Abstract
Carbon dioxide is the most commonly used gas for euthanasia of rodents. The current AVMA Guidelines recommend slowly filling the container with CO2 (SF) and now indicate that the practice of placing conscious animals in containers prefilled with CO2 (PF) is unacceptable. An investigator noted pulmonary hemorrhage (PH) in BALB/c mice euthanized by SF that was not observed after PF. Here we evaluated whether the air-displacement rate (SF compared with PF) influenced the development of PH or nasal hemorrhage (NH) in 2 commonly used mouse strains. In addition, we investigated the prevalence of PH and NH in mice euthanized by isoflurane overdose (IO). Male and female (age groups, 6 wk and 6 mo) BALB/c and C57BL/6 mice were euthanized by SF or PF. In addition, 6-mo-old BALB/c male mice were euthanized by IO. Lung, nasal turbinates, brain, and reproductive organs were collected for gross and histologic evaluation and scored for degree of hemorrhage (score, 0 to 3). Severity of hemorrhage did not differ according to mouse age or sex. PH in BALB/c mice was more severe after SF than PF, and SF and PF induced more severe PH in BALB/c than in C57BL/6 mice. PH in 6-mo-old male BALB/c mice was more severe after SF than IO. Neither SF, PF, nor IO influenced the prevalence of NH in any group. This study demonstrates that the method of euthanasia may need to be altered depending on the mouse strain used.
Abbreviations: IO, isoflurane overdose; NH, nasal hemorrhage; PF, prefilled with CO2; PH, pulmonary hemorrhage; SF, slowly filled with CO2
Carbon dioxide is the most commonly used gas for the euthanasia of rodents. The inhalation of CO2 causes respiratory acidosis and produces a reversible anesthetic state.6,24 A CO2 concentration of 30% or higher causes deep anesthesia and, with prolonged exposure, results in death.7 CO2 euthanasia methods include placing an animal into a chamber already prefilled with 100% CO2 (PF) or into a chamber that is slowly filled with CO2 at a fixed rate (SF). The anesthetic and rapid depressant effects of CO2 have been well established.3,4 CO2 is easily accessible, inexpensive, and does not cause accumulation of toxic tissue residues in animals.3,4 These advantages are especially striking when compared with other methods, such as isoflurane inhalation and injectable euthanasia solutions. Disadvantages of CO2 euthanasia include substantial and unpredictable differences in susceptibility to inhaled CO2 according to the species and age of the animal.9,27 In rats and mice, multiple reports of conflicting responses to different concentrations of CO2 are available.10,22,23,25,34 Moreover in rats, CO2 exposure can lead to pulmonary and upper respiratory tract lesions.11 Pulmonary hemorrhage (PH) due to CO2 euthanasia in rodents has been recognized as a background lesion,14,28 and the severity of pulmonary alveolar hemorrhages induced by CO2 inhalation is roughly proportional to the CO2 concentration inhaled.28 In contrast, we have noted extensive PH after low inhaled CO2 concentrations during SF euthanasia. Currently no studies demonstrating whether the presence of PH is positively or negatively correlated with the inhaled CO2 concentration used in euthanasia of mice are available. Likewise, we lack information regarding whether strain, age, or sex of mice affects the severity of CO2-induced PH.
Current guidelines recommend air displacement rate with CO2 of 10% to 30% per minute and advocate against placing conscious animals in prefilled chambers.2,4 The recommendation to expose conscious animals to decreased CO2 concentrations is based on human and animal responses to different concentrations of CO2.4,10,11 The filling rate of the chamber (10% to 30% CO2 per minute) is based on the time required to rapidly and successfully render animals unconscious with limited aversive stimuli. With this goal, many animal facilities have implemented the SF method and discontinued the PF method. After this change, an investigator at our animal facility noted that BALB/c mice euthanized by SF had PH, which was not previously observed with PF. Our subsequent pilot study indicated that, compared with the PF method, SF CO2 euthanasia method induced extensive PH and NH in BALB/c mice.
