Abstract
Mice are commonly anesthetized intraperitoneally with a ketamine-xylazine (KX) solution. Although this route of administration allows rapid uptake of the injected drugs, its disadvantages and potential risks include pain, peritoneal irritation, and perforation of an abdominal organ; some of the risks depend on the operator's experience. We compared the efficacy of intraperitoneal and subcutaneous administration of KX in HSD:ICR, BALB/cOlaHsd, and C57BL/6JOlaHsd mice in terms of time to onset and duration of surgical anesthesia, procedure safety, and mortality. Male and female mice (n = 20 each sex and strain) were anesthetized by using the same dose of intraperitoneal or subcutaneous KX. Time to onset and duration of immobilization and time to onset and duration of surgical anesthesia according to the pedal reflex differed significantly between strains. Within each strain, the durations of immobilization and surgical anesthesia were comparable between the routes of administration. The sex of the mouse but not the route of administration influenced whether surgical anesthesia was achieved. None of the subcutaneously-injected mice died. After intraperitoneal injections, 30% of the female mice died, compared with 3% of the male. In addition, fewer female mice achieved surgical anesthesia, suggesting a narrow therapeutic window for intraperitoneal KX in female mice. In conclusion, surgical anesthesia of mice with subcutaneous KX (K, 191.25 mg/kg; X, 4.25 mg/kg) seems to be safe, and the subcutaneous route is generally just as effective as the intraperitoneal route. The variability among mouse strains and between sexes requires further investigation to determine the optimal dosage.
Abbreviation: KX, ketamine–xylazine
Mice are commonly anesthetized by using a ketamine–xylazine (KX) cocktail.2-5,9-12,17,18,20,30,32 This combination is considered relatively safe in mice and achieves effective analgesia, muscle relaxation, and sedation.37
Ketamine is a dissociative anesthetic that produces catalepsy, analgesia, and immobilization characterized by CNS activation and sympathetic stimulation.26 This drug has a wide safety margin and a pronounced analgesic effect that prevents spinal sensitization (‘wind-up’) by inhibiting N-methyl-D-aspartate receptors.14 Xylazine is an α2-adrenergic agonist that produces species-dependent dose-related CNS depression and has powerful sedative, hypnotic, and analgesic effects.14 In rodents, the KX cocktail is generally administered through the intraperitoneal route. This injection method enables the operator to handle the animals relatively easily.
A substance's absorption, bioavailability, and metabolism are affected by its chemical and physical properties as well as by its dose and route of administration.33 In general, the rate of absorption is the fastest for the intravenous route and then decreases in the following order: intraperitoneal > intramuscular > subcutaneous > oral.36 Therefore, the intraperitoneal method of administration is commonly used in small laboratory animals in which the intravenous route is difficult to use. In addition, intraperitoneal administration is an alternative for drugs that might be degraded in the gastrointestinal tract if administered orally. Although the intraperitoneal route is considered a routine route of administration in rodents, it has several limitations and potential risks.4 These include pain, sensitivity of the peritoneum to irritating substances and to solutions of nonphysiologic pH, formation of fibrous tissue with adhesions within the abdominal cavity, needle perforation of an abdominal organ, and hemorrhage. In addition, repeated administration may result in cumulative irritant effects and needle-induced damage.33 Furthermore, intraperitoneal administration might result in injection into muscle, subcutaneous tissue, and the intestine and other organs rather than injection exclusively into the peritoneal cavity.13,15,21 All the complications mentioned can lead to discomfort, pain, and even death. Furthermore, careful aseptic conditions are needed, as well are operators that are well trained in the handling and restraint of laboratory animals.24 Therefore, identifying alternative routes for drug administration that preserve the drug's efficacy contributes to maintaining animal wellbeing.
Subcutaneous administration is easy and rarely painful in conscious mice;36 however the response to the administration of anesthesia by the subcutaneous route is considered to be less predictable than for other routes.14 Subcutaneous ketamine is occasionally used in humans for pain relief.8,29 Drug absorption through the subcutaneous route is thought to occur due to uptake by small capillaries, but its exact mechanism is unclear.33
We hypothesized that achieving surgical anesthesia with KX by using the subcutaneous route would be comparable to that after the intraperitoneal route and would therefore constitute a refinement to the procedure due to the less traumatic and less stressful injection. To that end, we compared the efficacy of KX administration by the subcutaneous route with that of the intraperitoneal route in 3 mice strains: HSD:ICR (CD1), BALB/cOlaHsd, and C57BL/6JOlaHsd. The effects of the KX administration route on the time to onset of immobilization, time to onset of surgical anesthesia, duration of surgical anesthesia, and duration of immobilization, as well as procedure safety and mortality were compared. The influence of strain and sex on each of these variables was investigated also.
Materials and Methods
This study was carried out at the Tel Aviv University Animal Facilities, which work under the Israeli state law for prevention of animal cruelty (animal experimentations) 19941 and a permit granted by the NIH. All experimental procedures were approved by the Tel Aviv University IACUC and followed the National Research Council's Guide for the Care and Use of Laboratory Animals16 and the Regulations of the Israeli Council for Experiments with Animals, 2001.34
Animals and housing conditions.
