Abstract
To survive inhospitable environments, tumor cells are forced to remodel their signaling pathways by altering transcription, translation, and post-translational modifications. This adaptation is regulated in a spatial and temporal manner and gives rise to individual tumor cells with distinct gene expression and metabolic signatures. Such phenotypic heterogeneity is the result of tumor cell plasticity, which—together with the genetic background of the tumor—determines whether cells resist environmental stress, enter dormancy, or metastasize. This review summarizes our understanding of how tumor cells exploit the cellular stress response to balance proliferation, differentiation, and survival signals, and to remodel local and distant environments. We focus in particular on tumor metastasis, which is the greatest impediment to clinical management of cancers today.
Keywords: Tumor cell plasticity, metastasis, dormancy, unfolded protein response (UPR)
Determinants of Metastatic Inefficiency
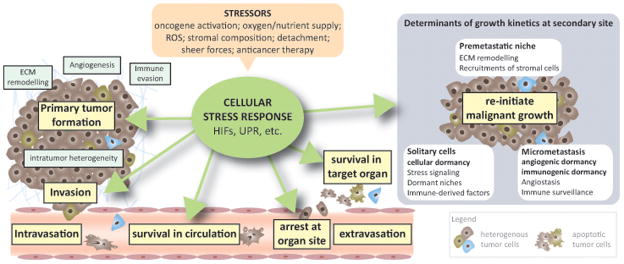
The development of clinically overt metastasis depends on the cancer cell’s ability to complete the invasion–metastasis cascade (Figure 1, Key Figure). Metastasis in itself is a highly inefficient process, and experimental models suggest that only ~80% of intravenously injected cancer cells survive and extravasate, and most (>90%) die within 24 h of entry into the target organ [1]. Of surviving disseminated tumor cells (DTCs), only few progress to macroscopic metastatic lesions, sometimes up to decades later following initial diagnosis. Metastatic latency may in some cases be explained by a slow doubling time of cancer cells (as seen in some breast cancer subtypes) [2]. Yet, growth of DTCs can also be actively restrained and DTCs may reside in a quiescent state (cellular dormancy; Box 1) [2–5] and microscopic lesions may fail to proceed due to angiogenic or immunogenic dormancy [3, 4] (Box 1; Figure 1). Thus, the survival of DTCs and the ability to resume growth at the target organ appear to be critical rate-limiting steps in metastasis.
Figure 1. Stress Signaling and the Metastatic Cascade (Key Figure).
The invasion–metastasis cascade comprises several steps: invasion, intravasation, survival in the circulation, arrest at the target organ site, extravasation, survival in the distant organ, dormancy, and re-initiation of malignant growth (colonization). Over the course of tumor progression, cancer cells are exposed to many kinds of stresses, ranging from genetic mutations to environmental changes and therapeutic drugs. To restore homeostasis, cells activate adaptive stress response pathways that not only increase stress tolerance but also actively remodel the microenvironment to relieve stress and fuel malignant growth. The spatial and temporal activation of these adaptive pathways contribute significantly to the heterogeneity of intratumoral phenotypes and, therefore, might impose metastatic traits on a subset of cancer cells. Activated stress signaling increases the capacity of cells to survive in the circulation, arrest at the target organ, and resist apoptosis upon entry into the target organ. When cells are incapable of restoring homeostasis, stress signaling induces apoptosis and thus contributes to the high attrition rates of CTCs/DTCs. The same pathways may also determine the growth kinetics of disseminated tumor cells at the metastatic site: Activation of stress signaling in primary tumor cells can lead to the secretion of factors that act systemically to create a hospitable environment for arriving tumor cells, known as the pre-metastatic niche. Activation of adaptive pathways is often coupled to transient growth arrest, suggesting that cells can enter dormancy to survive hostile environments.
Box 1. Mechanisms of Metastatic Dormancy.
Metastatic dormancy describes the lag time between tumor cell dissemination and clinical manifestation of overt metastasis. Tumor dormancy is thought to be mediated by two general, non-mutually exclusive mechanisms (for reviews see [2–5, 63]). In tumor mass dormancy, tumor cell proliferation is offset by cell death due to poor vascularization (angiogenic dormancy [103]) or the cytotoxic activity of immune cells (immunogenic dormancy [104]). Cellular dormancy [5], is the process whereby solitary or clustered cells enter quiescence (reversible growth arrest) in response to cell intrinsic or extrinsic factors. Clinical evidence suggest that DTCs preferentially reside in the BM as single cells that rarely express proliferation markers, suggesting that they enter cellular dormancy [4]. Crosstalk between dormancy modes becomes evident as experimental models have revealed that angiogenic or immunogenic factors (such as TSP1 [70] or TNFα and IFNγ [105], respectively) have direct growth inhibitory effects on tumor cells. Additionally, metastatic outgrowth of dormant cells that stochastically enter the cell cycle have been demonstrated to be eliminated by NK cell-mediated cytotoxicity, demonstrating how different “dormancy modes” cooperate to limit metastatic growth [76].
