Enterococcus faecium conferring antibiotic resistance (Arthur et al., 1993), with integration in the genomic locus yitF (BSU10970) in all cases (Figure 1b). The backbone of the transposon contains the transposase tnpA (showing 93% similarity at the nucleotide level with tnpA in E. faecium, but fragmented in all B. subtilis strains), a resolvase tnpR (present in only two B. subtilis strains), two 38 bp imperfect inverted repeats at the ends of the transposon and a direct repeat of 5 bp at the site of integration. Although the Tn1546 elements present in B. subtilis strains vary in length from 12 kb to 16 kb, they all contained the same five predicted transcriptional units (gene organization, putative functions and domains are shown in Figure 1b). As an example, the genes in the Tn1546 element present in strain B4146 can be found with the locus tags B4146_1165 to B4146_1182. The Tn1546 transposon found in B. subtilis includes the following genes: operon 1 encompasses a gene encoding a putative N-acetylmuramoyl-L-alanine amidase, ger(x)A and ger(x)C; operon 2 contains a gene with unknown function and a gene encoding a putative manganese catalase; operon 3 (designated spoVA2mob) carries one gene of unknown function with a predicted DUF1657 domain, one gene of unknown function with a predicted YhcN/YlaJ domain, spoVAC2mob, spoVAD2mob, spoVAEb2mob, one gene of unknown function with a predicted DUF1657 domain and one gene of unknown function with a predicted DUF421domain and a DUF1657 domain; gene 4 encodes a YetF N-terminal part and a YetF C-terminal part; and lastly gene 5 encodes a putative cardiolipin synthetase. Each of the five predicted transcriptional units is preceded by a sporulation-specific binding site for sigma factor G (σG) or K (σK), which are known to target RNA polymerase to specific promotor sequences that drive gene expression during spore development. Operons 1 and 2 were predicted to be under control of σK, and spoVA2mob, gene 4 and gene 5 were predicted to be under control of σG.
Figure 1.
(a) Time needed to achieve one decimal reduction at 100 °C of spores of 18 strains of B. subtilis (assessed for two independent spore crops). On the basis of heat resistance of spores, strains belonged to one of two significantly different groups. One group contained nine strains with low-level heat-resistant spores (168, B4055, B4056, B4057, B4058, B4059, B4060, B4061, B4143), the other group contained nine strains with high-level heat-resistant spores (B4067, B4068, B4069, B4070, B4071. B4072, B4073, B4145, B4146). (b) Overview of the Tn1546-like transposon exclusively present in B. subtilis strains producing high-level heat-resistant spores.
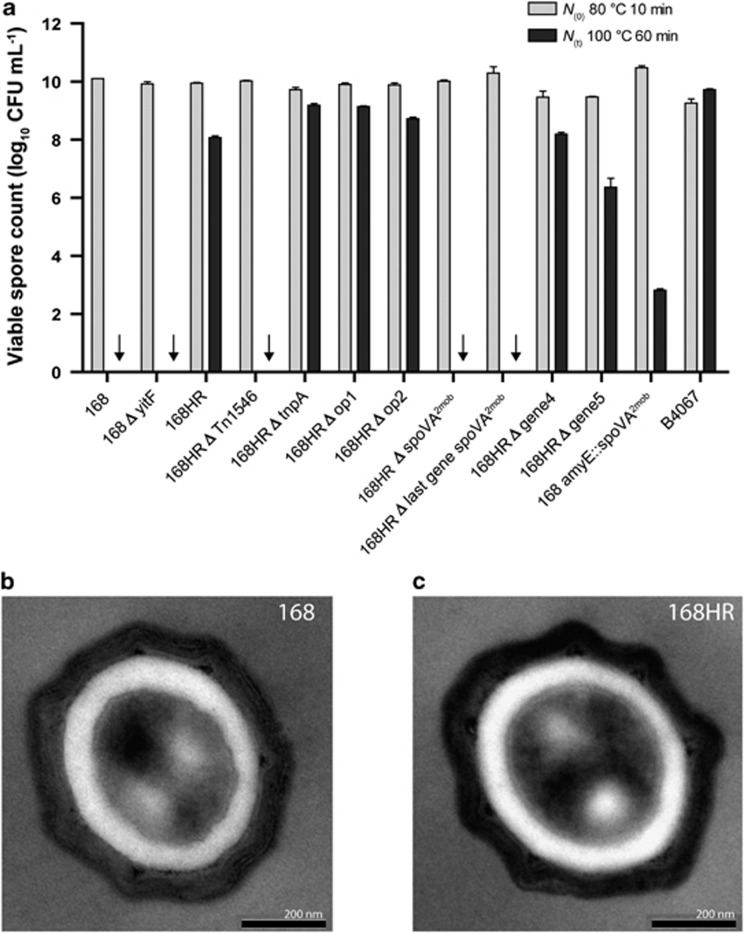
