Abstract
In many species, chronic stress due to overcrowding during the juvenile period triggers several metabolic and behavioral pathologies in adulthood. The aim of this study was to determine whether a chronic stress condition (overcrowding) induces changes in plasma and hair corticosterone concentrations, overall growth, and organ weights in young Wistar rats. The experimental subjects were divided into 2 groups (control and overcrowded); the overcrowded subjects were exposed to overcrowding during days 38 through 65 after birth. Plasma and hair corticosterone concentrations were higher in overcrowded rats compared with control subjects. In addition, overcrowding reduced body and organ weight gains. These results demonstrate that measuring the concentration of corticosterone in hair samples is an effective, noninvasive method for monitoring chronic stress in rats.
Overcrowding is defined as the coexistence of individuals in an overly confined space. In rats, overcrowded living conditions leads to changes in physical and psychologic health,55 increased HPA activity,26 and variations in the body and organ weights of juvenile58 and adult rats.20,37 Some studies have reported that overcrowding-related increases in blood corticosterone levels affect body weight and the weights of immunologic but not visceral organs;7,54 these changes can be related to somatic responses to the imposed stressor.8,26,58
The juvenile period is important as a transitional time from childhood to adulthood12 because this period involves physiologic maturation.45 Despite difficulty in defining the juvenile stage for male rats,29,52 most researchers agree that adult stage begins on the 60th day after birth, when animals reach physical maturity, implying increased gonadotropin releasing hormone and activation of the HPA.29
Researchers quantify glucocorticoids in various types of samples, including plasma, saliva, feces, urine and hair.1,21 Glucocorticoid deposition in hair has been proposed as an accurate indicator of chronic stress in several mammalian species.31 Quantifying glucocorticoid levels in hair is a noninvasive sampling method, and samples can be stored at room temperature, but the most relevant aspect is that hair samples indicate chronic (rather than acute) production of glucocorticoids.39
Hair follicles contain glucocorticoids, a peripheral component of the HPA.4,43 Corticosteroids potentially can be deposited in the hair shaft in 2 ways. One hypothesis is that steroids found in hair are dependent on bloodstream corticoid levels; another hypothesis suggests that steroid levels are directly related to the local production of glucocorticoids in follicles.42 However, the contribution of local glucocorticoid synthesis to the amount of hormone measured in hair is not yet clear.
A recent study41 showed that the exposure of adult male rats to chronic immobilization stress or chronic unpredictable stress resulted in increased corticosterone levels in the animals’ hair. Although that study was important for validating the use of hair to assess long-term HPA activity in rodents, further research is needed to confirm and extend those findings to other stressors and age groups. Therefore, the purposes of the present study were to determine corticosterone levels in the plasma and hair of juvenile male Wistar rats under overcrowded conditions and to determine how the stress of overcrowding influenced the body and organ weights of the animals.
Materials and Methods
The present research was conducted in the Neuroscience and Behavior Laboratory at the University of Los Andes (Bogotá, Colombia). The protocol was approved by the bioethics committee of the National University of Colombia and the IACUC of the Universidad de Los Andes.
Animals and housing conditions.
Male Wistar rats (age, 38 d; n = 18) bred inhouse at the university's laboratory animal facility were used in the study. Experimental subjects were maintained under a 12:12-h light:dark cycle (lights on, 0700), 21 ± 2 °C room temperature, and humidity control (40-60 %). Food (Laboratory Autoclavable Rodent Diet 5010, LabDiet, St Louis, MO) and water were freely available. Rats were allocated randomly into 2 groups (n = 9 each). The control group was housed in acrylic boxes (15 cm × 31cm × 18 cm; 3 rats per box; 155 cm2 per rat), whereas overcrowded rats were housed in similar but smaller boxes (12 cm × 12 cm × 18 cm; 3 rats per box; 48 cm2 per rat). Boxes contained sterilized poplar shavings (Anestcol, Bogotá, Colombia), which were replaced daily. All animals were handled briefly during each of the 3 days prior to blood and hair sampling to reduce the stress on the collection day. The experiment ended on postnatal day (PND) 65.
Blood samples and ELISA.
On PND 38 and 65, the rats were removed individually from their cages for approximately 5 min and transported to the next room to obtain blood samples. The rats were gently restrained while the veterinarian used ice-cold heparinized capillary tubes (Brand, Wertheim, Germany) and needles (21-gauge, 32 mm) to collect blood from the right saphenous vein. The samples were maintained at 0 °C, centrifuged for 10 min at 600 × g (MicroCL 17 Microcentrifuge, Thermo Scientific, Waltham, MA), and plasma extracted and stored at –20 °C. The plasma was evaluated by ELISA (catalog no. ADI-901-097, Enzo Life Sciences, Exeter, United Kingdom), plates were read at 490 nm, and corticosterone concentrations were determined by using the kit standards and Gen5 software (BioTek, Winooski, VT).
Hair samples and hormone analysis.
