Abstract
Evaluating the behavioral effects of enrichment on animals housed in biomedical facilities is necessary to effectively support their care and wellbeing. We tested the cumulative effects of an enhanced enrichment program on sooty mangabey behavior: locomotion, feeding and foraging, manipulating items in the enclosure, social affiliation, aggression, and abnormal behavior. The enhanced enrichment program included the addition of a substrate (timothy hay), widely distributing small pieces of produce and a forage mixture in the hay, adding structures and perching, and increasing the variety of food items, foraging devices, and other manipulable items. We tested 10 groups living in runs (n = 54) by using an ABA experimental design (phase A, standard enrichment; phase B, enhanced enrichment) and Wilcoxon signed-rank tests to compare behavior across phases. During phase B, subjects significantly increased feeding, foraging, and manipulation of items, and they decreased self-grooming, social affiliation, and aggression. Combined enrichment use increased from approximately 10% to 21% of the mangabeys’ time. Enhanced enrichment did not affect locomotion or abnormal behavior. The increases in feeding, foraging, and manipulation during enhanced enrichment were driven primarily by the subjects’ preference for foraging in the hay: it was the most effective component of the program in promoting feeding and foraging behavior, which comprises the majority of wild sooty mangabeys’ daily activity. Developing an effective, species-appropriate, and comprehensive enrichment program is essential to successfully promote the health and wellbeing of captive NHP.
Enrichment for captive animals involves changes to the environment intended to improve the animals’ biologic functioning and psychologic wellbeing.23,32 As a component of behavioral management, environmental enrichment often involves providing opportunities for animals to engage in species-typical foraging, locomotor, and problem-solving behaviors.23,36 Environmental enrichment includes structural, occupational, food and foraging, and sensory components.14 Using many different types of enrichment opportunities that are tailored to a species’ innate proclivities and natural history can encourage a wide range of species-typical behaviors, as well as lead to behavioral benefits like reduced aggression. For example, physical barriers providing visual privacy reduced aggression in groups of macaques,11,17 whereas the removal of perches for the arboreal gray-cheeked mangabey increased aggression.22 In addition, providing manipulable foraging devices and additional foods, particularly those that require processing time and cannot be monopolized by a single animal, can increase feeding and foraging and reduce inactivity, aggression, and abnormal behavior.3,4,7,30,33 Finally, adding a substrate to solid floors is a common and cost-effective strategy to increase foraging time in many NHP species.1,3,5-7,10,15
Most studies of the effects of enrichment on an animal's behavior focus on a single strategy, such as new foraging devices or the addition of substrate. In the present study, we examined the combined effects of multiple enrichment strategies, and this study is the first to evaluate enrichment for sooty mangabeys (Cercocebus atys). Sooty mangabeys are a West African NHP species characterized by large, predominantly terrestrial, multimale–multifemale troops (more than 100 animals); female philopatry; and a matrilineal dominance system that is less nepotistic than those found in macaque and baboon groups, particularly in captivity.12,20,21,26-28 Sooty mangabeys collect the majority of their diet from the forest floor in the leaf litter and shrubs,20,21,28 including fruits, leaves, fungi, invertebrates, and specialize on hard seeds and nuts.9,20,21 Mangabeys spend the majority of their day on the ground feeding and foraging (approximately 63%), making use of a relatively large area (approximately 100 m). They travel approximately 10% of their time, and they have a maximal home range of 6 to 7 square kilometers.19,20 Our goal was to provide an environment in which the mangabeys could more closely approximate the behavioral time budgets of their wild counterparts. Therefore, our methodologic approach stemmed from the natural ecology of the species as well as practical considerations inherent in a captive context.
The Yerkes National Primate Research Center maintains a colony of approximately 200 sooty mangabeys, which are an important animal model for HIV–AIDS research.34 As a natural host species of SIV, the vast majority of infected mangabeys do not become immunocompromised by the virus and remain healthy. In this study, a subset of the mangabey colony lived in groups of 2 to 14 conspecifics in run-style housing (22 to 45 m2 in area, depending on group size). Although the standard enrichment program compared favorably to requirements in the Guide13 and to professional practices across many primate facilities,2 we designed several additions to provide the run-housed mangabeys with a more naturalistic enclosure that afforded a wider variety of activities, particularly those that encouraged feeding and foraging, given that this task comprises the majority of wild sooty mangabeys’ daily activity.19 We compared the combined effects of several enrichment strategies to the standard program on several categories of sooty mangabey behavior.
The enhanced enrichment program included additional structural enrichment to increase locomotor opportunities, by using both rigid and flexible perching, spinning poles, and visual barriers to provide areas for privacy. We also provided additional manipulanda and items for chewing and gnawing (made of manzanita wood), because sooty mangabeys are hard-object feeders.9,21 To attempt to extend foraging time, we devised a variety of feeding devices and dispersed small food items more frequently into hay, which was provided to simulate foraging through leaf litter. This approach allowed us to measure the cumulative effect of a greatly enhanced, holistically designed enrichment program. To identify particularly effective features of the enhanced program, we looked at which behaviors were most changed during the enhanced enrichment phase and the subjects’ preferences for various food items and manipulanda.
Overall, we expected higher rates of feeding and foraging than locomotion and other behaviors, given that a wild troop of sooty mangabeys spent 63.3% of their time feeding and foraging, 18.5% resting, 10.3% traveling, and 7.9% engaged in unspecified social behavior.19 Compared with the standard enrichment program, we expected the enhanced enrichment program to stimulate behavioral changes in a species-appropriate direction, reflected by increases in feeding and foraging, object manipulation, and locomotion. Past studies that introduced a foraging substrate to various NHP species showed variable increases from baseline in feeding and foraging3,5-7,10,15 (for example, from 4% to 14%;10 from 1% to 7% to 13% to 35%;6 from 0% to 35% to 11% to 87%7). Therefore, we conservatively predicted an increase in feeding and foraging of at least 10% during the enhanced enrichment program. Several previous studies also observed decreases in social and self-directed behavior,3,5-7,10 although to a lesser extent. Therefore, we anticipated some decline in affiliation, aggression, self-grooming, and abnormal behavior during the enhanced enrichment program. As long as social relationships and group dynamics were not disrupted, we did not consider that a slight reduction in affiliation to be problematic. We did not expect sex- or age-associated differences in behavior.
