Abstract
Although oral gavage is the most straightforward approach to achieve precise enteric administration in rodents, it is associated with potential adverse consequences. Here we compare the effects of serial oral gavage in awake compared with anesthetized mice. Female C57BL/6J mice (n = 20 per group) were assigned to 1 of 3 treatment groups (control, awake gavage, or anesthetized gavage) and gavaged daily with 0.2 mL of saline (with no manipulation on weekends) for a total of 18 treatment days. Body weight and clinical appearance were monitored throughout the treatment period, after which mice were euthanized and necropsied. Endpoints evaluated included adrenal gland weight, plasma corticosterone, lymphocyte:neutrophil ratio, and esophageal histopathology. Mean body weight did not differ between groups. Compared with other groups, the awake gavage group had more mice removed (3 of 20) prior to study completion due to body weight loss greater than 10%, with corresponding gross and histopathologic lesions attributed to the gavage procedure. Mice gavaged when awake had an over 20-fold higher incidence of incomplete retention of the administered saline than did anesthetized mice. Of the mice that completed the study, esophageal inflammation was not apparent at necropsy regardless of treatment, with the exception of a single mouse in the awake gavage group. Although WBC and lymphocyte counts were lower in mice in the anesthetized gavage group compared with other groups, none of the endpoints measured to evaluate stress (adrenal gland weight, neutrophil:lymphocyte ratio, plasma corticosterone) differed. These findings support the use of brief isoflurane anesthesia when performing serial oral gavage in mice.
A common experimental technique performed on rodents is dosing by means of oral gavage, which involves passing a feeding needle through the mouth and into the esophagus. Oral gavage is the most straightforward approach to achieve precise enteric administration in rodents.19,35 However, the technique is associated with potential adverse consequences, including (but not limited to) esophageal trauma, aspiration pneumonia, and weight loss.4,10,15,23,27 In addition, stress has been associated with oral gavage performed in awake rodents, potentially confounding experimental endpoints.5,6,8,13 Restraint of awake rats6,8,12,32 and mice18 for oral gavage reportedly is stressful, and handling alone is a well-established stressor for mice.5 However, other studies concluded that rats were not unduly stressed after 28 consecutive days of oral gavage37 or 6 consecutive weeks of awake oral gavage.4 Several potential refinements and alternatives to oral gavage have been examined, including coating the gavage needle tip in palatable solutions,18 using a flexible catheter in place of a rigid gavage needle,39 and offering the dose in a flavored formulation that is voluntarily consumed by the rodent.11,16,38,40
To ameliorate the potential consequences of oral gavage, brief inhalant anesthesia has been proposed as a potential refinement compared with performing the procedure in awake animals.27 However, information is limited and in some cases conflicting regarding whether anesthetizing rodents for oral gavage results in reduced morbidity and mortality.1,25,27,33 To our knowledge, the effect of oral gavage dosing on anesthetized mice had not been studied previously. We performed the current study to assess the effects of serial oral gavage in awake compared with anesthetized mice. Our hypothesis was that histopathology would not differ between the groups but that daily anesthesia would result in increased stress (reflected by higher plasma corticosterone levels, adrenal gland weights, or neutrophil:lymphocyte ratios, or any combination of these effects) or reduced weight gain or both.
Materials and Methods
Animals.
All animal procedures performed in this study were approved by the Vanderbilt University IACUC. Animals were housed in an AAALAC-accredited facility in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals19 and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.29 On receipt from The Jackson Laboratory (Bar Harbor, ME), mice were maintained in a facility screened for and found free of the following pathogens: mouse hepatitis virus, mouse parvovirus, minute virus of mice, lymphocytic choriomeningitis virus, Sendai virus, pneumonia virus of mice, epizootic diarrhea of infant mice, Theiler mouse encephalomyelitis virus, mouse poxvirus, mouse adenovirus, mouse reovirus, mouse norovirus, Mycoplasma pulmonis, Helicobacter spp., endoparasites (Syphacia spp. and Aspicularis spp.), and ectoparasites (Myobia musculi, Radfordia afffinis, Mycoptes musculinus, and Psorergates simplex). Throughout the study, mice were maintained on a 12:12-h light:dark cycle and group-housed in autoclaved IVC with unrestricted access to both autoclaved water and PicoLab Irradiated Diet 5053 (LabDiet, St Louis, MO).
Oral gavage.
