Abstract
Rapid fragmentation and degradation of large undisturbed habitats constitute major threats to biodiversity. Several studies have shown that populations in small and highly isolated habitat patches are prone to strong environmental and demographic stochasticity and increased risk of extinction. Based on community assembly theory, we predict recent rapid forest fragmentation to cause a decline in species and functional guild richness of forest birds combined with a high species turnover among habitat patches, and well defined dominance structures, if competition is the major driver of community assembly. To test these predictions, we analysed species co-occurrence, nestedness, and competitive strength to infer effects of interspecific competition, habitat structure, and species′ traits on the assembly of bird species communities from 12 cloud forest fragments in southern Kenya. Our results do not point to a single ecological driver of variation in species composition. Interspecific competition does not appear to be a major driver of species segregation in small forest patches, while its relative importance appears to be higher in larger ones, which may be indicative for a generic shift from competition-dominated to colonisation-driven community structure with decreasing fragment size. Functional trait diversity was independent of fragment size after controlling for species richness. As fragmentation effects vary among feeding guilds and habitat generalists, in particular, tend to decline in low quality forest patches, we plead for taking species ecology fully into account when predicting tropical community responses to habitat change.
Introduction
Habitat fragmentation has profound and mainly negative effects on the long-term viability of indigenous animal and plant species [1–2], in particular in historically stable ecosystems such as tropical rainforests [3–5]. While habitat fragmentation mainly causes a decline in species richness at the regional level [6–7] (but see counterexamples in Schmiegelow et al. 1997 and Debinski & Holt 2000 [8–9]), it may trigger increased species richness (species density sensu Gotelli & Ellison 2002 [10]) and abundances at the local (i.e. fragment) level [11–13]. Such a “crowding” effect (sensu Collinge & Forman 1998 [14]) might intensify species interactions, particularly interspecific competition for resources and space [15–16], while at the same time reducing population sizes and genetic diversity at the species level [17]. Ultimately, this may affect long-term survival, food web structures, and ecosystem functioning [18].
Variation in species density, the average number of species per unit area, is closely related to the concept of species-area relationships (SAR). Given the common power function model of the SAR S = S0Az with S0 being the average number of species per unit area and z being the scaling constant [19] the species density SA becomes and is expected to be independent of patch area. The crowding effect [14] predicts increased species density in smaller habitat fragments. However, previous simulation studies showed that species densities in homogeneous landscapes only moderately increase at small patch sizes [20]. Hence, a positive deviation of observed species densities from those predicted under the neutral model may indicate a “crowding effect”, while a negative deviation may indicate that species numbers are mainly limited by environmental factors that correlate with fragment size.
Classic competition-based community assembly models [21] predict fragmented landscapes to exhibit a scattered pattern of species occurrences in which species with similar ecological niches occur in a segregated manner [22–24]. Indeed, competition-mediated species segregation has been found in a number of studies on landscape fragmentation [16, 25, 26]. However, species segregation is not the only possible outcome of fragmentation. If resource availability and mutualistic interactions outweigh competitive effects, habitat filtering may also lead to an aggregated pattern of species occurrences [26, 27]. Further, if key resources are unevenly distributed among fragments, patterns of species occurrences may follow the respective gradient in resource availability leading to a nested pattern of species occurrences [23, 28] where species assemblages in resource poor fragments are true subsamples of those in richer ones [28]. Due to the fact that nestedness and species segregation are opposing patterns, community organisation will often be intermediate between both extremes, depending on the respective pay-offs between species competition and habitat filtering [24]. Finally, ecological demands and behaviour of species may strongly affect species′ sensitivity to rapid changes in the habitat configuration. While habitat specialists (species with specific habitat demands and restricted movement behaviour) are assumed to suffer strongly under ongoing habitat degradation, habitat generalists (which can be found in various habitat types) are assumed to be able to better adapt to environmental changes [29].
Mechanisms underlying community assembly are still discussed even after more than half a century of research in the field. Standard analyses of community assembly are based on species occurrence and absence, however, these data are only rarely linked to trait- and environmental variation. When aiming to uncover mechanisms and constraints behind the pattern of species co-existence, there is a clear need to link the geometry of species occurrences with environmental and species functional trait data which can be expected to replace classic co-occurrence analysis that have been dominated the field since the pioneering work of Diamond (1975) [30]. Along these lines, Ulrich et al. (2014) [31] and Soliveres et al. (2015) [32] recently introduced a novel Markov chain-based approach to examine the frequency of intransitive competition in real-world communities and how they affect community diversity. We here apply this method to assess the role of intransitive competition on species coexistence in forest bird communities within and among 12 tropical forest fragments that vary in patch size and habitat quality. The indigenous forest of the Taita Hills of south-east Kenya, the northernmost outlier of the Eastern Arcs and part of the Eastern Afromontane biodiversity hotspot, has been subject to rapid loss, degradation and fragmentation of pristine habitats over the past decades (further details see material and methods) [33]. Despite this transition, indigenous forest remnants still harbour a typical cloud forest avifauna, including many endemic and endangered forest habitat specialists, but also a large number of habitat generalists that also occur in the non-indigenous landscape matrix.
The Taita bird community has been studied intensively over the past two decades, resulting in well supported knowledge about species richness, abundance, and ecological demands. Furthermore, the land use history of the Taita Hills is very well documented, and this combined information offers a strong framework to study how tropical avian communities are shaped in relation to species and landscape traits. Making use of this information, we here test the following three hypothesis: (i) Avian species density increases with decreasing fragment size, resulting in a high proportion of species surviving in forest fragments relative to intact forests; (ii) Species co-occurrence among small forest remnants is aggregated due to crowding effects; and (iii) Habitat specialists respond more strongly to habitat degradation compared to habitat generalists.
Material and Methods
Taita Hills study region
The Taita Hills cover an area of around 250 km2 and are geographically isolated from other mountain blocks to the south (90 km to the Usambara Mts.) and the north (80 km to the Chyulu Hills) (S1 Appendix). Semiarid plains in either direction constitute a strong dispersal barrier for species that depend on moist and cool cloud forest habitat, and this resulted in high levels of endemicity [34–35]. Degradation and fragmentation of the Taita forests started long before the colonial era, when slopes were cleared for agriculture up to the head of the streams [36]. Large-scale forest loss occurred during railway constructions between 1898 and 1924, while in more recent times, forest cover markedly decreased between 1955 and 2004. Even though half of the original indigenous forest has currently been lost, airborne remote sensing of spatio-temporal changes in forest cover [33] revealed that the total forest cover in the Taita landscape remained about the same between 1955 and 2004, mainly due to planting of exotic trees on rocky, barren or eroded areas, secondary bushlands and abandoned agricultural land. In addition to indigenous forest loss, the remaining patches also decreased in forest quality due to pit-sawing, charcoal manufacturing, firewood collection, pole removal and grazing [33]. Three larger forest fragments (Chawia (90 ha), Ngangao (147 ha) and Mbololo (179 ha)), nine smaller ones (< 15 ha), and several tiny patches of indigenous forest remained embedded in a fine-grained mosaic of human settlements and small-holder cultivation plots [33]. Small forest fragments, in particular, continue to suffer from cattle grazing and other forms of habitat disturbance, while the three larger forest fragments vary in the degree of habitat degradation too, being highest in Chawia forest, intermediate in Ngangao forest, and lowest in Mbololo forest [37].