Mice from 3 different strains—ICR, BALB/c and C57BL/6J (20 males and 20 females from each strain; age, 8 to 12 wk of age)—and free of viral, bacterial, and parasitic diseases in accordance with the Guidelines of the Federation of European Laboratory Animal Science Associations (FELASA)25 were purchased from Harlan Laboratories (Jerusalem, Israel).
The mice were housed in groups of 5 in open cages and maintained on a 12:12-h light:dark cycle. Each cage was provided with unrestricted access to municipal water and sterilized food (Rodent Diet 2018c, Harlan–Teklad, Madison, WI) and sterilized laboratory animal bedding (Sani-chips 7090, Harlan–Teklad). All the cages were supplied with cotton balls (Assouta, Petach Tikva, Israel) as nesting material for enrichment. Room temperature was maintained under climate-controlled conditions at 21 ± 2 °C, and the air-change rate was 15 to 20 times hourly. The mice were acclimated for at least 1 wk before the start of the study.
Experimental procedures.
The mice were randomly allocated into 2 groups according to injection route (subcutaneous or intraperitoneal) by using the ‘rand’ function of the Excel program (Microsoft, Redmond, WA); 10 male and 10 female mice from each strain were injected by using each route. The study was performed in a blinded manner: the researcher measuring the various parameters was unaware of the route of administration of the KX cocktail.
Anesthetic drugs.
The anesthetics used in this study were ketamine (100 mg/mL, Clorketam, Vetquinol, Lure, France) and xylazine (20 mg/mL, Sedaxylan Veterinary, Eurovet Animal Health, Bladel, Netherlands). To establish the most suitable dose for achieving surgical anesthesia by the intraperitoneal injection method, a pilot study was performed in 10 male BALB/c mice per dose. Reported doses of KX for surgical anesthesia in laboratory mice range from 80 to 200 mg/kg ketamine and 0.5 to 10 mg/kg xylazine.5 In the pilot study, we evaluated 4 KX doses: 80 mg/kg ketamine and 16 mg/kg xylazine; 100 mg/kg and 4 mg/kg; 100 mg/kg and 10 mg/kg; and 191.25 mg/kg and 4.25 mg/kg. These doses are routinely used by several laboratories at our institute. In the preliminary study, intraperitoneal doses of 80–16 mg/kg and 100–4 mg/kg KX failed to achieve surgical anesthesia in mice, whereas a dose of 100–10 mg/kg accomplished partial surgical anesthesia; all mice injected intraperitoneally with 191.25–4.25 mg/kg KX achieved surgical anesthesia. We therefore chose the dose of 191.25–4.25 mg/kg KX for the main study (data not shown).
The anesthetic solution was freshly prepared at each experimental time point by diluting 0.1 mL xylazine and 0.9 mL ketamine in 3 mL sterile saline (0.9% sodium chloride) in a plastic vial to provide a final volume of 4 mL; this mixture was used immediately to facilitate accurate dosing. A volume of 0.085 mL per 10 g of body weight was injected according to either the intraperitoneal or subcutaneous route, according to a randomization scheme. The mice were weighed on a balance (catalog no. N2B110, Navigator Balance, Ohaus, Nänikon, Switzerland) that was calibrated annually. The accuracy of the balance was checked daily by using a 20-g weight.
Injection techniques.
Intraperitoneal injection.
Mice were restrained by the scruff. The ventral side of the animal was exposed, and the head was tilted down at a slight angle. A sterile 25-gauge needle was placed, bevel up, in the lower right quadrant of the animal's abdomen. The needle was inserted at a 30° angle, to a depth of approximately 0.5 cm. Aspiration was used to confirm correct needle placement, and the anesthetic solution was injected.
Subcutaneous injection.
The mice were restrained by the scruff as they stood on the wire lid of the cage. A sterile 25-gauge needle was placed in a tent of skin over the scruff, bevel up, parallel to the skin and directed toward the posterior of the animal. The needle was inserted about 0.5 cm. Aspiration was used to confirm correct needle placement, and the anesthetic cocktail was injected.
Induction and monitoring of surgical anesthesia.
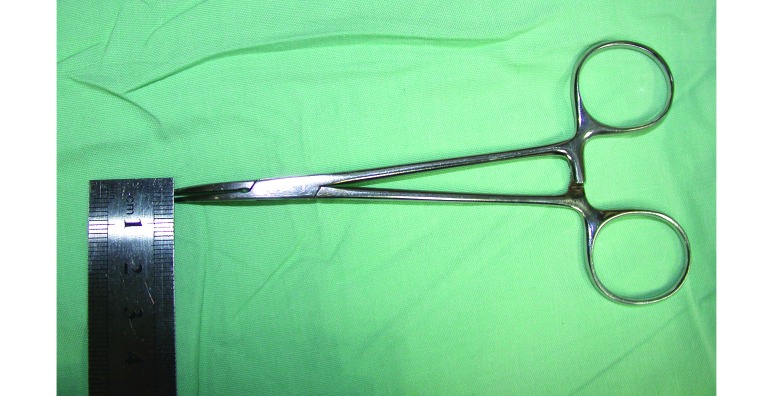
The time of injection was recorded, and the mice were immediately replaced in a cage. Once the righting reflex was lost, the animals were placed on a heated platform that accurately monitors core body temperature and automatically adjusts to maintain the correct body temperature (PhysioSuite, Kent Scientific, Torrington, CT); sterile lubricant eye drops containing carbomer (2 mg/g, Viscotears, Novartis, Basel, Switzerland) were applied to preserve eye moisture. The pedal withdrawal reflex3 was assessed on the hindlimbs and measured as the response to pressure from a modified curved 5-in. Halstead mosquito forceps closed to the first level. To minimize tissue trauma to the paw and to standardize the amount of pressure applied, the mosquito forceps were modified by bending one arm (Figure 1), thus precluding complete closure of the arms. Pedal withdrawal reflexes were recorded as fast (score, 1), moderate to low (score, 2), or absent (score, 3). The stimulus was repeated every 5 min on alternate legs until the animal displayed spontaneous locomotor activity (recorded as the recovery time). Mice with scores that did not meet the criteria (that is, did not achieve a score of 3) were considered to be in a light plane of anesthesia and thus unsuitable for surgery.