Female C57BL/6J mice (age, 6 wk; n = 60) were acclimated for 2 wk after receipt without any handling. Because study goals did not include examining sex-associated differences, only female mice were used here to minimize stress associated with intracage fighting. At 8 wk of age, mice were randomly assigned by body weight to 1 of 3 daily treatment groups (n = 20 per group): control (brief [less than 30 s] manual restraint), awake gavage (brief manual restraint and oral gavage), or anesthetized gavage (2% isoflurane for less than 5 min and oral gavage). A single experimenter (CPJ) with more than 2 y of experience in oral gavage of mice administered all treatments once daily between 0730 and 0900, with no manipulation on weekends, for a total of 18 treatment days. Body weights were measured twice each week. Mice were observed for at least 15 min immediately after oral gavage of 0.2 mL sterile 0.9% saline by using a reusable, straight, 20-gauge stainless steel feeding needle with a 2.25-mm ball (SouthPointe Surgical Supply, Coral Springs, FL). To ensure that the ball tip of the gavage needle passed the entire length of the esophagus, the gavage needle used was 3 in. long. The gavage needle was never forcibly advanced nor advanced further than the premeasured length from nose tip to costal margin (1.75 in. [4.5 cm]).
Clinical outcome.
Criteria for early removal from study included signs of labored breathing, hunched posture, lethargy, and greater than 10% loss of body weight. Clinical notes were made when any mouse's appearance or behavior immediately after dosing was abnormal. Each dose was scored for abnormal clinical outcome as none, gasping, or incomplete retention of the administered saline. Incomplete retention was defined as the appearance of saline in the mouth immediately after administration of the gavage dose.
Necropsy and histopathology.
Approximately 24 h after administration of the last gavage dose, mice were euthanized by carbon dioxide overdose in accordance with the AVMA Guidelines for the Euthanasia of Animals (2013 edition).2 Whole blood was collected from the caudal vena cava and preserved with EDTA for hematology analysis and corticosterone measurement. All blood samples were obtained between 0840 and 0945. Mice were randomized at necropsy, and 2 prosectors blinded to group composition performed all necropsies and dissections. Both adrenal glands were dissected and weighed as a pair for each mouse. The mouth, neck, thoracic cavity, and stomach were assessed for gross pathologic changes. The 3 mice removed early from study were evaluated grossly and microscopically but were excluded from analyses of adrenal gland weight, hematologic parameters, and plasma corticosterone.
Whole plucks (tongue, pharynx, trachea, lung, heart, and esophagus extending to and including the gastric cardia) were stretched on cardstock and fixed in 10% neutral buffer formalin. Once fixed, each esophagus was removed from the pluck, processed routinely, embedded in paraffin, sectioned longitudinally at 5 μm, and stained with hematoxylin and eosin. Sections were evaluated by an experienced veterinary pathologist (KLB) who was blinded to the composition of the study groups. Microscopic evidence of pathology was scored as present or absent for each animal.
Hematology and corticosterone assays.
CBC counts were performed on an automated analyzer (Forcyte Hematology Analyzer, Oxford Science, Oxford, CT). EDTA-preserved whole blood was spun down, and plasma submitted to the Vanderbilt University Medical Center Hormone Assay and Analytical Services Core for corticosterone measurement by using an ImmuChem 125I-corticosterone double-antibody radioimmunoassay (MP Biomedicals, Orangeburg, NY).
Statistical analyses.
Body weights over time were evaluated by linear regression, and one-way ANOVA was used to identify differences in final body weights, adrenal gland weights, hematologic parameters, and plasma corticosterone levels between groups. All values analyzed by one-way ANOVA were checked for Gaussian distributions and equal variances. One-way ANOVA analyses that resulted in significant differences between group means were further analyzed by using posthoc Tukey multiple-comparison tests. When possible given the data parameters, the Fisher exact test was the preferred contingency test. Clinical outcome per dose between groups was evaluated by using Fisher exact tests, and the incidence of histopathology (including animals removed early from study) was evaluated by using χ2 analysis. Statistical analyses were performed by using Prism 6 software (version 6.07, GraphPad, San Diego, CA).
Results
Body weight.
After randomization, the mean body weight for all 3 groups of mice was 18 g initially. Neither the rate of gain nor body weight at the end of the study differed between any of the groups (P = 0.9312, Figure 1, and F2,54 = 0.3391, P = 0.67, respectively).
Figure 1.
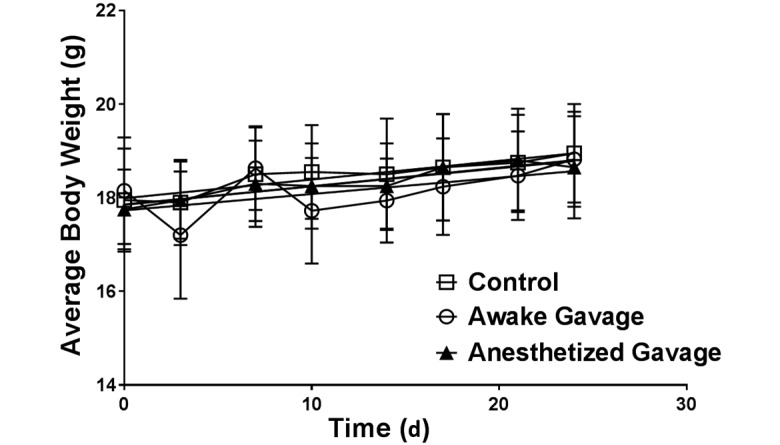
Body weight (mean ± SEM [error bars]) over time. Neither rate of weight gain nor final weight differed between groups (P = 0.93 and F2,54= 0.3391, P = 0.67, respectively).