Hundreds of inbred mouse strains are currently in use in biomedical research.21 Each strain of mice shows strain-specific genotypic and phenotypic characteristics, thus accommodating a wide range of research needs. For animal welfare, ethical, and legal reasons, it is essential to ensure that euthanasia is accomplished with minimal pain and distress. Therefore, differences between strains need to be identified, and euthanasia protocols might need to be modified to accommodate these differences. The aim of this study was to determine whether the air-displacement rate (SF compared with PF) influenced the development of PH and NH in 2 commonly used mouse strains, BALB/c and C57BL/6. In addition, PF and SF were compared with isoflurane overdose (IO) in 6-mo-old BABL/c male mice.
Materials and Methods
Animals.
CO2 euthanasia was evaluated in a total of 79 mice. BALB/cAnNcrl and C57BL/6NCrl mice (age groups, 6 wk and 6 mo; 43 female, 36 male) were purchased from Charles River Laboratories (Wilmington, MA); the SF and PF methods of euthanasia were alternated sequentially in these mice. In addition, 7 male 6-mo-old BALB/cAnNcrl mice were obtained from the same vendor and euthanized by the IO method. All mice were maintained in a AAALAC-accredited animal facility in groups of approximately 5 per cage, separated by sex, in IVC (7.70 in. × 12.17 in. × 5.25 in. [5.8 L]; no. 1 Small Mouse Cage, Thoren Caging Systems, Hazelton, P) on a 12:12-h light:dark cycle. Room temperature was maintained at 70 to 72 °C, and the air-exchange rate was maintained at 10 to 15 air-changes hourly in the room and 60 in the rack. Mice were acclimated for 2 wk prior to study. All procedures were reviewed and IACUC-approved. Organisms excluded from our facilities include Mycoplasma pulmonis,ectromelia virus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, mouse hepatitis virus, mouse norovirus, mouse parvovirus, minute virus of mice, PVM, reovirus 3, Sendai virus, Theiler encephalomyelitis virus, Aspiculuris tetraptera, Myocoptes spp., Radfordia and Myobia spp., Syphacia obvelata, fur mites, mesostigmatid mites, lice, Spironucleus muris, Giardia muris, large intestinal flagellates, amoebas, pinworms, and tapeworms.
Euthanasia method.
Mice were euthanized individually in a standard mouse IVC (no. 1 Small Mouse Cage, Thoren Caging Systems). This cage was not bedded and was not a home cage. For the SF method, mice were placed in the cage, and CO2 at a flow rate of 1.2 L/min was used to displace air at the rate of 21% per minute. Once the mice were unconscious (at approximately 3 to 4 min), the CO2 flow rate was increased to 4 L/min; this flow rate was maintained for 1 min, and then mice were removed from the cage. For the PF method, the cage was filled with CO2 at 4 L/min for 30 s, mice were placed in the cage, and the CO2 flow rate remained at 4 L/min for 90 s after mice were placed in the chamber. The CO2 entered from the top of the cage.
For the IO method, mice were placed in a clear, plastic induction box (10 in. × 4 in. × 4 in.; AB1, Braintree Scientific, Braintree, MA), and 5% isoflurane gas in O2 at a flow rate of 1.0 L/min was piped into the box. The mice remained in the induction box for about 3 to 4 min until deceased. One mouse was placed in the induction box at a time.
As mandated by institutional policy, cervical dislocation was performed in all mice immediately after euthanasia. Lung, nasal turbinates, brain, and reproductive organs were collected for gross and histologic evaluation (hematoxylin and eosin stain) by a blinded board-certified pathologist. Three transverse sections (cross sections) of lung and nasal cavity were scored from 0 to 3 according to the severity of PH and NH lesions: score of 0, no PH lesions noted; 1, PH involved less than 10% of cross-sectional area of the slide; 2, PH involved more than 10% but less than 20% of the cross-sectional area; 3, PH involved 20% or more of the cross-sectional area (Figure 1). The scoring scheme for nasal turbinate sections was: 0, no NH lesions noted; 1, free RBC occupied less than 5% of the total nasal sinus space on the slide; 2, free RBC occupied more than 5% but less than 10% of the total nasal sinus space; 3, free RBC occupied 10% or more of the nasal sinus space (Figure 2).