Figure 1.
Modified curved 5-in. Halstead mosquito forceps. Pedal withdrawal reflexes were measured in the hindlimbs as the response to curved 5-inch Halstead mosquito forceps closure on the first level. The mosquito forceps were modified by bending one arm, thus precluding complete closure of the arms.
The data recorded were injection time, time at loss of righting reflex, time at loss of the hindlimb pedal reflex, time at which a moderate or rapid pedal reflex was regained, and time at which the righting reflex was recovered. The variables analyzed were calculated from these times as follows: time to onset of immobilization (time from injection until loss of righting reflex), time to onset of surgical anesthesia (time from injection until loss of the pedal reflex) duration of surgical anesthesia (time from the loss of the pedal reflex to the time at which a moderate or rapid pedal withdrawal reflex was regained), and duration of immobilization (elapsed time from the loss of to recovery of the righting reflex).
Histology.
To assess whether injection by the subcutaneous route induces damage to the tissue surrounding the site of administration, BALB/c mice were injected intraperitoneally or subcutaneously as described (n = 4 for each route of administration). At 48 h after the injection, the mice were euthanized by CO2, and full-thickness samples (diameter, 1.5 cm) were excised from the tissue surrounding the site of injection for histopathology examination. In the mice injected subcutaneously, the section included the skin and subcutaneous tissue; in the mice injected intraperitoneally, the section included the skin, subcutaneous tissue, and abdominal wall.
Statistical analysis.
Results were summarized as percentages (for categorical data) or averages and standard deviations (for continuous data). The Fisher exact test and Pearson chi-squared test were used to compare categorical data between injection methods, sexes, and strains. ANOVA was conducted to compare the duration of immobilization by injection method, adjusted for sex and strain, with associated confidence intervals. Data for time to loss of righting reflex (that is, time to onset of immobilization) and duration of surgical anesthesia were not normally distributed and therefore were analyzed by using the Mann–Whitney and Kruskal–Wallis tests, with confidence intervals calculated by using the bootstrap method. Statistical analysis was performed by using SPSS 21 (IBM, Armonk, NY) and R 2.15.2 (www.r-project.org). Logistic regression was used to assess which factors were related to whether surgical anesthesia was achieved. Results were considered statistically significant when P values were 0.05 or less.
Results
Time to onset of immobilization.
The time to onset of immobilization ranged from 2.4 ± 0.5 min in C57BL/6J female mice that were injected intraperitoneally with KX to 3.1 ± 0.7 min in ICR females injected subcutaneously. In males, the time to onset of immobilization ranged from 2.3 ± 0.5 min in C57BL/6J mice injected intraperitoneally to 3.4 ± 0.5 min in BALB/c mice injected intraperitoneally (Table 1). Time to onset of immobilization differed (P < 0.001) among the strains, with C57BL/6J mice (males and females, pooled data) reaching immobilization most rapidly by both routes of administration (2.4 ± 0.5 min for mice injected intraperitoneally and 2.7 ± 0.5 min for mice injected subcutaneously) and BALB/c (males and females, pooled data) reaching immobilization the slowest for both routes of administration (3.1 ± 0.6 min for mice injected intraperitoneally and 3.0 ± 0.7 min for mice injected subcutaneously), whereas ICR mice (males and females, pooled data) reached immobilization in 2.9 ± 0.5 min when injected intraperitoneally and 2.8 ± 0.7 min when injected subcutaneously. Assessment of the time to onset of immobilization within strains showed that BALB/c female mice injected intraperitoneally reached immobilization significantly faster than did BALB/c males injected intraperitoneally (2.9 ± 0.6 min compared with 3.4 ± 0.6 min, P = 0.04), and C57BL/6J female mice injected intraperitoneally reached immobilization faster than did C57Bl/6J females injected subcutaneously (2.4 ± 0.5 min compared with 2.9 ± 0.3 min, P = 0.03). No other statistically significant differences within strains were observed for the time to onset of immobilization, either between males and females using the same route of administration or between routes of administration in the same sex (Table 1).
Table 1.