Several models have been proposed to explain when and why DTCs enter the dormant state. The cells might already be dormant when they leave the primary tumor, or they might disseminate in a “pre-malignant” state early in tumor progression [2]. Although these early DTCs are able to survive the dissemination process, additional (epi)genetic traits that enable growth in a different environment are likely lacking. Finally, the microenvironment at the distant organ may provide growth inhibitory signals (dormant niche), as has been proposed for the perivascular and hematopoietic stem cell niches [70, 106]. Several organ- and/or niche-specific microenvironmental factors reportedly determine the growth kinetics of DTCs at the target organ. These include hypoxia, biochemical and biophysical properties of the ECM, epigenetic regulators such as retinoic acid, growth inhibitory molecules such as GAS6, and members of the TGF superfamily [3–5, 63, 68, 70–75].
Among the signaling pathways associated with dormancy are the PI3K–AKT and AMPK pathways, both of which inhibit mTOR signaling. The MAPK pathway is often rewired in dormant cells and has been shown to activate the UPR and regulate the growth kinetics and survival of DTCs [85]. These pathways are all linked to autophagy regulation (for a comprehensive review of autophagy and dormancy, see [4]).
Early studies have demonstrated that only a small subpopulation of cells within a heterogeneous tumor carries metastasis-initiating potential [6]. More recently, extensive molecular characterization of metastasis-initiating cells (MICs) in colon cancer and triple-negative breast cancer has revealed that MICs arise from a subpopulation of tumor cells with stem-like properties [7, 8]. As such, MICs may be migrating cancer stem cells (CSCs) or they may acquire stem cell potential in a spatially and temporally regulated manner through phenotypic plasticity (reviewed in [9]). The question remains whether only DTCs with stem-like properties can initiate metastasis or whether any cancer cell can be reprogrammed into a MIC. A combination of genetic and epigenetic changes underlies the evolution of cells with MIC potential [9]. It has become increasingly evident that the local microenvironment affects a cancer cell’s propensity to metastasize. For example, tumor hypoxia positively correlates with metastasis and poor patient survival in several cancers [10]. Hypoxic cells activate adaptive survival pathways that stabilize the transcription factors hypoxia-inducible factor (HIF) 1 and 2, inhibit mammalian target of rapamycin (mTOR) signaling, and activate the homeostatic unfolded protein response (UPR) [10]. These adaptive pathways promote the epithelial-to-mesenchymal transition (EMT), stimulate secretion of factors that modify the extracellular matrix (ECM) both locally and systemically, and recruit stromal cells to the primary and metastatic sites (for comprehensive reviews, see [10–12]). Microenvironmental cues also impact dormancy, wherein cancer cells remain in a quiescent state at secondary sites for months, years, or even decades. Upon resuming malignant growth, they are often associated with properties that are distinct and advantageous over primary tumor cells [3, 4].
In this review, we emphasize the importance of tumor cell plasticity in successful dissemination and colonization of distant organs. We discuss how adaptive stress response pathways activated by microenvironmental conditions can (i) regulate cell survival during the metastatic cascade and (ii) determine metastatic growth or dormancy by reprogramming tumor cells and actively remodeling the environment. Finally, we propose possible therapeutic modalities to prevent or treat metastatic disease by targeting components of the cellular stress response.
Tumor Cell Plasticity: Implications for the Metastatic Cascade
Cellular plasticity is the ability of cancer cells to dedifferentiate into an embryonic stem-like state. In an extreme example, tumor cells transdifferentiate into endothelial-like cells that can form matrix-rich, vascular-like, and perfused channels; a phenomenon termed vascular mimicry [13]. These vascular-like networks likely form in response to the hypoxic microenvironment and are observed in different cancers where they correlate with metastasis and poor prognosis [13]. Interestingly, molecular barcoding of individual 4T1 breast cancer tumor cells has shown that subclones, which are able to enter the circulation and form metastasis, express the anticoagulants Serpine 2 and Slpi [14]. Expression of Serpine 2 and Slpi in breast cancer cell lines is sufficient to induce vascular mimicry and the anticoagulant activity of Serpine 2 and Slpi may contribute to intravasation and metastasis by increasing the perfusion of the newly formed vascular networks. This possibility is consistent with the findings of an earlier study of aggressive melanomas implicating tissue factor inhibitor 1 and 2 in vascular mimicry and regulation of the coagulation system to allow perfusion [15].