Genes encoded by the Tn1546 transposon were directly responsible for high-level heat resistance of spores as evidenced by the introduction of Tn1546 into the model laboratory strain B. subtilis 168. Active transposition of the Tn1546-like transposon was not possible as only remnants of the transposase gene (tnpA) were found in all nine strains that produce high-level heat-resistant spores, suggesting that the active transfer of Tn1546-like transposon is prone to evolutionary decay. Therefore, natural transfer of this element to B. subtilis 168 was achieved by generalized transduction, upon induction of a prophage (locus tags B4067_4636 to B4067_4698) in strain B4067 which produces spores with high-level heat resistance. A transductant was selected that produced spores with significantly higher heat resistance than spores of strain 168. Heat treatment of spores of this strain, designated 168HR, for 1 h at 100 °C resulted in less than 100-fold reduction in viable counts, while viable spores of strain 168 were reduced more than 10 billion-fold (Figure 2a). Sequencing of the genome of strain 168HR showed the presence of a 100 kb DNA fragment from B4067 that was recombined between metC and yitA with the Tn1546-like transposon inserted in yitF (Supplementary Figure 1). To exclude the potential effect of other mutations in the 100 kb region on the heat resistance of spores, the Tn1546-like transposon was deleted from the 168HR strain to verify its role in high-level heat resistance. Subsequent deletion of the Tn1546 transposon from strain 168HR rendered spores that were much more sensitive to heat treatment than those of 168HR, and similar to those of strain 168 (Figure 2a). The appearance of spores of strains 168 and 168HR was very similar and their core/sporoplast ratios were not significantly different (Figures 2b and c); thus, genes on the Tn1546 transposon do not seem to confer major structural changes. The core/sporoplast ratios were not significantly different for the analyzed spores of 168 and 168HR, with ratios of 0.52±0.06 and 0.55±0.07, respectively. Average dimensions of the spore core were 107 453±24 635 nm2 and 115 363±26 063 nm2, for 168 and 168HR, respectively. The average dimensions of the spore cortex were 97 088±19 098 nm2 and 94 668±20 591 nm2, for 168 and 168HR, respectively. Moreover, disruption of yitF due to Tn1546 insertion does not have a role in increased heat resistance of spores, with spores of constructed strain 168ΔyitF showing similar heat resistance characteristics as spores of the parental strain 168 (Figure 2a).
Figure 2.
(a) Survival of spores of B. subtilis strains. The initial counts of spores were determined following heating for 10 min at 80 °C (gray bars). Survival of spores after 60 min at 100 °C is indicated by black bars. A downward arrow means that counts were below the detection limit, i.e., 1.7 log units. Heating was applied to spores of the following strains: 168, 168ΔyitF, 168HR (which is 168 including the Tn1546 transposon encompassing five operons), 168HR without Tn1546 (168HRΔTn1546), 168HR without tnpA (168HRΔtnpA), 168HR without operon 1 (168HRΔop1), 168HR without operon 2 (168HRΔop2), 168HR without spoVA2mob (168HRΔspoVA2mob), 168HR without gene 4 (168HRΔgene 4), 168HR without gene 5 (168HRΔgene 5), strain 168 amyE::spoVA2mob, in which spoVA2mob was inserted on the amyE locus, and strain B4067, a food isolate producing high-level heat-resistant spores. (b and c) Representative pictures of transmisison electron microscopy cross sections of spores of B. subtilis strain 168 and 168HR, respectively.
