Abstract
Background
Dengue fever, caused by the dengue virus (DENV), is now the most common arbovirus transmitted disease globally. One novel approach to control DENV is to use the endosymbiotic bacterium, Wolbachia pipientis, to limit DENV replication inside the primary mosquito vector, Aedes aegypti. Wolbachia that is naturally present in a range of insects reduces the capacity for viruses, bacteria, parasites and fungi to replicate inside insects. Wolbachia’s mode of action is not well understood but may involve components of immune activation or competition with pathogens for limited host resources. The strength of Wolbachia-based anti DENV effects appear to correlate with bacterial density in the whole insect and in cell culture. Here we aimed to determine whether particular tissues, especially those with high Wolbachia densities or immune activity, play a greater role in mediating the anti DENV effect.
Methodology/findings
Ae. aegypti mosquito lines with and without Wolbachia (Wildtype) were orally fed DENV 3 and their viral loads subsequently measured over two time points post infection in the midgut, head, salivary glands, Malpighian tubules, fat body and carcass. We did not find correlations between Wolbachia densities and DENV loads in any tissue, nor with DENV loads in salivary glands, the endpoint of infection. This is in contrast with strong positive correlations between DENV loads in a range of tissues and salivary gland loads for Wildtype mosquitoes. Lastly, there was no evidence of a heightened role for tissues with known immune function including the fat body and the Malpighian tubules in Wolbachia’s limitation of DENV.
Conclusion/significance
We conclude that the efficacy of DENV blocking in Wolbachia infected mosquitoes is not reliant on any particular tissue. This work therefore suggests that the mechanism of Wolbachia-based antiviral effects is either systemic or acts locally via processes that are fundamental to diverse cell types. We further conclude that the relationship between DENV blocking and Wolbachia density is not linear in mosquito tissues
Author Summary
Dengue fever caused by the dengue virus (DENV) is transmitted by the mosquito, Aedes aegypti. To control the disease, an intracellular bacterium called Wolbachia has been introduced into Ae. aegypti where it blocks/limits success of infection of DENV. The mechanistic basis of blocking is not well understood but may involve Wolbachia activating the host immune system or competing with DENV for host resources. The strength of blocking appears to correlate with Wolbachia density. Here, we aimed to determine if any particular tissues inside the mosquito play a greater role in blocking. Tissues were chosen based on their Wolbachia density and their roles in infection and immunity. Wolbachia infected and uninfected mosquitoes were orally infected with DENV and Wolbachia density and DENV load were assessed in midgut, salivary gland, head, Malpighian tubules, fat body and carcass. Wolbachia density did not correlate with DENV loads in the same tissues nor with DENV loads in the salivary glands. We also showed that no one tissue appeared to play a greater role in blocking. In summary, these finding suggest that in the mosquito a threshold Wolbachia density may be required for DENV blocking. Our findings also suggest that blocking may involve mechanisms that are fundamental to all cells.
Introduction
Dengue fever, caused by the dengue virus (DENV), is the most prevalent arthropod transmitted virus, endemic in over 100 countries [1,2].The virus is comprised of four antigenically distinct serotypes (1–4) [3,4]. DENV is transmitted by Aedes aegypti and Ae. albopictus with the former being the principal vector [5]. With no specific antiviral drugs, management of the disease has mainly relied on relieving the associated symptoms of fever, headache and rash [6]. As the current tetravalent dengue vaccine offers incomplete protection [7], vector control remains the primary means of reducing disease prevalence.
One example of an emerging vector control strategy involves the use of a bacterial endosymbiont, Wolbachia pipentis that is naturally present in 40% of arthropods [8] and 28% of mosquito species, including Ae. albopictus, and Ae. notoscriptus. Interestingly, Ae. aegypti is not naturally infected with the symbiont [9]. Over the last decade, three different Wolbachia strains have been transinfected into Ae. aegypti where they form stable, inherited infections including; wMelPop-CLA and wMel, both from Drosophila melanogaster, wAlbB from Ae. Albopictus and wMelwAlbB, which is a superinfection from both host donors [10–13]. In these mosquito vectors, Wolbachia demonstrates an ability to limit or “block” the success of infection by viruses, nematodes and parasites [14–16]. This effect forms the basis of Wolbachia-based biocontrol trials to interrupt disease transmission in the human population via the vector [17]. The most advanced of such trials are focused on DENV control where the wMel strain has been released into wild Ae. aegypti mosquitoes and successfully spread [18].
Despite widespread field-testing, the mechanistic basis of Wolbachia-DENV blocking is poorly understood. Pathogen blocking has been partly attributed to the ability of the bacterium to increase the basal immune activity of the host thereby enabling it to resist subsequent DENV infection in a process known as ‘immune priming’ [19–21]. Wolbachia-DENV inhibition may also be as a result of competition between the symbiont and viruses for vital host nutrients such as cholesterol, as demonstrated in Drosophila [22]. Such competition may be expected given that the Wolbachia genome lacks a range of key genes in lipid biosynthesis pathways [23] and because viruses are heavily reliant on host cholesterol for replication [24,25]. Neither immune priming nor cholesterol competition however, can completely explain Wolbachia-DENV blocking.
The strength of blocking appears to correlate with Wolbachia density, whereby higher densities of the symbiont are associated with greater viral inhibition [11,26–28]. In mosquito cell lines, only highly infected cells show almost complete DENV inhibition [27,28]. The same relationship has been documented in other insects. In Drosophila simulans, the wMel, wAu and wRi strains grow to high densities and provide protection against Drosophila C virus (DCV). In contrast, the wHa and wNo strains that grow to very low densities show little blocking [29]. The fact that wAlbB is unable to block DENV in its natural host Ae. albopictus has also been attributed to low symbiont numbers. In Ae. aegypti where wAlbB has been introduced and hence grows to higher densities, DENV blocking is much stronger [28]. The correlation is further shown in Ae. aegypti by the disparity in blocking between the virulent wMelPop-CLA strain, which grows to very high densities compared to the wMel strain which grows to moderate densities [11].
Several studies have reported that Wolbachia is found at different densities in various tissues of the mosquito body, with the ovaries and Malpighian tubules tending to have high densities [14,21,28,30,31]. Osborne et al., [32] have suggested that Wolbachia density within the head, gut and Malpighian tubules correlated with the ability to mediate protection against DCV in D. simulans. These different tissues may be of varying importance for pathogen blocking as predicted by their Wolbachia densities or if they play a particular functional role in Wolbachia-based pathogen blocking. For example, the fat body is mainly involved in pathogen defence [33,34] and the Malpighian tubules, that happen to have very high Wolbachia densities now appear to have immune function [35]. It is unknown if there is a correlation between the Wolbachia encountered by DENV in these tissues and the subsequent progression of infection to the salivary glands as the endpoint of transmission.