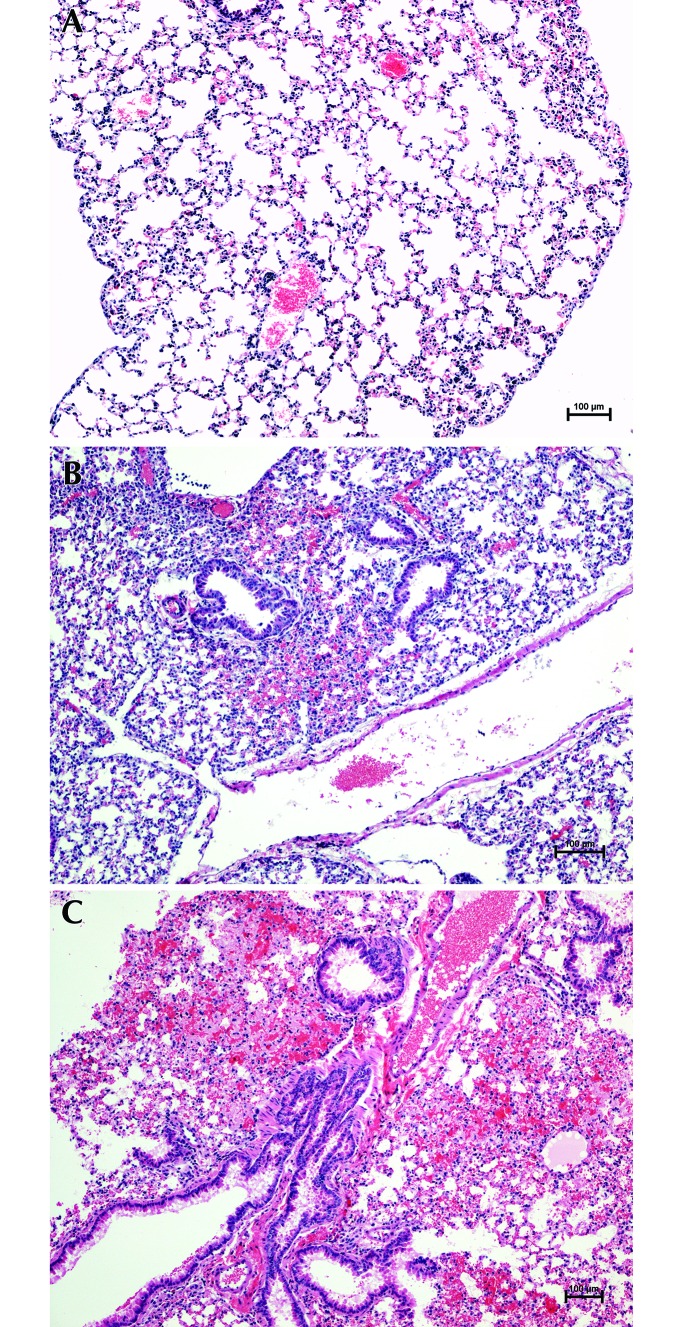
Figure 1.
Severity of PH. (A) Score, 0: no lesions (6-mo-old BALB/c female mouse euthanized by PF). (B) Score, 1 or 2: intermediate PH lesions involving less than 20% of the cross-sectional area (6-wk-old C57BL/6 female mouse euthanized by SF). (C) Score, 3: severe PH lesions involving 20% or more of the cross-sectional area (6-mo-old BALB/c male mouse euthanized by SF). Hematoxylin and eosin stain; magnification, 10×.
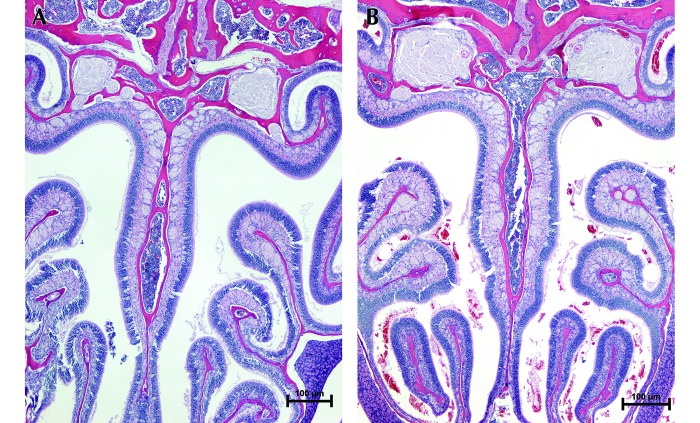
Figure 2.
