Abstract
Providing appropriate analgesia is essential in minimizing pain and maintaining optimal animal care and welfare in laboratory animals. Guinea pigs are common animal models in biomedical research, often requiring analgesic support. Here we evaluated the pharmacokinetics and efficacy of a sustained-release formulation of buprenorphine (Bup-SR) in this species. Guinea pigs (n = 7 each group) received either Bup-HCl (0.05 mg/kg BID for 3 d) or Bup-SR (0.3 mg/kg once). Plasma collection and measurement of paw-withdrawal pressure (PWP) was conducted at 0, 1, 3, 6, 12, 26, 48, and 72 h after treatment. Plasma levels of Bup-HCl peaked at 2331 pg/mL at 1 h after administration and declined to 165 pg/mL by 12 h. Plasma concentrations of Bup-SR peaked at 1344 pg/mL at 26 h after administration and declined to 429 pg/mL by 48 h. The PWP of the Bup-HCl–treated guinea pigs peaked at 674 g at 1 h and declined to 402 g at 6 h, whereas that of Bup-SR–treated guinea pigs at 1 h was 361 g, 555 g at 6 h (significantly higher than that after Bup-HCl), and peaked at 680 g at 12 h. The PWP of both treatments was similar from 24 to 72 h and ranged from 348 to 450 g. The plasma concentration and PWP showed good correlation. These results suggest that Bup-SR provides consistent analgesia equivalent to that of Bup-HCl for a prolonged period of time and that Bup-SR is an alternative method of analgesia in guinea pigs.
Abbreviations: Bup, buprenorphine; SR, sustained release; PWP, paw-withdrawal pressure; RS, Randall–Selitto
Providing appropriate analgesia is essential in minimizing pain and maintaining optimal animal care and welfare in laboratory animals. Roughly 170,000 guinea pigs were used in research in 2014,34 making them a common species used in research under the Animal Welfare Act, and more than 29,000 guinea pigs were listed as undergoing procedures in which pain or distress was mitigated.34 Buprenorphine (Bup) acts as a partial agonist at the μ-opioid receptor and as an antagonist at the κ-opioid receptor and is often used to reduce pain in laboratory rodents.14,21 Buprenorphine is 25 to 50 times more potent than morphine13 and is advantageous due to its relatively slow dissociation from the opioid receptors, minimal side effects, and decreased induction of drug dependence compared with other opioids.12,14,22 Buprenorphine hydrochloride (Bup-HCl) treatment is an effective analgesic in rodents, as demonstrated by the decreased changes in food and water intake, body weight, and ambient locomotor activity after experimental surgery.4,10,28 Despite the frequent use of the drug, the duration of action of Bup-HCl in rodents is a topic of discussion, with some suggesting that these species might need more frequent dosing than the frequently published intervals to maintain therapeutic levels.16,23 Multiple dosing may increase unwanted handling stress,3 leading to behavioral changes and altered research results. A single subcutaneous dose of sustained-release formulations of buprenorphine (Bup-SR) provide analgesia for 48 to 72 h in other rodent species,7,11,15,23,24 thereby decreasing animal handling and providing more consistent analgesia.
This study compares the pharmacokinetics of Bup-HCl and Bup-SR in guinea pigs over a 72-h period and assesses efficacy by using the Randall–Selitto (RS) assay. Our hypothesis was that a one-time administration of Bup-SR would provide equivalent, consistent, but longer lasting analgesia than Bup-HCl, according to withdrawal responses to linearly increasing force.
Materials and Methods
Animals.
Female Dunkin–Hartley guinea pigs (weight, 350 g) were obtained from Charles River Laboratories (Wilmington, MA). Guinea pigs were free of Sendai virus, lymphocytic choriomeningitis virus, pneumonia virus of mice, guinea pig adenovirus, guinea pig reovirus, Helicobacter spp., Mycoplasma pulmonis, and ectoparasites. Animals were group-housed at 4 or 5 per cage, with unrestricted access to Teklad Global Guinea Pig Diet 2040 (Envigo, Madison, WI) and filtered sterilized water. They were allowed to acclimate to housing conditions for 1 wk prior to 1 wk of training before initiating studies. Guinea pigs were maintained on a 12:12-h light:dark cycle at a room temperature of 21 to 24 °C. All experimental procedures were IACUC-approved.