Epithelial–Mesenchymal Plasticity
Epithelial–mesenchymal plasticity is a well-studied example of a reversible phenotypic change in cancer cells during the metastatic cascade [16]. The EMT is triggered by a variety of contextual signals in the tumor microenvironment, including hypoxia, growth factors, Wnt signaling components, and inflammatory mediators [17]. These signals induce transcription factors and posttranscriptional regulators that orchestrate the loss of epithelial properties, such as apical polarity and cell–cell junctions, while increasing mesenchymal characteristics such as migratory and invasive capabilities. It is commonly thought that the EMT enables intravasation of single epithelial tumor cells into the circulation. Consistent with this, gene expression analysis of circulating tumor cells (CTCs) isolated from mouse models or patient-derived samples of several cancers, as well as from early metastatic lesions of breast cancer orthotopic xenografts have demonstrated expression of mesenchymal markers [7, 16, 18–22], providing evidence that the EMT occurs in vivo. Importantly, rather than fully converting to a mesenchymal phenotype, CTCs undergo partial EMT and co-express epithelial and mesenchymal markers, reflecting the dynamic aspect of the process [21, 22]. Partial EMT is thought to maintain plasticity and enable reversion through mesenchymal-to-epithelial transition (MET) [16]. MET is considered a prerequisite for metastatic colonization because cells that have fully transitioned are unable to colonize distant organs, and epithelial cancer metastases generally display an epithelial phenotype [16, 23, 24].
The EMT also confers stem-like features on cancer cells, which likely augments their metastatic potential [16, 25]. However, these processes are not necessarily coupled, as they depend on the genetic/epigenetic background of the cells as well as contextual signals [9, 16]. Similarly, CTCs can express both stem cell and mesenchymal markers but their expression is not always linked [21].
Analysis of breast and pancreatic cancer in genetic mouse models suggests that the EMT, although conferring therapy resistance, may be dispensable for metastasis [26, 27]. This observation points to the need to further dissect the dynamics and functional role of the EMT in tumor progression.
Metastatic Seeding by Tumor Cell Clusters
As an alternative to single cell dissemination, groups of cells may leave the primary site as clusters and in mouse models of breast and pancreatic cancer, polyclonal metastasis is initiated by tumor cell clusters [28–30] with an efficiency higher than that of single disseminated cells [29]. Interestingly, tumor cell clusters isolated from a melanoma patient and a breast cancer mouse model were capable of passing through small constrictions (5–10μm) by rearranging into chain-like structures [31], further supporting their capacity to seed distant metastases. Both single and clustered cells within CTCs isolated from patients display a mesenchymal phenotype [22] that may aid in their collective invasion and enhance their survival in the circulation. However, these studies also demonstrate that metastasis may occur independent of an EMT, and cells may disperse while maintaining their epithelial integrity [30]. The relative importance of seeding by tumor cell clusters versus seeding by single cells remains to be determined. Among the cardinal questions arising from these studies is the mechanism(s) underlying the reported plasticity (see Outstanding Questions).
Outstanding Questions Box.
What are the mechanisms underlying tumor cell plasticity?
What mechanisms underlie tumor cell dissemination as single cells versus clusters? What is the relative contribution of seeding by tumor cell clusters versus seeding by single cells in metastasis formation?
What defines the importance of oxidative glucose metabolism in the metastatic cascade? To what extend is it linked to cellular ROS levels?
At what stage during tumor progression is the premetastatic niche formed and how long does it persist after surgery of primary lesions? How does the premetastatic niche affect dormancy?
How can we target plasticity? Is targeting components of the cellular stress response a therapeutic option? In what combinations should such therapies be applied?
Metabolic Plasticity as a Determinant of Successful Dissemination
Increased glucose uptake is a hallmark of primary tumors and metastatic lesions and is used as a diagnostic imaging method in 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. The enhanced glucose uptake by tumor cells is an example of the Warburg effect, in which cells preferentially produce energy by aerobic glycolysis while preserving glucose carbon to fuel biosynthetic pathways. Reprogramming of mitochondrial metabolism to use glutamine as a nitrogen and carbon source for biosynthetic reactions is a significant contributor to this phenotype [32]. However, it has become evident that tumors show a significant degree of metabolic heterogeneity, and elevated levels of both glycolysis and glucose oxidation have been observed in mouse tumor models and in metabolic flux analyses of patients with lung cancer [33, 34]. Interestingly, glucose oxidation seems to be the preferred pathway in less perfused regions of the tumor, irrespective of the genetic background of the analyzed lung tumors, suggesting that the metabolic activity is strongly influenced by the in vivo microenvironment [34]. The importance of mitochondrial metabolism in tumor progression has been appreciated and investigated by several groups, and the reader is referred to a recent review on this topic [35]. The emerging picture from these studies suggests that tumor cell subpopulations characterized by slow proliferative rates are dependent on mitochondrial respiration [35, 36].