The third operon on the Tn1546 element, carrying genes that encode SpoVA homologs and four other genes (designated spoVA2mob), was demonstrated to confer high-level heat resistance of spores. After deletion of only the spoVA2mob operon from strain 168HR (that is, 168HRΔspoVA2mob), the spores could be heat-inactivated under the same conditions as demonstrated for spores of strain 168 (Figure 2a). In addition, introduction of the spoVA2mob operon into the amyE locus of strain 168 rendered a strain (168 amyE::spoVA2mob) that produces spores with high-level heat resistance. Survival of these spores upon heating for 60 min at 100 °C was not quite as high as survival of spores of B. subtilis 168HR (containing the spoVA2mob operon as part of the Tn1546 transposon inserted in yitF), but significantly higher than survival of spores of B. subtilis 168; the latter were not recovered after this heat treatment, that is, showing more than 10 log units reduction (with a calculated reduction of 17.4 log units) (Figure 2a). The impact of the spoVA2mob element on spore heat resistance is much greater than some of the previously reported factors influencing Bacillus spore heat resistance, such as altered temperature, pH, salts and matrix composition during sporulation, which may lead to up to 10-fold increases in the times required to inactivate spores (Cazemier et al., 2001; Rose et al., 2007; Baril et al., 2012).
The other four transcriptional units present on the Tn1546 element were not required for high-level heat resistance as the spores retained high-level heat resistance following deletion of each of these transcriptional units in strain 168HR (Figure 2a). Sporulation-specific expression of all transcriptional units in the Tn1546 transposon except for the fourth transcriptional unit containing the fragmented gene yetF, was seen in strain 168HR and food isolate B4067 by RNA sequence analysis (data not shown). The encoded products may also determine spore properties other than heat resistance. Some of the encoded proteins were detected in extracts of spores of strain 168HR using mass spectrometry, revealing peptide fragments of the Mn catalase homolog and of proteins encoded by the first and the last gene on the spoVA2mob operon (Supplementary Table 5).
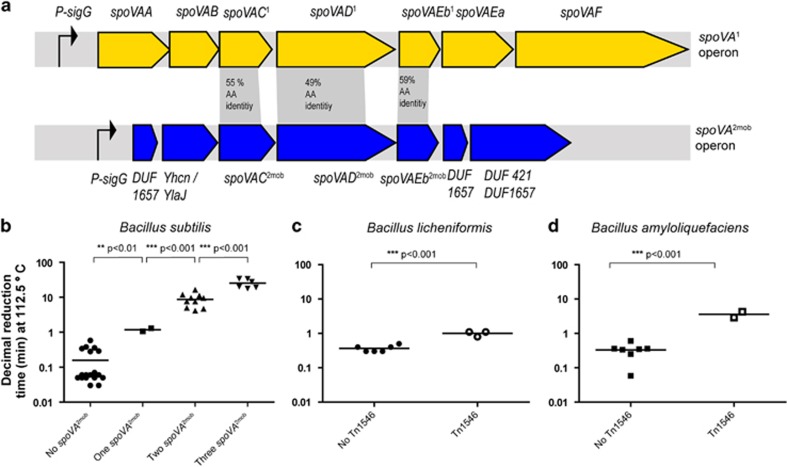
Additional evidence for the crucial role of spoVA2mob genes in high-level heat resistance of spores came from detailed genome analysis of the B. subtilis isolates from foods. The level of spore heat resistance was found to correlate with the number of spoVA2mob operons present in the chromosome. Spores of nine strains (168, B4055, B4056, B4057, B4058, B4059, B4060, B4061, B4143) that lack the spoVA2mob operon showed one decimal reduction in viable count after 0.2 min at 112.5 °C. Of the nine strains that produce high-level heat-resistant spores, one strain (B4146) carried one spoVA2mob operon on a Tn1546-like transposon element inserted in yitF, and the average time to achieve one decimal reduction of its spores was 1.2 min at 112.5 °C. Strains B4067, B4068, B4069, B4070, B4071, B4072, B4073 and B4145 contained the same element inserted in yitF and a second spoVA2mob operon on a Tn1546-like transposon between the two divergently transcribed genes yxjA (BSU39020) and yxjB (BSU39010) and their spores needed even longer average heating times of 8.8 min at 112.5 °C for the same inactivation. In addition to these two Tn1546-like transposons, three strains (B4067, B4070 and B4145) contained a third spoVA2mob operon, which was flanked by genes of another mobile genetic element, but further genomic context could not be determined. Spores of these strains required as much as 25.6 min on average at 112.5 °C for one decimal reduction of viable counts (Figure 3b).