Materials and Methods
Animals and housing.
This research was conducted with approval by the Emory University IACUC and complied with United States laws and regulations and various guidelines regarding the care and use of animals in research, including the Guide for the Care and Use of Laboratory Animals.13 The Yerkes National Primate Research Center is an AAALAC-accredited institution. The study population comprised 54 subjects including 48 adults (29 male; 19 female; age, 15 to 31 y) and 6 juveniles (2 male; 4 female; age, 2 to 4 y [mean, 3 y]). The average age of the study population was 20 y and the majority of subjects were middle-aged adults (16 males and 11 females were 15 to 24 y old), with the next biggest segment of the population considered upper-middle-aged (11 males and 5 females were 20 to 24 y old). Because sooty mangabeys at our facility typically live to their mid to late twenties, we considered our elderly subjects to be 25 y and older (2 males, 3 females). Thus, the majority of the study population was normal healthy adults, with 6 subjects at the young end of the age range and 5 subjects at the elderly end, providing a spectrum of activity levels.
The subjects lived in 10 groups ranging from 2 to 14 subjects; 4 groups were bachelor groups and 6 were mixed-sex (Figure 1). Subjects were housed in a building with 24 runs at the Yerkes National Primate Research Center Field Station (Lawrenceville, GA; run size, 3.66 × 3.05 × 2.13 m). Each group lived in 2 to 4 adjacent runs (22 to 45 m2) made of 2.54-cm mesh walls with polypropylene panel walls separating the runs; one side of each run was open-air, with garage doors that were closed in inclement weather. Adjacent runs were connected by a doorway and by tunnels that jutted out on the open-air side. Doorways between runs were closed by using an opaque panel to separate groups. Monkey chow was distributed twice daily and water was available at all times. When subjects experienced the enhanced enrichment schedule, the basic structural and feeding enrichment remained intact, and we added to the types and varieties available.
Figure 1.
Social group compositions.
Experimental design.
We tested the effects of the enhanced enrichment schedule by using an ABA-style design with 2-wk testing phases: A1, baseline phase (standard enrichment schedule); B, experimental phase (enhanced enrichment schedule); A2, baseline phase. Prior to collecting data in each phase, subjects were given 2-wk adjustment periods to adapt to the change in the enrichment schedule, so that novelty effects were diminished during the periods of data collection. This approach focused on the more long-term effects on behavior rather than on short-term responses. Although animals can habituate to enrichment, this approach is representative of real-world enrichment programs, which include multiple strategies to improve wellbeing over sustained periods of time. We based our 2-wk adjustment period on a previous study with the same subjects,8 in which the 2-wk adjustment period was sufficient in duration to allow novelty responses to decline. Because our focus was not on initial behavioral reactions to novel enrichment items, we did not collect data during the adjustment phases. Testing for each group was completed over 10 wk: A1 (baseline phase data collection) → Adj1 (enhanced enrichment schedule began) → B (experimental phase data collection) → Adj2 (return to standard enrichment schedule) → A2 (baseline phase data collection). We tested 6 runs at a time with the enhanced enrichment schedule (2 or 3 groups at a time; 4 blocks of testing) from August 2013 to February 2014.
Standard enrichment schedule.
The standard enrichment schedule implemented in phases A1 and A2 consisted of 4 built-in PVC perches per run, one half of a plastic 50-gallon barrel, a swing (polypropylene; thickness, 2 cm; 30 × 30 cm; hanging approximately 1 m from the ceiling on an inflexible metal rod), firehose running the length of the open-air side of the run, manipulable and food devices included foraging boards made of high-density polyethylene artificial turf (63.5 × 7.6 cm) and hard plastic toys (for example, Nylabones [PetEdge, Beverly, MA], Dental Stars [BioServ, Flemington, NJ). Oranges were distributed every day (1/2 orange per mangabey), and additional types of produce were distributed twice each week (for example, banana, carrot, onion, cabbage). In addition, 3 or 4 times weekly, a forage mixture made of dry cereal, grains, sunflower seeds, whole wheat, oats, dried peas, and uncooked pasta was scattered in each run and placed on the forage boards. Finally, destructibles (for example, butcher paper, shredded office paper, paper bags, wax-free cardboard boxes) were distributed twice each week.
Enhanced enrichment schedule.
The enhanced enrichment schedule maintained the standard schedule and added several components. We added timothy hay to an approximate depth of 15 cm as substrate to each run to stimulate foraging activity (Figure 2), a second firehose in a corner opposite to the main firehose, and a perch made from manzanita wood in the other corner (1 m long, 7 to 8 cm wide), and we alternated privacy walls and spinning poles every other run, so that each group had access to at least one of each (perching and structures were intended to stimulate locomotion). The privacy walls, installed to provide additional visual privacy, were made of polypropylene panels (thickness, 2 cm, 1.8 × 2.1 m) oriented parallel to the walls separating runs and extending from floor to ceiling, leaving approximately 0.5 m of space on either end for mangabeys to move around. The spinning poles were made from stainless steel and attached from floor to ceiling in the center of the run; they spun around the vertical-axis and had stationary loops on which the animals could sit and climb.
Figure 2.
Adult female sooty mangabey foraging in hay.