To obtain hair samples without hair follicles, the right flank area of each animal was shaved on the first and last days of the experiment (PND 38 and 65, respectively). Hair samples were processed as previously described.16 Briefly, the samples were rinsed 3 times (5 min each) in isopropanol (5 mL) at room temperature and dried for 4 d; 150- to 200-mg samples then were pulverized in a grinding ball mill (Retsch model MM200, Verder Scientific, Newtown, PA). Samples of powdered hair (49.5 to 50.5 mg each) were incubated overnight in methanol (1 mL) to extract corticosterone. Methanol was evaporated (SpeedVac, Savant, Thermo Fisher); samples were reconstituted in 5% methanol in deionized water (1 mL) and purified over C18 columns (Supelco Supel Select 30 mg × 1 mL, Sigma–Aldrich, St Louis, MO). The columns were activated by using methanol (1 mL) and deionized water (1 mL), samples were added to the columns, and columns were rinsed with 5% methanol (1 mL). Finally, samples were eluted into methanol (1 mL) and dried under vacuum for 3 h. This protocol was validated by demonstrating parallelism of authentic corticosterone standards with serially diluted purified rat hair extracts (data not shown). Corticosterone in the purified hair samples were quantified by using enzyme immunoassay kits (catalog no. K014-H1, DetectX, Arbor Assays, Ann Arbor, MI). Absorbancies were read at a wavelength of 450 nm, and corticosterone concentrations were determined by using the standard curve and Gen5 software (BioTek).
Euthanasia and organ collection.
To assess the effects of overcrowding stress on organ weight and to determine the relationship between organ weight changes and plasma and hair corticosterone levels, rats were anesthetized with ketamine (90 mg/kg IP) and xylazine (10 mg/kg IP) at the end of the study and then euthanized by means of cardiac perfusion (200 mL saline solution 0.9% followed by 200 mL 4% paraformaldehyde). Heart, lungs, kidneys, and adrenal glands were dissected and carefully weighed.
Statistical analysis.
For statistical analysis, corticosterone concentrations in hair (pg/mg) and plasma (ng/mL) were log-transformed to normalize the data. For hair corticosterone, plasma corticosterone, and body weight data, 2-way nested mixed-design ANOVA was used, with treatment as a between-subjects variable, day as a within-subjects variable, and cage as a nested variable within treatment group. Significant effects in the mixed ANOVA were followed by posthoc univariate ANOVA to determine specific differences related to treatment and day. Univariate ANOVA with treatment as a between-subjects variable and cage as a nested variable within treatment group were performed on the organ weight values expressed either as absolute weights or relative weights (organ weight divided by overall body weight). Right and left adrenal gland weights were combined to yield a single value for each animal for the purpose of statistical analysis. Due to technical problems, no organ weight data were available from one of the control rats, which can be seen in the adjusted error df values for the organ weight ANOVA. Hair or plasma corticosterone concentrations as predictors of organ weights were assessed by using a mixed linear-regression model with organ weight, corticosterone concentration (hair or plasma), and treatment as fixed effects variables and cage as a random effects variable. A similar analysis was performed to assess plasma corticosterone as a predictor of hair corticosterone. All statistical analyses were performed by using SPSS (IBM, Armonk, NY), and a P value less than 0.05 was considered significant. In the present experiment, all error bars indicate 1 SD.
Results
Corticosterone deposition in hair.
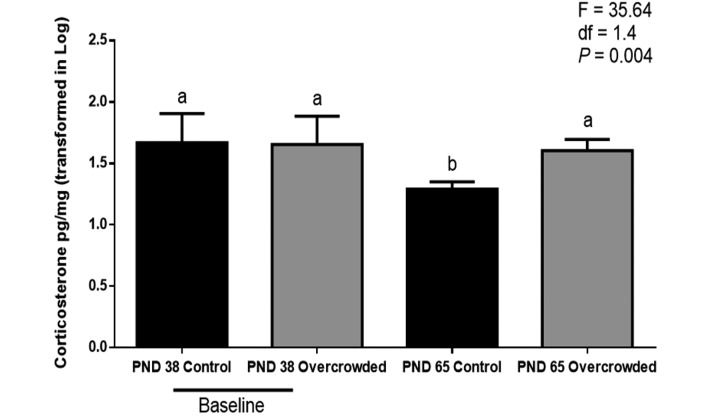
The assay results confirmed that the hair of male Wistar rats contains corticosterone. ANOVA revealed significant main effects of treatment (F = 10.69; df = 1.4; P = 0.031) and day (F = 13.56; df = 1.16; P = 0.002) and a significant treatment×day interaction (F = 7.94; df = 1.16; P = 0.012). Posthoc testing further revealed no significant difference between the control and overcrowded groups on PND 38 but significantly higher hair corticosterone concentrations in the overcrowded group (40.5 ± 9.8 pg/mg) compared with the control group (19.7 ± 9.8 pg/mg) on PND 65 (F = 35.64; df = 1.4; P = 0.004; Figure 1). The nested factor of cage within group had no significant effect on this or any other outcome measure.