Figure 3.
(a) Overview of the native spoVA operon (spoVA1) in B. subtilis 168 and the spoVA2mob operon found in B. subtilis strains producing spores with high-level heat resistance. (b) The calculated time to achieve a decimal reduction at 112.5 °C for spores of strains of B. subtilis that possess zero, one, two or three spoVA2mob operons. (c) The calculated time to achieve a decimal reduction at 112.5 °C of spores of nine strains of B. licheniformis. Three strains possess one Tn1546 transposon (including the spoVA2mob operon), and spores of these strains had significantly higher heat resistances than those of the six strains that did not contain this transposon. (d) The calculated time to achieve a decimal reduction at 112.5 °C of spores of nine strains of B. amyloliquefaciens. Two strains possess at least one Tn1546 transposon (including the spoVA2mob operon), and produce spores with significantly higher heat resistances than the seven strains that did not carry the transposon.
Despite extensive studies on many spo genes of B. subtilis 168, knowledge of the precise function of individual spoVA-encoded proteins is rather limited. The spoVA operon of B. subtilis 168 (for clarity reasons named spoVA1) encompasses spoVAA, spoVAB, spoVAC, spoVAD, spoVAEb, spoVAEa and spoVAF, of which the first five genes are essential for completion of sporulation (Tovar-Rojo et al., 2002). Only the functions of SpoVAC1 and SpoVAD1, which are associated with the inner membrane of the spore, are known (Vepachedu and Setlow, 2005; Li et al., 2012; Velasquez et al., 2014). Structural analysis of SpoVAD1 revealed a binding pocket that is important for uptake of pyridine-2,6-dicarboxylic acid (known as DPA) during sporulation (Li et al., 2012). SpoVAC1 was recently shown to function as a mechanosensitive channel during germination, with increased probability of opening at increased membrane tension (Velasquez et al., 2014). The spoVA2mob operon mediating high-level heat resistance carries spoVAC, spoVAD and spoVAEb (hereafter called spoVAC2mob, spoVAD2mob and spoVAEb2mob) and four genes with unknown functions (shown in Figure 3a). The SpoVAC, SpoVAD and SpoVAEb proteins encoded in the spoVA1 and spoVA2mob loci share 55%, 49% and 59% amino acid identity, respectively. Given the known roles of SpoVAC1 and SpoVAD1 in DPA uptake during sporulation, we hypothesize that proteins encoded by the spoVA2mob operon have an important auxiliary role in this process, ultimately leading to higher heat resistance of spores. This was indeed the case: the introduction of the spoVA2mob operon in strain 168 resulted in 50% higher DPA concentrations in B. subtilis spores. Spores of strains 168HR and 168 amyE::spoVA2mob contain 63.1±2.3 and 58.1±0.1 μg DPA per mg dry weight, respectively, both significantly higher than the concentration in spores of strain 168 (40.1±2.3 μg DPA per mg dry weight). Interestingly, high levels of DPA were previously reported in spores of a B. subtilis strain with high-level heat resistance (isolated from foods) (Kort et al., 2005), and this phenomenon can now be linked to the presence of spoVA2mob genes. At present, it has not been established which gene or which combinations of genes on the spoVA2mob operon are essential and sufficient to convey high-level heat resistance of spores, but we did find that deletion of the last gene of unknown function fully abolished high-level heat resistance of spores (Figure 2a), indicating at least its essential role. The last gene of the spoVA2mob operon encodes a protein that is predicted to be membrane bound by three transmembrane segments, and contains a DUF421 domain and a DUF1657 domain. Homologs of this protein were neither found in B. subtilis 168 nor in other Bacillus spp. unless they carried spoVA2 or spoVA2mob operons. Two other genes in the spoVA2mob operon encode proteins with DUF1657 domains, for which no homologs were found in B. subtilis 168 and other Bacillus spp. unless they contained the spoVA2 or spoVA2mob operon. The DUF421 encoded in the last gene in the spoVA2mob operon was found in other predicted proteins in the absence of DUF1657; in B. subtilis 168, this domain was encoded by five different genes, namely, yetF, yrbG, ykjA, ydfR and ydfS, but their functions have not been established or predicted. It is not clear at this stage what roles the proteins containing these domains have in heat resistance of spores.