Clinical outcome.
Nearly 7% of awake gavage doses (22 of 324 doses) were associated with incomplete saline retention, a significantly greater incidence than the 0.3% of anesthetized gavage doses (1 of 360 doses; P < 0.0001; Figure 2). In addition, gasping (1 or 2 gasps followed by recovery) occurred in 1.9% of awake gavage doses (6 of 324) compared with none in the anesthetized gavage group (P = 0.0110; Figure 2). When the total number of doses per group was considered, fewer than 1% of awake gavage doses (3 of 324 doses) were associated with adverse consequences resulting in early removal from study; this incidence did not differ significantly from that for anesthetized gavage doses (0 of 360 doses; P = 0.1058). This comparison assumes that the administration of a single dose resulted in the observed adverse consequence rather than the cumulative effect of multiple doses, an assumption supported by the acute loss (rather than a gradual decline) of body weight in individual mice (data not shown). When the total number of mice per group was considered, significantly more animals were lost over time from the awake gavage group (3 of 20 mice [15%]) compared with the anesthetized gavage group (0 of 20 mice; P = 0.0425).
Figure 2.
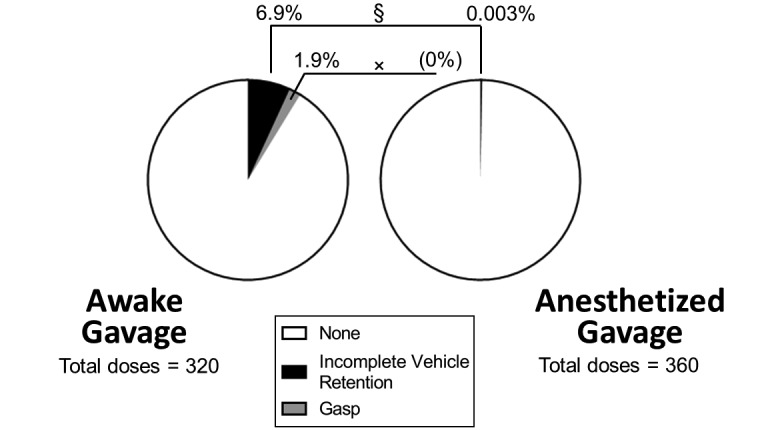
Abnormal clinical outcome per gavage dose. Compared with the anesthetized group, mice in the awake gavage group experienced increased incidences of incomplete vehicle retention (§, P < 0.0001; defined as the appearance of vehicle in the mouth immediately after administration) and gasping (×, P = 0.0110).
None of the mice in the control or anesthetized gavage groups exhibited criteria for early removal from study, whereas 3 mice in the awake gavage group were euthanized within the first 9 doses due to weight loss of greater than 10%. Of the 3 animals euthanized early, none was identified due to abnormal clinical signs during or immediately after gavage. For these individual animals, early removal from study did not correlate with previous clinical outcome per dose (that is, gasping and incomplete saline retention). At the time of early removal from study, one mouse was mildly hunched, another had a rough coat and was less active, and the remaining animal displayed no other sign but weight loss. None of the mice in any group met the criteria for early removal after study day 10.
Necropsy.
Of the 3 mice removed early from study, the 2 mice that displayed clinical signs both had complete esophageal tears with unilateral fibrinopleuritis. The mouse with only weight loss had a compressive salivary gland hematoma with a dilated, aerophagic esophagus. Other than those in the animals removed early from study, the only gross lesion observed during necropsy was mild esophageal dilation (esophagectasia) at the heart base (2 affected animals, one each from the awake and anesthetized oral gavage groups).
Histopathology.