When a mosquito takes a viremic blood meal, the virus first infects the midgut and then it disseminates to other tissues such as the Malpighian tubules, fat body, trachea and the salivary glands, where it can be transmitted to a human via the saliva on a subsequent bite [5]. The rate of DENV transmission correlates with the titre of virus in the salivary glands when studied in animal models [36] and mosquito infection rate is also known to correlate with virus infectious dose [37]. Even though several studies have suggested that intermediate mosquito tissues are infected by the virus differentially over time [38–41], it is not clear if there is a correlation between DENV infection in these tissues and that in the salivary glands.
Here we have examined the infectivity and viral load of a DENV serotype 3 strain in the tissues of Wildtype and wMel-infected Ae. aegypti. Specifically we have assessed whether Wolbachia densities predict DENV load in the same tissue and if densities in intermediate tissues predict subsequent DENV loads in the salivary glands. We found that there was a positive correlation between DENV loads in intermediate tissues and salivary glands in Wildtype but not Wolbachia-infected mosquitoes. There was also no correlation between Wolbachia densities and DENV loads in any particular tissue. Together, these findings suggest that no one tissue is particularly important for Wolbachia-based blocking and that Wolbachia may simply be limiting virus at the level of each individual cell, by fundamental processes shared by diverse cell types.
Materials and Methods
Mosquito rearing
Two mosquito lines were used for this experiment; Wolbachia infected [11] and Wolbachia uninfected Ae. aegypti mosquitoes designated wMel.F and Wildtype [31,42], respectively. The wMel.F mosquito line was collected in 2012 from field release sites in Cairns, Australia [18] while the Wildtype line was collected in 2014 from Babinda, Australia. The Wildtype mosquito line was used within four generations of field collection to limit inbreeding. At every generation, the wMel.F mosquito line was outcrossed with 20% Wildtype males to prevent genetic drift between the two lines. Adult mosquitoes were maintained on 10% sucrose while the larvae were fed TetraMin® fish food (Melle, Germany) ad libitum. Mosquitoes were reared under standard conditions of 25°C temperature, 65% relative humidity and photoperiod 12 hours light: dark.
Oral infection of mosquitoes with DENV 3
The DENV 3 strain used for this experiment was sampled from a patient during an outbreak in Cairns, Australia in 2008/2009 [43]. This strain was selected because it caused one of the largest dengue outbreaks in Australia [43] and because it has been demonstrated to infect both wMel and Wildtype mosquitoes at a high rate [44]. Passage 6 of DENV 3 (PFU 106) was propagated using the protocol by Ye et al., [45] and stored in single use aliquots of 1mL at -80°C. The virus was mixed with defibrinated sheep’s blood in the ratio 1:1 and fed through a membrane feeder to three to five day old mosquitoes. The mosquitoes were starved for 24 hours prior to oral infection. The wMel.F and Wildtype mosquitoes were both fed simultaneously over a period of three hours [44]. Mosquitoes were then anesthetized on ice and females that did not feed were sorted out and discarded. Engorged mosquitoes were maintained on 10% sucrose at 25°C until they were dissected.
Dissection of tissues
The midguts, salivary glands, head, fat body, Malpighian tubules and carcass were dissected from each individual mosquito. These tissues were chosen mainly based on their functional role in DENV infection, dissemination and transmission in the mosquito. DENV first infects and replicates in the midgut before being disseminated to other tissues [5]. The end point of disseminated DENV is the salivary glands from where it is transmitted to the human host through the saliva when the mosquito takes a blood meal [5]. Assessment of DENV dissemination in mosquitoes is commonly done using the head tissue given ease of dissection [21,42]. Tissues were dissected on 8 and 14 days post infection (dpi). These time points were chosen to reflect the early stage of infection where DENV would have disseminated from the midgut to other tissues and the late stage of infection where infection would have been well established [44]. Dissections were done in 1X phosphate buffered saline (PBS). Tissues of each individual mosquito were placed in 96-well PCR plates (VWR LabAdvantage, Australia) containing 200ul of extraction buffer (0.01M Trizma base, 0.001M EDTA, 0.05M NaCl and 2.5ul proteinase K) and 2-mm-diameter glass beads (Merck KGaA, Darmstadt, Germany). Ovaries were separated from the carcass and discarded to ensure that Wolbachia density was not unduly influenced by gravid females. To minimize contamination within mosquito lines the dissecting pins were immersed in 80% ethanol for ~10 seconds between individual mosquitoes and discarded after every 20 individuals. New dissecting pins were used for each line to avoid cross contamination between Wildtype and wMel.F mosquitoes. All tissues were stored at -80°C prior to RNA/DNA co-extraction. The entire experiment was replicated three times.
RNA/DNA extraction
Plates containing dissected tissues were homogenized for 1 min 30 seconds in a mini-Beadbeater (BioSpec Products, Bartlesville, OK). They were then incubated in a thermo cycler (C1000Tm Thermal cycler, Bio-Rad, California USA) at 56°C for 5 min, then 98°C for 5 min for the simultaneous extraction of RNA and DNA. The extracted RNA/DNA was stored at -80°C and subsequently used for the quantification of DENV 3 RNA copies and Wolbachia density.
Quantification of DENV 3 RNA copies
Taqman qPCR was used to quantify DENV 3 RNA copies in LightCycler480 (Roche, Applied Science, Switzerland). The RealTime Ready RNA Virus Master (©1996–2016 Roche Diagnostics) was used for concurrent cDNA synthesis and DENV 3 RNA copies quantification following manufacturer’s protocol. Primers for DENV were designed from the 3’UTR region with HEX labelled probes [46]. The following qPCR cycling conditions were used: reverse transcription at 50°C for 10 min, initial denaturation at 95°C for 30s, 45cycles of amplification at 95°C for 5s and 60°C for 30s and a final cooling step at 40°C for 10s. Absolute quantification of DENV 3 RNA copies for individual tissues was extrapolated from a standard curve as previously reported [14].
Quantification of Wolbachia density
Taqman multiplex qPCR was used for the quantification of the WD0513 Wolbachia gene [47] in LightCycler480 (Roche, Applied Science, Switzerland). The WD0513 gene was normalised to the mosquito housekeeping gene RPS17 [48,49] to account for different tissue sizes. The qPCR cycling conditions used are as follows: An initial incubation at 90°C for 5min followed by 45 cycles of amplification at 95°C for 10s, 60°C for 15s and 72°C for 1s and a final cooling step of 40°C for 10s. Relative quantification of Wolbachia was done using the inbuilt algorithm of LightCycler480.