Severity of NH. (A) Score, 0: no lesions (6-wk-old female C57BL/6 female mouse euthanized by SF). (B) Score, 3: severe NH lesions, with RBC occupying 10% or more of the total nasal sinus space (6-wk-old BALB/c female mouse euthanized by PF). Hematoxylin and eosin stain; magnification, 10×.
We used the Fisher's Exact test (SAS version 9.4, SAS Institute, NC; statistical significance: P < 0.05) to compare the prevalence of severe PH and NH between mouse strains and euthanasia methods. Logistic regression was used to determine whether age or sex played a role in the prevalences of severe PH and NH in both strains of mice euthanized by SF or PF.
Results
Figures 1 and 2 show examples of the histologic scores system for PH and NH.
BALB/c mice.
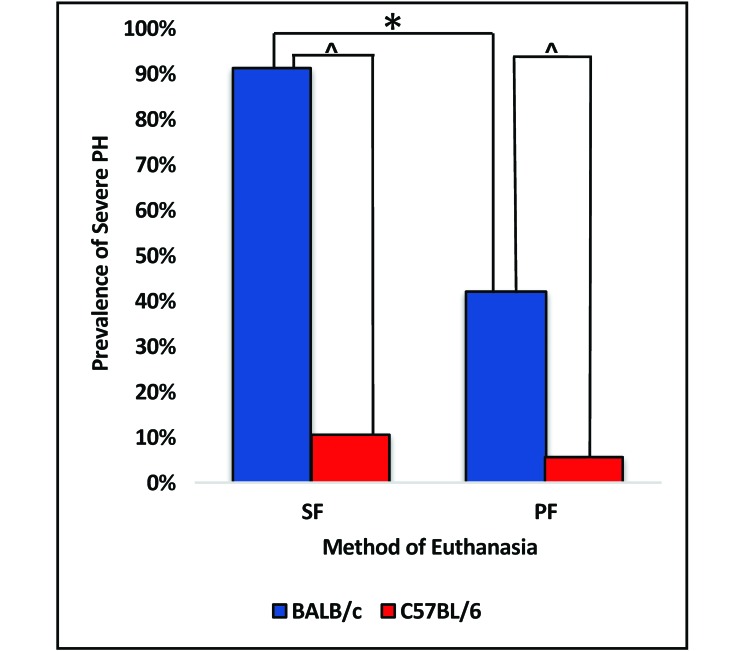
Of the 23 BALB/c mice euthanized by SF, 21 (91.3%) had severe PH, as did 8 of the 19 (42.1%) BALB/c mice euthanized by PF (Figure 3). SF induced significantly (P < 0.05) more severe PH in BALB/c mice than did PF. Of the 23 BALB/c mice euthanized by SF, 4 (17.4%) had severe NH, whereas 2 (10.5%) of the 19 BALB/c mice euthanized by PF had severe NH. The prevalence of severe NH in BALB/c mice did not differ between SF compared with PF (P = 0.6729).
Figure 3.
Prevalence of severe PH in BALB/c and C57BL/6 mice euthanized by SF or PF. SF induced significantly (*, P < 0.05) more severe PH in BALB/c mice compared with PF; SF and PF induced significantly (^, P < 0.05) more severe PH in BALB/c mice compared with C57BL/6 mice. The prevalence of severe PH did not differ in C57BL/6 mice euthanized by SF compared with PF (P = 0.1).
C57BL/6 mice.