Study design.
The 14 female guinea pigs were randomized into 2 equal groups of 7 animals. Prior to experimentation, guinea pigs were acclimated daily for 5 d to restraint for venipuncture and PWP restraint and testing. One group received Bup-HCl (0.05 mg/kg; 0.3 mg/mL, Ricket Benckiser Healthcare, London, England) twice daily for 60 h, whereas the other group received one injection of Bup-SR at 0.3 mg/kg (Buprenorphine SR-LAB 1 mg/mL, Zoopharm, Windsor, CO), followed by equal volumes of sterile saline every 12 h for 60 h. Injections were given subcutaneously in the interscapular region.
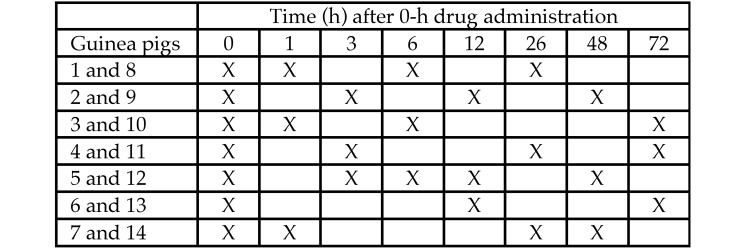
Blood collection and PWP testing were conducted on all guinea pigs at the 0-h time point and then on 3 guinea pigs per group at 1, 3, 6, 12, 26, 48, and 72 h after treatment with Bup-HCl or Bup-SR (Figure 1). Venipuncture and PWP was performed immediately prior to Bup-HCl or saline injection at the 12-, 48-, and 72-h time points. All guinea pigs were weighed at 0 and 72 h. A 2-wk washout period was given before conducting the second trial in a crossover design. The personnel collecting blood and performing PWP (BS, DW) were blinded to the study groups throughout the study.
Figure 1.
Time line of blood collection and PWP testing. Guinea pigs 1 through 7 received Bup-HCl; guinea pigs 8–14 received Bup-SR; 3 animals were tested per time point.
Plasma collection and analysis.
Immediately after PWP testing, 200 µL of blood was collected from the jugular vein, stored in a heparinized microcentrifuge tube (Becton Dickinson and Company, Franklin Lakes, NJ), and placed on ice until centrifugation. The blood was centrifuged at 10,000 × g for 10 min, and plasma was stored at –80 °C for later analysis. Anesthesia with isoflurane was used when manual restraint was insufficient; isoflurane was used on 5 separate occasions throughout the experiment.
Plasma concentrations of Bup were determined by performing liquid chromatography and tandem mass spectrometry on 50 µL of plasma.23 Briefly, samples were prepared by using a liquid–liquid extraction method with methyl tert-butyl ether and reconstitution in acetonitrile and ultrapurified water. Positive-ion electrospray ionization mass spectra were obtained by using a triple quadrupole mass spectrometer (AB SCIEX Q-TRAP 6500, SCIEX, Framingham, MA) with a turbo ionspray source interfaced with a Nextera MP Ultra HPLC with a SIL-30ACMP multiplate autosampler system (Shimadzu, Kyoto, Japan). The lower limit of detection for the analysis was 25 pg/mL. Pharmacokinetic analysis was performed by using Phoenix WinNonlin software (Pharsight, Cary, NC).
PWP testing.