In addition to the hard plastic toys that were always available, we provided hanging blocks made from manzanita wood for chewing and manipulation (0.3 m long). At 4 times per week, we provided 1 of 5 food devices for 24 h: foraging boards (the same as used during the standard enrichment schedule), frozen treat boards (polypropylene, 15 × 7.6 × 5.1 cm, with frozen juice, oatmeal, or yogurt with raisins), metal water bins (6.5 gallons, 48 × 15 × 10 cm, with food sprinkled in 3 cm-deep water), large rolling tubes (polyethylene; thickness, 1 cm; 15 × 45 cm, with holes in the sides) filled with shredded office paper and forage mix (Otto Environmental, Greenfield, WI), and hangers for mixed-berry–flavored Nutrablocks (Bio-Serv, Flemington, NJ), which were sandwiched between the mesh and a narrow piece of manzanita wood. The Nutrablock and manzanita could spin around a central a long bolt that was secured to the mesh of the run wall by using a wing nut (5 × 6.5 cm). We increased the rate of fresh produce distribution to twice daily, using smaller amounts chopped into smaller pieces, so that caloric intake was not increased over the baseline conditions. To reduce habituation to the typical enrichment foods, we diversified the menu substantially by including items like green beans, radishes, Brussels sprouts, and pecans in the shell, and we rotated through the various food options daily. Considering that sooty mangabeys have a more varied diet in the wild20,21 than they do in captivity, diversifying the subjects’ diet was appropriate even though some of the items may not be found in their natural habitat. The distribution of the forage mix was increased to 1 or 2 times daily, and destructibles (that is, paper products) were distributed once daily.
Behavioral data collection.
During each study phase, we collected behavioral data on each subject by using focal animal sampling, observing each subject for 12 min each day, 5 d per week, across each 2-wk phase (2 h total per subject per phase; 108 h of data per phase; 324 h total). We randomized the order of observations across subjects, balancing across time of day (0700 to 1600). Because we were interested in the sustained and cumulative effects of an entire program, we recorded behavior during times when some enrichment was freshly distributed and times when it had been distributed earlier in the day. This practice provided a comprehensive picture how the entire program affected behavior, which included quickly consumed foods and longer-lasting objects and structures.
During each observation session, we collected the frequency and duration of the following behaviors: locomotion, eating, rifling through the substrate (during enhanced enrichment [phase B] only), manipulation of enrichment items, self-grooming, affiliation, aggression, and abnormal behavior (we recorded frequency only for aggression and abnormal behavior; Figure 3). Both the rate and duration of each behavior were analyzed because they show different aspects of behavior; rate shows the frequency of behavior onset whereas duration indicates the overall time devoted to the behavior. Including both measures provides a fuller account of activity within each behavior category.
Figure 3.
Ethogram. *, Number of episodes and duration recorded; behavior must occur for at least 3 s for recording of duration to begin.
Statistical analyses.
For each subject, we summed the number of episodes and duration of each behavior in each phase and converted them to hourly rates (episodes per hour) and the total number of minutes per hour devoted to each behavior. From the mean duration per hour, we calculated the percentage of time spent engaged in each behavior. All statistical tests were performed by using SPSS software version 22 (IBM, Armonk, NY). We compared the subjects’ hourly rate and duration of each behavior across all experimental phases using the nonparametric Wilcoxon signed-rank test, because the data within each phase were not normally distributed for both frequency- and duration-based behaviors (Shapiro–Wilk tests, P ≤ 0.038). Because we made 3 pairwise comparisons to assess behavioral changes across study phases (that is, phase A1 compared with B, B compared with A2, A1 compared with A2) for 12 behaviors (that is, locomotion hourly rate, locomotion hourly duration, eating hourly rate [Figure 3 and Table 1]), we applied a conservative α level of 0.001 to determine statistical significance (by using the Bonferroni correction, in which the standard α level of 0.05 was divided by the number of comparisons made; in this case, 0.05 / 36 total comparisons). Marginally significant P values were considered to be between α = 0.05 and the adjusted α. To assess behavioral differences between sexes and age groups, each subject's data was averaged across phases, and we used Mann–Whitney U tests to compare male with female mangabeys and adults with juveniles in regard to hourly rate and duration of each of the 7 behavior categories (Bonferroni-corrected α = 0.007 [that is, 0.05 / 7 behavioral comparisons for both sex and age group]). Further comparisons among age groups were not warranted on the basis of visual inspection of the data. We compared juveniles with the rest of the adult population, including the elderly subjects, because the juveniles’ means in each behavioral category were starkly different from the adults’ and elderly adults’ in each study phase. For example, the mean rate of locomotion ranged from 35 to 42 times per hour across phases for adults, whereas it was 92 to 105 times per hour for juveniles. Although elderly subjects were expected to represent the other end of the spectrum, means of their behavior across phases were close to the rates and durations of the middle- and upper-middle–aged adults (for example, elderly locomotion rate ranged from 28 to 32 times per hour across phases).
Table 1.