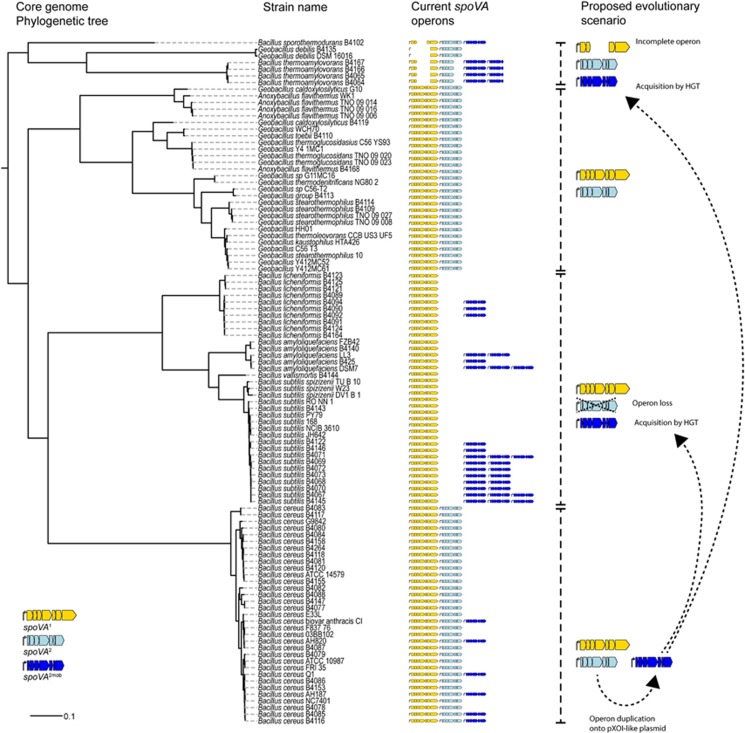
It is conceivable that the Tn1546 transposon found in the B. subtilis group originates from B. cereus pXO1-like plasmids that can carry this transposon including the spoVA2mob operon (Figure 4; Rasko et al., 2007), given similarities in gene presence and GC content (Supplementary Table 6) and because the Tn3-like transposon requires a plasmid intermediate for active transposition (Arthur et al., 1993). It is not clear whether the presence of the spoVA2mob operon in B. cereus strains has an influence on the heat resistance of these spores. Limited sequence variation in key genes in the spoVA2mob operon found in the B. subtilis group strains suggests that genomic incorporation of the Tn1546 transposon, including the spoVA2mob operon, involves a recent evolutionary event.
Figure 4.
Maximum likelihood core genome phylogenetic tree of 103 spore-forming Bacillaceae, with indication of the number and type of spoVA operons present in the genomes, and proposed evolutionary scenarios. Three types of spoVA operons were identified in this analysis and are indicated in the tree. First, a spoVA1 operon, encompassing spoVAA, spoVAB, spoVAC1, spoVAD1, spoVAEb1, spoVAEa and spoVAF. Second, a spoVA2 operon, encompassing a gene with a predicted DUF1657 domain, a gene with a YhcN/YljA domain, spoVAC2, spoVAD2, spoVAEb2, a gene with a predicted DUF1657 domain and a gene with a predicted DUF 421 domain and DUF1657 domain. Third, a spoVA2mob operon, which is a duplication of the spoVA2 operon, but present on a mobile genetic element, e.g., Tn1546 in B. subtilis strains. The proposed evolutionary scenarios were based on protein trees of SpoVAC and SpoVAD and the genomic context of the spoVA operons. Strains of B. cereus, Geobacillus spp. and A. flavithermus all carry spoVA1 and spoVA2 operons. Six strains of B. cereus carry spoVA2mob on a pXO1-like plasmid, as part of a Tn1546 transposon. Members of the B. subtilis group (B. subtilis, B. vallismortis, B. amyloliquefaciens, B. licheniformis) lost the spoVA2 operon during evolution, but the spoVA2mob operon re-entered in some strains as part of a Tn1546 transposon. Similarly, spoVA2mob entered strains of B. thermoamylovorans and B. sporothermodurans. Incomplete spoVA1 and spoVA2 operons were observed in strains of B. thermoamylovorans, B. sporothermodurans and C. debilis.