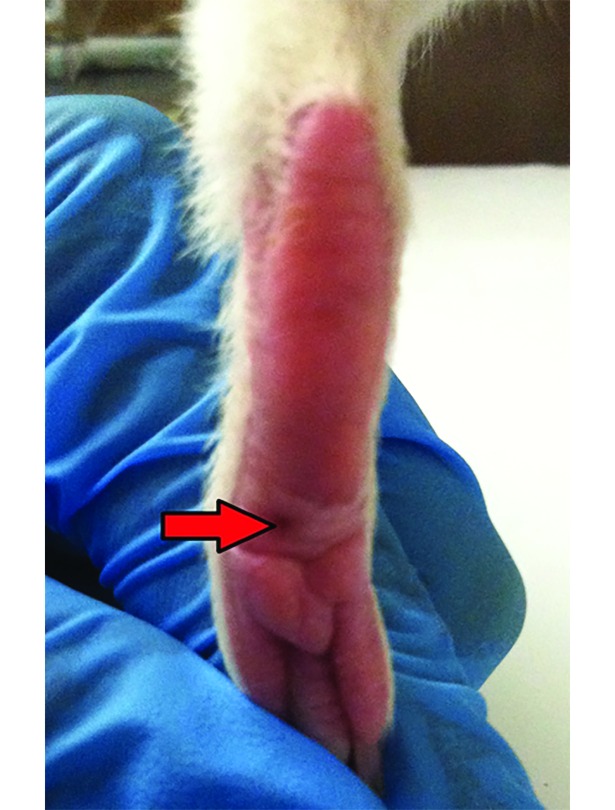
PWP testing was conducted by using a modified RS assay. Guinea pigs were fully restrained in a modified large rat sling suit (IITC Life Science, Woodland Hills, CA). Prior to toe pinching, guinea pigs were given 1 min to acclimate to the sling. Pressure to the plantar surface of the left hindpaw was gradually and constantly increased by using an RS electronic algesimeter (IITC 2500 Digital Paw Pressure Meter, IITC Life Science) until the animal withdrew the paw. The point of application on the hindpaw (Figure 2) and the experimenter (BS) were consistent throughout the study. The paw pressure applicator is labeled for a maximum force of 800 g and accuracy of 0.5%. The maximum amount of force (g) at the time the animal flinched was recorded. To prevent equipment damage, application was discontinued after 750 g of force, even when the animal never flinched.
Figure 2.
The site at which the algesimeter was applied on the left hindpaw.
Statistical analysis.
Repeated-measures analysis was done by using the Proc Mixed protocol of SAS (SAS Institute, Cary, NC). Separate models were fit with paw pressure or pharmacokinetics as the response. Fixed effects included treatment (Bup-HCl or Bup-SR), time (0, 1, 3, 6, 12, 26, 48, or 72 h) and group×treatment interaction. The animal's identification number was included as a random effect to account for repeated measures. Pharmacokinetic values below the limit of detection were replaced with the minimal value of 12.5. For PWP analysis, a random treatment×animal interaction term was added to the model to account for the crossover design. When comparing PWP data at various time points with baseline for each group, Dunnett adjusted P values were used. P values less than 0.10 were considered statistically significant. Spearman rank correlation was used to determine whether plasma concentration correlated with PWP.
Results
Pharmacokinetics.
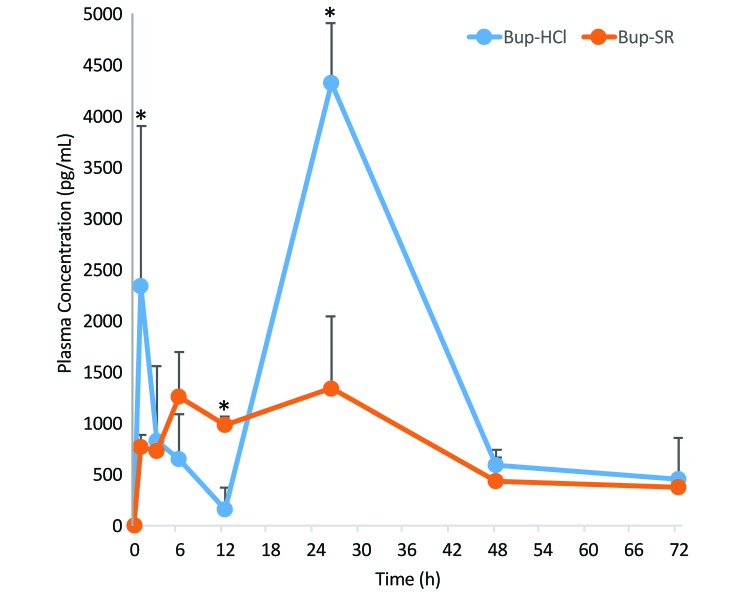
The average Bup plasma concentrations of guinea pigs treated with Bup-HCl every 12 h and Bup-SR over the 72 h period are shown in Figure 3. Baseline values (0 h) of all guinea pigs were below the lower limit of detection. At 1 h, the plasma concentrations (mean ± 1 SD) of Bup-HCl reached 2331 ± 1573 pg/mL and declined to 165 ± 205 pg/mL by 12 h. As expected, the concentration of the sample collected 2 h after administration was high, at 4323 ± 586 pg/mL, and samples collected at 48 and 72 h were near the 12-h nadir. The plasma concentrations of Bup-SR steadily increased from 777 ± 109 pg/mL at 1 h to 1261 ± 435 pg/mL at 6 h and peaked at 1344 ± 701 pg/mL at 26 h. Plasma concentrations of Bup-SR–treated guinea pigs at 48 and 72 h were near the 12-h nadir of Bup-HCl. Plasma