Land-cover information was derived from airborne true-colour images, converted to orthomosaics at a spatial resolution of 0.5 m [33]. Brightness variations were removed by corrections for light falloff and bidirectional effects using the methods developed by Pellikka (1998) [38] after which frames were mosaicked using the EnsoMOSAIC [39]. The resulting mosaics were orthorectified, projected to Transverse Mercator projection with a Clarke 1880 [40] spheroid and Arc 1960 datum, and resampled to 0.5 m ground resolution. The resulting geometric accuracy was within 2 m as verified in the field using GPS. The land cover model was subsequently ground-truthed, revised and fine-tuned during field visits in 2007 and 2008, confirming the correct remote-sensing classification of large patches of closed—canopy forest, exotic plantations, and non-forested habitat [33]. Based on this land cover model, we calculated the following four landscape characteristics using Fragstats v. 3.3 and ArcView 3.2 (ESRI 2013): (i) indigenous forest patch size, (ii) indigenous forest patch perimeter; (iii) percentage of closed-canopy forest cover within 800 m of each indigenous forest patch, and (iv) patch proximity, a distance-weighted, area-based isolation index (PPI) [40]. We related observed species richness to these landscape characteristics to test the first and third hypothesis.
Bird assessments
Understorey bird community metrics were derived from a long-term (1996 to 2010) bird ringing program using standard mist-netting procedures as described in Karr (1981) [41]. Mist netting was conducted in collaboration with the Ornithology Section of the National Museums of Kenya, Ornithology Section. Permission for bird collection was issued by the National Museums of Kenya. Permits to access the forest fragments were provided by the Kenyan Forest Service. As endangered, Taita endemic bird species were involved in this study, its collection was approved and mainly conducted by members of the ethics committee of the National Museums of Kenya personally (P Njoroge, RK Mulwa, O Kioko). Birds were collected using mist-nets, which were regularly controlled, to prevent any negative effects on trapped birds. This activity was approved by the animal ethics committee of the National Museums of Kenya. Mist-net lines were operated in one to seven plots per fragment (depending on fragment size) and were evenly spaced out to sample entire plots, while net positions, net lengths (120m/plot) and daily trapping efforts (06-18h) were kept constant between trapping sessions. Nets were routinely checked at 30-minute intervals so as to promptly remove, process, and release the birds. Time intervals between subsequent ringing sessions varied between 1.0 and 4.6 months, and the number of ringing sessions per fragment ranged between 20 and 32 over the 15 year study period. While mist nets are regarded as likely the best technique for assessing the relative abundances of tropical understorey birds [41, 42], habitat modifications such as removal of canopy trees and clearing of the understorey, in particular, may alter flight height of some species, thereby changing their susceptibility to mist-net capture [43]. To minimize this possible bias in the assessment of species richness, we restricted our analysis to the understorey bird community, i.e. species that are reliably caught in mist nets. Therefore, our data, covering 15 years of bird observation, are believed to be highly appropriate to assess total species richness and as well as relative abundances in the smaller fragments sampled with identical sampling effort.
For each bird species we assembled data on body mass (g), average bill culmen length, depth and width (cm), tarsus, tail, and wing length (cm), and average hand-wing index. The dominant principal component of the three bill measures was used to assess bill characteristics, and the respective dominant eigenvector of the wing, tarsus, and tail measures served as a proxy to the type of locomotion [44]. Following Claramunt et al. (2012) [45], we used the hand-wing index to quantify dispersal ability. Each species was assigned to one of four feeding guilds, insectivore, seed-eater, fruit-nectar feeder, and omnivore. We also assembled dietary (coded into ten categories) and foraging stratum preferences (coded into eight categories), respectively [46]. To reduce dimensionality, we calculated the dominant principal components of the matrices and used these in subsequent analyses. The complete species list of bird species, respective abundances per forest fragment, and species traits are given in the S1 Appendix.
Statistical analysis
Analyses were based on matrices containing species relative abundances with species in rows and forest fragments in columns. We composed matrices for each fragment separately and then grouped the three larger (> 90 ha) and nine smaller (<15 ha) fragments in two additional matrices. We calculated the following three metrics of species co-occurrence. First, we estimated species segregation among fragments (negative species associations) by the C-score [24, 47, 48] that is a normalized count of the number of checkerboard submatrices ({{1,0},{0,1}} or {{0,1},{1,0}}). As an auxiliary metric of species segregation that focuses on the pattern of species turnover among sites we used the standard proportional turnover ; where alpha refers to the average richness per fragment site and gamma tom the total observed species richness [49]. Third, we performed a nestedness analysis to identify gradients in species occurrences and richness across fragments [23] using the NODF (nestedness by overlap and decreasing fill) metric of Almeida-Neto et al. (2008) [48]. For the nestedness analysis, rows were always sorted according to species occurrence totals. Finally, we calculated the functional diversity of each feeding guild based on five measured functional traits (bill characteristics, body size, dispersal, locomotion, stratum) using the functional attribute metric FAD [49, 50], a measure of total trait space encompassed by the species of a given community calculated as the sum of the Euclidean distances between species in trait space. For comparability, trait expressions were Z-transformed prior to calculation. Co-occurrence analyses were done using the freely available Fortran application NODF [51] and Turnover [52]. Source code is available from WU by request.
For statistical inference of NODF, C-score, and betaP, we used a null model approach and compared the observed co-occurrence metric scores with those obtained from 1000 null matrices each that were randomized using a null model that resamples the matrix with placement probabilities proportional to observed total abundances of rows and columns (proportional abundance model [53]). This is a conservative null model that has been shown to account well for inherent site differences and unequal species colonization probabilities (the mass effect) that are not directly linked to the pattern of interest [24]. Note that this model is equivalent to a neutral model without dispersal limitation and speciation. To account for richness effects [54], raw FAD scores were compared to a null model in which the trait expressions for each single trait were randomly reshuffled among species. Statistical significance was estimated from the respective tail distributions at the two-sided 5% error level. Additionally, we calculated standardized effect sizes (SES = Obs—Exp) / StDevExp; Obs and Exp: observed and expected scores, StDevExp: standard deviation of expectation). SES scores should have values below –1.96 and above +1.96 at the two-sided 5% error level under the assumption that the respective null distribution is approximately normal. To account for multiple testing, all significance levels were Bonferroni corrected.