Median rate (number of episodes), median duration (min), and the duration (min [% of total time]) per 60 min at which mangabeys engaged in various behaviors
| Phase |
||||
| Behavior | Measure | A1 | B | A2 |
| Locomotion | Median rate | 34.3 | 35.3 | 41.3 |
| Median duration | 4.3 | 4.5 | 5.1 | |
| Mean duration (%) | 5.8 (9.7) | 5.3 (8.8) | 6.5 (10.8) | |
| Eating | Median rate | 14.0 | 29.5 | 12.8 |
| Median duration | 4.0 | 6.9 | 2.7 | |
| Mean duration (%) | 4.1 (6.8) | 7.6 (12.7) | 3.6 (6.0) | |
| Rifling | Median rate | 12.0 | ||
| substrate | Median duration | 1.9 | ||
| Mean duration (%) | 2.4 (4.0) | |||
| Manipulation | Median rate | 2.3 | 4.8 | 3.0 |
| Median duration | 0.6 | 1.8 | 1.1 | |
| Mean duration (%) | 1.8 (3.0) | 2.7 (4.5) | 2.0 (3.3) | |
| Total | Median rate | 17.8 | 51.8 | 16.8 |
| enrichment | Median duration | 5.4 | 11.3 | 5.1 |
| use | Mean duration (%) | 5.9 (9.8) | 12.7 (21.2) | 5.5 (9.2) |
| Self-groom | Median rate | 7.5 | 3.8 | 6.8 |
| Median duration | 3.1 | 1.5 | 3.4 | |
| Mean duration (%) | 5.2 (8.7) | 3.6 (6) | 5.1 (8.5) | |
| Affiliation | Median rate | 10.3 | 7.5 | 8.3 |
| Median duration | 5.8 | 2.5 | 5.1 | |
| Mean duration (%) | 6.8 (11.3) | 4.5 (7.5) | 5.3 (8.8) | |
| Aggression | Median rate | 4.0 | 3.0 | 4.3 |
| Abnormal | Median rate | 0 | 0 | 0 |
| behavior | ||||
Total enrichment use is the sum of the eat, rifling substrate, and manipulation behavior categories.
We assessed the subjects’ preferences for items in the eat and manipulation categories by calculating the mean duration (minutes per hour) that subjects engaged with each item in phases A1 (baseline) and B (enhanced; A1 and A2 were not significantly different for these behavior categories). Using Wilcoxon signed-rank tests, we compared preferences across the 7 items in each behavior category within phase (α = 0.002), as well as the subjects’ preferences across phases for 2 items in the eat category and 2 items in the manipulation category that were available to the same extents in both phases A1 and B (α = 0.025; eat items, chow and oranges; manipulation items, plastic barrels and toys).
Results
Behavior across phases.
Table 1 summarizes the median hourly rates and durations for all behaviors and the mean durations with associated percentage of time that sooty mangabeys spent engaged in each behavior (medians are displayed because we used nonparametric tests to compare behavior across phases). Locomotion hourly rate and duration during phase A2 (baseline enrichment) was significantly higher than those for phases B (enhanced enrichment; both P ≤ 0.001; Wilcoxon signed-rank tests) and A1 (hourly rate, P = 0.001; duration, P = 0.006 [marginally significant]; α = 0.001). The percentage of time spent in locomotion ranged from 8.8% to 10.8% across phases. The hourly rate and duration of eating increased significantly during the enhanced enrichment phase compared with the baseline phases (both P < 0.001; α = 0.001). During enhanced enrichment, the hourly rate of manipulation increased significantly (P < 0.001), and the hourly duration of manipulation increased marginally significantly (both P ≤ 0.007; α = 0.001). The mean percentage of time spent eating increased from 6.4% during baseline enrichment to 12.7% with enhancement, and time spent manipulating items increased from 3.0% to 4.5% (averaging A1 and A2 for all behavior categories). There were no significant differences between the baseline enrichment phases in these categories. The behavior of rifling substrate comprised 4% of time in phase B, but could not be compared across phases since there was no substrate in the baseline phases. Overall, total enrichment use (eating, manipulation, and rifling substrate) increased from 9.5% to 21.2% of the mangabeys’ time during the enhanced phase (Table 1). In the locomotion and enrichment-related categories, hourly rate (number of episodes) was consistently higher than the hourly duration. For example, the median hourly rate of total enrichment use was approximately 17 times per hour during baseline enrichment and 52 times per hour during the enhanced enrichment phase, whereas median hourly duration went from approximately 5 to 11 min per hour. Although neither locomotion nor manipulation showed sex-associated differences, female mangabeys had a higher hourly rate of eating than did males (P = 0.006; α = 0.007). Overall, juveniles displayed higher hourly rates and durations of locomotion, eating, and manipulation than did adults (P ≤ 0.002; α = 0.007).
Self-grooming hourly rate and duration decreased during enhanced enrichment (phase A1 compared with B, P = 0.002 [marginally significant]; phase B compared with A2: P = 0.001; α = 0.001), declining from 8.6% of the mangabeys’ time during baseline to 6.0% during the enhanced enrichment phase (Table 1). There was no significant difference in self-grooming between the baseline phases. Across phases, the hourly rate of self-grooming was higher than the hourly duration, but the difference was not as pronounced as it was for locomotion and enrichment-related behavior. There were no sex-associated differences in self-grooming, but juveniles displayed a lower hourly rate (P = 0.003) and lower duration per hour (P = 0.004) of self-grooming than did adults (α = 0.007).
The hourly rate of affiliative behavior declined marginally significantly in enhanced enrichment compared with the first baseline phase (P ≤ 0.001) but not the second baseline phase, and there was a marginally significant difference between the 2 baseline phases (P = 0.015). Hourly duration of affiliative behavior declined marginally significantly during the enhanced phase compared with the baseline phases (P ≤ 0.006; α = 0.001). The average percentage of time spent affiliating with others dropped from 10% in baseline phases to 7.5% in the enhanced enrichment phase. As with self-grooming, the hourly rate of affiliative behavior was higher than its duration, and the difference was not as pronounced as it was for locomotion and other enrichment-related activities. Similarly, the hourly rate of aggressive behavior was lower during enhanced enrichment compared with the baseline phases, albeit marginally (phase A1 compared with B, P = 0.032; phase A2 compared with B, P = 0.021; α = 0.001). There were no differences in aggressive behavior between phases A1 and A2. Although there were no sex-associated differences in aggressive behavior, the overall hourly rate of affiliative behavior was significantly higher in female mangabeys than male (P < 0.001; α = 0.001). There were no differences between adults and juveniles in the hourly rates or durations of affiliative or aggressive behavior.