Figure 1.
Hair corticosterone levels of control rats (n = 9) and overcrowded rats (n = 9). Baseline values are those before overcrowding (postnatal day [PND] 38); those for PND 65 represent 27 d of overcrowding. Concentration data were log-transformed. For hair corticosterone analyses, 2-way nested mixed design ANOVA was used; different letters indicate that the values differ significantly (P < 0.01). Error bars indicate 1 SD. Posthoc univariate ANOVA were used to determinate specific group differences after overall ANOVA.
Effect of overcrowding during the juvenile period on plasma corticosterone.
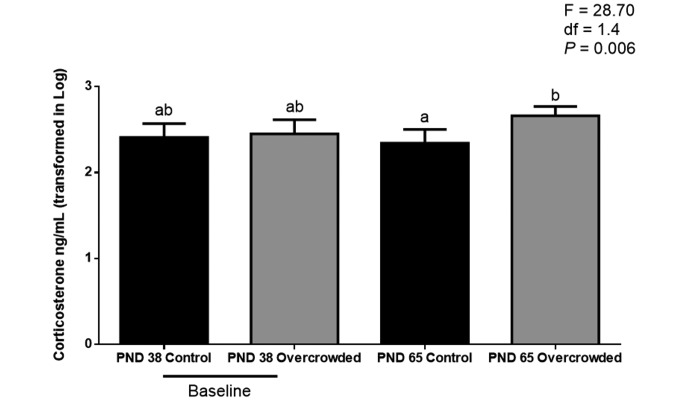
Plasma corticosterone showed a significant main effect of treatment (F = 9.80; df = 1.4; P = 0.035) and a significant treatment×day interaction (F = 9.26; df = 1.16; P = 0.008). Posthoc testing showed that the groups did not differ on PND 38, whereas the overcrowded group had significantly higher plasma corticosterone concentrations (470.2 ± 113.2 ng/mL) than did the control group (232.6 ± 78.7 ng/mL) on PND 65 (F = 28.70; df = 1.4; P = 0.006 (Figure 2).
Figure 2.
Plasma corticosterone levels of control rats (n = 9) and overcrowded rats (n = 9). Baseline values are those before overcrowding (postnatal day [PND] 38); those for PND 65 represent 27 d of overcrowding. Concentration data were log-transformed. For plasma corticosterone analyses, 2-way nested mixed design ANOVA was used; different letters indicate that the values differ significantly (P < 0.01). Error bars indicate 1 SD. Posthoc univariate ANOVA were used to determinate specific group differences after overall ANOVA.
Relationships between plasma and hair corticosterone concentrations.
Multiple linear regression analyses of data from control and overcrowded rats showed that plasma corticosterone concentrations did not significantly predict hair corticosterone at either PND 38 or PND 65.
Effect of overcrowded group on body weight.
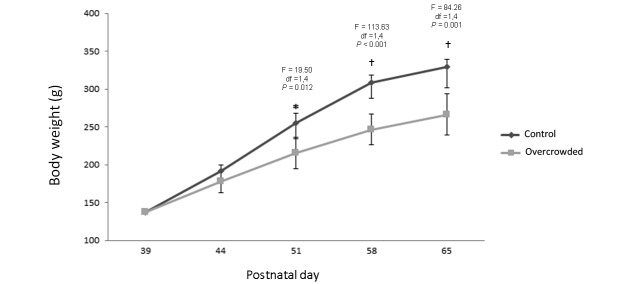
Analysis of body weight data showed significant main effects of treatment (F = 42.48; df = 1.4; P = 0.003) and day (F = 955.29; df = 4, 64; P < 0.001) and a significant treatment×day interaction (F = 46.52; df = 4, 64; P < 0.001). Posthoc analyses further revealed no statistically significant group differences in weight on PND 39 or PND 44, but weight in the overcrowded group was significantly lower than that of the control group on PND 52 (F = 19.50; df = 1, 4; P = 0.012), PND 58 (F = 113.63; df = 1.4; P < 0.001), and PND 65 (F = 84.26; df = 1.4; P = 0.001; Figure 3). Mean body weight at PND 65 (the end of the study) was 347.5 ± 26.371 g for the control group compared with 266.5 ± 9.634 g for the overcrowded group.
Figure 3.
Body weight of male juvenile Wistar rats on postnatal day (PND) 38 (before overcrowding); PND 44, 51, and 58 (during overcrowding); and PND 65 (after overcrowding). Gray line corresponds to the control group (n = 9); the green line corresponds to the overcrowded group (n = 9). For analysis of body weight, 2-way nested mixed-design ANOVA was used; *, P < 0.05; †, P < 0.01. Error bars indicate 1 SD. Posthoc univariate ANOVA were used to determinate specific group differences at each time point.