Genes encoding SpoVAC, SpoVAD and SpoVAEb are conserved among spore-forming Bacillaceae and Clostridium spp. (Galperin et al., 2012), and were also present in the analyzed genomes of 103 Bacillaceae species (Figure 4). Three types of spoVA operons could be distinguished: spoVA1, spoVA2 and spoVA2mob (where mob indicates presence on a mobile genetic element). The division between spoVA1 and spoVA2 is based on the difference in operon structure (Figure 3a) and separate clustering of the SpoVAC and SpoVAD proteins in the evolutionary trees (Supplementary Figure 2).
Both the spoVA1 and spoVA2 operons are present in the spore-forming Geobacillus spp., Anoxybacillus flavithermus, and species of the B. cereus group sensu strictu, but not as parts of mobile genetic elements (Figure 4). Interestingly, all evaluated strains belonging to the B. subtilis group possess the spoVA1 operon while lacking the spoVA2 operon. However, some strains gained spoVA2mob on the Tn1546 transposon (Figure 4). The determining role of the spoVA2mob element in high-level spore heat resistance was experimentally confirmed for strains of B. licheniformis and B. amyloliquefaciens (Figures 3c and d).
Interestingly, the genomes of species notorious for very high-level heat resistance of their spores, namely B. thermoamylovorans, B. sporothermodurans and Caldibacillus debilis (Scheldeman et al., 2005, 2006; Berendsen et al., 2015a), showed diverse compositions of their spoVA operons. The exact roles of the spoVA1, spoVA2 and spoVA2mob operons in determining spore properties in these species remains to be established.
Horizontal gene transfer has an important role in bacteria to acquire resistance against selective pressures (Ochman et al., 2000). The transfer of the spoVA2mob operon to sporeformers occurs in the vegetative growth phase, subsequently leading to production of highly heat-resistant spores that can survive heat treatments routinely used in food processing. The acquisition of the spoVA2mob operon in food isolates may take place during growth in a food-processing environment, but it is also possible that such events occur during growth in other niches, such as soil or compost. The competitive advantage of acquisition of these genes may also be related to properties other than merely heat resistance of spores.
Conclusions
This study shows that horizontal gene transfer can profoundly affect heat resistance characteristics of spores. Our finding that the spoVA2mob operon on a Tn1546-like transposon has an important role in high-level heat resistance of Bacillus spores offers new opportunities for dealing with the problem of highly heat-resistant spores in food and health. Studying phenotypic properties of strains other than the well-studied laboratory strain in conjunction with analysis of their genomes proved to be a powerful approach to match phenotypes with underlying genetic traits.