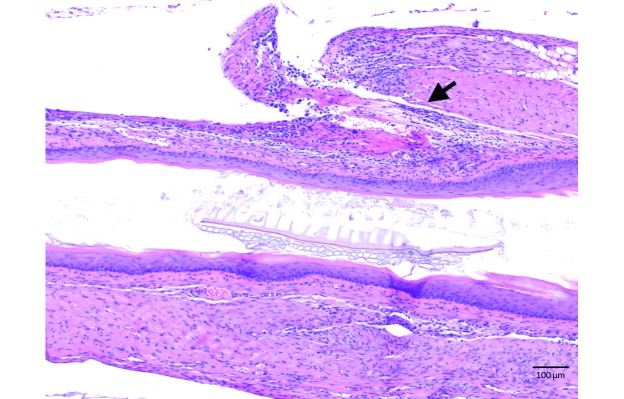
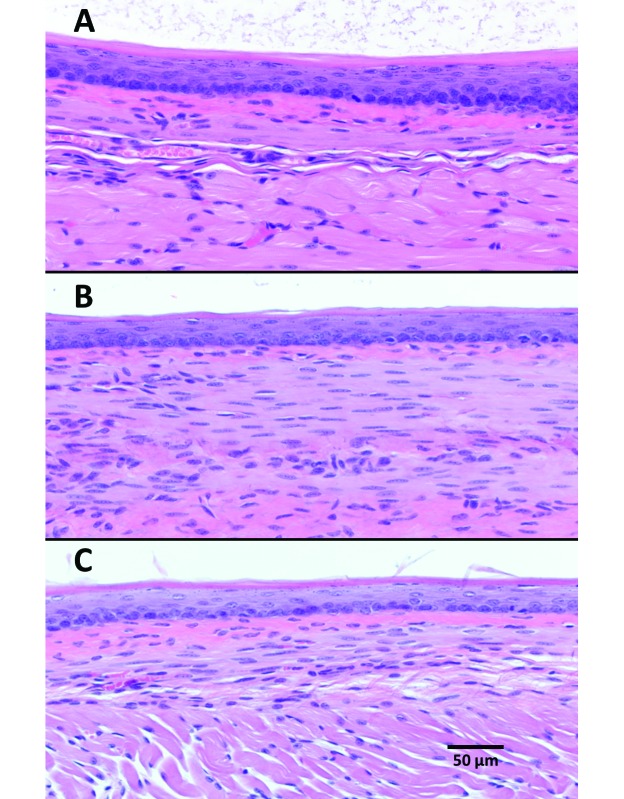
There was no corresponding abnormal histopathology for the 2 cases of esophagectasia (one from each gavage group) observed on gross necropsy. None of the animals that completed the study had abnormal histopathology, with the exception of one mouse in the awake gavage group that was found to have periesophagitis consistent with a partial esophageal tear (Figure 3) despite a lack of clinical signs throughout the treatment period and no gross findings at necropsy. All mice removed early from study had corresponding abnormal histopathology. Specifically, the 2 mice with complete esophageal tears had acute, unilateral, fibrinous pleuritis with exogenous vegetal material present and an acute, fibrinous inflammation of esophageal connective tissue, whereas the mouse with the compressive salivary gland hematoma had interstitial hemorrhage and congested blood vessels within the gland. Significantly more animals in the awake gavage group had histologic lesions associated with the procedure when compared with the anesthetized gavage group (4 of 20 compared with 0 of 20, respectively; P = 0.0138; Table 1). Aside from these findings, there was no evidence of esophageal inflammation for any other mice in any of the 3 groups (Figure 4).
Figure 3.
Esophageal histopathology of a mouse in the awake gavage group, with marked periesophagitis within the esophageal wall (arrow) suggestive of a healed, partial esophageal tear. Hematoxylin and eosin stain; magnification, 10×.
Table 1.
Necropsy results
| Control | Awake gavage | Anesthetized gavage | |
| Mild gross esophagectasia | 0 | 1 | 1 |
| Histopathology | 0 | 4a | 0 |
| Esophageal tear | 0 | 3a,b | 0 |
| Salivary gland hematoma | 0 | 1 | 0 |
Data are given as the number of mice affected (n = 20 per group)
Significantly (P < 0.05) different from values for control and anesthetized gavage groups
Includes one partial esophageal tear
Figure 4.
Representative esophageal histology from (A) control, (B) awake gavage, and (C) anesthetized gavage treatment groups. Hematoxylin and eosin stain; magnification, 20×.
Measures of stress.
None of the endpoints measured to evaluate stress differed significantly between groups, including adrenal gland weight (F2,54 = 0.9386, P = 0.0719), neutrophil:lymphocyte ratio (F2,54 = 0.3361, P = 0.4802), and plasma corticosterone (F2,54 = 2.956, P = 0.0556). However, one-way ANOVA indicated significantly different means between groups for both total WBC (F2,54 = 0.08944, P = 0.0170) and lymphocyte (F2,54 = 0.03715, P = 0.0172) counts, and posthoc Tukey analyses revealed that mice in the anesthetized group had lower total WBC and lymphocyte counts on average when compared with either the control group or the awake gavage group (Table 2).
Table 2.