Possible competitive interactions among species within the seven feeding guilds were assessed following Ulrich et al. (2014) [31] and Soliveres et al. (2015) [32]. For each guild, we calculated 100,000 random species × species competitive strengths matrices, translated these into a column stochastic transition matrix, and used a Markov chain model to predict relative species abundances from this transition matrix within the 12 fragments. We compared predicted and observed relative species abundances by rank order correlation (rC) and chose the best fitting competition matrix to assess the maximum impact of interspecific competition on community assembly. High values of rC point to the possibility that interspecific competition is a major driver for observed species distributions while low rC values point towards a minor impact of competition [55].
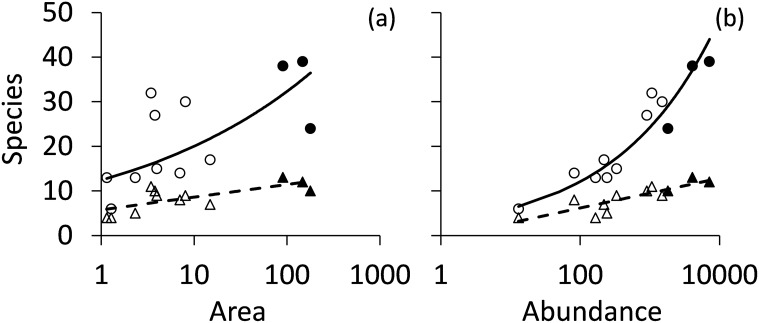
Using the empirical species—area relationship S = 12.4A0.21;r2 = 0.44 (Fig 1a) we assessed average species densities SA per ha area by SA = S/A0.21, with S reflecting the total species richness and A the fragment area. We used a general linear models with identity link function and normal error structure to relate FAD to the categorical variables feeding guild, functional traits, and remnant size group (large—small), and to species richness as the continuous co-variate.
Fig 1. Species—area (a) and species—abundance (b) relationships of all species (circles) and of forest specialist species (triangles) of the East African Taita forest fragments.
Power function ordinary least squares regressions to all species: a: S = 12.4A0.21, r2 = 0.44, P < 0.01; b: S = 3.03 I0.30, r2 = 0.92, P < 0.001. Logarithmic regression to specialist species a: S = 1.23 ln A + 5.78, r2 = 0.53, P < 0.01; b: S = 1.45 ln I– 0.49, r2 = 0.73, P < 0.01.
Results
We recorded a total of 17,520 individuals from 69 bird species in the 12 Taita forest fragments (Table 1), of which 36 species belonged to the insectivorous guild and 12 species to the frugi/nectarivorous guild (Table 2). Species richness increased moderately with area (Fig 1a) and perimeter (r = 0.61, P = 0.03). The best predictor of fragment richness was the total number of individuals caught (Fig 1b). Richness did not significantly vary with fragment isolation (Pearson r = 0.27, P > 0.3) and increased weakly with forest cover within the matrix (Pearson r = 0.51, P = 0.09). In fragments below 15 ha, species richness was independent of fragment area (Fig 1a, r = 0.17, P > 0.5) but tended to be positively related to fragment perimeter, albeit not statistically significant (r = 0.61, P = 0.08). Most species rich were the Ngangao (39 species) and Chawia (38 species) forest fragments, while the large and predominately pristine Mbololo fragment was comparatively poor in species richness (32 species). Of the smaller fragments, Fururu and Macha were most species rich (30 and 32 species, respectively).
Table 1. Area, perimeter, degree of isolation, percentage of closed-canopy forest cover within 800 m, and total number of species and individuals, of 12 indigenous forest fragments.
| Fragment | Area (ha) | Perimeter (ha) | Isolation | Cover | All Species | Forest specialists | Individuals |
|---|---|---|---|---|---|---|---|
| Mbololo | 178.79 | 9980 | 0.39 | 46.2 | 24 | 10 | 1797 |
| Ngangao | 146.93 | 11529 | 0.54 | 48.6 | 39 | 12 | 7179 |
| Chawia | 90.25 | 5291 | 0.37 | 42.0 | 38 | 13 | 4066 |
| Ronge | 14.81 | 2035 | 0.33 | 36.4 | 17 | 7 | 219 |
| Fururu | 8.04 | 1495 | 0.53 | 5.1 | 30 | 9 | 1492 |
| Vuria | 6.99 | 1099 | 0.18 | 18.0 | 14 | 8 | 78 |
| Yale | 3.94 | 897 | 0.56 | 6.1 | 15 | 9 | 330 |
| Ndiwenyi | 3.76 | 893 | 0.53 | 4.4 | 27 | 10 | 898 |
| Macha | 3.42 | 1728 | 0.56 | 2.1 | 32 | 10 | 1054 |
| Mwachora | 2.31 | 606 | 0.25 | 2.0 | 13 | 5 | 239 |
| Kichuchenyi | 1.28 | 514 | 0.53 | 1.1 | 6 | 4 | 13 |
| Wundanyi | 1.14 | 455 | 0.50 | 1.6 | 13 | 4 | 166 |
Table 2. Competition impact (rC), SES scores (proportional abundance null model) and species co-occurrences metrics (C-score, NODF, proportional species turnover beta) for three large and nine small East African forest fragments (cf. Table 1).
Significant SES score (P < 0.05) are marked in bold. The single granivore forest specialist species made it impossible to calculate respective co-occurrence metrices.