concentrations at 1, 12, and 26 h differed significantly (P = 0.003, P = 0.098, and P < 0.001, respectively). Noncompartmental analysis (Table 1) was performed on Bup-HCl for the first 12 h only because the plasma concentration reached a nadir at that point. As expected, Bup-HCl had a much shorter half-life (6.6 h) than that of Bup-SR (25.3 h). In addition, exposure (as determined by AUC) during the first 12 h was increased nearly 3-fold with Bup-SR compared with Bup-HCl. Clearance times were shorter for Bup-SR. The pharmacokinetic data are derived from the second trial only because data from the first trial were obtained and stored inconsistently.
Figure 3.
Plasma concentration (mean ± 1 SD [error bars]) of Bup after dosing with Bup-HCl (0.5 mg/kg BID) or Bup-SR (0.3 mg/kg once) in guinea pigs. Three guinea pigs were sampled at each time point. Bup-HCl samples were analyzed 12 h after administration, representing the nadir plasma levels; except at the 26-h time point, samples were collected 2 h after the 24-h drug administration. *, P < 0.09.
Table 1.
Noncompartmental analysis of Bup-HCl and Bup-SR in guinea pigs
| Cmax (ng/mL) | Tmax (h) | t1/2 (h) | AUClast (h × ng/mL) | Tlast (h) | Cl/Fpred (mL/h/kg) | |
| Bup-SR (overall) | 1344 | 26 | 25.3 | 56,996 | 72 | 4.24 |
| Bup-HCl (0–12 h) | 2331 | 1 | 6.63 | 4,050 | 12 | 8.38 |
| Bup-SR (0–12 h) | 1261 | 6 | n.d. | 11,593 | 12 | not done |
Cmax, maximal concentration measured; Tmax, time of Cmax; t1/2, terminal half-life; AUClast, AUC from time 0 to Tlast; Tlast, time point to which AUClast was calculated; Cl/Fpred, predicted clearance.
PWP analysis.
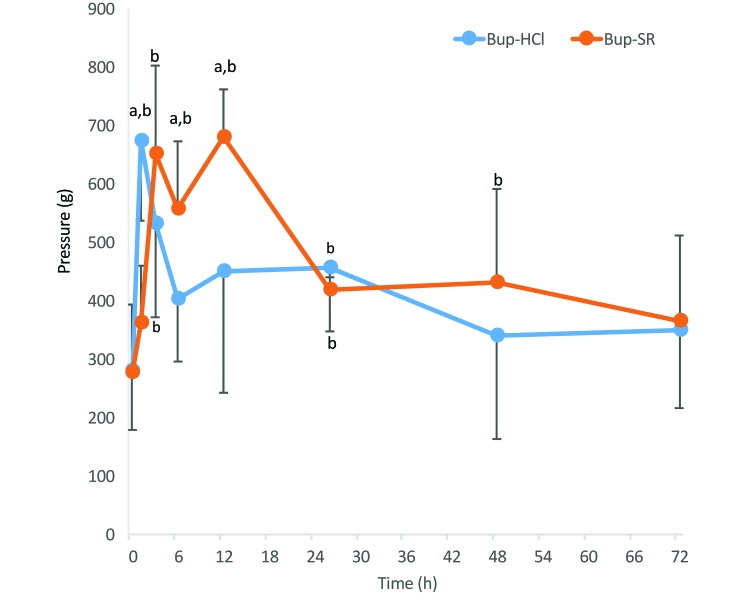
The amount of pressure applied to the paw correlated well (r = 0.62) with plasma Bup concentrations. Guinea pigs treated with Bup-HCl had significantly (P < 0.01) higher PWP measurements at 1, 3, and 26 h compared with baseline (0 h), and those treated with Bup-SR had significantly increased PWP measurements at 3, 6, 12 (P < 0.01), 26 (P = 0.04), and 48 h (P = 0.07; Figure 4). The PWP of guinea pigs treated with Bup-HCl was greatest at 1 h after administration at 674 ± 137 g with a rapid decline by 6 h to 402 ± 106 g and a continued decline back to baseline by 72 h at 348 ± 131 g. In comparison, guinea pigs treated with Bup-SR had a slight increase in PWP (compared with baseline values) at 1 h after administration at 361 ± 99 g, which further increased to 650 ± 151 g at 3 h and peaked at 680 ± 81 g at 12 h. The PWP declined at the following 26, 48 and 72 h time points. The PWP of Bup-HCl–treated guinea pigs at 1 h was significantly greater (P < 0.001) than that of Bup-SR–treated guinea pigs. The PWP of Bup-SR–treated guinea pigs was greater than that of Bup-HCl treated animals at both the 6-h (P = 0.09) and 12-h (P < 0.003) time points.