Effects of overcrowding on absolute and relative organ weights.
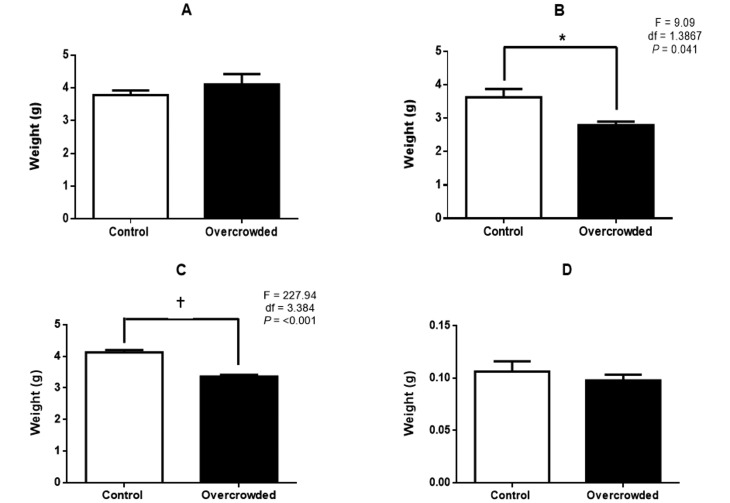
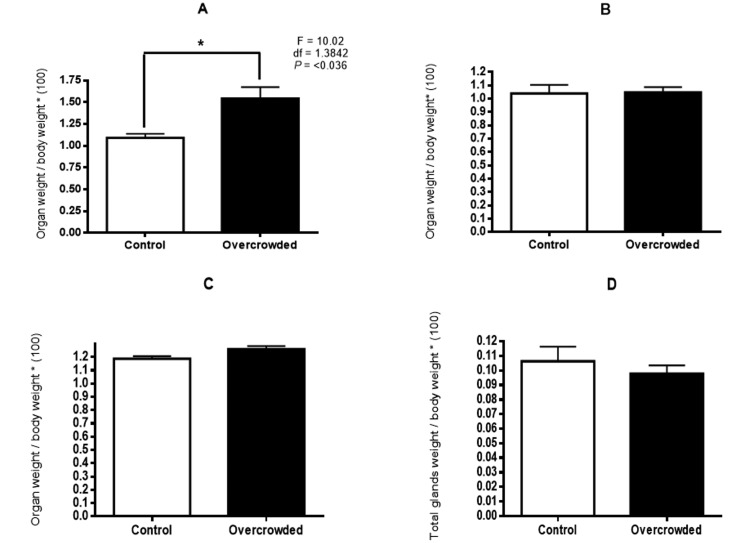
Treatment effects on organ weights varied by organ and depended on whether absolute or relative (that is, organ weight divided by body weight) weight was analyzed (Figures 4 and 5). For heart and kidney, organ weight was significantly lower in the overcrowded compared with the control group when expressed as absolute values (heart: F = 9.09; df = 1.3867; P = 0.041; kidney: F = 227.94; df = 1.3384; P < 0.001) but not when expressed as relative weights. A different pattern occurred with the lungs, where absolute weight did not differ between groups but was significantly higher in the overcrowded group than the control group when relative values were analyzed (F = 10.02; df = 1.3842; P = 0.036). Finally, neither absolute nor relative adrenal gland weight differed between groups.
Figure 4.
Absolute weight of (A) lung, (B) heart, (C) kidneys, and (D) adrenal glands of control rats (n = 8) and overcrowded rats (n = 9) on postnatal day 65 (after overcrowding). *, P < 0.05; †, P < 0.01; error bars, 1 SD.
Figure 5.
Relative weight of (A) lung, (B) heart, (C) kidneys, and (D) adrenal glands of control rats (n = 8) and overcrowded rats (n = 9) on postnatal day 65 (after overcrowding). *, P < 0.05; †, P < 0.01; error bars, 1 SD.
Relationships between organ weights and plasma and hair corticosterone concentrations.
Multiple linear-regression analyses revealed that neither plasma nor hair corticosterone concentration predicted organ weight (absolute or relative) at PND 38 or 65.
Discussion
Social hierarchy as well as territory defense determines individual vulnerability when an animal is exposed to stressful conditions.5 Accordingly, we used overcrowded housing as a stress model in young rats. A previous study has demonstrated the utility of quantifying plasma glucocorticoids for assessing stress over short periods of time.47 In addition, the glucocorticoid concentration in plasma displays a circadian rhythm and is susceptible to environmental perturbations.31 Measuring cortisol or corticosterone levels in plasma, saliva, urine, or feces reflects glucocorticoid concentrations over short time frames (minutes, hours, or days) after exposure to a stressor event. To assess the long-term effects of events, new tools that have been validated for monitoring include glucocorticoid levels in hair, which provide a noninvasive and effective method for diagnosing chronic stress.16,39, 41,44,59 Our current results clearly show that overcrowded housing from PND 38 to 65 significantly increased the hair corticosterone concentration of juvenile male Wistar rats.