Effect of injection route on anesthetic parameters (mean ± SEM) in mice
| Sex | Route | n | Weight (g) | Died | Time to onset of immobilization (min) | Achieved surgical anesthesiaa | Time to onset of surgical anesthesia (min) | Duration of surgical anesthesia (min) | Duration of immobilization (min) | |
| BALB/c | ||||||||||
| F | IP | 10 | 20.1 ± 1.1 | 5 (50) | 2.9 ± 0.6 | 5/5 (100) | 6.7 ± 2.6 | 34.0 ± 5.5 | 67.0 ± 12.4 | |
| SC | 10 | 19.8 ± 1.3 | 0 | 2.8 ± 0.4 | 8/10 (80) | 12.5 ± 4.6 | 29.4 ± 9.0 | 67.8 ± 12.0 | ||
| M | IP | 10 | 24.8 ± 1.7 | 1(10) | 3.4 ± 0.5 | 8/9 (89) | 13.8 ± 4.4 | 28.8 ± 11.6 | 68.8 ± 14.2 | |
| SC | 10 | 25.0 ± 1.6 | 0 | 3.1 ± 0.9 | 8/10 (80) | 16.9 ± 8.0 | 25.0 ± 15.1 | 74.3 ± 16.1 | ||
| ICR | ||||||||||
| F | IP | 10 | 26.4 ± 1.9 | 2 (20) | 3.0 ± 0.5 | 2/8 (25) | 20.0 ± 10.0 | 15.0 ± 0.0 | 46.9 ± 6.3 | |
| SC | 10 | 24.7 ± 3.3 | 0 | 3.1 ± 0.7 | 6/10 (60) | 20.0 ± 7.8 | 17.5 ± 8.2 | 53.0 ± 8.0 | ||
| M | IP | 10 | 30.1 ± 1.9 | 0 | 2.7 ± 0.5 | 9/10 (90) | 6.7 ± 5.0 | 30.0 ± 7.1 | 51.7 ± 5.2 | |
| SC | 10 | 29.8 ± 1.8 | 0 | 2.4 ± 0.5 | 9/10 (90) | 15.6 ± 6.4 | 26.7 ± 12.0 | 59.1 ± 5.5 | ||
| C57BL/6J | ||||||||||
| F | IP | 10 | 18.0 ± 1.2 | 2 (20) | 2.4 ± 0.5 | 8/8 (100) | 15.0 ± 6.9 | 25.0 ± 11.0 | 72.6 ± 10.3 | |
| SCb | 9 | 19.1 ± 1.4 | 0 | 2.9 ± 0.3 | 6/9 (67) | 25.0 ± 6.7 | 27.5 ± 7.6 | 70.2 ± 19.6 | ||
| M | IP | 10 | 20.4 ± 1.3 | 0 | 2.3 ± 0.5 | 10/10(100) | 10.0 ± 7.8 | 30.5 ± 16.1 | 70.6 ± 9.1 | |
| SC | 10 | 21.0 ± 1.3 | 0 | 2.5 ± 0.5 | 8/10 (80) | 19.4 ± 15.0 | 25.6 ± 10.2 | 73.6 ± 10.4 | ||
| Total | ||||||||||
| F | IP | 30 | 21.4 ± 3.9 | 9 (30) | 2.8 ± 0.6 | 15/21 (71) | 14.4 ± 8.1 | 26.7 ± 10.5 | 61.5 ± 15.0 | |
| SCb | 29 | 21.4 ± 3.4 | 0 | 2.9 ± 0.5 | 20/29 (69) | 16.8 ± 6.9 | 25.3 ± 9.5 | 63.5 ± 15.4 | ||
| M | IP | 30 | 25.1 ± 4.3 | 1 (3) | 2.8 ± 0.6 | 27/29 (93) | 12.3 ± 6.7 | 29.8 ± 11.9 | 63.5 ± 13.0 | |
| SC | 30 | 25.3 ± 3.9 | 0 | 2.7 ± 0.7 | 25/30 (83) | 17.2 ± 6.5 | 21.5 ± 14.7 | 69.0 ± 13.2 | ||
| all | IP | 60 | 23.3 ± 4.5 | 10 (17) | 2.8 ± 0.6 | 42/50 (84) | 13.0 ± 7.3 | 28.0 ± 12.1 | 62.7 ± 13.8 | |
| SC | 59 | 23.4 ± 4.2 | 0 | 2.8 ± 0.6 | 45/59 (76) | 17.0 ± 6.6 | 25.6 ± 10.9 | 66.3 ± 14.5 | ||
F, female; IP, intraperitoneal; M, male; SC, subcutaneous
Continuous variables are displayed as mean ± 1 SD, and categorical variables are displayed as n (%).
Mice that died were excluded from analysis. Data are given as no. anesthetized/no. injected (% anesthetized)
One female C57BL/6J was excluded from the experiment due to low body weight.
Duration of immobilization.