Abnormal behavior was observed in 4 of the 54 subjects: 3 adult males (age, 18 to 20 y) and one juvenile male (3 y). All subjects that displayed abnormal behavior were nursery-reared at birth (all other subjects were mother-reared). There was no overall difference in the hourly rate of abnormal behavior across phases. According to visual inspection of the data, the enhanced enrichment phase did not appear to affect the frequency with which the 3 adult males engaged in any type of abnormal behavior, but the frequency of abnormal behavior appeared to decline during enhanced enrichment in the juvenile male (Table 2).
Table 2.
Number of episodes of abnormal behavior observed during each phase; the types of abnormal behavior observed per subject (with number of episodes observed in parentheses)
| Any abnormal behavior | |||||
| Subject | A1 | B | A2 | Total | Types of abnormal behavior observed |
| 1 (adult) | 23 | 18 | 20 | 61 | Self-directed (clapping/clasping, 48) |
| Feces-smearing (1) | |||||
| Stereotypic locomotion (2) | |||||
| Self-biting (10) | |||||
| 2 (adult) | 0 | 0 | 4 | 4 | Feces-smearing (4) |
| 3 (adult) | 0 | 0 | 1 | 1 | Feces-smearing (1) |
| 4 (juvenile) | 79 | 27 | 66 | 172 | Self-directed (digit-sucking, 162) |
| Self-directed locomotor (1) | |||||
| Self-biting (9) | |||||
Enrichment preferences.
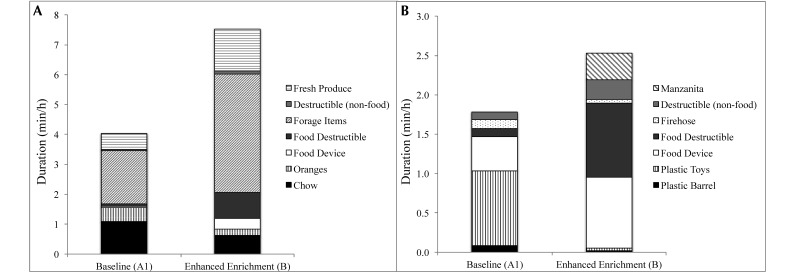
Wilcoxon signed-rank tests showed that items available in both phases to the same extent (eat: chow, oranges; manipulation: toys and plastic barrels) were consumed or manipulated for lower durations during enhanced enrichment, likely in favor of other items that became available or more numerous in phase B, such as forage, food devices, and food destructibles (chow, P = 0.007; oranges, P = 0.012 [marginally significant]; toys, P < 0.001; barrels, P = 0.03; α = 0.002; Figure 4 A and B). Mangabeys showed a general preference for forage items in both the first baseline and the enhanced phases, with time spent eating forage significantly longer than all other food items within phase (phase A1: all P < 0.001, except for a marginally significant difference between the duration spent eating chow and forage items, P = 0.008; phase B: P < 0.001; α = 0.002; Figure 4). The preference for forage items was particularly high during the enhanced phase, when they were scattered in the hay.
Figure 4.
Enrichment preferences during phases A1 and B. (A) Eating preferences. (B) Manipulation preferences.
Although the manipulation of enrichment items was relatively less frequent than other behaviors (Table 1), the relative frequency and duration with which the mangabeys engaged with various objects can reveal preferences. The relatively stronger preference for manipulating toys during the first baseline phase was replaced by preferences for food devices and food destructibles in the enhanced phase. In phase A1, subjects spent more time manipulating toys compared with the foraging boards (albeit nonsignificantly, according to Wilcoxon signed-rank tests [P = 0.14]), which were always available but only sprinkled with forage 3 or 4 times per week. Aside from the food device, time spent manipulating toys was significantly longer than for all other manipulable items (P ≤ 0.002, except for a marginally significant difference between toy and firehose [P = 0.006]; α = 0.002). However, when the diversity and availability of food devices and food destructibles were increased during enhanced enrichment, time spent manipulating food-related items increased significantly compared with other items (P ≤ 0.002, except for a marginally significant difference between food device and nonfood destructibles [P = 0.003]; α = 0.002; Figure 4 B).
Discussion
Although the magnitude of behavioral changes in the sooty mangabeys observed in this study were not large within each behavioral category, we found consistent, statistically significant increases in feeding, foraging, and manipulation and decreases in self-grooming, affiliative behavior, and aggressive behavior (marginally so for affiliation and aggression). Because these behavioral changes remained after the mangabeys had 2 wk of exposure to the additional types of enrichment, they were the cumulative, sustained effects of a holistically designed enrichment program. The program we evaluated here was more extensive than the standard program for laboratory NHP in the United States, according to a survey of 22 facilities.2 For example, only 36% of respondents reported providing feeding enrichment one or more times daily, whereas the enhanced enrichment program tested here involved produce and forage distribution twice daily each, with additional foraging options available at least once each day (for example, food devices and food destructibles). Most facilities reported that they gave enrichment devices (for example, puzzle balls, fleece boards) to less than half of their NHP, whereas all of our mangabeys had access to 4 different devices each week. The use of a substrate in the current study is also not commonly provided for run-housed laboratory NHP, even though it is one of the most effective forms of foraging enrichment.7
Overall, like wild mangabeys,19 the subjects in the present study spent the majority of their time feeding and foraging, followed by locomotion and social interactions. As expected, the enhanced enrichment phase had a greater effect on feeding and foraging than it did on social behaviors. In most cases, the behavioral effects observed during the enhanced enrichment phase did not carry over when the enrichment schedule returned to baseline, bolstering the conclusion that behavioral effects were the direct result of the enhanced enrichment program. Both the hourly rate and duration of most behaviors showed the same patterns across phases: enrichment-related activity increased during enhanced enrichment whereas self-grooming and social behavior declined slightly. The discrepancies between hourly rate and duration within behavior categories indicate that the mangabeys locomoted and ate frequently for short bouts at a time, whereas self-grooming and affiliative behaviors were sustained for longer bouts. For example, subjects engaged in locomotion and affiliation for similar percentages of time, but they initiated locomotion between 34 to 41 times in an hour and initiated affiliative behavior with others 7 to 10 times each hour (Table 1). This finding is in line with their preference for searching for small forage items (Figure 4 A), which do not require much processing time to consume and involved short bouts of movement from one patch of substrate to the next. In addition, living in run-housing may have restricted the duration of any one bout of locomotion.