Data analysis
Tissue infectivity (proportion infected) of DENV 3 was analysed using the binary logistic function in a generalized linear model with presence or absence of DENV 3 infection as the response variable and tissue type and time as predicting factors. DENV 3 RNA copies (DENV load) in tissues was analysed using the tweedie distribution with log link function in a generalized linear model with DENV load as the response variable and tissue type and time as predicting factors. Wolbachia density in tissues was analysed using the tweedie distribution with log link function in a generalized linear model with Wolbachia density as the response variable and time and tissue as the predictive factors. Models were run separately for the Wildtype and wMel.F mosquito lines. Non-Parametric Spearman correlation co-efficient was used to test for correlation between the following: (1) DENV loads in intermediate tissues and salivary glands, (2) Wolbachia density in tissues and DENV load in salivary glands and (3) DENV load and Wolbachia density in the same tissue. All statistical analyses were performed in SPSS® (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY)
Results
DENV infectivity by tissue over time
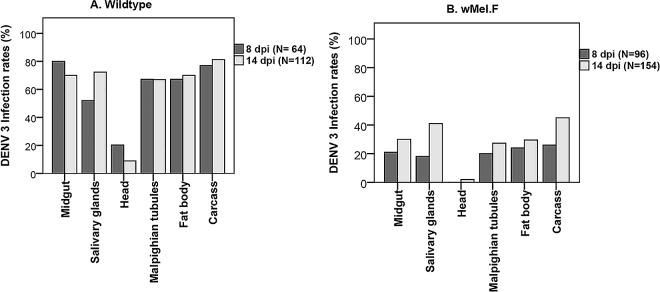
To determine if time post infection and tissue type had an effect on DENV 3 infectivity, we examined head, salivary glands, midgut, Malpighian tubules, fat body and carcass at 8 and 14 dpi. There was a significant effect of tissue for both Wildtype (Wald = 139.60; df = 5; p< 0.0001) and wMel.F (Wald = 40; df = 5; p< 0.0001) mosquitoes (Fig 1). The head was the least infected tissue in both Wildtype and wMel.F mosquitoes, failing to recapitulate patterns of infection in other disseminated tissues including the salivary glands. In a previous study [42] where DENV 3 infection rates in the mosquito head and body were examined, head infection rates were significantly lower than that of the body at 7 dpi in wMel.F mosquitoes. However by 14 dpi in the same study there was no difference between head and body infections in wMel.F mosquitoes. Furthermore, in the Wildtype mosquitoes, head infection rates were lower than that of the body at both 7 and 14 dpi but these differences were not significant [42]. The disparity observed in head infection rates between the present and previous study could possibly be due to the comparatively small sample size used by the previous study. Midgut and carcass were the most highly infected tissues in both Wildtype and wMel.F mosquitoes, respectively. In Wildtype mosquitoes (Fig 1A) there was a significant interaction between tissue and time (Wald = 15; df = 5; p = 0.011,). Midgut infections decline with time, becoming less of a source of infection beyond 8 days. Conversely, salivary glands are still becoming increasingly infected post 8 days. Interestingly, the pattern of infection across hemocoel-associated tissues indicates early dissemination and a plateau of infection rates as well as a similarity in the capacity for these tissues to support DENV replication. There was a clear effect of time (Wald = 10; df = 5; p = 0.002) in wMel.F mosquitoes with infectivity increasing from 8 to 14 dpi across all tissues (Fig 1B). Across the board, tissue infection rates are reduced in wMel.F mosquitoes as expected [14,42] but unlike in Wildtype mosquitoes, more tissues show rising infection rates with time, suggesting the power of blocking is strongest early in infection.
Fig 1.
DENV infection rates in Ae. aegypti tissues for Wildtype (A) and wMel.F (B) at 8 and 14 dpi.
DENV load in tissues over time
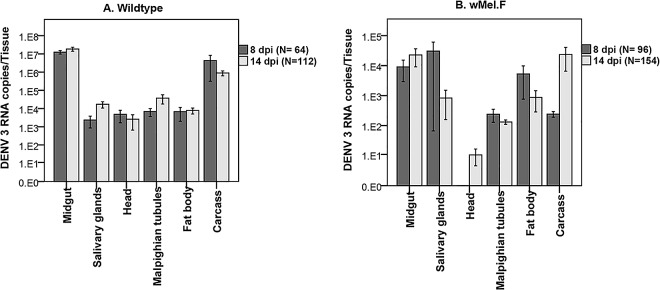
To determine if time post infection and tissue type had an effect on DENV load in tissues, the DENV loads of all the tissues were compared at 8 and 14 dpi. There was a significant variation in DENV loads in tissues of both Wildtype (Wald = 1497; df = 5; p< 0.0001) and wMel.F (Wald = 50; df = 5; p<0.0001) mosquitoes. Midguts had the highest DENV load in the Wildtype mosquitoes while head had the lowest load in the wMel.F mosquitoes (Fig 2). Even though time did not have a significant effect on DENV loads in tissues of both Wildtype (Wald = 2.1; df = 1; p = 0.144) and wMel.F (Wald = 1.9; df = 1; p = 0.166) mosquitoes, there was a significant interaction between time and tissue for both Wildtype (Wald = 131; df = 5; p< 0.0001) and wMel.F mosquitoes (Wald = 180; df = 5; p<0.0001). For instance while DENV load in the carcass decreased over time that of the salivary glands increased in Wildtype mosquitoes (Fig 2A). On the other hand, DENV loads in the carcass increase over time while that of the salivary glands decreased in the wMel.F mosquitoes (Fig 2B). For the most part, however, DENV appears to infect tissues early, reach a peak DENV load and remain relatively stable in Wildtype mosquitoes. In general, wMel.F mosquitoes, exhibited greater variation in DENV load across time and tissues and between individual mosquitoes than is seen for Wildtype possibly demonstrating variation in the efficacy of blocking.
Fig 2.
Mean ± sem DENV load in Ae. aegypti tissues for Wildtype (A) and wMel.F (B) at 8 and 14 dpi.