Hematologic parameters, plasma corticosterone concentrations, and adrenal gland weights of mice
| Control | Awake gavage | Anesthetized gavage | |
| WBC (×103/μL) | 6.40 ± 0.63 | 6.42 ± 0.57 | 5.38 ± 0.59a |
| Neutrophils (×103/μL) | 1.17 ± 0.12 | 1.13 ± 0.12 | 1.01 ± 0.12 |
| Lymphocytes (×103/μL) | 4.68 ± 0.49 | 4.79 ± 0.47 | 3.93 ± 0.46a |
| Neutrophil:lymphocyte ratio | 0.25 ± 0.02 | 0.24 ± 0.03 | 0.26 ± 0.03 |
| Plasma corticosterone concentration (ng/mL) | 284.86 ± 50.20 | 370.57 ± 129.10 | 381.13 ± 85.50 |
| Adrenal gland weight (mg) | 9.35 ± 1.00 | 10.65 ± 1.96 | 11.65 ± 1.53 |
Data are given as mean ± SEM (n = 20 per group)
Significantly different (P < 0.05) compared with values for control and awake gavage groups
Discussion
Support for the use of brief anesthesia as an oral gavage refinement includes the observation that stress has been associated with the technique in awake rodents.5,6,8,13 In addition, a higher rate of adverse consequences when performing gavage in awake compared with anesthetized rats was seen previously.28 The AALAS Learning Library lesson entitled “Working with the Laboratory Mouse”1 advises considering anesthesia for oral gavage to reduce the risk of esophageal trauma (citing reference28) and then contradicts the use of anesthesia by describing that an awake mouse will facilitate administration by swallowing the feeding needle.1,35 Letters to the editor in response to the previous study28 reported lower morbidity and mortality in awake rats and mice and suggested that the results might reflect insufficient training,25,33 although the authors’ response (published alongside the letters to the editor) defended personnel experience, training, and technique and proposed that the true incidence of adverse consequences associated with oral gavage is underreported.27 Arguments for performing oral gavage in awake animals include published neuronal effects of long-term (greater than 2 h) isoflurane anesthesia,7,9,24,41,42 potential drug interactions,28 and a higher incidence of incomplete saline retention in rats when anesthetized for the procedure.28 We performed the current study to assess the effects of serial oral gavage performed on awake compared with anesthetized mice.
In our study, the mean body weight of neither gavage treatment group (awake nor anesthetized) differed significantly from that of the control group over the 4-wk course of daily gavage treatment. The most consistent criterion that resulted in early removal from study was loss of more than 10% of body weight. Although other clinical signs were seen variably in affected mice, signs were mild and nonspecific, emphasizing the importance of recording individual body weight over time when monitoring the health of animals undergoing oral gavage. Given that adverse consequences were not readily apparent after the associated dose administration, definitively attributing animal loss to the oral gavage procedure itself would have been difficult without necropsy. This observation supports the hypothesis that adverse consequences secondary to awake oral gavage are potentially underrecognized and underreported,27 particularly when concurrent with other study manipulations.
Esophageal tear was the most common adverse consequence associated with gavage dosing, exclusively found here among mice undergoing awake gavage. In addition, we found 2 cases of esophagectasia, one from each gavage group (awake and anesthetized). These cases were mild and subclinical and had no corresponding histopathology, but esophagectasia associated with oral gavage might be a factor to consider in a subset of scientific studies for its potential effect on mouse physiology (for example, gastrointestinal transit time). One case of salivary gland hematoma occurred (in the awake gavage group). Although granulomatous inflammation of the salivary gland occurred in a gavage-dosed control animal,30 to our knowledge salivary gland hemorrhage secondary to oral gavage has not been reported previously. This unexpected adverse consequence is likely very rare and of much less concern than is the risk of esophageal tear when performing oral gavage in mice. Although we initially intended to score esophageal inflammation on a scale of 0 to 4, other than animals that were removed early due to adverse consequences and one mouse with mild periesophagitis discovered histologically (all within the awake gavage group), esophageal inflammation was not apparent (score, 0) in mice dosed by oral gavage—awake or anesthetized. When all doses were assessed collectively, adverse consequences were not significantly different in awake or anesthetized mice. However, cumulative loss of mice dosed serially was significantly higher in those that were gavaged when awake compared with anesthetized.