Overall, there were very few sex-associated differences in behavior, with females exhibiting higher overall hourly rates of eating and affiliative behavior than males. The 6 juvenile subjects were more active than the adults in terms of locomotion, eating, and manipulation, and they engaged in less self-grooming than adults. The juveniles showed no differences in affiliative, aggressive, or abnormal behavior from adults, and their patterns of behavior across phases were the same as the those of the adults. To be cautious, given the much higher activity rates of juveniles in some behavioral categories, we ran all statistical tests without the 6 juveniles and verified the overall pattern of results. Because the other age groups (such as the 5 elderly adults) did not differ from one another as distinctly as did the juveniles, we did not check the pattern of results after excluding them.
Contrary to our expectations, locomotion did not increase under the enhanced enrichment schedule despite the addition of more structural enrichment that offered more opportunity to use the 3D space in the enclosure. Interestingly, a previous study3 found that red-capped mangabeys given seeds with a substrate showed decreased rates of locomotion, as well. Overall, the mangabeys spent about 4 to 5 min per hour locomoting in each phase (approximately 9% to 10% of their time). Although locomotion did not increase as expected, the percentage of time the mangabeys spent moving around their enclosure matched what was reported for a wild group of mangabeys19 (approximately 10% of time) and may explain the inflexibility of this behavior. It is probably unreasonable to expect run-housed sooty mangabeys to locomote more than their wild counterparts, who have no spatial restrictions (although we have only a single report of their activity budget for comparison19).
During the enhanced phase, the mangabeys doubled the time spent eating, from 6.8% to 12.7% of their time, which was in a species-appropriate direction. Time spent searching through the substrate for food contributed about 4% of additional time engaged with enrichment during the enhanced phase, totaling about 17% of time feeding and foraging during the enhanced enrichment schedule (eating and rifling through the substrate). This result is comparable to findings in other studies of feeding enrichment; for example, in a similarly designed study, feeding behavior increased from 3% to 16% in chimpanzees given a wide variety of feeding enrichment throughout the day.4 Another study saw an increase from 4% to 14% in the time rhesus monkeys spent foraging in a substrate.10 Therefore, although the enhanced enrichment schedule did not increase the subjects’ feeding and foraging time to levels seen in a wild group of sooty mangabeys (63% of their time),19 our findings are in line with what others have seen in captive primates. Nevertheless, additional improvements to feeding and foraging opportunities are still needed. Specifically, we added manzanita wood blocks and pecans in the shell for the mangabeys to eat and gnaw on, but offering harder nuts and seeds may have further expanded their feeding duration. Because of health and safety concerns at our facility, pecans in the shell and manzanita wood were included as a first step toward providing more naturalistic foraging items, with the hope that more hard-shelled seeds and nuts could be provided in the future.
Although the time spent manipulating items in the environment was relatively low, there was a consistent, statistically detectable increase in the time subjects spent manipulating food and nonfood items (food devices, paper destructibles, firehose, manzanita wood), from 3.0% to 4.5% of their time. This increase may be due the variety of devices offered throughout the week. Together these findings show that the mangabeys increased their engagement with the available enrichment (eating, foraging, and manipulating) from about 10% of their time in baseline to 21% under the enhanced enrichment schedule (an increase of approximately 11% points). Although this increase met our expectations, more is needed to facilitate feeding and foraging times that better approximate those observed in wild sooty mangabeys.19
Our assessment of the mangabeys’ preferences for eating and manipulating various forms of enrichment showed that the forage mixture was the most preferred item, particularly when it was scattered in the substrate. Food devices were also preferred items, and manzanita was the most preferred nonfood manipulable item. As expected, most items that were increased or otherwise made available to a greater extent during the enhanced enrichment routine showed an increase in use from baseline to the enriched phase. However, the items that were available to the same extent under both enrichment schedules were used less during the enhanced period, likely because the monkeys attended to more food-related items available in phase B. This effect was particularly striking for toys, which were the most preferred manipulable item during baseline, even compared with food devices. Their preferences indicate that offering substrate seeded with food, a variety of manipulable foraging devices, and a hard wood for chewing are of particular interest to sooty mangabeys and should be incorporated into their enrichment protocols. In light of our mangabeys’ demonstrated preferences and the benefits of substrate shown in many other studies,3,5-7,10,15 a foraging substrate is a top priority for run-housed primates.