| Guild | Species (all) | Species (large) | Species (small) | Competition metric | SES scores | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All species | |||||||||||
| rC (large) | rC (small) | C-score (large) | C-score (small) | NODF (large) | NODF (small) | Beta (large) | Beta (small) | ||||
| Frugi-/Nectarivores | 12 | 8 | 10 | 0.10 | 0.40 | 2.91 | -1.16 | -1.41 | 0.15 | 3.34 | 1.63 |
| Insectivores | 36 | 33 | 26 | 0.56 | 0.19 | 1.42 | 1.72 | -3.01 | -2.89 | 3.16 | 5.82 |
| Omnivores | 11 | 10 | 10 | 0.71 | 0.36 | -0.56 | 1.5 | 1.46 | -2.46 | 0.48 | 2.34 |
| Granivores | 10 | 5 | 9 | 0.08 | 0.01 | 1.23 | 1.11 | -0.74 | -1.55 | 0.58 | 4.24 |
| Forest specialists | |||||||||||
| rC (large) | rC (small) | C-score (large) | C-score (small) | NODF (large) | NODF (small) | Beta (large) | Beta (small) | ||||
| Frugi-/Nectarivores | 4 | 4 | 3 | 0.63 | 0.88 | 0.07 | -0.45 | -0.07 | 0.41 | 0.00 | -0.22 |
| Insectivores | 10 | 9 | 7 | 0.78 | 0.55 | 0.05 | 0.98 | 0.19 | 0.67 | -0.11 | 0.77 |
| Omnivores | 3 | 3 | 3 | 0.67 | 0.66 | 0.00 | 0.57 | 0.00 | 0.49 | 0.00 | 0.14 |
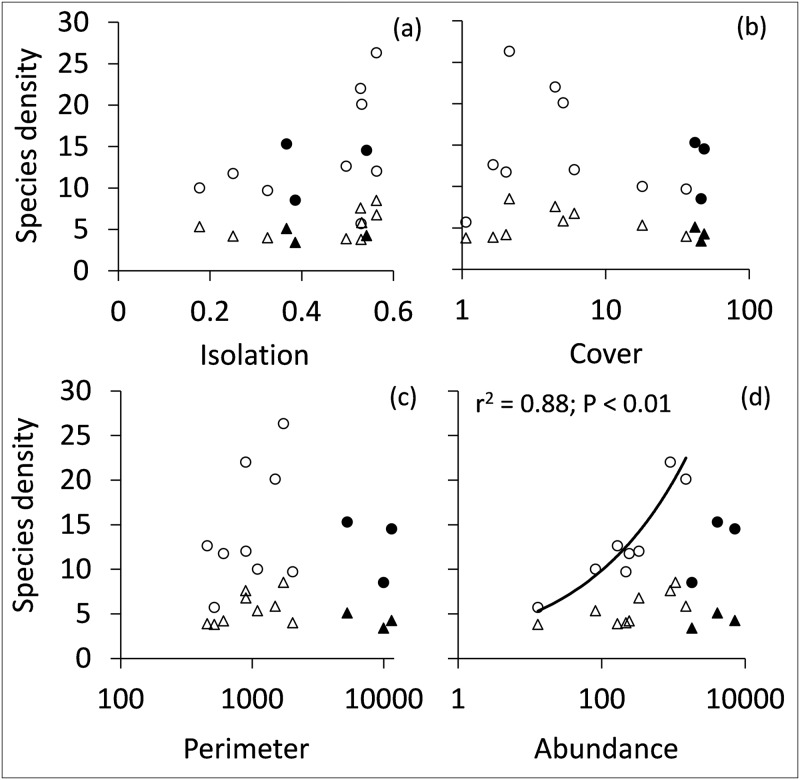
Average species density was independent of fragment isolation (Fig 2a), forest cover (Fig 2b), and fragment perimeter (Fig 2c). In the smaller fragments, species density increased with total abundance (Fig 2d). In the larger fragments, species density was at an intermediate level compared to the smaller fragments (Fig 2d). A comparison of the relative abundances between the smaller and the larger fragments revealed a significant shift in relative abundance between the two fragment types. Of the 22 species with relative abundances below 0.001 in the larger fragments, 18 achieved higher relative abundances in the smaller fragments (not shown). This shift was accompanied by a sharp decline of five species (Columba larvata, Phyllastrephus placidus, Phylloscopus ruficapillus, Turdus olivaceus, Zoothera gurneyi) in the smaller fragments.
Fig 2. Bird species density per forest fragment of all species (circles) and of forest specialist species (triangles) was independent of fragment isolation (a), percentage of forest cover outside the fragments (b), and fragment perimeter (c), but increased with abundance in small fragments (d).
coefficients of determination and associated parametric significance levels in (d) refer to a power function model.
A separate analysis based on forest specialist species only (Table 1) also revealed an increase in richness with fragment size (Fig 1a) and abundance (Fig 1b), however, less strong so compared to an analysis with all species included (Fig 1). The proportion of forest specialists decreased with fragment area (r = -0.32, P = 0.32) and abundance (r = -0.73, P < 0.01), while specialist species density was not significantly related to habitat isolation (Fig 2a), forest cover (Fig 2b), fragment perimeter (Fig 2c), or specialist abundances (Fig 2d).
A comparison of species richness between the three larger (total area 416 ha, 56 species) and nine smaller fragments (total area 46 ha, 55 species) (Table 1) revealed a loss of 14 species (25%) and a gain of 13 species (23.6%) in the latter. The larger fragments contained 17 species of forest specialists, the smaller ones 14 species. Despite the fact that the nine smaller fragments comprised only 11% of the area of the larger fragments, total species richness decreased by 2% (1 species) only. The prevalence of negative nestedness and positive betaP SES scores (Table 2) further indicated spatial turnover in species composition among the larger and smaller fragments, however, we did not find direct evidence that the latter was caused by competitive interactions. Indeed, correlations between the competitive interaction matrix and the observed distribution of abundances (Table 2) were on average weak and explained at most 50% of variance in abundance.
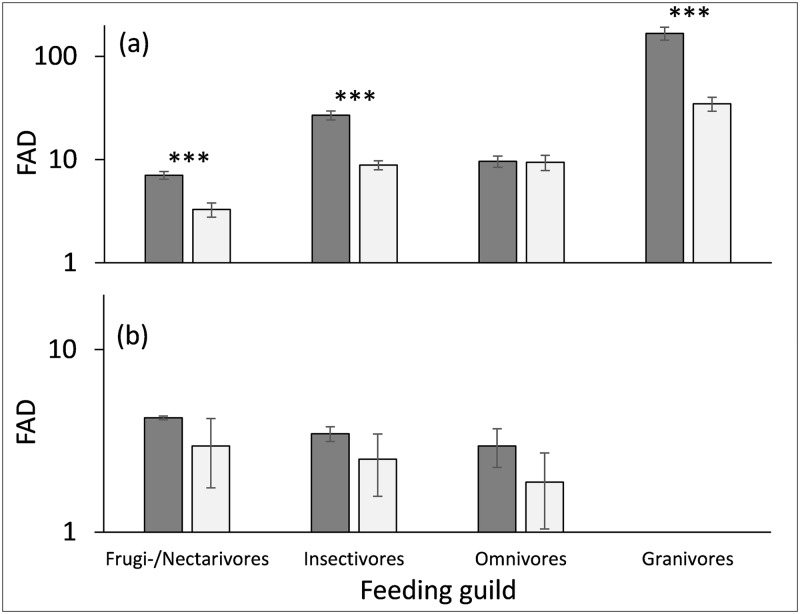
Finally, we compared functional diversity between feeding guilds and between larger and smaller fragments (Fig 3a, Table 3). Except for omnivores, functional attribute diversity significantly differed between feeding guilds and was always larger in the large fragments (Fig 3a). We observed the same pattern for forest specialists (Fig 3b) although the differences were statistically not significant due to the small number of species. Linear modelling accounting for differences in species richness between fragments (Table 3) revealed that these differences were mainly caused by co-variation with species richness. Although FAD significantly differed between feeding guilds and guild specific differences in species traits (Table 2), FAD did not vary with fragment size (Table 3). FAD also did not significantly vary with any of the categorical variables (guild, trait, size) when using standardized effect scores (Table 3).