Our data indicate that hair corticosterone concentrations in rats follow a similar dynamic as cortisol in hair from other mammals.13,15,17, 28,31,33,44,48,49,60 A recent study confirmed the presence of corticosterone in the hair of adult Sprague–Dawley rats.41,59 Hair corticosterone levels were higher in rats exposed to restraint, chronic unpredictable stress, and chronic immobilization than in control animals 59. In addition, hair corticosterone concentrations were elevated in rats that underwent electrode implantation, and variations in serum and hair corticosterone levels occurred in mice exposed for 30 d to repeated social defeat.59 To our knowledge, our current results are the first evidence of corticosterone deposition in the hair of juvenile male Wistar rats, and are important for extending previous findings to a different type of social stressor (that is, overcrowding) in a rodent species.
We found no relationship between plasma and hair corticosterone levels at either of 2 time points (PND 38 and 65). Recent experiments in rats and mice reported correlations between blood and hair corticosterone levels,59 but studies in other species found no correlation between blood and hair or feather corticosteroid concentrations in humans,40 dogs with chronic exposure to glucocorticoids,36 and birds.19 Perhaps this lack of correlation is simply because plasma levels represent a ‘snapshot’ of acute HPA activity, whereas hair and feathers provide measures of chronic activity. Hair glucocorticoid concentrations vary more gradually than does the plasma concentration and therefore might represent a better method of monitoring glucocorticoid concentrations over prolonged time periods.36
Corticosterone measured in growing hair after it has been shaved might come from the surrounding plasma or might be synthesized in response to a local-type HPA axis.24,42 Although adrenalectomy of Sprague–Dawley rats significantly reduced hair corticosterone levels, the hormone was still detected41. This finding first prompts consideration of the length of time necessary for complete clearance of all corticosterone from the skin and hair follicles. Second, low levels of corticosterone in the plasma might come from organs other than adrenal glands51 to potentially accumulate in hair. Third, the residual hair corticosterone in the adrenalectomized rats might reflect local synthesis, as has been suggested regarding cortisol levels in human hair.24 Additional research is needed to test these hypotheses, and the mechanism underlying corticosterone synthesis in the rat hair shaft is unknown currently.
In primates, hair cortisol levels are higher during infancy and decrease over time until adulthood.18 These findings support our results showing higher corticosterone levels on PND 38 than PND 65 in control rats. However hair corticosterone levels should be monitored throughout life to confirm a similar pattern in rats as in primates.
The high variability in hair corticosterone concentrations on PND 38 might reflect interindividual variation in the expression of the glucocorticoid-metabolizing enzyme 11β-hydroxysteroid dehydrogenase 2 within the skin.14 Another potential source of variability is prenatal stress,3,32 which might enhance fetal exposure to corticosterone through increased concentrations in amniotic fluid50 and placental transport.57 Social roles and group restructuring are important factors that influence variability in plasma corticosterone levels.11,13 The plasma level variability on PND 65 corroborates that physiologic dynamics are constantly altered by physical and social factors,5 such as the overcrowded conditions in our study.
The scientific literature contains numerous examples of significant changes in the body weights of subjects that underwent chronic social stress2,5,30 and of overcrowding stress in Wistar rats.9,27,34 The present study found differences in body weight between the control and overcrowded groups that might reflect social (social defeat, overcrowding), physical (minimum living space, handling), or environmental (temperature, humidity, ammonia) factors involved in overcrowded housing. In addition, we found differences in the weights of several organs, although the nature of the difference depended on whether absolute or relative weight was compared. The appropriateness of analyzing organ weight results as absolute data or indexed by body weight has been an issue in several studies,,35,56and different approaches may be appropriate for different organs.6 Here we observed that overcrowding caused significant decreases in absolute (but not relative) heart and kidney weights, no change in absolute weight but a significant decrease in the relative weight of lung tissue, and no change in either absolute or relative adrenal weights. Therefore, the growth of the heart and kidneys was affected to the same degree as was overall body growth, whereas the lungs seem to have been somewhat protected from the effects of overcrowding because they had a different pattern of weight reduction from heart and kidneys. A previous study similarly reported a decrease in kidney weight that closely paralleled the reduction in body weight in young chronically stressed Wistar rats.8 Those authors further associated the weight decrease with less glomerular volume and fewer nephrons per kidney;8 however, the responses of kidneys exposed to overcrowding have not been studied extensively. Differences in heart weight between the overcrowded and control groups might reflect differences in physical activity, housing area, and opportunity to express natural behavior.46 The failure of lung weight to respond to overcrowding was surprising, especially considering that a previous study found reduced lung weight in rats exposed to high-density caging.58 One possible reason for this difference is that the group size was much greater in the previous study (18 subjects per cage) than in the current investigation (3 rats per cage). Although we did not monitor ammonia concentrations in air, they should be considered given that previous studies have documented deleterious effects of ammonia on health in rats.10,23 The effect of overcrowding on adrenal gland weight likely depends on the particular experimental conditions. Whereas studies of chronic stress have often documented increases in adrenal weight,38,53 other authors found no such increase due to proportional changes (increase compared with decrease) in different zones of the adrenal cortex.26 The same phenomenon may have occurred in the present study.