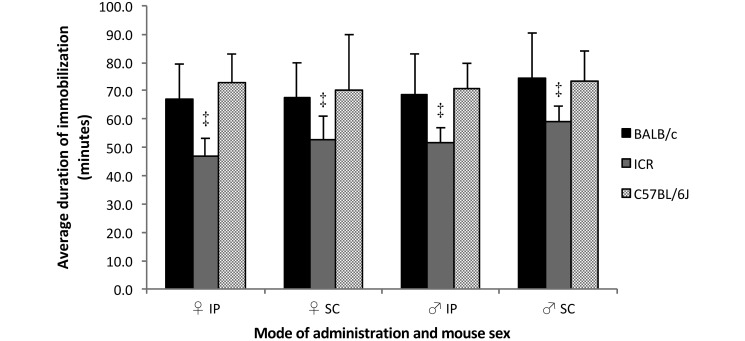
All mice became immobilized. The average duration of immobilization in female mice ranged from 46.9 ± 6.3 min in ICR females injected intraperitoneally to 72.6 ± 10.3 min in C57BL/6J females injected intraperitoneally. In males, the average duration of immobilization ranged from 51.7 ± 5.2 min in ICR males injected intraperitoneally to 74.3 ± 16.1 min in BALB/c males injected subcutaneously (Table 1). The average duration of immobilization differed significantly by strain (pooled data from all mice of a particular strain injected by both methods): specifically, ICR mice had a significantly shorter duration of immobilization than did BALB/c and C57BL/6J mice (ICR, 53.0 ± 7.5 min; BALB/c, 69.9 ± 13.3 min; C57BL/6J, 71.8 ± 12.5 min, P < 0.001, Figure 2). Within the C57BL/6J and BALB/c groups, administration of KX by either the intraperitoneal or the subcutaneous route resulted in similar average duration of immobilization, whereas ICR males injected intraperitoneally had a significantly shorter duration of immobilization than did those injected subcutaneously (51.7 ± 5.2 min compared with 59.1 ± 5.5 min, P = 0.006).
Figure 2.
Duration of immobilization among mice after subcutaneous compared with intraperitoneal administration of KX. Duration of immobilization was measured in 10 male and 10 female mice from each strain as the time from loss of to recovery of the righting reflex. ‡ P < 0.001.
The sex of the mice did not affect the average duration of immobilization within strains, regardless of the route of administration.
Achievement of surgical anesthesia.
A larger proportion of pooled male and female data from BALB/c (13 of 14 mice, 93%) and C57BL/6J (18 of 18 mice, 100%) mice injected by the intraperitoneal route achieved surgical anesthesia as compared with the subcutaneously injected counterparts (16 of 20 mice [80%] and 14 of 19 mice [74%], respectively). In contrast, more ICR mice (pooled male and female data) achieved surgical anesthesia after subcutaneous compared with intraperitoneal injection of KX (15 of 20 mice [75%] compared with 11 of 18 mice [61%]).
Notably, significantly more male mice in both administration-route groups achieved surgical anesthesia than did female mice (52 of 59 males [88%] compared with 35 of 50 females [70%], P = 0.03). Indeed, analysis by logistic regression showed that mouse sex—but not mode of administration or mouse strain—affected whether surgical anesthesia was achieved (P = 0.044).
Time to onset of surgical anesthesia.
The average time to onset of surgical anesthesia in female mice ranged from 6.7 ±2.6 min in BALB/c females injected intraperitoneally to 25.0 ± 6.7 min in C57BL/6J females injected subcutaneously. In males, the average duration of surgical anesthesia ranged from 6.7 ± 5.0 min observed in ICR mice injected intraperitoneally to 19.4 ± 15.0 min in C57BL/6J males injected subcutaneously (Table 1).
The average time to onset of surgical anesthesia differed significantly by strain: it was shortest in BALB/c mice (12.8 ± 6.3 min, pooled data of all mice of this strain injected by both methods) and longest in C57BL/6J mice (18.1 ± 6.6 min), whereas it was 13.9 ± 7.8 min in ICR mice (P < 0.0014 for the difference among the strains). In addition, the average time to onset of surgical anesthesia differed significantly by injection method (13.0 ± 7.3 min for all mice injected intraperitoneally compared with 17.0 ± 6.6 min for all mice injected subcutaneously, P < 0.0120).
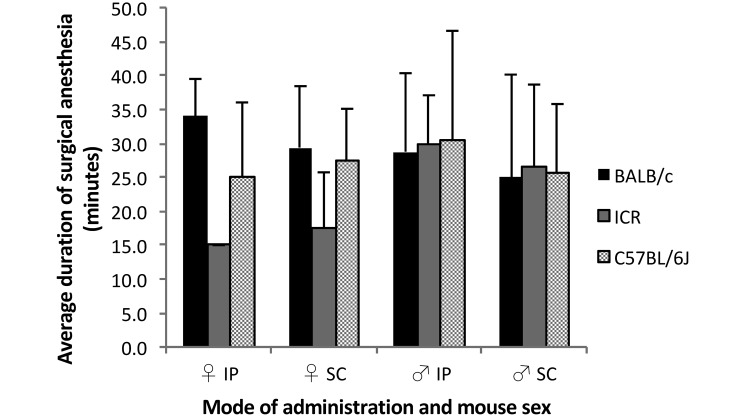
Duration of surgical anesthesia.
The average duration of surgical anesthesia in female mice ranged from 15.0 min in ICR females injected intraperitoneally (notably, only 2 of the10 ICR females injected intraperitoneally achieved surgical anesthesia) to 34.0 ± 5.5 min in BALB/c females injected intraperitoneally. In males, the average duration of surgical anesthesia ranged from 25.0 ± 15.1 min observed in BALB/c males injected subcutaneously to 30.5 ± 16.1 min in C57BL/6J males injected intraperitoneally (Table 1). Within the BALB/c and C57BL/6J groups (pooled data for males and females), the average duration of surgical anesthesia was similar after administration of KX by the intraperitoneal and subcutaneous routes (Table 1 and Figure 3). Because only 20% (2 of 10) of ICR female mice injected intraperitoneally achieved surgical anesthesia, the average duration of surgical anesthesia of ICR females injected intraperitoneally could not be compared statistically with that of the females injected subcutaneously or with that of males injected intraperitoneally within the same strain.