Fig 3. Functional attribute diversity (a: all species, b: forest specialists only) of four bird feeding guilds differed significantly (***: parametric P(F) < 0.001) between large (dark grey columns) and small fragments (light grey columns dots).
The single granivore forest specialist species made it impossible to calculate FAD.
Table 3. General linear mixed modelling detected significant (*: parametric P(F) < 0.05; ***: P < 0.001) differences of functional attribute diversity (FAD) among feeding guilds, and guild×functional trait combinations when using raw FAD as dependent variable, but not when using SES transformed values (traits reshuffling null model).
Fragment species richness served as metric covariate. Given are partial eta2 values.
| Variable | df | FAD | SES FAD |
|---|---|---|---|
| Feeding guild | 3 | 0.06*** | 0.03 |
| Functional trait | 5 | 0.02 | 0.02 |
| Remnant size | 1 | <0.01 | <0.01 |
| Guild×trait | 15 | 0.11*** | 0.09 |
| Guild×size | 3 | 0.04 | <0.01 |
| Trait×size | 5 | 0.03 | 0.03 |
| Species | 1 | 0.04* | <0.01 |
| Squared species | 1 | 0.65*** | <0.01 |
| Error | 229 | ||
| r2 (model) | 0.95*** | 0.18 |
Discussion
Within an island biogeographic framework [56], species richness is predicted to vary positively with patch size and negatively with patch isolation. Along these lines, Canale et al. (2012) [57] reported a strong decline in species richness in tropical forest fragments of less than 10 ha (equivalent to a lowered species density), approximately the upper size limit of our small forest fragments. Hanski et al. (2013) [58] predicted such a decline while demonstrating that SARs that only account for area effects tend to overestimate species richness in fragmented landscapes if fragments are highly isolated, and they proposed an extension of the power function SAR that downsizes species richness in small fragments. In turn, a number of empirical studies reported a small island effect [12] where species density in very small islands or habitat patches increased [59–60]. Our results do not support either prediction. Species richness in the smallest fragments did not negatively deviate from the observed SAR (Fig 1a) although we observed a tendency to independence of area below 10 ha. However, richness was very closely related to the number of individuals indicating that habitat capacity is probably the main trigger of species richness. Contrary to Hanski et al. (2013) [58], area corrected richness (species density) was not linked to fragment isolation (Fig 2a).
The counter-intuitive finding that species richness in small fragments was only 2% lower than in large ones, is in line with other studies that reported patch connectivity to be of higher importance than patch size [61], in particular for generalist species with a broad ecological amplitude that can easily cross the landscape matrix and (re)colonise small forest patches [62, 63]. This generalist-focused explanation is corroborated by the fact that specialist species increased less in richness in the larger fragments (Fig 1). Bird mobility in heterogeneous landscapes tends to vary among feeding guilds [64–66], with small understorey insectivores often showing the highest sensitivity to fragment isolation [67]. The resulting high species turnover (i.e. partial replacement of sedentary, forest-restricted specialists by mobile, matrix-tolerant generalists in small, degraded forest fragments) might be responsible for the weak SAR observed in our study.
Previous studies showed that bird assemblages strongly vary in composition with habitat area and structure (such as the degree of fragmentation), and predicted higher species richness in large, connected forest patches [68–69]. Yet, Trzinski et al. (1999) [70], Banks-Leite et al. (2012) [62], and Neuschulz et al. (2013) [71] documented no significant impact of habitat heterogeneity on bird community structure in tropical forest fragments. Banks-Leite et al. (2012) [62] argued that loss of tropical species is mainly driven by habitat destruction, rather than fragmentation. According to these authors, species with broad ecological amplitude can readily exploit the landscape matrix in which forest fragments are embedded and may even gain from habitat fragmentation. The high species turnover between isolated forest fragments (independent of fragment size) observed in our study is in line with these predictions.
Various studies have reported increasing mobility with decreasing habitat integrity [72–74]. Yet, Price (2006) [75] showed that frugivorous species conducted shorter, rather than longer, movements in fragmented forests, despite their high intrinsic mobility. Based on earlier studies of mobility [76–77] and gene flow [78] in the same study area, bird species currently surviving in the Taita Hills forest also appear to differ in metapopulation dynamics. Results of this study add to the effect of functional connectivity [79] and support the need to define species- (or guild-) specific fragmentation thresholds [80] in conservation. Indeed, even though we found species richness to be only marginally affected by patch area, there are documented cases of local species extinctions in small Taita forest fragments [42]. In this respect, our results are also relevant for the ongoing SLOSS debate [81–82]. Although each large fragment was at least twice as large as all smaller fragments together, they hosted at most 75% of the total species richness of the small fragments (Table 2). As such, our findings corroborate the theoretical expectation of Lasky & Keitt (2013) [26] that networks of small habitat remnants would be able to maintain higher total species richness than homogenous habitat blocks of the same size. Yet, contrary to these authors, we did not find reduced fragment (alpha) diversities within each of the smaller fragments. We interpret these findings as evidence that tropical forest birds respond to increased habitat fragmentation by higher mobility [78], or that there is a debt between former habitat destruction and ongoing local extinction [83].
We were surprised to see that functional trait identity was not linked to the pattern of species—co-occurrence. Species interactions are mediated by traits and classical community assembly theory predicts co-existing species to show low levels of trait similarity [84]. Yet, we did not find strong evidence that the bird communities in our study fragments are shaped by competitive interactions (Table 2), which might explain why trait expression was not significantly linked to co-occurrence. This finding contrasts to recent evidence by Bregman et al. (2015) [16] who reported increased levels of interspecific competition within bird guilds at decreasing fragment size. However, these authors used indirect evidence within the community assembly framework that assumes that overdispersion of phylogenetic and functional traits is a consequence of competitive interactions (Darwin’s competition-relatedness hypothesis, reviewed in Allan et al. 2013, Götzenberger et al. 2012) [85–86]. However, Cahill et al. (2008) [87] found little evidence for this assertion and it is now well known that overdispersion (spatial segregation) might stem from different processes, particularly from filter effects within heterogeneous landscapes [88] and even from dispersal limited neutral community assembly [89]. Here we used a direct way to assess whether any set of competitive strength relationships between species is able to predict observed abundance distributions. For the smaller fragments this relation was highest in omnivores where competition explained at most 13% of variance in species relative abundances (rC = 0.36, Table 2) comparing to 50% in the larger fragments. Apparently species density in the smaller fragments yet did not reach the threshold for intense competitive effects and thus is of rather marginal importance for community assembly.