DENV loads in intermediate tissues predict DENV loads in Wildtype salivary glands
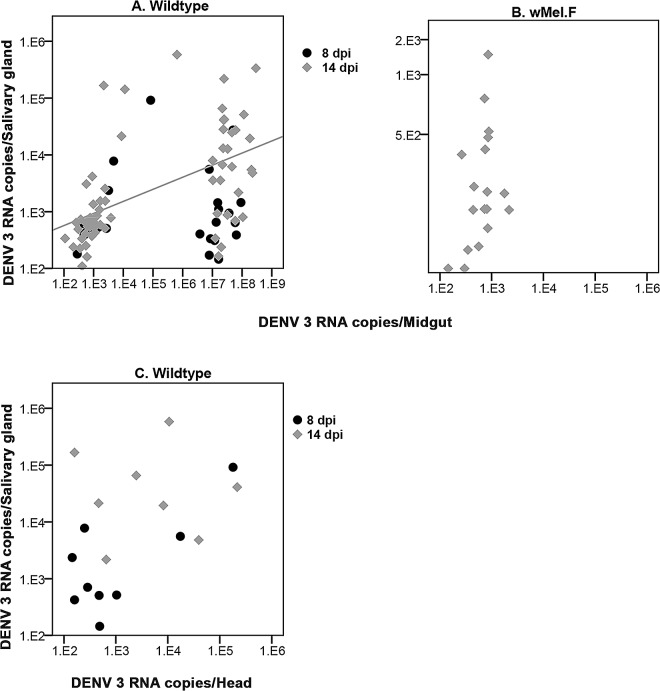
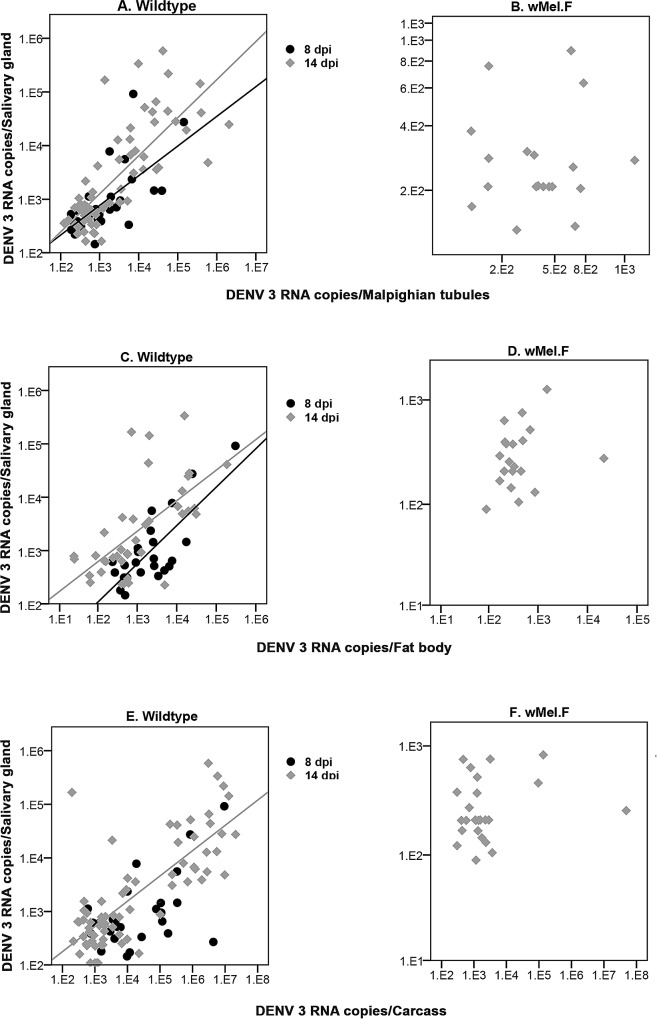
We examined if DENV load in a range of tissues early in the infection process was predictive of loads in the salivary glands by testing for correlations. In the wMel.F mosquito line, the efficacy of blocking effect rendered many mosquitoes uninfected. As such sufficient numbers of DENV positive heads were not obtained for either time point and all tissues at 8 dpi had to be excluded. For Wildtype mosquitoes head DENV loads were not predictive of salivary gland DENV loads at either time point, 8dpi (r = 0.250; p = 0.516) or 14dpi (r = -0.071; p = 0.867) (Fig 3C). There was a significant correlation between midgut and salivary gland DENV loads only at 14 dpi (r = 0.701; p<0.0001) (Fig 3A) in the Wildtype but not in the wMel.F mosquitoes (r = 0.460; p = 0.550) at 14 dpi (Fig 3B). In the Wildtype, salivary glands DENV loads were positively correlated to that of the Malpighian tubules at both 8 (r = 0.684; p<0.0001) and 14 (r = 0.783; p<0.0001) dpi (Fig 4A). A positive correlation was also found between fat body and salivary gland DENV loads at both 8 (r = 0.594; p = 0.002) and 14 (r = 0.684; p<0.0001) dpi (Fig 4C). Carcass DENV loads were positively correlated to salivary gland DENV loads only at 14 dpi (r = 0.701; p<0.0001) (Fig 4E). Malpighian tubule (r = -0.260; p = 0.917), fat body (r = 0.299; p = 0.188) and carcass (r = 0.127; p = 0.545) DENV loads were not predictive of DENV loads in wMel.F mosquito salivary gland (Fig 4B, Fig 4D and Fig 4F). In summary, these findings show that DENV load in upstream tissues may predict salivary gland loads in Wildtype but not wMel.F infected Ae. aegypti.
Fig 3. Correlation between DENV 3 load in salivary gland and that of midgut and head in Ae. aegypti.
(A) Correlation significant at 14 dpi only (R2 Linear = 0.28 at 14 dpi) for Wildtype midgut. (B) No correlation at 14 dpi for wMel.F midgut. (C) No correlation at either 8 or 14 dpi for Wildtype head.
Fig 4. Correlation between DENV load in salivary gland and Malpighian tubules, fat body and carcass and in Ae. aegypti.
(A) Correlation significant at both 8dpi (R2 Linear = 0.428) and 14dpi (R2 Linear = 0.582) for Wildtype Malpighian tubules. (B) No correlation at 14 dpi for wMel.F Malpighian tubules. (C) Significant correlation at both 8dpi (R2 Linear = 0.597) and 14dpi (R2 Linear = 0.399) for Wildtype fat body. (D) No correlation at 14 dpi for wMel.F fat body. (E) Significant correlation at14 dpi only (R2 Linear = 0.593) for Wildtype carcass. (F) No correlation at 14 dpi for wMel.F carcass.