Of the 19 C57BL/6 mice euthanized by SF, 2 (10.5%) had severe PH, as did 1 (5.6%) of the of 18 C57BL/6 mice euthanized by PF (Figure 3). The prevalence of severe PH in C57BL/6 mice did not differ between SF compared with PF (P = 0.1). None of the 19 C57BL/6 mice euthanized by SF had severe NH, whereas 2 (11.1%) of the 18 C57BL/6 mice euthanized by the PF method had severe NH. The prevalence of severe NH in C57BL/6 mice did not differ between SF and PF (P = 0.2297).
BALB/c compared with C57BL/6.
SF and PF induced significantly (P < 0.05) more severe PH in BALB/c mice compared with C57BL/6 mice (Figure 3). The prevalence of severe NH due to the SF method (P = 0.1137) or PF method (P = 1) did not differ between the strains. Logistic regression revealed that the prevalence of PH and NH were independent of sex and age for both methods of CO2 euthanasia in BALB/c and C57BL/6 mice.
IO compared with SF and PF in 6-mo-old BALB/c male mice.
None of the seven 6-mo-old BALB/c mice euthanized by IO had severe PH, whereas 7 of the 8 (87.5%) mice euthanized by SF and 1 of the 6 (16.7%) euthanized by PF had severe PH. SF induced significantly (P < 0.05) more severe PH in 6-mo-old male BALB/c mice compared with IO, but the prevalence of severe PH did not differ between IO and PF (P = 0.4615).
None out the seven 6-mo-old BABL/c mice euthanized by IO had severe NH, whereas 1 (12.5%) of the 8 euthanized by SF and 1 (16.7%) of the 6 euthanized by PF had severe NH. The prevalence of severe NH did not differ between these 3 methods of euthanasia in these mice (IO compared with SF, P = 1; IO compared with PF, P = 0.4615).
NH was limited to the nasal sinus and no damage to nasal mucosa in tissue samples was identified. There were no significant gross or histologic findings in other organs (brain and reproductive organs) in any mice.
Discussion
In this study, we demonstrated that: 1) BALB/c mice had more severe PH when euthanized by SF compared with PF; 2) compared with C57BL/6 mice, BALB/c mice had more severe PH when euthanized by SF; 3) 6-mo-old male BALB/c mice had more severe PH when euthanized by SF compared with IO; 4) there was no significant relationship between development of severe NH with either SF or PF in BALB/c and C57BL/6; and 5) the prevalence of PH and NH were independent of sex and age for both methods of CO2 euthanasia.
Immune responses to infection and inflammation are known to differ between mouse strains: for example, BALB/c mice predominantly produce a Th2-type response, whereas C57BL/6 mice predominantly produce Th1 responses.15,18,30,32 Furthermore, different strains of mice show different susceptibilities to various pathogens, such as Burkholderia pseudomallei and Trypanosoma cruzi, depending on their strain-specific immunologic responses.5,16,17,18,19 Other commonly used inbred mouse strains such as 129 and C3H/He mice express Klra genes (a part of the natural killer cell receptor that aids in antigen presentation), whereas C57BL/6 mice do not express them at all.30 Because the inhalation of CO2 is an acute event during a euthanasia procedure, whether strain-specific differences in immune responses play a role in the development of CO2-induced PH requires further investigation. Limited knowledge is available regarding the molecular mechanism of acute respiratory injury induced by inhalation toxicity. However, growing evidence suggests the involvement of nicotinamide adenine dinucleotide-phosphate oxidase and reactive oxygen species in driving pulmonary inflammation and compromising the integrity of pulmonary vessels in acute pulmonary injuries.12,13,26 Studies investigating the differences in molecular mechanisms between different mouse strains are needed to further define the pathogenesis underlying our current findings.
We also studied whether IO euthanasia led to severe PH or NH. Isoflurane is not used for euthanasia as widely as is CO2 because isoflurane is less cost effective and is a potential health hazard to humans. One previous study demonstrated that rats display more aversive behavior toward CO2 than toward isoflurane,34 however, repeated exposure of rats to isoflurane has been shown to be as aversive as CO2.8,34 In our study, 6-mo-old male BALB/c mice euthanized by SF had more severe PH than did the IO group. We did not test the effects of isoflurane in female or younger mice, but logistic regression revealed that the prevalence of PH and NH was independent of sex and age for both methods of CO2 euthanasia. From this finding, we speculate that the effects of IO on PH and NH may apply across all ages and sexes in BALB/c mice, and we surmise that isoflurane might represent a reasonable alternative euthanasia method for studies where pulmonary pathologic evaluation is important. Researchers using isoflurane need to remember to scavenge waste isoflurane gas appropriately to avoid human exposures.