Total trait space, as expressed by FAD, increases with species richness [52]. However, various authors found homogenization effects in fragmented landscapes where smaller fragments are devoid of habitat specialists and regionally rare species [90–92]. Consequently, this selective pattern of species extinction should translate into a reduced effective functional diversity that is the degree of FAD after accounting for richness effects. Rather than observing such a pattern, FAD remained constant after correction for richness differences (Table 3). While small tropical forest fragments are hence able to maintain a high effective functional diversity, communities will inevitably collapse when fragment areas become exceedingly small, which raises the question about a minimal tropical forest fragment size. SES-transformed FAD scores of the three smallest fragments (Mwachora, Kichuchenyi, Wundanyi, Table 1) were indeed smaller (average SES FAD = -0.26±0.14) than those of the three large fragments (average SES FAD = 0.13±0.15), although not statistically significant. A one ha area is probably at the lower boundary for a functioning understorey bird community in the Taita forest archipelago.
Conclusion
In conclusion, results of this study do not point to a single ecological driver of the observed variation in avian species composition among indigenous fragments of the Taita forest archipelago. Interspecific competition does not appear to be a major driver of species segregation, since in small forest fragments no single competitive strength hierarchy was able to predict at least a major part of the observed species abundances distributions. In larger fragments, however, the relative importance of competition might be higher. We did not find strong evidence for habitat filtering either, and community-wide species co-occurrences and joint occurrences between pairs of species were neither nested nor segregated, as would be expected if competition would be the main driver of species occurrences. The frequency of pairwise species segregation was even much below the level expected under random association. However, the large differences in species density and species richness between the two smallest fragments highlight that local peculiarities might heavily constrain species richness, irrespective of subsequent patterns of co-occurrences. Such variable, and partly opposing, responses of single guilds to fragmentation suggest that it is vital to take species ecology into consideration when predicting community-wide responses to habitat change in tropical forests.
Supporting Information
(XLSX)
Acknowledgments
We thank M. Githiru, T. Callens, T. Spanhove, V. Lehouck, L. De Neve, D. Van de Loock, M. Chovu, S. Karimi, T. Brooks, D. Gitau, T. Imboma, J. Kageche and P. Kariuki for field assistance, H. Matheve for help with the database.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Fieldwork was funded by research grants G.0258.01N, G.0055.08N, G.0149.09N and G.0308.13N of Research Foundation Flanders (FWO), by Flemish Interuniversity Council project 02 ⁄ 6 ⁄ 7-338-607, and through contacts facilitated by FWO research community WO.037.10N. Biometric data collection was funded by Natural Environment Research Council (grant NE/I028068/1 to JAT); data analyses were supported by Polish National Science Centre (grant NCN 2014/13/B/NZ8/04681 to WU).
References
- 1.André H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: A review. Oikos 71 355–366. [Google Scholar]
- 2.Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN et al. (2013) The biodiversity of species and their rates of extinction, distribution, and protection. Science 344: 1246–1252. [DOI] [PubMed] [Google Scholar]
- 3.Laurance SGW, Stouffer PC, Laurance WE (2004) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol 16: 605–618. [Google Scholar]
- 4.Waltert M, Bobo KS, Sainge NM, Fermon H, Mühlenberg M (2005) From forest to farmland: habitat effects on afrotropical forest bird diversity. Ecol Appl 15: 1351–1366. [Google Scholar]
- 5.Kirika JM, Farwig N, Böhning-Gaese K (2008) Effects of local disturbance of tropical forests on frugivores and seed removal of a small-seeded afrotropical tree. Conserv Biol 22: 318–328. 10.1111/j.1523-1739.2007.00874.x [DOI] [PubMed] [Google Scholar]
- 6.Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371: 65–66. [Google Scholar]
- 7.Henle K, Lindenmayer DB, Margules CR, Saunders DA, Wissel C (2004) Species survival in fragmented landscapes: where are we now? Biod Conserv 13: 1–8. [Google Scholar]
- 8.Schmiegelow FKA, Machtans CS, Hannon SJ (1997) Are boreal birds resilient to forest fragmentation? An experimental study of short term community responses. Ecology 78: 1914–1932. [Google Scholar]
- 9.Debinski DM, Holt RD (2000) A survey and overview of habitat fragmentation experiments. Conserv Biol 14: 342–355. [Google Scholar]
- 10.Gotelli NJ, Ellison AM (2002) Biogeography at a regional scale: determinants of ant species density in New England bogs and forests. Ecology 83: 1604–1609. [Google Scholar]
- 11.Hoyle M, Harborne AR (2005) Mixed effects of habitat fragmentation on species richness and community structure in a microarthropod microecosystem. Ecol Entomol 30: 684–691. [Google Scholar]
- 12.Dengler J (2010) Robust methods for detecting a small island effect. Div Distr 16: 256–266. [Google Scholar]
- 13.Farmilo BJ, Melbourne BA, Camac JS, Morgan JW (2014) Changes in plant species density in an experimentally fragmented forest landscape: are the effects scale-dependent? Australian J Ecol 39: 416–23. [Google Scholar]
- 14.Collinge SK, Forman RTT (1998) A conceptual model of land conversion processes: predictions and evidence from a micro-landscape experiment with grassland insects. Oikos 82: 66–84. [Google Scholar]
- 15.Weiher E, Keddy P (eds.) (1999) Ecological assembly rules: perspectives, advances, retreats. Cambridge Univ. Press: Cambridge. [Google Scholar]
- 16.Bregman TP, Lees AC, Seddon N, MacGregor HEA, Darski B, Aleixo A et al. (2015) Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology 96: 2692–2704. [DOI] [PubMed] [Google Scholar]
- 17.Dixo M, Metzger JP, Morgante JS, Zamudio KR (2009) Habitat fragmentation reduces genetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biol Conserv 142: 1560–1569. [Google Scholar]