Figure 3.
Duration of surgical anesthesia among mice after subcutaneous compared with intraperitoneal administration of KX. Duration of surgical anesthesia was measured in male (n = 8–10) and female (n = 2–8) mice from each strain as the time from complete absence of until regaining of a moderate or rapid pedal withdrawal reflex.
Mortality.
No deaths occurred in the subcutaneous-administration groups, whereas 16.7% (10 of 60 mice) of mice injected intraperitoneally died (P = 0.0007). Significantly more females died after intraperitoneal injection (9 of 30 mice, 30%) compared with males (1 of 30 mice, 3%, P = 0.006). Specifically by strain, 50% (5 of 10) of BALB/c females, 10% (1 of 10) of BALB/c males, 20% (2 of 10) of ICR females, and 20% of (2 of 10) C57BL/6J females died after receiving the anesthetic by the intraperitoneal injection route (Table 1).
Histology.
No abnormal pathology was observed at the site of injection in the mice injected intraperitoneally. In the mice injected subcutaneously, mild edema with mixed infiltration of neutrophils and lymphocytes was present. In one case, mild infiltration of inflammatory cells between muscle fibers was noted.
Discussion
The requirement to maintain animal welfare and safety falls under the ‘Refinement’ category of the ‘3 Rs’ and constitutes an important goal in laboratory animal practice.28 Therefore, when considering an anesthetic regimen for mice undergoing a study, the invasiveness of the route and its potential effect on animal wellbeing should be considered along with other factors such as bioavailability, depth and duration of anesthesia desired, drug and equipment availability, the technical skill of the anesthetist, and the strain and sex of the animals. Any change in technique that leads to a more refined and less invasive procedure is preferable.
In the current study, we compared the efficacy of intraperitoneal administration of KX for inducing surgical anesthesia with that of subcutaneous administration of KX in 3 common laboratory mouse strains. Our results show that after injection of KX by the intraperitoneal route compared with subcutaneous dosing, statistically significant differences among strains emerged regarding the times to onset and average durations of immobilization and surgical anesthesia. Within each strain, however, both methods of administration—intraperitoneal and subcutaneous—resulted in comparable time to onset of immobilization. Exceptions were the time to onset of immobilization in BALB/c female mice injected intraperitoneally and in C57BL/6J female mice injected intraperitoneally: these times were significantly shorter than those of BALB/c males injected intraperitoneally and of C57Bl/6J females injected subcutaneously, respectively. However, these differences in the time to onset of immobilization are not clinically meaningful.
The durations of immobilization and surgical anesthesia within each strain were generally comparable between the routes of administration. An exception was the significantly shorter duration of immobilization observed for ICR males injected intraperitoneally compared with males of the same strain injected subcutaneously. Our findings are in line with those described in a previous study, which did not find statistical differences between routes of administration in mice when comparing the onset and depth of anesthesia and changes in vital signs after intraperitoneal or subcutaneous administration of ketamine (75 mg/kg) combined with medetomidine (1 mg/kg) or dexmedetomidine (0.5 mg/kg).6 Importantly, none of the mice we anesthetized by the subcutaneous route died, indicating that this administration route might be safer than intraperitoneal injection when using a dose of 191.25 mg/kg ketamine and 4.25 mg/kg xylazine.
Subcutaneous injection has many advantages. First, the animal experiences less trauma because the needle penetrates only the skin, whereas when the intraperitoneal route of administration is used, the needle penetrates the skin, muscle, and peritoneum and carries the risk of damage to internal organs.21 Second, in contrast to intraperitoneal injection, subcutaneous injection does not require turning the mouse on its back and tilting the head downward, potentially causing increased distress. Subcutaneous administration requires less technical skill than does intraperitoneal dosing, it avoids potential damage to internal organs and peritonitis due to chemical irritancy or poor technique, and, depending on the dose chosen, may result in lower mortality. The disadvantages of the subcutaneous route include the presence of a tissue reaction at the site of injection and increased variability in several of the measured parameters. For example, in the current study, fewer animals reached a surgical plane of anesthesia with subcutaneous dosing than with intraperitoneal dosing. This difference could be problematic for investigators wanting to perform surgery in KX-anesthetized animals.
Sex, strain, and the route of administration can influence the response to surgical anesthesia induced by KX and its effectiveness. Strain-associated differences regarding the response to surgical anesthesia can be expected, especially between inbred strains and between outbred compared with inbred strains.19,22,23,26,31 In our study, sex and strain appeared to influence the parameters of surgical anesthesia. This effect was particularly notable as the smaller proportions of ICR females injected intraperitoneally or subcutaneously that achieved surgical anesthesia (25% and 60%, respectively), compared with 90% of ICR males injected by either route of administration. Moreover, the time to onset of surgical anesthesia was relatively longer in the ICR females who did achieve surgical anesthesia, whereas the duration of anesthesia was relatively short. Mouse sex and strain affected mouse mortality after KX injection by the intraperitoneal route, with BALB/c female mice showing the highest rate of mortality. The observation that more females died after intraperitoneal injection than did males injected by the same route coupled with the observation that fewer female than male mice achieved surgical anesthesia suggests a narrow therapeutic window for KX in female mice when administered intraperitoneally. Strain, body weight, age, and sex contributed to anesthetic variability in rabbits15 possibly due to differences in plasma corticosteroids, sex hormones, or hepatic enzymes.17 The influence of the sex of the animal on anesthetic agents remains poorly researched and understood.7 Studies in humans have shown that women seem to be more sensitive to opioid receptor agonists than are men and may experience respiratory depression and other adverse effects when given the same dosage as that for males.7,27 In addition, women have a 20% to 30% greater sensitivity to muscle relaxants.27 No definitive evidence has been found in the laboratory animal literature to support our finding. The observed differences among strains imply that further experiments should examine the specific dose for each mouse strain and sex, given that the ideal dose of KX for subcutaneous administration may be higher or different doses of the individual components of the cocktail may be required compared with those for intraperitoneal administration. It is important that investigators realize that an animal's sex and strain can affect the efficacy of KX anesthesia and account for these variabilities when planning their studies.