In a previous study, PH occurred before the animal lost consciousness.1 In that study, the authors sampled pulmonary tissue every 30 s from 0 to 240 s after exposure to 30% CO2 (with or without 20% O2) and compared alveolar consolidation in BALB/c and nude mice (age and sex unknown).1 As the CO2 exposure time increased, cumulative lung consolidation increased, alveolar consolidation was more extensive in BALB/c than in nude mice.1 Only the right caudal lung lobe was evaluated, because it was affected in rats euthanized with CO2.11 In our study, all lung lobes were cut transversally, and the accumulation of alveolar hemorrhagic lesions in all lung lobes was scored from 0 to 3 by a board-certified anatomic pathologist. Our study showed that PH is not limited to the right caudal lung lobe; rather it tended to occur randomly in all lung lobes. Unlike the earlier study,1 the mice in our study continued to be exposed to 100% CO2 after they lost consciousness and were not removed from the chamber until the involuntary agonal breathing movements had ceased, which exceeded 300 s for the SF group compared with 120 s for the PF group. Similarly, our results showed that prolonged exposure time to CO2 at a low concentration initially (SF group) caused more severe hemorrhagic lesions compared those in the PF group in BALB/c mice. The negative correlation between the concentration of CO2 and severity of PH lesions seen in our study also was present in another study in rats11 and is probably due to the fact that a lower concentration of CO2 causes the mice to be exposed to the gas for a prolonged period.
The pathogenesis of PH and NH due to CO2 exposure is unknown. CO2 gas inhalation leads to decreased arterial oxygen tension (PaO2) and increased CO2 tension (PaCO2), which then cause respiratory acidosis and hypercapnia.31,33 Our assumption is that the acute respiratory acidosis disrupts the alveolar capillary endothelium, leading to leakage of fluid and hemorrhage into the alveolar spaces. Without compensation, prolonged exposure to or inhalation of high concentrations of CO2 causes CNS depression, followed by death.20,24,29 Alveolar consolidation can occur before the animal becomes unconscious,1 and the authors surmised that the conscious mice experience a feeling of “drowning.”1 Limiting this stress and identifying the strain-associated differences in response to various concentrations of CO2 need to be considered for the wellbeing of the animals.
We used cervical dislocation as a secondary method of euthanasia after the mice were removed from the chamber. A previous review paper mentioned cervical dislocation as a cause of PH in mice.2 If cervical dislocation alone were to cause PH, it would have been present in all mice across all groups in our study. However, PH was absent in most of our mice; in particular all 7 of the BALB/c mice euthanized by IO followed by cervical dislocation lacked significant PH.
We chose to account only for severe PH lesions (score, 3) in our statistical evaluation, given that severe alveolar hemorrhagic lesions would likely confound the interpretation of pulmonary disease study results. In our study, severe PH involved only intraalveolar hemorrhagic lesions and was distinguished from congestion, in which RBC remained confined within the vasculature. The occurrence of severe PH in conjunction with severe NH is notable. Of the 8 mice that had severe NH, 5 also had severe PH. All 5 animals that had severe PH and NH were BALB/c mice, and 4 of these 5 mice were euthanized by SF. This pattern further supports our findings that the likelihood of severe PH in BALB/c mice increases when they are euthanized by SF. It is also important to note that NH was limited to the nasal cavity lumen (sinus), and the mucosa of the nasal cavity was free of damage and hemorrhage. This observation may indicate that PH started first and the blood flowed up from the lung and into the nasal cavity, in contrast to the current supposition that mucosal irritation and damage from high CO2 concentrations lead to NH.11 In addition, no inflammatory infiltrates surrounded the PH or NH lesions, thus confirming that the hemorrhagic lesions were associated with acute gas inhalation.