An important point is that measurement of glucocorticoid concentrations in hair cannot detect acute stress-associated effects;22 therefore concentrations of glucocorticoids in hair should be measured as a complementary method to salivary or plasma quantification.25 Nevertheless, noninvasive methods to study chronic stress are important tools for monitoring physiologic conditions that might influence health and wellbeing.39 Further studies should address the effects of larger cages and the relation of overcrowding on the hair corticosterone concentrations, body weight, and organ weights of both juvenile and adult animals.
In conclusion, corticosterone is present in the hair of Wistar rats during the juvenile period. The current data indicate that our technique for quantifying the levels of corticosterone in hair can be used to monitor corticosterone in Wistar rats over long periods of time. Overcrowding induced significant differences in the hair and plasma concentrations of corticosterone and lung, kidney, and heart weights of male juvenile Wistar rats. Future investigations are necessary to understand the relations between cage size, housing conditions, and hair glucocorticoids in other laboratory species. Nevertheless, measurement of hair corticosterone can be a useful approach for researchers, veterinarians, and animal colony managers to assess chronic stress in laboratory rodents.
Acknowledgments
This research was funded by the graduate student program and the Direction of Research Office–Bogotá (DIB) of the Universidad Nacional de Colombia. We thank Christina Gagliardi (University of Massachusetts Amherst) for her assistance in developing the protocol for the rat hair corticosterone assay and Lisa Fiorenzo (University of Massachusetts Amherst) for expert statistical assistance.
References
- 1.Accorsi PA, Carloni E, Valsecchi P, Viggiani R, Gamberoni M, Tamanini C, Seren E. 2008. Cortisol determination in hair and faeces from domestic cats and dogs. Gen Comp Endocrinol 155:398–402. [DOI] [PubMed] [Google Scholar]
- 2.Aioi A, Okuda M, Matsui M, Tonogaito H, Hamada K. 2001. Effect of high population density environment on skin barrier function in mice. J Dermatol Sci 25:189–197. [DOI] [PubMed] [Google Scholar]
- 3.Amugongo SK, Hlusko LJ. 2013. Impact of maternal prenatal stress on growth of the offspring. Aging Dis 5:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. 2006. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol 126:1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. 2004. Behavioral and physiologic characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology 29:899–910. [DOI] [PubMed] [Google Scholar]
- 6.Bailey SA, Zidell RH, Perry RW. 2004. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol 32:448–466. [DOI] [PubMed] [Google Scholar]
- 7.Beckett PR, Fiorotto ML, Davis TA, Reeds PJ. 1996. Corticosterone has independent effects on tissue maturation and growth in the suckling rat. Pediatr Res 39:395–400. [DOI] [PubMed] [Google Scholar]
- 8.Benchimol de Souza D, Silva D, Marinho Costa Silva C, Barcellos Sampaio FJ, Silva Costa W, Martins Cortez C. 2011. Effects of immobilization stress on kidneys of Wistar male rats: a morphometrical and stereological analysis. Kidney Blood Press Res 34:424–429. [DOI] [PubMed] [Google Scholar]
- 9.Bernátová I, Puzserova A, Navarova J, Csizmadiova Z, Zeman M. 2007. Crowding-induced alterations in vascular system of Wistar–Kyoto rats: role of nitric oxide. Physiol Res 56:667–669. [DOI] [PubMed] [Google Scholar]
- 10.Burn CC, Peters A, Day MJ, Mason GJ. 2006. Long-term effects of cage-cleaning frequency and bedding type on laboratory rat health, welfare, and handleability: a cross-laboratory study. Lab Anim 40:353–370. [DOI] [PubMed] [Google Scholar]
- 11.Buwalda B, Geerdink M, Vidal J, Koolhaas JM. 2011. Social behavior and social stress in adolescence: a focus on animal models. Neurosci Biobehav Rev 35:1713–1721. [DOI] [PubMed] [Google Scholar]
- 12.Cavigelli SA, Yee JR, McClintock MK. 2006. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav 50:454–462. [DOI] [PubMed] [Google Scholar]
- 13.Cavigelli SA, Chaudhry HS. 2012. Social status, glucocorticoids, immune function, and health: can animal studies help us understand human socioeconomic-status–related health disparities? Horm Behav 62:295–313. [DOI] [PubMed] [Google Scholar]