Histology analysis of the injection sites revealed only mild damage to the surrounding tissues in the mice injected subcutaneously. This finding should be further explored to confirm that subcutaneous can replace intraperitoneal as a route of administration for inducing surgical anesthesia with KX. Although the intraperitoneal injection is generally presumed to not induce damage to the surrounding tissue, it is difficult to evaluate the site of injection in the peritoneum because the internal organs change locations, whereas the specific site of injection after subcutaneous administration is easier to locate. One group35 reported that findings at the intraperitoneal injection sites of a KX combination consisted of inflammation and adipose necrosis, which were interpreted as being secondary to, or extensions of, muscle necrosis.
In conclusion, surgical anesthesia of mice with 191.25 mg/kg ketamine and 4.25 mg/kg xylazine by using the subcutaneous route of administration seems to be safe and, in some cases, just as effective as the intraperitoneal route. Ideal doses for inducing surgical anesthesia by using KX injected subcutaneously have yet to be determined and may well differ among various strain–sex combinations. The dose we used in the current study worked well for BALB/c males by the intraperitoneal route but appeared too high for BALB/c females when given by the intraperitoneal route and too low for ICR females when given by either the subcutaneous or intraperitoneal route.
We presume that the wellbeing of the mice is enhanced with the subcutaneous route of administration because it may cause less distress and does not involve turning the animal on its back. Furthermore, subcutaneous administration avoids the risk of puncture of abdominal organs. The subcutaneous route may therefore be interpreted as a potential refinement of the use of murine models in biomedical research and provide an effective alternative to intraperitoneal dosing when anesthetizing mice with KX. Because we noted considerable variability between mouse strains and between sexes in several of the parameters measured, further studies should investigate the influence of variables such as body composition, metabolic parameters, and hormonal differences on anesthetic parameters after subcutaneous administration of KX. In addition, safe and effective KX doses for strain- and sex-specific subgroups should be determined.
Acknowledgments
We thank Dr Sharon Furman-Assaf for assisting with the preparation of the manuscript.
References
- 1.AAALAC [Internet]. 2005. Prevention of cruelty to animals law (experiments on animals) 5754–1994. [Cited 06 October 2016]. Available at:https://aaalac.org/intlRefs/Israel/Israel-Law.pdf
- 2.Alves HC, Valentim AM, Olsson IA, Antunes LM. 2009. Intraperitoneal anaesthesia with propofol, medetomidine, and fentanyl in mice. Lab Anim 43:27–33. [DOI] [PubMed] [Google Scholar]
- 3.Arras M, Autenried P, Rettich A, Spaeni D, Rulicke T. 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 51:443–456. [PubMed] [Google Scholar]
- 4.Borchard RE, Barnes CD, Eltherington LG. 1990. Drug dosage in laboratory animals: a handbook. Caldwell (NJ): Telford Press. [Google Scholar]
- 5.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 6.Burnside WM, Flecknell PA, Cameron AI, Thomas AA. 2013. A comparison of medetomidine and its active enantiomer dexmedetomidine when administered with ketamine in mice. BMC Vet Res 9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campesi I, Fois M, Franconi F.2012. Sex and gender aspects in anesthetics and pain medication. Handb Exp Pharmacol 214:265 –278.PubMed. [DOI] [PubMed]
- 8.Eide K, Stubhaug A, Oye I, Breivik H. 1995. Continuous subcutaneous administration of the N-methyl-d-aspartic acid (NMDA) receptor antagonist ketamine in the treatment of postherpetic neuralgia. Pain 61:221–228. [DOI] [PubMed] [Google Scholar]
- 9.Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blumel G. 1984. A comparative study with various anesthetics in mice (pentobarbitone, ketamine–xylazine, carfentanyl–etomidate). Res Exp Med (Berl) 184:159–169. [DOI] [PubMed] [Google Scholar]
- 10.Flecknell P. 2009. Anaesthesia of common laboratory species: special considerations. p 181–234. In: Laboratory Animal Anaesthesia 3rd ed. London (UK): Academic Press. [Google Scholar]
- 11.Flecknell PA, Richardson CA, Popovoc A. 2007. Laboratory animals. p 765–784. In: Tranquilli WJ, Thurmon JC, Grimm KA. Lumb and Jones’ veterinary anesthesia, 4th ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 12.Gaertner DJ, Hallman TM, Hankenson FC, Batcheder MA. 2008. Anesthesia and analgesia for laboratory rodents, chapter 10 p 239–297. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals. New York (NY): Academic Press. [Google Scholar]