Figure 4.
Amount of pressure (mean ± 1 SD [error bars]) applied to the left hindpaw to measure the withdrawal response of guinea pigs treated with either Bup-HCl or Bup-SR. Six guinea pigs were sampled at each time point. a, significant (P < 0.09) difference between treatment groups; b, significant (P < 0.07) difference compared with baseline value within the group.
Body weight.
Body weight was recorded prior to initiating studies and at the end of the 72-h period. The weight change over the 72-h period did not differ between groups. Bup-HCl–treated guinea pigs had an average weight loss of 0.28 g, and Bup-SR–treated guinea pigs had an average weight loss of 0.22 g.
Discussion
Buprenorphine is a common analgesic used to mitigate pain in rodent research models, including guinea pigs.13,14,22,36 However, very little data have been published on the pharmacokinetics and efficacy of Bup in guinea pigs. Sustained-release formulations of Bup have recently become available, providing reduced dosing frequency, prolonged plasma concentrations, and efficacy in rat and mouse models.15,23 The suggested dosage range for Bup in guinea pigs is 0.05 mg/kg every 8 to 12 h.14 This dosing regimen is similar to the recommended dosing regimen for mice, which our previous studies showed to lead to plasma concentrations that fell below therapeutic levels between doses.23 The Bup plasma concentration for the relief of moderate to severe pain in rodent species is between 500 to 1000 pg/mL.18,23 Although the therapeutic plasma concentration of Bup for guinea pigs is unknown, we targeted a therapeutic level of 500 pg/mL in the current study on the basis of previous findings from mouse, rat, and human studies.13,18,23,24 The average plasma concentrations for Bup-HCl administered every 12 h fell below 500 pg/mL between 6 and 12 h, suggesting that dosing every 8 h may be more appropriate for maintaining analgesia in guinea pigs. The response in the PWP assay had a sharp decline after 1 h and did not differ from baseline at 6 and 12 h. This finding suggests the analgesic effects in guinea pigs had diminished during the interdosing interval; however, additional investigation is needed to accurately determine the therapeutic plasma concentrations and dosing regimen in guinea pigs. In comparison, Bup-SR maintained plasma concentrations of more than 500 pg/mL until 26 h, which then fell below 500 pg/mL between 26 and 48 h. Given that the plasma concentration at 48 h was 429 pg/mL, a dosing interval of 24 to 48 h may be appropriate for Bup-SR in guinea pigs. More thorough study is required to determine the time point when plasma concentrations fall below therapeutic levels.