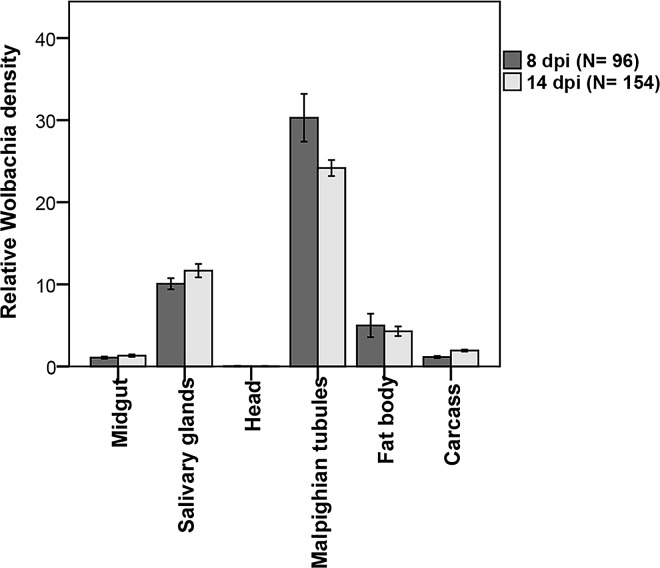
Wolbachia density in tissues over time
To determine if time and tissue type affect Wolbachia density in wMel.F mosquitoes, we compared Wolbachia density in the head, salivary glands, midgut, Malpighian tubules, fat body and carcass over two time points (8 and 14 dpi) (Fig 5). We observed that time had no effect (Wald = 0.18; df = 1; p = 0.671) on Wolbachia density in tissues. There was a significant tissue effect (Wald = 3423; df = 5; p<0.0001) demonstrating that Wolbachia density varied across tissue types. For instance, Wolbachia was most abundant in the Malpighian tubules with the head having the lowest bacterial density. There was an interaction between time and tissue type (WALD = 28; df = 5; p<0.0001). For example in the Malpighian tubules, Wolbachia density decreased from 8 dpi to 14 dpi while that of the carcass increased from 8 to 14 dpi (Fig 5). In summary, Wolbachia density varied across different tissue types with the Malpighian tubules and head harbouring the highest and the least number of Wolbachia respectively.
Fig 5. Wolbachia tissue densities in Ae aegypti.
Malpighian tubules had the highest density and head the lowest density.
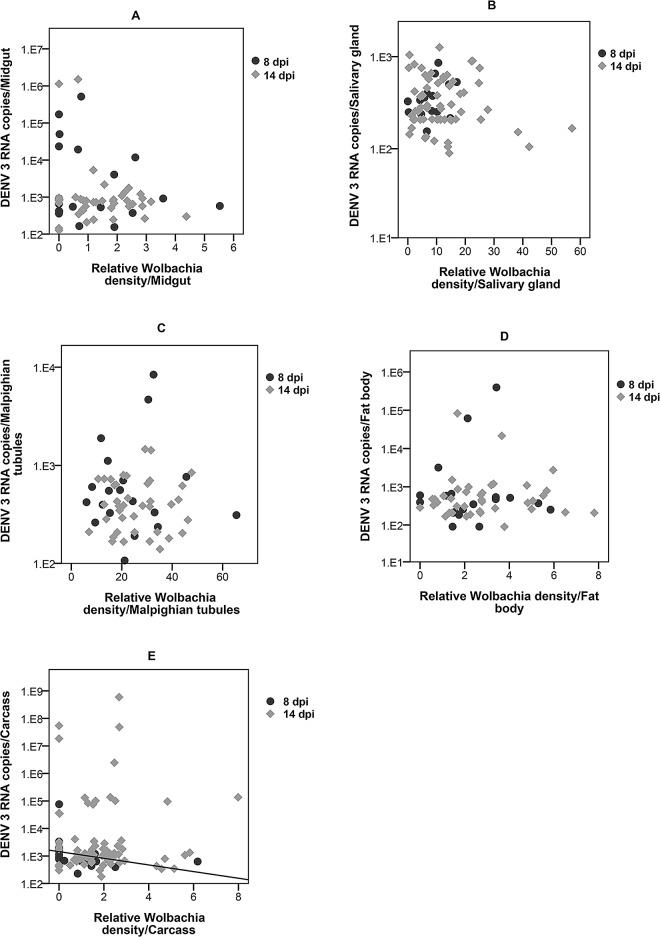
Correlation between Wolbachia density and DENV load in individual tissues
We first examined if the Wolbachia density in particular tissues was predictive of DENV load in that same tissue. In the carcass Wolbachia density was negatively correlated (r = -0.580; p = 0.005) with DENV load at 8 dpi, but this was not the case at 14 dpi (r = 0.160; p = 0.898) (Fig 6E). At 8 dpi Wolbachia density in the midgut (r = -0.125; p = 0.601), salivary glands (r = 0.453; p = 0.0680), Malpighian tubules (r = -0.095; p = 0.700) and fat body (r = 0.070; p = 0.765) were not correlated with DENV load (Fig 6A–6D). Neither was there a significant correlation between Wolbachia density in the midgut (r = -0.060; p = 0.702), salivary glands (r = -0.063; p = 0.626), Malpighian tubules (r = -0.026; p = 0.873) and fat body (r = 0.0044; p = 0.784) and DENV load at 14 dpi (Fig 6A–6D). Infection rates for both DENV and Wolbachia were too low to be statistically analysed in the head for both 8 and 14 dpi. These findings demonstrate that Wolbachia density is not predictive of DENV load within any of tissue types tested.
Fig 6. Correlation between Wolbachia density and DENV load in wMel.F tissues.
No correlation between Wolbachia density and DENV load in the midgut (A), salivary glands (B) Malpighian tubules (C), fat body (D) at 8 and 14 dpi. (E) DENV load decreased as Wolbachia density increased (Linear R2 = 0.125) in the carcass at 8 dpi but not at 14dpi.