Several factors might have influenced our findings, and results are likely multifactorial. First, the size of the gavage needle we chose for this study (20-gauge with a 2.25-mm ball) was one size larger than that AALAS recommends for mice weighing 18 g (the average initial body weight).1 However, we based our choice of needle size on other oral gavage studies, which used the same size feeding needle on mice of the same strain and age or younger without reported morbidity or mortality.10,18 In addition, the Kent Scientific product catalog lists a gavage needle with an even larger tip (2.4 mm) for mice weighing 15 to 20 g.22 Furthermore, the same gavage needle was used for all gavage dose administrations for both awake and anesthetized dosing. If gavage needle size was the only reason for injury, adverse consequences would be expected to occur in both awake and anesthetized animals. Second, as we described in the introduction section, training and retraining of rodent oral gavage technique is an important component of personnel competency.25,33 Although the person that performed all gavage procedures for this study has more than 2 y of experience dosing more than 60 mice daily in a preclinical setting, this person had not recently performed daily oral gavage of mice prior to the start of this study. All early removals due to adverse consequences took place in the first 2 wk of the study, so it is possible that the experimenter became more proficient in oral dosing by the third week of study. However, this person had performed oral gavage regularly prior to beginning this study, and no appreciable change in technique was observed over the 4-wk study period. Last, overall, we did not find histopathologic changes in mice esophaguses at the end of our 4-wk study period. However, another study found a similar pattern of adverse consequences when performing awake gavage in mice, with 15% mortality within the first 2 of 6 wk of daily gavage dosing;4 and another study reported altered immunotolerance after 14 d of consecutive gavage.23 These findings suggest that, within the first 2 wk, 1) a physical change may occur in the esophageal epithelium and/or 2) mice become behaviorally acclimated to the awake gavage procedure. Regardless of putative contributing factors, the highest risk of adverse consequence secondary to gavage appears to be within the first 2 wk of daily awake oral gavage.
Because one of the arguments for performing this procedure in anesthetized animals is the associated stress of handling and restraint of awake rodents, we measured several endpoints to evaluate stress in the treatment groups studied. These measures included adrenal gland weight,13 plasma corticosterone,26 and neutrophil:lymphocyte ratio.36 None of the measures differed significantly from the control group results (Table 1), indicating that within the study period we evaluated (approximately 4 wk), mice may become acclimated to the stress associated with daily oral gavage and daily brief anesthesia. Because the concentration of circulating glucocorticoids is not generally a sensitive indicator of stress, due to interanimal variability and normal diurnal cycles of glucocorticoid production, we measured adrenal gland weight as an anatomic pathology biomarker of subacute to chronic stress.13 In addition, the neutrophil:lymphocyte ratio has been suggested as a potentially sensitive biomarker of chronic stress in rodents.36 Although the neutrophil:lymphocyte ratios did not differ between our treatment groups, mice undergoing daily brief anesthesia for oral gavage on average had significantly fewer WBC and lymphocytes. Although lymphocyte counts in mice can decrease with handling or other stressors,31,34 several studies suggest that anesthetics (including isoflurane in mice) have immunomodulatory effects3,14,20 and isoflurane anesthesia has been associated with altered WBC counts in small mammals. One study found decreased total WBC and neutrophil counts in mice 2 d after more than 30 min of isoflurane anesthesia,20 and decreased lymphocytes have been observed in island flying foxes (Pteropus hypomelanus) after brief restraint and isoflurane anesthesia.17 The decreased lymphocyte counts observed here for mice anesthetized daily might be indicators of mildly increased stress, but none of the measures of stress we assessed indicated significantly increased stress for any particular group evaluated.
The incidence of incomplete saline retention was more than 20 times higher in mice gavaged when awake than in those gavaged when anesthetized with isoflurane, in contrast to the opposite trend in rats anesthetized with halothane for gavage.28 Incomplete retention of the administered substance is undesirable and can lead to numerous confounders for experimental procedures because the precise dose is not delivered.
Oral gavage provides precise control of dose volume and timing21,35 which are critical factors in many studies. Refinements and alternatives to oral gavage (for example, voluntary oral consumption, flexible catheters) should be considered when selecting materials and methods for any study requiring oral dosing. In addition, training and retraining of personnel is important to minimize the risk of adverse consequences during rodent oral gavage. In the current study, awake gavage was associated with both a higher incidence of incomplete saline retention and more frequent esophageal trauma, resulting in removal of animals from the study. In addition, as compared with other groups, we found no evidence of greater stress after anesthesia, as reflected by corticosterone levels, neutrophil:lymphocyte ratio, and adrenal weight. Finally, we found that body weight was not affected by 18 d of daily brief anesthesia for gavage. In conclusion, our findings support the use of brief isoflurane anesthesia when performing serial oral gavage in mice.
Acknowledgments
The Vanderbilt University Medical Center Hormone Assay and Analytical Services Core is supported by NIH grants DK059637 and DK020593, and we thank Dr Dale Edgerton for his assistance.
The Translational Pathology Shared Resource is supported by NCI/NIH Cancer Center Support Grant 2P30 CA068485-14 and the Vanderbilt Mouse Metabolic Phenotyping Center Grant 5U24DK059637-13, and we are grateful to Atef Khalil and Tania Dawant for their assistance with necropsy and blood collection.