We used the RS assay to evaluate the efficacy of Bup analgesia in guinea pigs. This assay measures the withdrawal response of a hindpaw to the application of a linearly increasing mechanical force1,27 and has been used in many experiments to evaluate analgesics in rats.2,5,6,8,9,17,29,31,33 The RS assay is considered to be the most predictive among available acute pain models.17,26 Furthermore, tests that measure tonic pain, such as paw and tail pressure tests, are particularly appropriate for assessing postsurgical pain because they activate similar c-polymodal nociceptors.28 These tests are more effective and more appropriate than are assays that use heat for determining relative analgesic potencies of opioid drugs in guinea pigs and other rodents.19 Conditioning animals for at least 4 d prior to conducting RS assays improves the test's sensitivity,1,32 and testing the plantar compared with the dorsal surface of the paw increased repeatability and withdrawal thresholds in rats.30
Few reports address the use of the RS assay in guinea pigs. In one report, the assay was conducted similarly to what we describe.35 To increase the sensitivity of the assay, the guinea pig sows in our study were trained for 5 d before testing began and were tested on the plantar surface of a hindpaw. In previous rat studies, using the last of 3 paw-pressure measurements in the RS assay enlarged the differences between study groups,1 and the previous guinea pig study used an average of 5 tests.35 However, in the current study, we found that the first of 3 attempts was the most sensitive—by the third measurement, the guinea pigs anticipated the pressure and withdraw the paw prior to reaching the maximal force, causing a falsely low value. We reduced the anticipated response from the guinea pigs by not repeating the assay at consecutive time points. The previous study allowed for a 6-h rest between test sessions;35 we provided a rest period of 5 to 24 h between tests, with the exception of 2 guinea pigs during the Bup-SR testing. This modification minimized the anticipated response (that is, the development of tolerance) to PWP testing. The increased frequency of testing and the consequently shortened (3 h) rest period did not seem to affect the PWP results from the 2 guinea pigs that received additional testing.
We found the RS assay to be effective for evaluating the pain response in guinea pigs, because the PWP coincided with the Bup plasma concentrations in the Bup-HCl– and Bup-SR–treated animals. Elevated plasma concentrations were associated with an increase in PWP response, which declined as concentrations waned. This association is evident at the early time points after Bup administration. Bup-HCl showed a spike in plasma concentration and PWP response at 1 h, which fell sharply the first 12 h; in contrast, Bup-SR required some time to be released from the matrix to reach elevated plasma levels, and the PWP increased as the plasma levels increased over the first 12 h. The exception was the 26-h time point of the Bup-HCl treatment. Although the response exceeded baseline, it was lower than expected compared with the plasma levels. Pain perception varies markedly between subjects.25 Although the responses of individual guinea pigs showed similar trends in each group, interindividual variation might explain the large standard deviations between the groups in our study. For example, one particular guinea pig in the Bup-HCl group showed a maximal PWP response at all time points evaluated (3, 12, and 48 h), whereas the responses of the other guinea pigs declined back to baseline. These atypical responses from the outlier animal were likely anomalous but were included in the analysis. The sensitivity of the RS assay might have been increased by inducing an inflammatory reaction on the plantar surface of the foot by using a strong adjuvant, such as complete Freund adjuvant.28
Both groups of animals showed similar weight changes over the 72-h period, suggesting that increased pica or inappetence may not be a concern with Bup-SR compared with Bup-HCl at these dosages in guinea pigs. In other studies involving mice and rats, Bup-SR was advantageous and led to decreased weight loss compared with Bup-HCl.20 This result could simply be due to species-associated differences between mice, rats, and guinea pigs; between SR formulations, or in study methods.
The 0.3-mg/kg loading dose for Bup-SR was based on the cumulative dose of Bup-HCl of 0.05 mg/kg every 12 h for 60 h. Sedation was not noted after Bup administration. In addition, the guinea pigs that received Bup-SR developed injection-site reactions that were nonulcerative, nonpainful, roughly 1 cm in diameter, and did not require medical intervention. These lesions were less severe than were the ulcerative lesions reported in previous rodent studies using Bup-SR,7,15,20 likely because the manufacturer reformulated the matrix to reduce reactivity.