In conclusion, when euthanized by the currently recommended SF CO2 method, BALB/c mice are more prone to develop severe PH than were C57BL/6 mice. This strain-associated difference in response to a euthanasia method needs to be addressed when using BALB/c mice to study pulmonary pathology, and this consideration will serve as an important refinement for animal wellbeing. In addition, the IO method can be considered as an alternative to SF CO2 euthanasia method in BALB/c mice to limit background PH. Further studies are needed to determine whether other strains of mice experience similar side effects SF CO2 euthanasia.
Acknowledgments
We thank Dr Ann Hess for assisting us with statistical analysis, Dr Tawfik Aboellail for providing histologic images, and Dr Jessica Ayers for manuscript editing.
References
- 1.Ambrose N, Wadham J, Morton DB. 2000. Refinement of euthanasia, p 1159–1170. In: Balls M, Halder ME, van Zeller A-M. editors. Progress in reduction, refinement, and replacement of animal experimentation. Oxford (UK):Elsevier. [Google Scholar]
- 2.Artwohl J, Brown P, Corning B, Stein S. [Internet]. 2005. Public Statements: Report of the ACLAM task force on rodent euthanasia. [Cited 15 November 2015]. Available at: http://www.aclam.org/Content/files/files/Public/Active/report_rodent_euth.pdf.
- 3.AVMA [Internet]. 2007. AVMA Guidelines for the euthanasia of animals: 2007 ed. [Cited 15 October 2015]. Available at: https://www.avma.org/KB/Policies/Pages/Euthanasia-Guidelines.aspx
- 4.AVMA [Internet]. 2013. Guidelines for the euthanasia of animals: 2013 ed. [Cited 15 October 2015]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf
- 5.Breitbach K, Klocke S, Tschernig T, van Rooijen N, Baumann U, Steinmetz I. 2006. Role of inducible nitric oxide synthase and NADPH oxidase in early control of Burkholderia pseudomallei infection in mice. Infect Immun 74:6300–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosnan RJ, Pham TL. 2008. Carbon dioxide negatively modulates N-methyl-d-aspartate receptors. Br J Anaesth 101:673–679. [DOI] [PubMed] [Google Scholar]
- 7.Cartner SC, Barlow SC, Ness TJ. 2007. Loss of cortical function in mice after decapitation, cervical dislocation, postassium chloride injection, and CO2 inhalation. Comp Med 57:570–573. [PubMed] [Google Scholar]
- 8.Chisholm J, De Rantere D, Fernandez NJ, Krajacic A, Pang DS. 2013. Carbon dioxide, but not isoflurane, elicits ultrasonic vocalizations in female rats. Lab Anim 47:324–327. [DOI] [PubMed] [Google Scholar]
- 9.Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C. 1996. Recommendations for euthanasia of experimental animals: Part 1. DGXI of the European Commission. Lab Anim 30:293–316. [DOI] [PubMed] [Google Scholar]
- 10.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161. [DOI] [PubMed] [Google Scholar]
- 11.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385. [PubMed] [Google Scholar]
- 12.Frazziano G, Moreno L, Moral-Sanz J, Menendez C, Escolano L, Gonzalez C, Villamor E, Alvarez-Sala JL, Cogolludo AL, Perez-Vizcaino F. 2011. Neutral sphingomyelinase, NADPH oxidase and reactive oxygen species. Role in acute hypoxic pulmonary vasoconstriction. J Cell Physiol 226:2633–2640. [DOI] [PubMed] [Google Scholar]
- 13.Frazziano G, Champion HC, Pagano PJ. 2012. NADPH oxidase-derived ROS and the regulation of pulmonary vessel tone. Am J Physiol Heart Circ Physiol 302:H2166–H2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frith CH, Ward JM. 1988. Respiratory system. Color atlas of neoplastic and nonneoplastic lesions in aging mice. New York (NY): Elsevier. [Google Scholar]
- 15.Garcia-Pelayo MC, Bachy VS, Kaveh DA, Hogarth PJ. 2015. BALB/c mice display more enhanced BCG vaccine induced Th1 and Th17 response than C57BL/6 mice but have equivalent protection. Tuberculosis (Edinb) 95:48–53. [DOI] [PubMed] [Google Scholar]
- 16.Gessner A, Blum H, Rollinghoff M. 1993. Differential regulation of IL9 expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology 189:419–435. [DOI] [PubMed] [Google Scholar]
- 17.Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noel A, Cataldo DD. 2009. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res 58:845–854. [DOI] [PubMed] [Google Scholar]
- 18.Guyach SE, Bryan MA, Norris KA. 2009. Differences in antibody and immune responses between BALB/c and C57BL/6 mice infected with Trypanosoma cruzi. J Immunol 182 (Suppl 1):129.5. [abstract] [Google Scholar]
- 19.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. 1989. Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T-cell subsets. J Exp Med 169:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin RS, Rippe JM. 2008. Irwin and Rippe's intensive care medicine, 6th ed. Philadelphia (PA): Lippincott Williams and Wilkins. [Google Scholar]
- 21.Isogenic [Internet]. 2005. Mouse strains. [Cited 15 December 2015]. Available at: http://isogenic.info/html/mouse_strains.html.