We thank Dr William Dupont (Department of Biostatistics, Vanderbilt University) for consultation on sample size and study design.
References
- 1.American Association for Laboratory Animal Science. [Internet] 2005. AALAS learning library animal care and use courses. Working with the laboratory mouse. Lesson 12. Oral gavage. [Cited 13 October 2016]. Available at: https://www.aalaslearninglibrary.org/Pages/Courses/Lessons.aspx?intCourseID=2451&intTrackID=0. [Google Scholar]
- 2.American Veterinary Medical Association (AVMA). [Internet] 2013. AVMA guidelines for the euthanasia of animals, 2013 ed. [Cited 18 December 2015]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
- 3.Anderson SL, Duke-Novakovski T, Singh B. 2014. The immune response to anesthesia: part 1. Vet Anaesth Analg 41:113–126. [DOI] [PubMed] [Google Scholar]
- 4.Arantes-Rodrigues R, Henriques A, Pinto-Leite R, Faustino-Rocha A, Pinho-Oliveira J, Teixeira-Guedes C, Seixas F, Gama A, Colaco B, Colaco A, Oliveira PA. 2012. The effects of repeated oral gavage on the health of male CD1 mice. Lab Anim (NY) 41:129–134. [DOI] [PubMed] [Google Scholar]
- 5.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 6.Bonnichsen M, Dragsted N, Hansen AK. 2005. The welfare impact of gavaging laboratory rats. Anim Welf 14:223–227. [Google Scholar]
- 7.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. 2010. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 112:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AP, Dinger N, Levine BS. 2000. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci 39:17–21. [PubMed] [Google Scholar]
- 9.Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM. 2014. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology 120:626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Meijer VE, Le HD, Meisel JA, Puder M. 2010. Repetitive orogastric gavage affects the phenotype of diet-induced obese mice. Physiol Behav 100:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diogo LN, Faustino IV, Afonso RA, Pereira SA, Monteiro EC, Santos AI. 2015. Voluntary oral administration of losartan in rats. J Am Assoc Lab Anim Sci 54:549–556. [PMC free article] [PubMed] [Google Scholar]
- 12.Dobrakovova M, Jurcovicova J. 2009. Corticosterone and prolactin responses to repeated handling and transfer of male rats. Exp Clin Endocrinol 83:21–27. [DOI] [PubMed] [Google Scholar]
- 13.Everds NE, Snyder PW, Bailey KL, Bolon B, Creasy DM, Foley GL, Rosol TJ, Sellers T. 2013. Interpreting stress responses during routine toxicity studies: a review of the biology, impact, and assessment. Toxicol Pathol 41:560–614. [DOI] [PubMed] [Google Scholar]
- 14.Faller S, Strosing KM, Ryter SW, Buerkle H, Loop T, Schmidt R, Hoetzel A. 2012. The volatile anesthetic isoflurane prevents ventilator-induced lung injury via phosphoinositide 3-kinase–Akt signaling in mice. Anesth Analg 114:747–756. [DOI] [PubMed] [Google Scholar]
- 15.Germann PG, Ockert D. 1994. Granulomatous inflammation of the oropharyngeal cavity as a possible cause for unexpected high mortality in a Fischer 344 rat carcinogenicity study. Lab Anim Sci 44:338–343. [PubMed] [Google Scholar]
- 16.Gonzales C, Zaleska MM, Riddell DR, Atchison KP, Robshaw A, Zhou H, Sukoff-Rizzo SJ. 2014. Alternative method of oral administration by peanut butter pellet formulation results in target engagement of BACE1 and attenuation of gavage-induced stress responses in mice. Pharmacol Biochem Behav 126:28–35. [DOI] [PubMed] [Google Scholar]
- 17.Heard DJ, Huft VJ. 1998. The effects of short-term physical restraint and isoflurane anesthesia on hematology and plasma biochemistry in the island flying fox (Pteropus hypomelanus). J Zoo Wildl Med 29:14–17. [PubMed] [Google Scholar]
- 18.Hoggatt AF, Hoggatt J, Honerlaw M, Pelus LM. 2010. A spoonful of sugar helps the medicine go down: a novel technique to improve oral gavage in mice. J Am Assoc Lab Anim Sci 49:329–334. [PMC free article] [PubMed] [Google Scholar]
- 19.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 20.Jacobsen KO, Villa V, Miner VL, Whitnall MH. 2004. Effects of anesthesia and vehicle injection on circulating blood elements in C3H/HeN male mice. Contemp Top Lab Anim Sci 43:8–12. [PubMed] [Google Scholar]
- 21.Johnson MD, Cox Gad S, Kemper CJ. 2015. The rat, p 153–284. In: Gad SC. Animal models in toxicology. Boca Raton (FL): CRC Press. [Google Scholar]
- 22.Kent Scientific Corporation.[Internet]. 2016Surgical instruments—feeding needles for laboratory rats and mice. [Cited 07 January 2016]. Available at: https://www.kentscientific.com/products/productView.asp?productID=6224&Mouse_Rat=Surgical&Products=Feeding+Needles.