- 14.Chapman K, Holmes M, Seckl J. 2013. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93:1139–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comin A, Veronesi MC, Montillo M, Faustini M, Valentini S, Cairoli F, Prandi A. 2012. Hair cortisol level as a retrospective marker of hypothalamic–pituitary–adrenal axis activity in horse foals. Vet J 194:131–132. [DOI] [PubMed] [Google Scholar]
- 16.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147:255–261. [DOI] [PubMed] [Google Scholar]
- 17.Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. 2008. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biol Psychiatry 63:990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dettmer AM, Novak MA, Meyer JS, Suomi SJ. 2014. Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology 42:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairhurst GD, Marchant TA, Soos C, Machin KL, Clark RG. 2013. Experimental relationships between levels of corticosterone in plasma and feathers in a free-living bird. J Exp Biol 216:4071–4081. [DOI] [PubMed] [Google Scholar]
- 20.Fournier S, Joseph V, Kinkead R. 2011. Influence of juvenile housing conditions on the ventilatory, thermoregulatory, and endocrine responses to hypoxia of adult male rats. J Appl Physiol (1985) 111:516–523. [DOI] [PubMed] [Google Scholar]
- 21.Gow R, Thomson S, Rieder M, Van Uum S, Koren G. 2010. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int 196:32–37. [DOI] [PubMed] [Google Scholar]
- 22.Haverbeke A, Diederich C, Depiereux E, Giffroy JM. 2008. Cortisol and behavioral responses of working dogs to environmental challenges. Physiol Behav 93:59–67. [DOI] [PubMed] [Google Scholar]
- 23.Horn MJ, Hudson SV, Bostrom LA, Cooper DM. 2012. Effects of cage density, sanitation frequency, and bedding type on animal wellbeing and health and cage environment in mice and rats. J Am Assoc Lab Anim Sci 51:781–788. [PMC free article] [PubMed] [Google Scholar]
- 24.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. 2005. Human hair follicles display a functional equivalent of the hypothalamic–pituitary–adrenal (HPA) axis and synthesize cortisol. FASEB J 19:1332–1334. [DOI] [PubMed] [Google Scholar]
- 25.Jensen KH, Pedersen LJ, Nielsen EK, Heller KE, Ladewig J, Jorgensen E. 1996. Intermittent stress in pigs: effects on behavior, pituitary–adrenocortical axis, growth, and gastric ulceration. Physiol Behav 59:741–748. [DOI] [PubMed] [Google Scholar]
- 26.Kirillov OI, Khasina EI, Durkina VB. 2003. [Effect of stress on postnatal growth in weight of rat body and adrenal gland.] Ontogenez 34:371–376. [Article in Russian]. [PubMed] [Google Scholar]
- 27.Knyazeva SI, Loginova NA, Loseva EV. 2012. Anxiety level and body weight changes in rats living in overpopulated cages. Bull Exp Biol Med 154:3–6. [Article in English, Russian]. [DOI] [PubMed] [Google Scholar]
- 28.Luo H, Hu X, Liu X, Ma X, Guo W, Qiu C, Wang Y, Wang Q, Zhang X, Zhang W, Hannum G, Zhang K, Liu X, Li T. 2012. Hair cortisol level as a biomarker for altered hypothalamic–pituitary–adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biol Psychiatry 72:65–69. [DOI] [PubMed] [Google Scholar]
- 29.McCormick CM, Mathews IZ. 2007. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav 86:220–233. [DOI] [PubMed] [Google Scholar]
- 30.McCormick CM, Smith C, Mathews IZ. 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res 187:228–238. [DOI] [PubMed] [Google Scholar]
- 31.Meyer JS, Novak MA. 2012. Minireview. Hair cortisol: a novel biomarker of hypothalamic–pituitary–adrenocortical activity. Endocrinology 153:4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modir F, Elahdadi Salmani SM, Goudarzi I, Lashkarboluki T, Abrari K. 2014. Prenatal stress decreases spatial learning and memory retrieval of the adult male offspring of rats. Physiol Behav 129:104–109. [DOI] [PubMed] [Google Scholar]
- 33.Morris CA, Amyes NC, Hickey SM. 2010. Responses of prolactin and hair growth to selection for age at puberty in Angus cattle. Animal 5:198–201. [DOI] [PubMed] [Google Scholar]
- 34.Nagaraja HS, Jeganathan PS. 2002. Voluntary alcohol drinking and caloric intake in rats exposed to crowding stress. Indian J Med Res 116:111–116. [PubMed] [Google Scholar]
- 35.Onyeanusi BI, Adeniyi AA, Onyeanusi CG, Ayo JO, Ibe CC. 2009. A study of the kidney of the Wistar rat in Northern Guinea savannah zone: the morphometric aspect. Pakistan journal of nutrition:PJN 8:1040–1042. [Google Scholar]
- 36.Ouschan C, Kuchar A, Mostl E. 2013. Measurement of cortisol in dog hair: a noninvasive tool for the diagnosis of hypercortisolism. Vet Dermatol 24:428–431. [DOI] [PubMed] [Google Scholar]
- 37.Romeo RD. 2010. Pubertal maturation and programming of hypothalamic–pituitary–adrenal reactivity. Front Neuroendocrinol 31:232–240. [DOI] [PubMed] [Google Scholar]