- 22.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Aversion to gaseous euthanasia agents in rats and mice. Comp Med 52:249–257. [PubMed] [Google Scholar]
- 23.Makowska JI, Vickers L, Mancell J, Weary DM. 2009. Evaluating methods of gas euthanasia for laboratory mice. Appl Anim Behav Sci 121:230–235. [Google Scholar]
- 24.Martoft L, Lomholt L, Kolthoff C, Rodriguez BE, Jensen EW, Jorgensen PF, Pedersen HD, Forslid A. 2002. Effects of CO2 anaesthesia on central nervous system activity in swine. Lab Anim 36:115–126. [DOI] [PubMed] [Google Scholar]
- 25.Moody CM, Chua B, Weary DM. 2014. The effect of carbon dioxide flow rate on the euthanasia of laboratory mice. Lab Anim 48:298–304. [DOI] [PubMed] [Google Scholar]
- 26.Perng DW, Chang TM, Wang JY, Lee CC, Lu SH, Shyue SK, Lee TS, Kou YR. 2013. Inflammatory role of AMP-activated protein kinase signaling in an experimental model of toxic smoke inhalation injury. Crit Care Med 41:120–132. [DOI] [PubMed] [Google Scholar]
- 27.Pritchett-Corning KR. 2009. Euthanasia of neonatal rats with carbon dioxide. J Am Assoc Lab Anim Sci 48:23–27. [PMC free article] [PubMed] [Google Scholar]
- 28.Renne R, Brix A, Harkema J, Herbert R, Kittel B, Lewis D, March T, Nagano K, Pino M, Rittinghausen S, Rosenbruch M, Tellier P, Wohrmann T. 2009. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol Pathol 37 7 Suppl:5S–73S. [DOI] [PubMed] [Google Scholar]
- 29.Rice SA. [Internet].2004. Human health risk assessment of CO2: survivors of acute high-level exposure and population sensitive to prolonged low-level exposure. Presented at the 3rd annual conference on carbon sequestration. Alexandria, Virginia, 3–6 May 2004 [Cited 15 November 2015]. Available at: https://www.netl.doe.gov/publications/proceedings/04/carbon-seq/169.pdf [Google Scholar]
- 30.Sellers RS, Clifford CB, Treuting PM, Brayton C. 2011. Immunologic variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol 49:32–43. [DOI] [PubMed] [Google Scholar]
- 31.Sieker HO, Hickam JB. 1956. Carbon dioxide intoxication: the clinical syndrome, its etiology, and management with particular reference to the use of mechanical respirators. Medicine (Baltimore) 35:389–424. [PubMed] [Google Scholar]
- 32.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. 2004. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22:460–466. [DOI] [PubMed] [Google Scholar]
- 33.Westlake EK. 1958. Respiratory failure and carbon dioxide narcosis. Postgrad Med J 34:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong D, Makowska IJ, Weary DM. 2012. Rat aversion to isoflurane versus carbon dioxide. Biol Lett 9:20121000. [DOI] [PMC free article] [PubMed] [Google Scholar]