The results of our current study suggest that a single dose of Bup-SR at 0.3 mg/kg provides comparable, or better, analgesia for a prolonged period of time compared with Bup-HCl at 0.05 mg/kg administered every 12 h for 60 h. Although the therapeutic concentration of Bup in guinea pigs is unknown, the Bup-SR provided plasma concentrations that exceeded 500 pg/mL for as long as 24 h. The response of the guinea pigs to the paw-withdrawal stimulus paralleled the plasma concentrations, suggesting analgesic efficacy when plasma levels exceed 500 pg/mL. In addition, Bup-SR decreases animal handling and personnel involvement due to fewer doses, making Bup-SR an acceptable alternative to Bup-HCl for analgesia in guinea pigs. Additional studies to further evaluate the dose–response curve of Bup-SR in guinea pigs will further refine the dosing regimen of Bup-SR, with the potential to sustain plasma concentrations beyond 24 to 48 h.
References
- 1.Anseloni VCZ, Ennis M, Lidow M. 2003. Optimization of the mechanical nociceptive threshold testing with the Randall–Selitto assay. J Neurosci Methods 131:93–97. [DOI] [PubMed] [Google Scholar]
- 2.Apaydin S, Goldeli E, Uyar M, Erhan E, Yegul I, Tuglular I. 2001. The antinociceptive effect of moclobemide on the vocalization threshold to paw pressure in a rat model of unilateral mononeuropathy. Pharmacol Res 44:503–507. [DOI] [PubMed] [Google Scholar]
- 3.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 4.Bourque SL, Adams MA, Nakatsu K, Winterborn A. 2010. Comparison of buprenorphine and meloxicam for postsurgical analgesia in rats: effects on body weight, locomotor activity, and hemodynamic parameters. J Am Assoc Lab Anim Sci 49:617–622. [PMC free article] [PubMed] [Google Scholar]
- 5.Bujalska M, Gumulka SW. 2001. Effect of cyclooxygenase and NO synthase inhibitors on antinociceptive action of acetaminophen. Pol J Pharmacol 53:341–350. [PubMed] [Google Scholar]
- 6.Bujalska M, Tatarkiewicz J, de Cordé A, Gumułka SW. 2007. Effect of cyclooxygenase and nitric oxide synthase inhibitors on streptozotocin-induced hyperalgesia in rats. Pharmacology 81:151–157. [DOI] [PubMed] [Google Scholar]
- 7.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 8.Cegielska-Perun K, Bujalska-Zadrożny M, Gąsińska E, Makulska- Nowak HE. 2014. Enhancement of antinociceptive effect of morphine by antidepressants in diabetic neuropathic pain model. Pharmacol Rep 66:228–234. [DOI] [PubMed] [Google Scholar]
- 9.Chipkin RE, Latranyi MB, Iorio LC, Barnett A. 1983. Determination of analgesic drug efficacies by modification of the Randall and Selitto rat yeast paw test. J Pharmacol Methods 10:223–229. [DOI] [PubMed] [Google Scholar]
- 10.Christoph T, Kogel B, Schiene K, Meen M, De Vry J, Friderichs E. 2005. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 507:87–98. [DOI] [PubMed] [Google Scholar]
- 11.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan A. 2003. Buprenorphine: new pharmacological aspects.. Int J Clin Pract Suppl. 133:3-8. [PubMed] [Google Scholar]
- 13.Evans HC, Easthope SE. 2003. Transdermal buprenorphine. Drugs 63:1999–2010. [DOI] [PubMed] [Google Scholar]
- 14.Flecknell PA. 2009. Laboratory animal anaesthesia, 3rd ed. London (UK): Elsevier. [Google Scholar]
- 15.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 16.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butrophanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13. [PubMed] [Google Scholar]
- 17.Gainok J, Daniels R, Golembiowski D, Kindred P, Post L, Strickland R, Garrett N. 2011. Investigation of the antiinflammatory, antinociceptive effect of ellagic acid as measured by digital paw pressure via the Randall–Selitto meter in male Sprague–Dawley rats. Am Assoc Nurse Anesth J 79 4 Suppl:S28–S34. [PubMed] [Google Scholar]