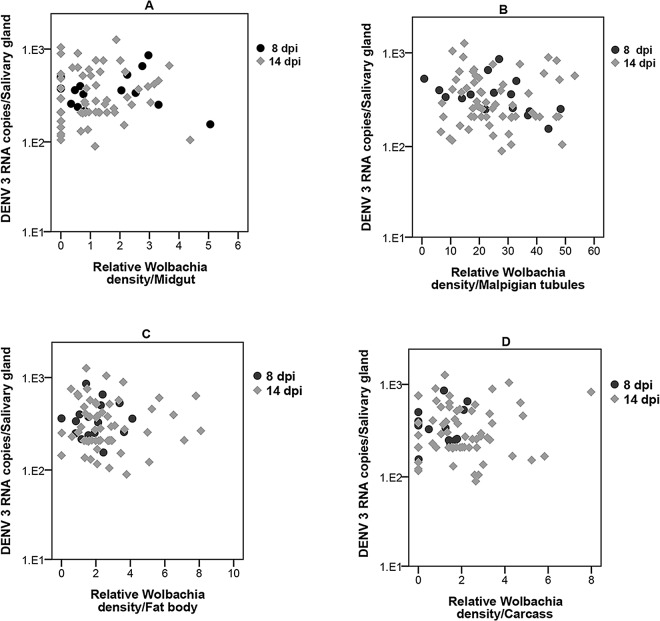
Correlation between Wolbachia density in intermediate tissues and DENV load in salivary glands
To determine if Wolbachia density in any particular tissue has an effect on DENV load in the salivary gland, we compared Wolbachia density in all the five dissected tissues to DENV load in the salivary glands. At 8 dpi there was no correlation between Wolbachia density in midgut (r = 0.21; p = 0.940), Malpighian tubules (r = 0.412; p = 0.101), fat body (r = 0.221; p = 0.395) and carcass (r = 0.051; p = 0.844) and DENV load in the salivary glands (Fig 7A–7D). Neither was there a significant correlation at 14 dp between the Wolbachia density in midgut (r = 0.155; p = 0.232), Malpighian tubules (r = 0.046; p = 0.732), fat body (r = 0.060; p = 0.642) and carcass (r = 0.32; p = 0.801) (Fig 7A–7D). Infection rates for both Wolbachia and DENV were too low to be statistically analysed in the head at both 8 and 14 dpi. These results show that Wolbachia density in intermediate tissues does not predict salivary gland DENV load.
Fig 7. Correlation between Wolbachia density in tissues and DENV loads in salivary glands.
No correlation between salivary glands DENV loads and Wolbachia density in (A) midgut, (B) Malpighian tubules, (C) fat body, and (D) carcass.
Discussion
This study investigated whether particular tissues, especially those with high Wolbachia densities or immune function, are important in Wolbachia-mediated DENV blocking. Specifically, we assessed whether DENV loads or Wolbachia densities encountered in intermediate tissues predict subsequent infection in the salivary glands. All tissues examined were susceptible to DENV 3 infection. As expected, the tissues of Wolbachia infected mosquitoes had considerably lower DENV infection rates compared to the Wildtype, due to pathogen inhibition [11,14,42]. Interestingly, we observed that the strength of blocking was strongest early in the infection process but then declined with time. This is consistent with observations in Wolbachia infected flies [29] where DCV numbers are initially low but progressively climb from 2 to 30 days post infection. Moreira et al., and Ye et al., [14,44] also observed slight increases in infection rates over time in wMelPop-CLA and wMel infected mosquitoes respectively, but only when DENV titres in the blood meal were high. The differences in blocking between early and late stages of infection raise the question whether the midgut may be playing a particular role in Wolbachia-DENV inhibition.
The midgut, not surprisingly had a high initial infection rate in Wildtype mosquitoes but as observed in other studies [41], the infection rate declined as the salivary glands became increasingly infected. This increasing infection suggests that salivary glands may be a site of DENV replication as well as accumulation. The very low infection rates in the head for both Wildtype and wMel.F mosquitoes were unexpected due to the fact that the head is generally used as a proxy for DENV dissemination. Our finding is contrary to previous studies that found higher head infection using immunofluorescence assays and RNA estimates of DENV 1, 2 and 3 [39,41,50]. However infection rates may be affected by the specific DENV and mosquito genotypes studied [51] or environmental effects that often vary across studies [52,53]. Lastly, head infection rates could also be inflated if head tissues become contaminated with the salivary gland during dissections.
While it is known that infection of the midgut is dependent on the amount of DENV the mosquito ingests [54], whether this affects virus dissemination from the midgut to other tissues including the salivary glands is not well understood. Salazar et al., [41] have reported that the trachea may facilitate DENV 2 dissemination from the midgut. However other studies suggest that virus disseminates to mosquito tissues through the hemolymph [55,56]. DENV loads in almost all hemocoel-associated tissues of Wildtype mosquitoes were similar to one another and predictive of salivary gland loads at 8 dpi. This pattern suggests that DENV infects these tissues in parallel and then seeps into the hemolymph and then makes its way to the salivary glands. Interestingly, midgut DENV loads in Wildtype mosquitoes were only predictive of salivary gland DENV loads late in the infection process. This is likely explained by incomplete dissemination of DENV out of the midgut in the early stages of infection.
Our findings demonstrate that the infection dynamics of the fat body is not different from other hemocoel-associated tissues in Wildtype mosquitoes. This finding does not support a special role of this immune active tissue [34,57] in DENV inhibition. Innate immune genes, particularly in the TOLL pathway, have been shown to decline in the fat body after 3 days when challenged with DENV [33]. Therefore the capacity for DENV to successfully infect this tissue may reflect declines in transcription of immunity genes over time. Another tissue that has been reported to be involved in insect immunity is the Malpighian tubules. In Drosophila Malpighian tubules exhibit basal expression of antimicrobial peptides (AMPs) that then increase in response to immune challenge [58]. Malpighian tubules have further been shown to fight infection independent of the fat body in Drosophila [59] and melanise larvae of the dog heartworm, Dirofilaria immitis in Ae.sollicitans [60]. Regardless, the Malpighian tubules had similar DENV loads to other tissues in Wildtype mosquitoes. This suggests that the Malpighian tubules are unlikely to be playing an immune role in modulating DENV replication and transmission in Ae. aegypti.
Unlike in Wildtype mosquitoes, DENV loads in the wMel.F mosquito midgut, Malpighian tubules, fat body and carcass did not significantly influence that of the salivary glands. There was no correlation between DENV load in the salivary glands and Wolbachia density in any of the tissues studied. In almost all cases, Wolbachia densities in particular tissues were also not predictive of DENV loads in those same tissues. This is true even for the Malpighian tubules that harbour extremely high densities of Wolbachia. This unique tropism may relate to access to nitrogen given Wolbachia’s reliance on host amino acids for nutrition [61]. Our findings are contrary to those for Drosophila [32] where Wolbachia density in the head, gut, and Malpighian tubules correlated with DCV inhibition in the whole fly. Drosophila, however, is naturally infected with Wolbachia unlike Ae. aegypti [11]. Native hosts for Wolbachia appear to have more restricted tissue distributions and reduced bacterial densities compared to novel hosts that may be the result of coadaptation [62]. Therefore the relationships between tissue densities and blocking in flies may not be the same as in novelly infected mosquitoes.