- 18.Ebenman B, Law R, Borrvall C (2004) Community viability analysis: the response of ecological communities to species loss. Ecology 85: 2591–2600. [Google Scholar]
- 19.Rosenzweig ML (1995) Species diversity in space and time. Cambridge University Press: Cambridge. [Google Scholar]
- 20.Rosindell J, Hubbell SP, He F, Harmon LJ, Etienne RS (2012) The case for ecological neutral theory. Trends Ecol Evol 27: 203–208. 10.1016/j.tree.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Diamond JM (1975a) Assembly of species communities In: Cody ML, Diamond JM (eds) Ecology and evolution of communities, Harvard Univ. Press: Princeton, pp. 342–444. [Google Scholar]
- 22.Holyoak M, Leibold MA, Holt RD (eds.) (2005) Metacommunities: spatial dynamics and ecological communities. University Chicago Press: Chicago. [Google Scholar]
- 23.Ulrich W, Gotelli NJ (2007) Disentangling community patterns of nestedness and species co-occurrence. Oikos 116: 2053–2061. [Google Scholar]
- 24.Ulrich W, Gotelli NJ (2013) A null model algorithm for presence—absence matrices based on proportional resampling. Ecol Modelling 244: 20–27. [Google Scholar]
- 25.Nupp TE, Swihart RK (2001) Assessing competition between forest rodents in a fragmented landscape of Midwestern USA. Mamm Biol 66: 345–356. [Google Scholar]
- 26.Lasky JR, Keitt TH (2013) Reserve size and fragmentation alter community assembly, diversity, and dynamics. Am Nat 182: E142–E160. 10.1086/673205 [DOI] [PubMed] [Google Scholar]
- 27.Horner-Devine MC, Silver JM, Leibold MA, Bohannan BJM, Colwell RK, Furhman JA et al. (2007) A comparison of taxon co-occurrence patterns for macro- and microorganisms. Ecology 88: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 28.Patterson BD, Atmar W (1986) Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol J Linn Soc 28: 65–82. [Google Scholar]
- 29.Pandit SN, Kolasa J, Cottenie K (2009) Contrasts between habitat generalists and specialists: an empirical extension to the basic metacommunity framework. Ecology 90: 2253–2262. [DOI] [PubMed] [Google Scholar]
- 30.Diamond JM (1975a) Assembly of species communities In: Cody ML, Diamond JM (eds) Ecology and evolution of communities, Harvard Univ. Press: Princeton, pp. 342–444. [Google Scholar]
- 31.Ulrich W, Soliveres S, Kryszewski W, Maestre FM, Gotelli NJ (2014) Matrix models for quantifying competitive intransitivity from species abundance data. Oikos 123: 1057–1070. 10.1111/oik.01217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soliveres S, Maestre FT, Ulrich W, Manning P, Boch S, Bowker M et al. (2015) Intransitive competition is widespread in plant communities and maintains species richness. Ecol Lett 18: 790–798. 10.1111/ele.12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellikka PKE, Lotjonen M, Sijander M, Lens L (2009) Airborne remote sensing of spatiotemporal change (1955–2004) in indigenous and exotic forest cover in the Taita Hills, Kenya. International J Appl Earth Observ Geoinf 11: 221–232. [Google Scholar]
- 34.Zimmermann DA, Turner DA, Pearson DJ (1996) Birds of Kenya and Northern Tanzania Christopher Helm: London. [Google Scholar]
- 35.Beentje HJ, Hdiangúi N, Mutangah J (1987) Forest islands in the mist. Swara 10: 20–21. [Google Scholar]
- 36.Hildebrandt JM (1877) Von Mombassa nach Kitui. Zeitschrift der Gesellschaft für Erdkunde 14: 321–350. [Google Scholar]
- 37.Chege J, Bytebier B (2005) Vegetation structure of four small forest fragments in Taita Hills, Kenya. J East African Nat Hist 94: 231–234. [Google Scholar]
- 38.Pellikka P (1998) Development of correction chain for multispectral airborne video camera data for natural resource assessment. Fennia 176: 1–110. [Google Scholar]
- 39.Holm M, Lohi A, Rantasuo M, Väatäinen S, Höyhtyä T, Puumalainen J et al. (1999) Creation of large image mosaics of airborne digital camera imagery. In: Proceedings of the 4th International Airborne Remote Sensing Conference and Exhibition, vol. II, Ottawa, Canada, 21–24 June, pp. 520–526.
- 40.Clark BJF, Pellikka PKE (2010) Landscape analysis using multiscale segmentation and object orientated classification In: Röder A (ed.), Recent advances in remote sensing and geoinformation processing for land degradation assessment. Taylor & Francis. [Google Scholar]
- 41.Karr JR (1981) Surveying birds with mist nets. Stud. J Avian Biol 6: 62–67. [Google Scholar]
- 42.Callens T (2012) Genetic and demographic signatures of population fragmentation in a cooperatively breeding bird from south-east Kenya. PhD thesis, Ghent University.
- 43.Remsen JV, Good DA (1996) Misuse of data from mist-net captures to assess relative abundance in bird populations. Auk 113: 381–398. [Google Scholar]
- 44.Trisos CH, Petchey OL, Tobias JA (2014) Unraveling the interplay of community assembly processes acting on multiple niche axes across spatial scales. Am Nat 184: 593–608. 10.1086/678233 [DOI] [PubMed] [Google Scholar]
- 45.Claramunt S, Derryberry EP, Remsen JV, Brumfield RT (2012) High dispersal ability inhibits speciation in a continental radiation of passerine birds. Proc Roy Soc B 279: 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pigot A, Trisos CH, Tobias JA (2016) Functional traits reveal the expansion and packing of ecological niche space underlying an elevational diversity gradient in passerine birds. Proc Roy Soc B 283: 20152013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone L, Roberts A (1990) The checkerboard score and species distributions. Oecologia 85: 74–79. [DOI] [PubMed] [Google Scholar]
- 48.Almeida-Neto M, Guimarães P, Guimarães PR Jr, Loyola RD, Ulrich W (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117: 1227–1239. [Google Scholar]
- 49.Tuomisto H (2010) A consistent terminology for quantifying species diversity? Yes, it does exist. Oecologia 164: 853–860 10.1007/s00442-010-1812-0 [DOI] [PubMed] [Google Scholar]
- 50.Walker B, Kinzig A, Langridge J (1999) Plant attribute diversity, resilience, and ecosystem function: The nature and significance of dominant and minor species. Ecosys 2: 95–113. [Google Scholar]
- 51.Ulrich W (2011) NODF—a Fortran program for nestedness analysis. www.umk.pl/~ulrichw.
- 52.Ulrich W (2011) Turnover—a Fortran program for the analysis of species associations. www.umk.pl/~ulrichw.