- 23.Kinder JM, Then JE, Hansel PM, Molinero LL, Bruns HA. 2014. Long-term repeated daily use of intragastric gavage hinders induction of oral tolerance to ovalbumin in mice. Comp Med 64:369–376. [PMC free article] [PubMed] [Google Scholar]
- 24.Makaryus R, Lee H, Yu M, Zhang S, Smith SD, Rebecchi M, Glass PS, Benveniste H. 2011. The metabolomic profile during isoflurane anesthesia differs from propofol anesthesia in the live rodent brain. J Cereb Blood Flow Metab 31:1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntyre K. 2001. Rodent gavage technique concerns: avoiding excess mortality. Contemp Top Lab Anim Sci 40:7–7. [PubMed] [Google Scholar]
- 26.Möstl E, Palme R. 2002. Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74. [DOI] [PubMed] [Google Scholar]
- 27.Murphy S. 2001. Training could prevent deaths due to rodent gavage procedure. [Author response to the letter to the editor.] Contemp Top Lab Anim Sci 40:8. [PubMed] [Google Scholar]
- 28.Murphy SJ, Smith P, Shaivitz AB, Rossberg MI, Hurn PD. 2001. The effect of brief halothane anesthesia during daily gavage on complications and body weight in rats. Contemp Top Lab Anim Sci 40:9–12. [PubMed] [Google Scholar]
- 29.National Institutes of Health. [Internet] 2002. PHS policy on humane care and use of laboratory animals. [Cited 18 September 2015]. Available at: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-02-062.html
- 30.National Toxicology Program 2008. Toxicology and carcinogenesis studies of formamide (Cas No. 75-12-7) in F344/N rats and B6C3F1 mice (gavage studies). Natl Toxicol Program Tech Rep Ser 541:1–192. [PubMed] [Google Scholar]
- 31.O'Connell KE, Mikkola AM, Stepanek AM, Vernet A, Hall CD, Sun CC, Yildirim E, Staropoli JF, Lee JT, Brown DE. 2015. Practical murine hematopathology: a comparative review and implications for research. Comp Med 65:96–113. [PMC free article] [PubMed] [Google Scholar]
- 32.Pace TWW, Spencer RL. 2005. Disruption of mineralocorticoid receptor function increases corticosterone responding to a mild, but not moderate, psychologic stressor. Am J Physiol Endocrinol Metab 288:E1082–E1088. [DOI] [PubMed] [Google Scholar]
- 33.Rao GN, Peace TA, Hoskins DE. 2001. Training could prevent deaths due to rodent gavage procedure. Contemp Top Lab Anim Sci 40:7–8. [PubMed] [Google Scholar]
- 34.Schwab CL, Fan R, Zheng Q, Myers LP, Hébert P, Pruett SB. 2005. Modeling and predicting stress-induced immunosuppression in mice using blood parameters. Toxicol Sci 83:101–113. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu S.2004. Routes of administration. p 527–542. In: Hedrich HJ, Bullock G. The handbook of experimental animals: the laboratory mouse. San Diego (CA): Academic Press. [Google Scholar]
- 36.Swan MP, Hickman DL. 2014. Evaluation of the neutrophil–lymphocyte ratio as a measure of distress in rats. Lab Anim (NY) 43:276–282. [DOI] [PubMed] [Google Scholar]
- 37.Turner PV, Vaughn E, Sunohara-Neilson J, Ovari J, Leri F. 2012. Oral gavage in rats: animal welfare evaluation. J Am Assoc Lab Anim Sci 51:25–30. [PMC free article] [PubMed] [Google Scholar]
- 38.Walker MK, Boberg JR, Walsh MT, Wolf V, Trujillo A, Duke MS, Palme R, Felton LA. 2012. A less stressful alternative to oral gavage for pharmacologic and toxicological studies in mice. Toxicol Appl Pharmacol 260:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheatley JL. 2002. A gavage dosing apparatus with flexible catheter provides a less stressful gavage technique in the rat. Lab Anim (NY) 31:53–56. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler TL, Eppolito AK, Smith LN, Huff TB, Smith RF. 2007. A novel method for oral stimulant administration in the neonate rat and similar species. J Neurosci Methods 159:282–285. [DOI] [PubMed] [Google Scholar]
- 41.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. 2008. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid β protein level in vivo. Ann Neurol 64:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. 2012. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 71:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]