Across a range of studies Wolbachia density appears to correlate with the strength of DENV blocking/viral inhibition [11,26–29]. There are several possible models for this relationship. Firstly, in the simplest case there is a negative linear relationship between the two. In whole mosquitoes this hypothesis is supported based on a comparison of DENV blocking between the wMelPop strain, which grows to very high densities, and the wMel strain, which grows to moderate densities [11]. Secondly, blocking may become apparent only after particular thresholds of Wolbachia densities are reached. In an Ae. albopictus cell line, a minimum density of ~960 Wolbachia per host cell (wsp/actin) was required for complete blocking of DENV [28] with no obvious correlations at lower Wolbachia densities. We observed the highest density of ~30 Wolbachia per host cell (WD0513/RPS17) in the Malpighian tubules demonstrating that Wolbachia does not normally grow to such high densities in wMel.F Ae aegypti tissues. Our work therefore confirms the observations in cell lines [28] that at lower densities, there is no correlation between Wolbachia densities and blocking. Given different functional roles of particular tissues, especially with regards to immunity, we also hypothesized there may be tissue specific contributions to DENV blocking. Our work suggests, however, that none of the tissues we examined played a greater role in the expression of blocking. Instead, efficacy of blocking may be determined at the level of the individual cell. Our work does not rule out the involvement of immunity[19–21] or nutrient competition for key resources [22] in the mechanism of inhibition, but suggests Wolbachia must act through aspects of host cell biology that are either systemic or fundamental to diverse cell types.
Conclusion
Our findings in Wildtype mosquitoes demonstrate that DENV disseminates from the midgut and infects mosquito hemocoel-associated tissues equally through time. They also suggest that infection of the mosquito head is not an accurate proxy for the assessment of dissemination. In terms of Wolbachia-based blocking of DENV this study reports two main findings. Firstly, the Wolbachia tissue densities in the mosquito are not linear predictors of DENV load as has been reported in cell lines where densities are usually very high. This may be related to the much lower densities naturally present in insect tissues. Secondly, DENV inhibition is unlikely to be explained by tissue specific mechanisms. Future studies seeking to dissect the involvement of either immunity, resource competition or other unknown contributors to mechanism, should focus on aspects of host cell biology that are fundamental across tissues. Generalisations from cell line based-studies are likely to be more biologically meaningful when Wolbachia densities are lower and more reflective of those found in insect tissues.
Acknowledgments
We are grateful to Alison Carrasco, Cassandra Koh, James Griffiths and Henry Ye for technical support.
Data Availability
All raw data files are available from the Figshare database (DOI 10.4225/03/57BFAE2B85A57).
Funding Statement
This research was supported by the National Health and Medical Research Council of Australia through a project (APP1020607) and a program (1037003) grant to EAM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Gething WP, Brady JO, Messina PJ, Farlow WA, Moyes CL, et al. (2013) The global distribution and burden of dengue. Nature 496: 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. (2012) Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Neglect Trop Dis 6: e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes EC, Burch SS (2000) The causes and consequences of genetic variation in dengue virus. Trends Microbiol 8: 74–77. [DOI] [PubMed] [Google Scholar]
- 4.Holmes EC, Twiddy SS (2003) The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 3: 19–28. [DOI] [PubMed] [Google Scholar]
- 5.Black WCt, Bennett KE, Gorrochotegui-Escalante N, Barillas-Mury CV, Fernandez-Salas I, de Lourdes Muñoz M, et al. (2002) Flavivirus susceptibility in Aedes aegypti. Arch Med Res 33: 379–388. [DOI] [PubMed] [Google Scholar]
- 6.Thomas SJ, Endy TP (2011) Critical issues in dengue vaccine development. Curr Opin Infect Dis 24: 442–450. 10.1097/QCO.0b013e32834a1b0b [DOI] [PubMed] [Google Scholar]
- 7.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, et al. (2015) Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372: 113–123. 10.1056/NEJMoa1411037 [DOI] [PubMed] [Google Scholar]
- 8.Zug R, Hammerstein P (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7: e38544 10.1371/journal.pone.0038544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittayapong P, Baisley KJ, Baimai V, O'Neill SL (2000) Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J Med Entomol 37: 340–345. [DOI] [PubMed] [Google Scholar]
- 10.McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang Y, et al. (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323: 141–144. 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- 11.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476: 450–453. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 12.Joubert DA, Walker T, Carrington LB, De Bruyne JT, Kien DHT, Hoang NLT, et al. (2016) Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog 12: e1005434 10.1371/journal.ppat.1005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi Z, Khoo CC, Dobson SL (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310: 326–328. 10.1126/science.1117607 [DOI] [PubMed] [Google Scholar]
- 14.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 15.Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, et al. (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340: 748–751. 10.1126/science.1236192 [DOI] [PubMed] [Google Scholar]
- 16.Kambris Z, Cook PE, Phuc HK, Sinkins SP (2009) Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326: 134–136. 10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iturbe-Ormaetxe I, Walker T, SL ON (2011) Wolbachia and the biological control of mosquito-borne disease. EMBO Rep 12: 508–518. 10.1038/embor.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–457. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 19.Rances E, Ye YH, Woolfit M, McGraw EA, O'Neill SL (2012) The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog 8: e1002548 10.1371/journal.ppat.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, et al. (2012) Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 109: E23–31. 10.1073/pnas.1116932108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bian G, Xu Y, Lu P, Xie Y, Xi Z (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6: e1000833 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caragata EP, Rances E, Hedges LM, Gofton AW, Johnson KN, O'Neill SL, et al. (2013) Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9: e1003459 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, et al. (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2: e69 10.1371/journal.pbio.0020069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu YE, Cassese T, Kielian M (1999) The cholesterol requirement for Sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J Virol 73: 4272–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie JM, Khromykh AA, Parton RG (2007) Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe 2: 229–239. 10.1016/j.chom.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 26.Ye YH, Woolfit M, Rances E, O'Neill SL, McGraw EA (2013) Wolbachia-associated bacterial protection in the mosquito Aedes aegypti. PLoS Neglect Trop Dis 7: e2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL (2010) Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One 5: e13398 10.1371/journal.pone.0013398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu P, Bian G, Pan X, Xi Z (2012) Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Neglect Trop Dis 6: e1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne SE, San Leong Y, O'Neill SL, Johnson KN (2009) Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog 5: e1000656 10.1371/journal.ppat.1000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, Rousset F, et al. (1999) Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol 29: 153–160. [DOI] [PubMed] [Google Scholar]