In line others’ findings3,5-7,10 and our predictions, the mangabeys showed reduced self-grooming and slight reductions in affiliative and aggressive behaviors during the enhanced enrichment phase. Decreases in self-grooming have similarly been observed in rhesus monkeys given feeding enrichment.10,30 Although self-grooming was not excessive to begin with (approximately 3 min per hour), its reduction during the enhanced phase likely reflects a preference to engage in enrichment-related activities over self-directed activities. In addition, if self-grooming serves to reduce an animals’ anxiety,16,18,31 the additional enrichment might have produced a less stressful environment overall. The slight reduction in affiliative behavior during enhanced enrichment of sooty mangabeys has been observed in other NPH species given a foraging substrate (chimpanzees4 and rhesus macaques6,10). Like self-grooming, subjects in the previous studies might have reduced affiliation in favor of enrichment-related activities, to some extent. However, we note that the effects of the enhanced enrichment phase on affiliative behavior were not very pronounced, given that the decline in hourly duration was marginal and hourly rate did not follow exactly the same pattern. Affiliative interactions did not cease completely, and we saw no change to the general dynamics of the group (such as increased aggression or changes in dominance ranks). The marginally significant reduction in aggression that we observed during the enhanced phase has been seen in other studies4,7 as well and supports a previous study in captive sooty mangabeys showing that distributed food enrichment is associated with less aggression than is clumped enrichment.33 The privacy walls may have contributed to the declines in affiliative and aggressive behavior, given that visual barriers have been shown to have such effects on captive NHP, possibly because when an animal is out of visual contact the likelihood of any type of encounter is reduced.11,17
Although enrichment can reduce the occurrence of abnormal behavior,4,5 its effects are not always long-lasting.25 We observed no overall change in abnormal behavior across phases, likely because only a small percentage of our population exhibited any at all – just 4 of the 54 subjects. Notably, each of subjects that showed abnormal behavior was nursery-reared at birth and subsequently singly housed for varying intervals. Among these subjects, only the juvenile subject showed an apparent reduction in abnormal behavior (primarily digit sucking) during the enhanced enrichment phase (Table 2). However, with so few subjects showing abnormal behavior overall, we cannot draw any general conclusions about the effects of the enhanced enrichment program on abnormal behavior. It was not surprising to observe abnormal behavior in these particular subjects, as many studies suggest that physiologic changes stemming from nursery rearing may lead some animals to develop and maintain abnormal behavior throughout their lives,24,29,35 despite long periods of time living in enriched circumstances.
In conclusion, by adding various forms of enrichment to run housing, we affected species-appropriate changes in sooty mangabey behavior. Like many other primate species,1,5-7,10,15 sooty mangabeys benefited from the addition of a foraging substrate: it was the most effective form of enrichment in terms of stimulating feeding and foraging behavior. Nevertheless, more environmental enhancement is needed to better approximate the time wild mangabeys spend feeding and foraging.19 In addition, locomotion was unchanged by the addition of structural enrichment, although the time our subjects spent moving around the enclosure closely matched what has been observed in wild mangabeys. Although we might not dramatically increase locomotion from baseline levels, encouraging some amount of additional locomotion is beneficial for captive animals that do not need to travel in search of food. Like other studies,3,5-7,10 the decreases in self-grooming and social behavior were not unexpected, and there were no changes to group dynamics. Finally, with just 4 of 54 subjects that exhibited abnormal behavior, any effect of the enhanced enrichment schedule on their behavior was inconclusive.
The next steps in evaluating the effects of enrichment on the behavior of sooty mangabeys include investigating whether scattering hard seeds and nuts (items on which wild mangabeys specialize9,21) into a substrate further increases feeding and foraging durations to levels approaching those in a free-ranging group.19 In addition, determining the effects of various forms of enrichment on abnormal behavior in sooty mangabeys would be valuable for devising effective behavioral treatments. Because several new enrichment procedures were simultaneously tested in the current study, possible interactions among their effects cannot be discounted. Further investigation is needed to compare the effects of each new type of enrichment independently. Our study underscores the importance of evaluating enrichment and identifying effective strategies for each species of laboratory-housed animal to successfully support their psychologic wellbeing and care.
Acknowledgments
We greatly appreciate the assistance of the Animal Care, Colony Management, and Facilities Management departments at the Yerkes National Primate Research Center Field Station. Animal care staff members were instrumental in assisting our execution of this research, and Facilities Management designed and built many of the enrichment structures and devices used in this study. This project was funded by the National Center for Research Resources (grant P51 RR000165) and supported by the Office of Research Infrastructure Programs (grant P51 OD011132). All aspects of management and research use conformed to the requirements of the Emory University IACUC and United States federal regulations and guidelines. The Yerkes Center is fully AAALAC-accredited.
References
- 1.Bennett AJ, Corcoran CA, Hardy VA, Miller LR, Pierre PJ. 2010. Multidimensional cost–benefit analysis to guide evidence-based environmental enrichment: providing bedding and foraging substrate to pen-housed monkeys. J Am Assoc Lab Anim Sci 49:571–577. [PMC free article] [PubMed] [Google Scholar]
- 2.Baker KC, Weed JL, Crockett CM, Bloomsmith MA. 2007. Survey of environmental enrichment programs for laboratory primates. Am J Primatol 69:377–394. [DOI] [PubMed] [Google Scholar]