Acknowledgments
We would like to thank Rosella Koning for technical assistance with the heat inactivation of spores, Antonina Krawczyk, Anne de Jong and Robyn Eijlander for sharing of RNA sequencing data and valuable discussions, and Professor Michiel Kleerebezem and Professor Jerry Wells for critical reading of the manuscript. This work was supported by the Top Institute Food and Nutrition, The Netherlands.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Arthur M, Molinas C, Depardieu F, Courvalin P. (1993). Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol 175: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchtung JM, Lee CA, Monson RE, Lehman AP, Grossman AD. (2005). Regulation of a Bacillus subtilis mobile genetic element by intercellular signaling and the global DNA damage response. Proc Natl Acad Sci USA 102: 12554–12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril E, Coroller L, Couvert O, Leguérinel I, Postollec F, Boulais C et al. (2012). Modeling heat resistance of Bacillus weihenstephanensis and Bacillus licheniformis spores as function of sporulation temperature and pH. Food Microbiol 30: 29–36. [DOI] [PubMed] [Google Scholar]
- Bayjanov JR, Molenaar D, Tzeneva V, Siezen RJ, van Hijum SA. (2012). PhenoLink—a web-tool for linking phenotype to ~omics data for bacteria: application to gene-trait matching for Lactobacillus plantarum strains. BMC Genomics 13: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen EM, Krawczyk AO, Klaus V, de Jong A, Boekhorst J, Eijlander RT et al. (2015. a). Spores of Bacillus thermoamylovorans with very high heat resistances germinate poorly in rich media despite the presence of ger clusters, but efficiently upon non-nutrient Ca-DPA exposure. Appl Environ Microbiol 81: 7791–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen EM, Zwietering MH, Kuipers OP, Wells-Bennik MHJ. (2015. b). Two distinct groups within the Bacillus subtilis group display significantly different spore heat resistance properties. Food Microbiol 45(Part A): 18–25. [DOI] [PubMed] [Google Scholar]
- Berendsen EM, Wells-Bennik MHJ, Krawczyk AO, de Jong A, van Heel A, Eijlander RT et al. (2016). Draft genome sequences of ten Bacillus subtilis strains that form spores with a high or low heat-resistance. Genome Announce 4: pii: e00124–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brul S, van Beilen J, Caspers M, O'Brien A, de Koster C, Oomes S et al. (2011). Challenges and advances in systems biology analysis of Bacillus spore physiology; molecular differences between an extreme heat resistant spore forming Bacillus subtilis food isolate and a laboratory strain. Food Microbiol 28: 221–227. [DOI] [PubMed] [Google Scholar]
- Cano R, Borucki M. (1995). Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science 268: 1060–1064. [DOI] [PubMed] [Google Scholar]
- Carver T, Harris SR, Berriman M, Parkhill J, McQuillan JA. (2012). Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, Parkhill J. (2005). ACT: the Artemis Comparison Tool. Bioinformatics 21: 3422–3423. [DOI] [PubMed] [Google Scholar]
- Cazemier AE, Wagenaars SF, Ter Steeg PF. (2001). Effect of sporulation and recovery medium on the heat resistance and amount of injury of spores from spoilage bacilli. J Applied Microbiol 90: 761–770. [DOI] [PubMed] [Google Scholar]
- Cutting SM. (2011). Bacillus probiotics. Food Microbiol 28: 214–220. [DOI] [PubMed] [Google Scholar]
- Duc LH, Hong HA, Fairweather N, Ricca E, Cutting SM. (2003). Bacterial spores as vaccine vehicles. Infect Immun 71: 2810–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. (2003). Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol 1: 117–126. [DOI] [PubMed] [Google Scholar]
- Galperin MY, Mekhedov SL, Puigbo P, Smirnov S, Wolf YI, Rigden DJ. (2012). Genomic determinants of sporulation in bacilli and clostridia: towards the minimal set of sporulation-specific genes. Environ Microbiol 14: 2870–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GW. (2006). History of science – spores. J Applied Microbiol 101: 507–513. [DOI] [PubMed] [Google Scholar]
- Guerout-Fleury AM, Frandsen N, Stragier P. (1996). Plasmids for ectopic integration in Bacillus subtilis. Gene 180: 57–61. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Ivanova N, Sorokin A, Anderson I, Galleron N, Candelon B, Kapatral V et al. (2003). Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423: 87–91. [DOI] [PubMed] [Google Scholar]