- 31.Amuzu HE, Simmons CP, McGraw EA (2015) Effect of repeat human blood feeding on Wolbachia density and dengue virus infection in Aedes aegypti. Parasit Vectors 8: 246 10.1186/s13071-015-0853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborne SE, Iturbe-Ormaetxe I, Brownlie JC, O'Neill SL, Johnson KN (2012) Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl Environ Microbiol 78: 6922–6929. 10.1128/AEM.01727-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez JL, Dimopoulos G (2010) The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Devel Comp Immunol 34: 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann JA (1995) Innate immunity of insects. Curr Opin Immunol 7: 4–10. [DOI] [PubMed] [Google Scholar]
- 35.Dow JA (2009) Insights into the Malpighian tubule from functional genomics. J Exp Biol 212: 435–445. 10.1242/jeb.024224 [DOI] [PubMed] [Google Scholar]
- 36.Gubler DJ, Rosen L (1976) A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am J Trop Med Hyg 25: 146–150. [DOI] [PubMed] [Google Scholar]
- 37.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A (1986) Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. DTIC Document. [DOI] [PubMed]
- 38.Kuberski T (1979) Fluorescent antibody studies on the development of dengue-2 virus in Aedes albopictus (Diptera: Culicidae). J Med Entomol 16: 343–349. [DOI] [PubMed] [Google Scholar]
- 39.Linthicum K, Platt K, Myint K, Lerdthusnee K, INNI BL, GHN DW (1996) Dengue 3 virus distribution in the mosquito Aedes aegypti: an immunocytochemical study. Med Vet Entomol 10: 87–92. [DOI] [PubMed] [Google Scholar]
- 40.Chen W-J, Wei H-L, Hsu E-L, Chen E-R (1993) Vector competence of Aedes albopictus and Ae. aegypti (Diptera: Culicidae) to dengue 1 virus on Taiwan: development of the virus in orally and parenterally infected mosquitoes. J Med Entomol 30: 524–530. [DOI] [PubMed] [Google Scholar]
- 41.Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ (2007) Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol 7: 9 10.1186/1471-2180-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A et al. (2014) Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Neglect Trop Dis 8: e2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, Christofferson RC, et al. (2013) An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One 8: e68137 10.1371/journal.pone.0068137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yixin HY, Carrasco AM, Frentiu FD, Chenoweth SF, Beebe NW, van den Hurk AF, et al. (2015) Wolbachia Reduces the Transmission Potential of Dengue-Infected Aedes aegypti. PLoS Neglect Trop Dis 9: e0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yixin HY, Ng TS, Frentiu FD, Walker T, van den Hurk AF, O'Neill SL, et al. (2014) Comparative susceptibility of mosquito populations in North Queensland, Australia to oral infection with dengue virus. Am J Trop Med Hyg 90: 422–430. 10.4269/ajtmh.13-0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warrilow D, Northill JA, Pyke A, Smith GA (2002) Single rapid TaqMan fluorogenic probe based PCR assay that detects all four dengue serotypes. J Med Virol 66: 524–528. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson NM, Kien DTH, Clapham H, Aguas R, Trung VT, Chau TN, et al. (2015) Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 7: 279ra237–279ra237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook PE, Hugo LE, Iturbe-Ormaetxe I, Williams CR, Chenoweth SF, Ritchie SA, et al. (2006) The use of transcriptional profiles to predict adult mosquito age under field conditions. Proc Natl Acad Sci U S A 103: 18060–18065. 10.1073/pnas.0604875103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, et al. (1999) Housekeeping genes as internal standards: use and limits. J Biotechnol 75: 291–295. [DOI] [PubMed] [Google Scholar]
- 50.Fontaine A, Jiolle D, Moltini-Conclois I, Lequime S, Lambrechts L (2016) Excretion of dengue virus RNA by Aedes aegypti allows non-destructive monitoring of viral dissemination in individual mosquitoes. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambrechts L, Chevillon C, Albright RG, Thaisomboonsuk B, Richardson JH, Jarman RG, et al. (2009) Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol Biol 9: 1 10.1186/1471-2148-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yixin HY, Carrasco AM, Dong Y, Sgrò CM, McGraw EA (2016) The Effect of Temperature on Wolbachia-Mediated Dengue Virus Blocking in Aedes aegypti. Am J Trop Med Hyg: 15–0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alto BW, Lounibos LP, Mores CN, Reiskind MH (2008) Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc R Soc Lond B 275: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett KE, Olson KE, de Lourdes Muñoz M, Fernandez-Salas I, Farfan-Ale JA, Higgs S, et al. (2002) Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg 67: 85–92. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto N, Kimura T, Ohyama A (1987) Multiplication and distribution of type 2 dengue and Japanese encephalitis viruses in Toxorhynchites splendens after intrathoracic inoculation. Arch Virol 97: 37–47. [DOI] [PubMed] [Google Scholar]
- 56.LaMotte LC Jr (1960) Japanese B encephalitis virus in the organs of infected mosquitoes. Am J Hyg 72: 73–87. [DOI] [PubMed] [Google Scholar]
- 57.Hultmark D (1993) Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet 9: 178–183. [DOI] [PubMed] [Google Scholar]
- 58.Tapadia MG, Verma P (2012) Immune response and anti-microbial peptides expression in Malpighian tubules of Drosophila melanogaster is under developmental regulation. PLoS One 7: e40714 10.1371/journal.pone.0040714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGettigan J, McLennan R, Broderick K, Kean L, Allan AK, Cabrero P, et al. (2005) Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem Mol Biol 35: 741–754. 10.1016/j.ibmb.2005.02.017 [DOI] [PubMed] [Google Scholar]
- 60.Bradley TJ, Nayar JK (1985) Intracellular melanization of the larvae of Dirofilaria immitis in the Malpighian tubules of the mosquito, Aedes sollicitans. J Invertebr Pathol 45: 339–345. [DOI] [PubMed] [Google Scholar]
- 61.Caragata EP, Rances E, O'Neill SL, McGraw EA (2014) Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb Ecol 67: 205–218. 10.1007/s00248-013-0339-4 [DOI] [PubMed] [Google Scholar]
- 62.McGraw EA, O’Neill SL (2004) Wolbachia pipientis: intracellular infection and pathogenesis in Drosophila. Curr Opin Microbiol 7: 67–70. 10.1016/j.mib.2003.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data files are available from the Figshare database (DOI 10.4225/03/57BFAE2B85A57).