- 18.Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 41:337–343. [DOI] [PubMed] [Google Scholar]
- 19.Hayes AG, Sheehan MJ, Tyers MB. 1987. Differential sensitivity of models of antinociception in the rat, mouse and guinea pig to μ and κ opioid receptor agonists. Br J Pharmacol 91:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healy JR, Tonkin JL, Kamarec SR, Saludes MA, Ibrahim SY, Matsumoto RR, Wimsatt JH. 2014. Evaluation of an improved sustained-release buprenorphine formulation for use in mice. Am J Vet Res 75:619–625. [DOI] [PubMed] [Google Scholar]
- 21.Heavaner JE, Cooper DM. 2008. Pharmacology of analgesics, p 97-123. In: Fisher RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego (CA): Academic Press. [Google Scholar]
- 22.Johnson RE, Fudala PJ, Payne R. 2005. Buprenorphine: considerations for pain management. J Pain Symptom Manage 29:297–326. [DOI] [PubMed] [Google Scholar]
- 23.Kendall LV, Hansen RJ, Dorsey K, Kang S, Lunghofer PJ, Gustafson DL. 2014. Pharmacokinetics of sustained-release analgesics in mice. J Am Assoc Lab Anim Sci 53:478–484. [PMC free article] [PubMed] [Google Scholar]
- 24.Kendall LV, Wegenast DJ, Smith BJ, Dorsey KM, Kang S, Lee NY, Hess AM. 2016. Efficacy of sustained-release buprenorphine in an experimental laparotomy model in female mice. J Am Assoc Lab Anim Sci 55:66–73. [PMC free article] [PubMed] [Google Scholar]
- 25.Latasch L, Probst S, Dudziak R. 1984. Reversal by nalbuphine of respiratory depression caused by fentanyl. Anesth Analg 63:814–816. [PubMed] [Google Scholar]
- 26.Le Bars D, Gozariu M, Cadden SW. 2001. [Critical analysis of animal models of acute pain. II] Ann Fr Anesth Reanim 20:452–470. [Article in French]. [DOI] [PubMed] [Google Scholar]
- 27.Randall LO, Selitto JJ. 1957. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 111:409–419. [PubMed] [Google Scholar]
- 28.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343. [DOI] [PubMed] [Google Scholar]
- 29.Rovati AL, Vidal y Plana RR, Casula PL, Bizzarri D, Makovec F, Setnikar I. 1979. Pharmacological study of a new nonsteroidal antiinflammatory drug: protacine (CR 604). Arzneimittelforschung 29:1116–1122. [PubMed] [Google Scholar]
- 30.Santos-Nogueira E, Redondo Castro E, Mancuso R, Navarro X. 2012. Randall–Selitto test: a new approach for the detection of neuropathic pain after spinal cord injury. J Neurotrauma 29:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. 1989. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of µ, δ, and κ receptors. J Pharmacol Exp Ther 248:1269–1275. [PubMed] [Google Scholar]
- 32.Taiwo YO, Coderre TJ, Levine JD. 1989. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res 487:148–151. [DOI] [PubMed] [Google Scholar]
- 33.Tzschentke TM, Christoph T, Kögel B, Schiene K, Hennies HH, Englberger W, Haurand M, Jahnel U, Cremers TI, Friderichs E, De Vry J. 2007. (–)-(1R, 2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel µ-opioid receptor agonist–norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 323:265–276. [DOI] [PubMed] [Google Scholar]
- 34.US Department of Agriculture [Internet]. 2014 Annual report animal usage by fiscal year. [Cited 07 October 2016]. Available at: https://www.aphis.usda.gov/animal_welfare/downloads/7023/Animals%20Used%20In%20Research%202014.pdf
- 35.Ward BB, Huang B, Desai A, Cheng XM, Vartanian M, Zong H, Shi X, Thomas TP, Kotlyar AE, Van Der Spek A, Leroueil PR, Baker JR., Jr 2012. Sustained analgesia achieved through esterase-activated morphine prodrugs complexed with PAMAM dendrimer. Pharm Res 30:247–256. [DOI] [PubMed] [Google Scholar]
- 36.Wu SZ, Liu KS, Chu KS, Cheng KI, Kuei CH, Wang JJ, Tzeng JI. 2006. The depot of buprenorphine decanoate produced a dose-related long-lasting antinociceptive effect in guinea pigs. Acta Anaesthesiol Taiwan 44:161–168. [PubMed] [Google Scholar]