Increasing evidence suggests that, similar to the differences in the biosynthetic and bioenergetic needs of proliferating and non-proliferating cells, migrating cells and primary tumor cells may utilize distinct metabolic pathways [19, 35–38]. Analysis of gene expression signatures in an orthotopic breast cancer model indicated that CTCs are enriched in factors that regulate mitochondrial respiration and biogenesis (including the master regulator of mitochondrial biogenesis peroxisome proliferator-activated receptor gamma coactivator 1-alpha [PGC-1α]) compared with primary and metastatic lesions [19]. Accordingly, CTCs display increased levels of PGC-1α-related activities, such as oxidative phosphorylation, mitochondrial biogenesis, and oxygen consumption, and silencing PGC-1α decreased invasion [19]. Mitochondrial bioenergetics are also expected to support energy-intensive activities associated with cell motility, such as focal adhesion dynamics [37, 38]. Of note, a recent report implicates PGC-1α as a tumor and metastasis suppressor in prostate cancer [39]. Loss of PGC-1α was shown to promote metastasis, and although the underlying mechanism remains elusive, enforced expression of this metabolic master regulator locked cell metabolism in a catabolic state, limiting primary tumor growth and preventing metastasis [39]. The contradictory effects of such metabolic regulators on the metastatic cascade may well be lineage- and context-dependent, but this finding illustrates that metabolic flexibility is an integral metastatic trait. Along these lines, an in vivo selected highly metastatic prostate cancer cell line was found to exhibit metabolic plasticity [36].
ROS: A Balancing Act
Mitochondrial respiration is a major generator of cellular reactive oxygen species (ROS), coupled with metabolic reactions in peroxisomes and the endoplasmic reticulum (ER). While ROS contribute to genomic instability and regulate important pro-tumorigenic pathways (e.g., HIFs, MAPK, AKT) [40], elevated levels of ROS can induce cell death or senescence programs [40]. Accordingly, cancer cells often require a potent antioxidant defense to limit oxidative stress [40] and, thus, inhibition of the antioxidant response may offer potential for anti-cancer therapy [41–43].
Metastatic activity is also influenced by ROS. In xenograft experiments, mitochondrial DNA mutations that affect complex I activity contribute to metastasis of murine and human cancer cells, and pretreatment with ROS scavengers diminishes their metastatic potential [44]. Increased mitochondrial ROS have also been found to enhance the migration of “superinvasive” B16F10 mouse melanoma cells in vivo [45].
Several lines of evidence support a contrasting role for ROS in limiting metastasis [43, 46–48]. One possible explanation of how ROS limit metastasis may be associated with the regulation of detachment-induced cell death (anoikis), as excessive oxidative stress was suggested to be the major cause of anoikis [49]. However, it remains to be elucidated to what extent anoikis determines the attrition of CTCs, given their relatively short circulation time. Notably, CTCs can form tumor cell clusters [30] or are associated with platelets [18], which could enhance their survival and prevent anoikis. Indeed, increased antioxidant capacity has been associated with enhanced metastasis in vivo [43, 46–48]. Antioxidant treatment of mice harboring the melanocyte-specific BRAFV600E mutation and PTEN deletion [48] or of mice inoculated with human melanoma cells [43] significantly accelerates melanoma metastasis, indicating that certain levels of ROS limit metastasis. Mechanistically, elevated carbon flux through the folate pathway (a potent producer of NADPH in addition to the pentose phosphate pathway), observed in CTCs and metastatic lesions, increases NADPH levels and enables replenishment of endogenous antioxidants [43], demonstrating that oxidative stress affects metastatic traits (see Outstanding Questions).
Collectively, these studies support the notion that the ability of CTCs/DTCs to switch between metabolic states strongly depends on their ability to simultaneously control oxidative stress.
Integration of Microenvironment and Metabolism in Determining the Fate of Metastasis
It is well established that most microenvironments are hostile to the majority of tumor cells (including stem-like cells, [43]). The mechanisms underlying the high attrition rates of tumor cells entering secondary sites and factors that enable their persistence and ultimately drive metastatic growth are being identified [4, 9].