- 53.Ulrich W, Gotelli NJ (2013) Pattern detection in null model analysis. Oikos 122: 2–18. [Google Scholar]
- 54.Mouchet MA, Villéger S, Mason MWH, Mouillot D (2010) Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol 24: 867–876. [Google Scholar]
- 55.Laird RA, Schamp BS (2006) Competitive intransitivity promotes species co-existence. Am Nat 168: 182–193. 10.1086/506259 [DOI] [PubMed] [Google Scholar]
- 56.MacArthur RH, Wilson EO (1963) An equilibrium theory of insular zoogeography. Evolution 17: 373–87. [Google Scholar]
- 57.Canale GR, Peres CA, Guidorizzi CE, Gatto CAF, Kierulff MCM (2012) Pervasive defaunation of forest remnants in a tropical biodiversity hotspot. PLoS ONE 7: e41671 10.1371/journal.pone.0041671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanski I, Zurita GA, Bellocq MI, Rybicki J (2013) Species—fragmented area relationship. Proc Nat Acad Sci USA 113: 12715–12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrison LW (2014) The small-island effect: empty islands, temporal variability and the importance of species composition. J Biogeogr 41: 1007–1017. [Google Scholar]
- 60.Wang Y, Wu Q, Wang X, Liu C, Wu L, Chen C et al. (2015) Small-island effect in snake communities on islands of an inundated lake: The need to include zeroes. Basic Appl Ecol 16: 19. [Google Scholar]
- 61.Martensen AC, Pimentel RG, Metzger JP (2008) Relative effects of fragment size and connectivity on bird community in the Atlantic rain forest: Implications for conservation. Biol Conserv 141: 2184–2192. [Google Scholar]
- 62.Banks-Leite C, Ewers RM, Metzger JP (2012) Unravelling the drivers of community dissimilarity and species extinction in fragmented landscapes. Ecology 93: 2560–2569. [DOI] [PubMed] [Google Scholar]
- 63.Banks-Leite C, Pardini R, Tambosi LR, Pearse WD, Bueno AA, Bruscagin RT et al. (2014) Using ecological thresholds to evaluate the costs and benefits of set-asides in a biodiversity hotspot. Science 345: 1041–1045. 10.1126/science.1255768 [DOI] [PubMed] [Google Scholar]
- 64.Harris RJ, Reed JM (2002) Behavioral barriers to non-migratory movements of birds. Ann Zool Fennici 39: 275–290. [Google Scholar]
- 65.Gillies CS, St. Clair CC (2008) Riparian corridors enhance movement of a forest specialist bird in fragmented tropical forest. Proc Nat Acad Sci 105: 19774–19779. 10.1073/pnas.0803530105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lees AC, Peres CA (2008) Avian life-history determinants of local extinction risk in a hyper-fragmented neotropical forest landscape. Anim Conserv 11: 128–137. [Google Scholar]
- 67.Şekercioğlu ÇH (2012) Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J Ornithology 153: 153–161. [Google Scholar]
- 68.Banks-Leite C, Ewers RM, Metzger JP (2010) Edge effects as the principal cause of area effects on birds in fragmented secondary forest. Oikos 119: 918–926. [Google Scholar]
- 69.Kennedy CM, Marra PP, Fagan WF, Neel MC (2010) Landscape matrix and species traits mediate responses of neotropical resident birds to forest fragmentation in Jamaica. Ecol Monogr 80: 651–669. [Google Scholar]
- 70.Trzcinski MK, Fahrig L, Merriam G (1999) Independent effects of forest cover and fragmentation in the distribution of forest breeding birds. Ecol Appl 9: 586–593. [Google Scholar]
- 71.Neuschulz EL, Brown M, Farwig N (2013) Frequent bird movements across a highly fragmented landscape: the role of species traits and forest matrix. Anim Conserv 16: 170–179. [Google Scholar]
- 72.Carey AB, Reid JA, Horton SP (1990) Spotted Owl home range and habitat use in Southern Oregon Coast Ranges. Journal of Wildlife Management 54: 11–17. [Google Scholar]
- 73.Wiktander U, Olsson O, Nilsson SG (2001) Seasonal variation in home-range size, and habitat area requirement of the Lesser Spotted Woodpecker (Dendrocopos minor) in southern Sweden. Biol Conserv 100: 387–395. [Google Scholar]
- 74.Hansbauer MM, Storch I, Pimentel RG, Metzger JP (2008) Comparative range use by three Atlantic Forest understorey bird species in relation to forest fragmentation. J Trop Ecol 24: 291–299. [Google Scholar]
- 75.Price OF (2006) Movements of frugivorous birds among fragmented rainforests in Northern Territory, Australia. Wildlife Research 33: 521–528. [Google Scholar]
- 76.Lens L, Van Dongen S, Norris K, Githiru M, Matthysen E (2002) Avian persistence in fragmented rainforest. Science 298: 1236–1238. 10.1126/science.1075664 [DOI] [PubMed] [Google Scholar]
- 77.Aben J, Adriaensen F, Thijs K, Pellikka P, Siljander M, Lens L et al. (2012) Effects of matrix composition and configuration on forest bird movements in a fragmented Afromontane biodiversity hotspot. Animal Conserv 15: 658–668. [Google Scholar]
- 78.Callens T, Galbusera P, Matthysen E, Durand EY, Githiru M, Huyghe JR et al. (2011) Genetic signature of population fragmentation varies with mobility in seven bird species of a fragmented Kenyan cloud forest. Mol Ecol 20: 1829–1844. 10.1111/j.1365-294X.2011.05028.x [DOI] [PubMed] [Google Scholar]
- 79.Villard M-A, Metzger JP (2014) Beyond the fragmentation debate: a conceptual model to predict when habitat configuration really matters. J Appl Ecol 51: 301–318. [Google Scholar]
- 80.Martensen AC, Ribeiro MC, Banks-Leite C, Prado PI, Metzger JP (2012) Associations of forest cover, fragment area, and connectivity with neotropical understory bird species richness and abundance. Conserv Biol 26: 1100–1111. 10.1111/j.1523-1739.2012.01940.x [DOI] [PubMed] [Google Scholar]
- 81.Diamond JM (1975b) The island dilemma: Lessons of modern biogeographic studies for the design of natural reserves. Biol Conserv 7: 129–146. [Google Scholar]
- 82.Tjørve E (2010) How to resolve the SLOSS debate: Lessons from species-diversity models. J Theoretical Biol 264: 604–612. [DOI] [PubMed] [Google Scholar]
- 83.Jackson ST, Sax DF (2010) Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends Ecol Evol 25: 153–160. 10.1016/j.tree.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 84.MacArthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101: 377–385. [Google Scholar]
- 85.Allan E, Jenkins T, Fergus AJF, Roscher C, Fischer M, Petermann J et al. (2013) Experimental plant communities develop phylogenetically overdispersed abundance distributions during assembly. Ecology 94: 465–477. [DOI] [PubMed] [Google Scholar]
- 86.Götzenberger L, de Bello F, Bråthen KA, Davison J, Dubuis A, Guisan A et al. (2012) Ecological assembly rules in plant communities—approaches, patterns and prospects. Biol Rev 87: 111–127. 10.1111/j.1469-185X.2011.00187.x [DOI] [PubMed] [Google Scholar]
- 87.Cahill JF, Kembel SW, Lamb EG, Keddy P (2008) Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspectives Plant Ecol Evol Sys 10: 41–50. [Google Scholar]
- 88.Blois JL, Gotelli NG, Behrensmeyer AK, Faith JT, Lyons SK, Williams JW et al. (2014) A framework for evaluating the influence of climate, dispersal limitation, and biotic interactions using fossil pollen associations across the late Quaternary. Ecography 37: 1095–1108. [Google Scholar]
- 89.Ulrich W, Jabot F, Gotelli N (2016) Competitive interactions change the pattern of species co-occurrences under neutral dispersal. Oikos. [Google Scholar]
- 90.Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117: 507–514. [Google Scholar]
- 91.Watson DM (2003) Long-term consequences of habitat fragmentation—highland birds in Oaxaca, Mexico. Biol. Conserv. 111: 283–303. [Google Scholar]
- 92.Watson DM (2002) A conceptual framework for studying species composition in fragments, islands and other patchy ecosystems. J Biogeogr 29: 823–834. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.