- Johnson LS, Eddy S, Portugaly E. (2010). Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinformatics 11: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort R, O'Brien AC, van Stokkum IH, Oomes SJ, Crielaard W, Hellingwerf KJ et al. (2005). Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl Environ Microbiol 71: 3556–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk AO, Berendsen EM, Eijlander RT, de Jong A, Wells-Bennik MHJ, Kuipers OP. (2015). Draft genome sequences of four Bacillus thermoamylovorans strains isolated from milk and acacia gum, a food ingredient. Genome Announce 3: pii: e00165–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JM, Bongers RS, Kleerebezem M. (2007). Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl Environ Microbiol 73: 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stoeckert CJ Jr., Roos DS. (2003). OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13: 2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Davis A, Korza G, Zhang P, Li YQ, Setlow B et al. (2012). Role of a SpoVA protein in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 194: 1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LJR, Kamphuis HJ, Nout MJR, Zwietering MH. (2011). Microbiota of cocoa powder with particular reference to aerobic thermoresistant spore-formers. Food Microbiol 28: 573–582. [DOI] [PubMed] [Google Scholar]
- Lima LSR. (2012). Microbial ecology of the cocoa chain: Quality aspects and insight into heat-resistant bacterial spores. Wageningen University: Wageningen, PhD thesis. [Google Scholar]
- Moineau S, Pandian S, Klaenhammer TR. (1994). Evolution of a lytic bacteriophage via DNA acquisition from the Lactococcus lactis chromosome. Appl Environ Microbiol 60: 1832–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. (2000). Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64: 548–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Lawrence JG, Groisman EA. (2000). Lateral gene transfer and the nature of bacterial innovation. Nature 405: 299–304. [DOI] [PubMed] [Google Scholar]
- Oomes SJCM, van Zuijlen AC, Hehenkamp JO, Witsenboer H, van der Vossen JM, Brul S. (2007). The characterisation of Bacillus spores occurring in the manufacturing of (low acid) canned products. Int J Food Microbiol 120: 85–94. [DOI] [PubMed] [Google Scholar]
- Peck MW, Stringer SC, Carter AT. (2011). Clostridium botulinum in the post-genomic era. Food Microbiol 28: 183–191. [DOI] [PubMed] [Google Scholar]
- Rasko DA, Rosovitz MJ, Okstad OA, Fouts DE, Jiang L, Cer RZ et al. (2007). Complete sequence analysis of novel plasmids from emetic and periodontal Bacillus cereus isolates reveals a common evolutionary history among the B. cereus-group plasmids, including Bacillus anthracis pXO1. J Bacteriol 189: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read TD, Peterson SN, Tourasse N, Baillie LW, Paulsen IT, Nelson KE et al. (2003). The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 423: 81–86. [DOI] [PubMed] [Google Scholar]
- Rose R, Setlow B, Monroe A, Mallozzi M, Driks A, Setlow P. (2007). Comparison of the properties of Bacillus subtilis spores made in liquid or on agar plates. J Appl Microbiol 103: 691–699. [DOI] [PubMed] [Google Scholar]
- Rupnik M, Wilcox MH, Gerding DN. (2009). Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 7: 526–536. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL et al. (2011). Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheldeman P, Pil A, Herman L, De Vos P, Heyndrickx M. (2005). Incidence and diversity of potentially highly heat-resistant spores isolated at dairy farms. Appl Environ Microbiol 71: 1480–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheldeman P, Herman L, Foster S, Heyndrickx M. (2006). Bacillus sporothermodurans and other highly heat-resistant spore formers in milk. J Appl Microbiol 101: 542–555. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. (2006). Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Applied Microbiol 101: 514–525. [DOI] [PubMed] [Google Scholar]
- Sunde EP, Setlow P, Hederstedt L, Halle B. (2009). The physical state of water in bacterial spores. Proc Natl Acad Sci USA 106: 19334–19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Rojo F, Chander M, Setlow B, Setlow P. (2002). The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 184: 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez J, Schuurman-Wolters G, Birkner JP, Abee T, Poolman B. (2014). Bacillus subtilis spore protein SpoVAC functions as a mechanosensitive channel. Mol Microbiol 92: 813–823. [DOI] [PubMed] [Google Scholar]
- Vepachedu VR, Setlow P. (2005). Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J Bacteriol 187: 5677–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeland RH, Rosenzweig WD, Powers DW. (2000). Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407: 897–900. [DOI] [PubMed] [Google Scholar]
- Wells-Bennik MHJ, Eijlander RT, den Besten HMW, Berendsen EM, Warda AK, Krawczyk AO et al. (2016). Bacterial spores in food: survival, emergence and outgrowth. Annu Rev Food Sci Technol 7: 457–482. [DOI] [PubMed] [Google Scholar]
- Yan X, Yu HJ, Hong Q, Li SP. (2008). Cre/lox system and PCR-based genome engineering in Bacillus subtilis. Appl Environ Microbiol 74: 5556–5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.