- 3.Blois-Heulin C, Jubin R. 2004. Influence of the presence of seeds and litter on the behavior of captive red-capped mangabeys Cercocebus torquatus torquatus. Appl Anim Behav Sci 85: 349–362. [Google Scholar]
- 4.Bloomsmith MA, Alford PL, Maple TL. 1988. Successful feeding enrichment for captive chimpanzees. Am J Primatol 16:155–164. [DOI] [PubMed] [Google Scholar]
- 5.Brent L. 1992. Woodchip bedding as enrichment for captive chimpanzees in an outdoor enclosure. Anim Welf 1:161–170. [Google Scholar]
- 6.Byrne GD, Suomi SJ. 1991. Effects of woodchips and buried food on behavior patterns and psychologic wellbeing of captive rhesus monkeys. Am J Primatol 23:141–151. [DOI] [PubMed] [Google Scholar]
- 7.Chamove AS, Anderson JR, Morgan-Jones SC, Jones SP. 1982. Deep woodchip litter: hygiene, feeding, and behavioral enhancement in 8 primate species. International Journal for the study of animal problems 3:308–318. [Google Scholar]
- 8.Crast J, Jonesteller TJ, Bloomsmith MA. 2014. Abnormal behavior in captive sooty mangabeys. Anim Welf 23:167–177. [Google Scholar]
- 9.Daegling DJ, McGraw WS, Ungar PS, Pampush JD, Vick AE, Bitty EA. 2011. Hard-object feeding in sooty mangabeys (Cercocebus atys) and interpretation of early hominin feeding ecology. PLoS One 6:e23095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doane CJ, Andrews K, Schaefer LJ, Morelli N, McAllister S, Coleman K. 2013. Dry bedding provides cost-effective enrichment for group-housed rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 52:247–252. [PMC free article] [PubMed] [Google Scholar]
- 11.Estep DQ, Baker SC. 1991. The effects of temporary cover on the behavior of socially housed stumptailed macaques (Macaca arctoides). Zoo Biol 10:465–472. [Google Scholar]
- 12.Gust DA, Gordon TP. 1994. The absence of a matrilineally based dominance system in sooty mangabeys, Cercocebus torquatus atys. Anim Behav 47:589–594. [Google Scholar]
- 13.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 14.Keeling ME, Alford PL, Bloomsmith MA. 1991. Decision analysis for developing programs of psychological wellbeing: a bias-for-action approach, p 57–65. In: Novak MA, Petto AJ. Through the looking glass. Washington (DC): American Psychological Association. [Google Scholar]
- 15.Ludes E, Anderson JR. 1996. Comparison of the behaviour of captive white-faced capuchin monkeys (Cebus capucinus) in the presence of 4 kinds of deep litter. Appl Anim Behav Sci 49:293–303. [Google Scholar]
- 16.Maestripieri D, Schino G, Aureli F, Troisi A. 1992. A modest proposal: displacement activities as an indicator of emotions in primates. Anim Behav 44:967–979. [Google Scholar]
- 17.Maninger N, Kim J, Ruppenthal G. 1998. The presence of visual barriers decreases agonism in group housed pigtail macaques (Macaca nemestrina). Am J Primatol 45:193–194. [Google Scholar]
- 18.Mason GJ. 1991. Stereotypies: a critical review. Anim Behav 41:1015–1037. [Google Scholar]
- 19.McGraw WS. 1998. Comparative locomotion and habitat use of 6 monkeys in the Tai Forest, Ivory Coast. Am J Phys Anthropol 105:493–510. [DOI] [PubMed] [Google Scholar]
- 20.McGraw WS, Vick AE, Daegling DJ. 2010. Sex and age differences in the diet and ingestive behaviors of sooty mangabeys (Cercocebus atys) in the Tai Forest, Ivory Coast. Am J Phys Anthropol 144:140–153. [DOI] [PubMed] [Google Scholar]
- 21.McGraw WS, Vick AE, Daegling DJ. 2014. Diet variation and food hardness in sooty mangabeys (Cercocebus atys): implications for fallback foods and dental adaptation. Am J Phys Anthropol 154:413–423. [DOI] [PubMed] [Google Scholar]
- 22.Neveu H, Deputte BL. 1996. Influence of availability of perches on the behavioral wellbeing of captive, group-living mangabeys. Am J Primatol 38:175–185. [DOI] [PubMed] [Google Scholar]
- 23.Newberry RC. 1995. Environmental enrichment: increasing the biologic relevance of captive environments. Appl Anim Behav Sci 44:229–243. [Google Scholar]
- 24.Novak MA. 2003. Self-injurious behavior in rhesus macaques: new insights into its etiology, physiology, and treatment. Am J Primatol 59:3–19. [DOI] [PubMed] [Google Scholar]
- 25.Novak MA, Kinsey JH, Jorgensen MJ, Hazen TJ. 1998. Effects of puzzle feeders on pathologic behavior in individually housed rhesus monkeys. Am J Primatol 46:213–227. [DOI] [PubMed] [Google Scholar]
- 26.Range F. 2006. Social behavior of free-ranging juvenile sooty mangabeys (Cercocebus torquatus atys). Behav Ecol Sociobiol 59: 511–520. [Google Scholar]
- 27.Range F, Noë R. 2002. Familiarity and dominance relations among female sooty mangabeys in the Taï National Park. Am J Primatol 56:137–153. [DOI] [PubMed] [Google Scholar]
- 28.Range F, Förderer T, Storrer-Meystre Y, Benetton C, Fruteau C. 2007. The structure of social relationships among sooty mangabeys in Taï, p 109–130. In: McGraw WS Zuberbühler K Noë R, editors. Monkeys of the Taï Forest. Cambridge (UK): Cambridge University Press. [Google Scholar]
- 29.Sánchez MM. 2006. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav 50:623–631. [DOI] [PubMed] [Google Scholar]
- 30.Schapiro SJ, Suarez SA, Porter LM, Bloomsmith MA. 1996. The effects of different types of feeding enhancements on the behaviour of single-caged, yearling rhesus macaques. Anim Welf 5:129–138. [Google Scholar]
- 31.Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. 1996. Primate displacement activities as an ethopharmacologic model of anxiety. Anxiety 2:186–191. [DOI] [PubMed] [Google Scholar]
- 32.Shepherdson DJ. 1998. Introduction: tracing the path of environmental enrichment in zoos, p 1–14. In: Shepherdson DJ Mellen JD Hutchins M, editors. Second nature: environmental enrichment for captive animals. Washington (DC): Smithsonian Institution Press. [Google Scholar]
- 33.Stahl D, Kaumanns W. 2003. Food competition in captive sooty mangabeys (Cercocebus torquatus atys). Primates 44:203–216. [DOI] [PubMed] [Google Scholar]
- 34.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. 2007. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest 117:3148–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. 2005. The physiology and neurochemistry of self-injurious behavior: a nonhuman primate model. Front Biosci 10:1–11. [DOI] [PubMed] [Google Scholar]
- 36.Young RJ, editor. 2003. Environmental enrichment for captive animals. Oxford (UK): Blackwell Publishing. [Google Scholar]