- 38.Rostamkhani F, Zardooz H, Zahediasl S, Farrokhi B. 2012. Comparison of the effects of acute and chronic psychologic stress on metabolic features in rats. J Zhejiang Univ Sci B 13:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell E, Koren G, Rieder M, Van Uum US. 2012. Hair cortisol as a biologic marker of chronic stress: current status, future directions, and unanswered questions. Psychoneuroendocrinology 37:589–601. [DOI] [PubMed] [Google Scholar]
- 40.Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. 2007. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 30:E183–E191. [DOI] [PubMed] [Google Scholar]
- 41.Scorrano F, Carrasco J, Pastor-Ciurana J, Belda X, Rami-Bastante A, Bacci ML, Armario A. 2014. Validation of the long-term assessment of hypothalamic–pituitary–adrenal activity in rats using hair corticosterone as a biomarker. FASEB J 29:859–867. [DOI] [PubMed] [Google Scholar]
- 42.Sharpley CF, Kauter KG, McFarlane JR. 2009. An initial exploration of in vivo hair cortisol responses to a brief pain stressor: latency, localization, and independence effects. Physiol Res 58:757–761. [DOI] [PubMed] [Google Scholar]
- 43.Sharpley CF, McFarlane JR, Slominski A. 2011. Stress-linked cortisol concentrations in hair: what we know and what we need to know. Rev Neurosci 23:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siniscalchi M, McFarlane JR, Kauter KG, Quaranta A, Rogers LJ. 2013. Cortisol levels in hair reflect behavioural reactivity of dogs to acoustic stimuli. Res Vet Sci 94:49–54. [DOI] [PubMed] [Google Scholar]
- 45.Sisk CL, Foster DL. 2004. The neural basis of puberty and adolescence. Nat Neurosci 7:1040–1047. [DOI] [PubMed] [Google Scholar]
- 46.Spangenberg EM, Augustsson H, Dahlborn K, Essen-Gustavsson B, Cvek K. 2005. Housing-related activity in rats: effects on body weight, urinary corticosterone levels, muscle properties, and performance. Lab Anim 39:45–57. [DOI] [PubMed] [Google Scholar]
- 47.Stalder T, Kirschbaum C. 2012. Analysis of cortisol in hair—state of the art and future directions. Brain Behav Immun 26: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 48.Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. 2013. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 38:1220–1235. [DOI] [PubMed] [Google Scholar]
- 49.Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. 2011. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res 186:310–314. [DOI] [PubMed] [Google Scholar]
- 50.Tanswell AK, Smith BT. 1978. The relationship of amniotic membrane 11-oxidoreductase activity to lung maturation in the human fetus. Pediatr Res 12:957–960. [DOI] [PubMed] [Google Scholar]
- 51.Taves MD, Gomez-Sanchez CE, Soma KK. 2011. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab 301:E11–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tirelli E, Laviola G, Adriani W. 2003. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev 27:163–178. [DOI] [PubMed] [Google Scholar]
- 53.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. 2006. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291:E965–E973. [DOI] [PubMed] [Google Scholar]
- 54.Vahdatpour T, Adl K, Nezhad Y, Sis N, Riyazi SR, Vahdatpour S. 2009. Effects of corticosterone intake as stress-alternative hormone on broiler chickens: performance and blood parameters. Asian J Anim Vet Adv 4:16–21. [Google Scholar]
- 55.Valencia-Alfonso CE, Feria-Velasco A, Luquin S, Diaz-Burke Y, Garcia Estrada J. 2004. [The effects of the social environment on the brain.] Rev Neurol 38:869–878. [Article in Spanish]. [PubMed] [Google Scholar]
- 56.Webster SH, Liljegreen ET, Zimicen DJ. 1947. Organ:body weight ratios for liver, kidneys, and spleen of laboratory animals: albino rat. Am J Anat 81:477–513. [DOI] [PubMed] [Google Scholar]
- 57.Weinstock M. 2005. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun 19:296–308. [DOI] [PubMed] [Google Scholar]
- 58.Yıldız A, Hayirli A, Okumus Z, Kaynar O, Kisa F. 2007. Physiologic profile of juvenile rats: effects of cage size and cage density. Lab Anim (NY) 36:28–38. [DOI] [PubMed] [Google Scholar]
- 59.Yu T, Xu H, Wang W, Li S, Chen Z, Deng H. 2015. Determination of endogenous corticosterone in rodent's blood, brain, and hair with LC-APCI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 1002:267–276. [DOI] [PubMed] [Google Scholar]
- 60.Zoccola PM, Dickerson SS. 2012. Assessing the relationship between rumination and cortisol: a review. J Psychosom Res 73:1–9. [DOI] [PubMed] [Google Scholar]