- 13.Gaines Das R, North D. 2007. Implications of experimental technique for analysis and interpretation of data from animal experiments: outliers and increased variability resulting from failure of intraperitoneal injection procedures. Lab Anim 41:312–320. [DOI] [PubMed] [Google Scholar]
- 14.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. 2012. Mice anesthesia, analgesia, and care: part I. Anesthetic considerations in preclinical research. ILAR J 53:E55–E69. [DOI] [PubMed] [Google Scholar]
- 15.Green CJ, Knight J, Precious S, Simpkin S. 1981. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10-y experience. Lab Anim 15:163–170. [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.Ishizaka S, Sievers RE, Zhu BQ, Rodrigo MC, Joho S, Foster E, Simpson PC, Grossman W. 2003. New technique for measurement of left ventricular pressure in conscious mice. Am J Physiol Heart Circ Physiol 286:H1208–H1215. [DOI] [PubMed] [Google Scholar]
- 18.Kawahara Y, Tanonaka K, Daicho T, Nawa M, Oikawa R, Nasa Y, Takeo S. 2005. Preferable anesthetic conditions for echocardiographic determination of murine cardiac function. J Pharmacol Sci 99:95–104. [DOI] [PubMed] [Google Scholar]
- 19.Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. 2002. Genetic variation in morphine analgesic tolerance: a survey of 11 inbred mouse strains. Pharmacol Biochem Behav 73:821–828. [DOI] [PubMed] [Google Scholar]
- 20.Meyer RE, Fish RE. 2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers, p 27–82. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals. San Diego (CA): Academic Press. [Google Scholar]
- 21.Miner NA, Koehler J, Greenaway L. 1969. Intraperitoneal injection of mice. Appl Microbiol 17:250–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogil JS, Smith SB, O'Reilly MK, Plourde G. 2005. Influence of nociception and stress-induced antinociception on genetic variation in isoflurane anesthetic potency among mouse strains. Anesthesiology 103:751–758. [DOI] [PubMed] [Google Scholar]
- 23.Moskowitz AS, Terman GW, Carter KR, Morgan MJ, Liebeskind JC. 1985. Analgesic, locomotor, and lethal effects of morphine in the mouse: strain comparisons. Brain Res 361:46–51. [DOI] [PubMed] [Google Scholar]
- 24.Nevalainen T, Dontas I, Forslid A, Howard BR, Klusa V, Kasermann HP, Melloni E, Nebendahl K, Stafleu FR, Vergara P, Verstegen J, FELESA. 2000. FELASA recommendations for the education and training of persons carrying out animal experiments (category B). Report of the Federation of European Laboratory Animal Science Associations Working Group on Education of Persons Carrying out Animal Experiments (Category B) accepted by the FELASA Board of Management. Lab Anim 34:229–235. [DOI] [PubMed] [Google Scholar]
- 25.Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B, FELESA (Federation of European Laboratory Animal Science Associations Working Group on Health Monitoring of Rodent and Rabbit Colonies). 2002. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36:20–42. [DOI] [PubMed] [Google Scholar]
- 26.Paddleford R. 2000. Small animal anesthesia. Milano-Cremona (Italy): Masson. [Google Scholar]
- 27.Pleym H, Spigset O, Kharasch ED, Dale O. 2003. Gender differences in drug effects: implications for anesthesiologists. Acta Anaesthesiol Scand 47:241–259. [DOI] [PubMed] [Google Scholar]
- 28.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (United Kingdom): Methuen. [Google Scholar]
- 29.Slatkin NE, Rhiner M. 2003. Ketamine in the treatment of refractory cancer pain: case report, rationale, and methodology. J Support Oncol 1:287–293. [PubMed] [Google Scholar]
- 30.Smith W. 1993. Responses of laboratory animals to some injectable anaesthetics. Lab Anim 27:30–39. [DOI] [PubMed] [Google Scholar]
- 31.Sonner JM, Gong D, Eger EI., 2nd 2000. Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg 91:720–726. [DOI] [PubMed] [Google Scholar]
- 32.Spikes SE, Hoogstraten-Miller SL, Miller GF. 1996. Comparison of 5 anesthetic agents administered intraperitoneally in the laboratory rat. Contemp Top Lab Anim Sci 35:53–56. [PubMed] [Google Scholar]
- 33.Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 34.Weizmann Institute of Science [Internet]. 2001. [Regulations ofthe Israeli Council for experiments with animals\ 2001.] [Cited6 October 2016.] https://www.weizmann.ac.il/vet/Takanot.pdf[Article in Hebrew [Google Scholar]
- 35.Wellington D, Mikaelian I, Singer L. 2013. Comparison of ketamine–xylazine and ketamine–dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci 52:481–487. [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfensohn SE, Lloyd MH. 1994. Aleutian disease in laboratory ferrets. Vet Rec 134:100. [DOI] [PubMed] [Google Scholar]
- 37.Wyatt JD, Scott RA, Richardson ME. 1989. The effects of prolonged ketamine–xylazine intravenous infusion on arterial blood pH, blood gases, mean arterial blood pressure, heart and respiratory rates, rectal temperature and reflexes in the rabbit. Lab Anim Sci 39:411–416. [PubMed] [Google Scholar]