Metabolic Flexibility in Target Organs
Metabolic flexibility is just as critical for DTCs after they reach the target organ as it is in enabling them to adapt to the new microenvironment [50–52]. Mitochondrial metabolism is higher in metastatic breast cancer cells than non-metastatic cells, and this is linked to organ tropism: cells preferentially migrating to lung or bone rely on elevated oxidative phosphorylation, while those migrating to liver show increased glycolytic metabolism [50], indicating that adjustment to the metabolic milieu of the target organ is a prerequisite for metastasis. The liver microenvironment for example is characterized by metabolic zonation, and an oxygen gradient that exists between the periportal and perivenous blood creates metabolic compartments for oxidative or oxygen-independent metabolic reactions [53]. Accordingly, as evidenced by activation of the HIF1α luciferase reporter system, colorectal cancer cells activate hypoxia-signaling pathways soon after they exit the portal circulation and enter the liver [51]. As part of their adaptation, DTCs in the liver downregulate miR-483-5p and miR-551a, microRNAs that target mRNA encoding creatine kinase (brain type). This kinase is released into the extracellular space where it converts creatine to phosphocreatine [51]. Phosphocreatine is taken up and rapidly consumed by liver DTCs to generate ATP, enabling tumor cell survival [51]. Likewise, HIF1α/PDK1-dependent metabolic reprogramming towards a glycolytic phenotype favors metastasis of breast cancer cells to the liver, but not the skin, where such reprogramming is absent [50].
Metabolic adaption to specialized environments has also been reported in the brain, where brain metastases of diverse cancers are able to utilize acetate as an alternative fuel in vivo, demonstrating that tumors hijack the metabolic programs of the target organs for metastatic growth [54]. Additionally, adipocytes have been shown to attract ovarian cancer cells from the peritoneal fluid to the omentum and provide lipids to fuel metastatic growth [55]. These examples highlight the diverse metabolic changes through which tumor cells adapt to different environmental conditions, a prerequisite for establishing the metastatic niche. Precisely how the temporal and spatial switches to distinct metabolic pathways are controlled remains elusive (see Outstanding Questions).
Tumor Cell Influence on Stromal Metabolism
Metastasis is facilitated by the pre-metastatic niche, emphasizing the importance of the local microenvironment for DTC survival and metastatic colonization [56, 57]. The formation of such niches is associated with a high degree of hypoxia in primary tumors, and the hypoxia-induced gene lysyl oxidase (LOX) has emerged as an important regulator of this process [58–60]. Systemic LOX and LOX-like proteins secreted from primary breast tumors modify pre-metastatic lung tissue by collagen I crosslinking [58] and remodeling of the basement membrane, which facilitates recruitment of bone marrow (BM)-derived cells (BMDCs) [60] and metastasis in orthotopic breast cancer models. Secreted LOX also increases osteoclastogenesis, and the resulting focal osteolytic bone lesions provide a niche for CTCs and increase bone metastasis in estrogen receptor-negative breast cancers [59]. Additional factors, such as monocyte chemotactic protein 1, contribute to hypoxia-dependent recruitment of BMDCs to the pre-metastatic site, which has been shown to reduce immune surveillance and enhance lung colonization of intravenously injected breast tumor cells in mice [61]. Circulating miRNAs are also thought to contribute to pre-metastatic niche formation. Circulating vesicular miR-122 is taken up by fibroblasts and astrocytes in the lung and brain, respectively [62]. Downregulation of the miR122 target genes pyruvate kinase and glucose transporter 1, results in decreased glucose uptake by niche cells and leaves more available for arriving DTCs [62].
As metastatic burden may be induced by injection of either conditioned media from hypoxic tumor cells or miRNA-containing vesicles into tumor-naïve mice [59–62], it is plausible that metastatic seeding is preceded by preparation of the distant tissue. Of note, the experimental models used in these studies do not recapitulate the clinical situation (surgical removal of primary tumor with metastases that developed at a later time point) and thus cannot identify when the pre-metastatic niche is formed, or for how long it may persist once the primary tumor is removed. Even when tumors are diagnosed before signs of invasion, DTCs can be detected in the BM [2] and may contribute to pre-metastatic niche formation [4]. Indeed, mouse and human pancreatic cancer CTCs abundantly express a diverse set of stromal ECM genes [21], demonstrating that they at least have the ability to modify the host environment [21]. It will thus be important to decipher the relative contributions of the primary tumor “secretome” in modifying the future metastatic site versus the contribution of DTCs. This will require experimental models that closely mimic the clinical situation.
Dormancy may be an Escape Route from Metabolic Stress Imposed by the Microenvironment
Understanding the kinetics of tumor metastasis is particularly important for the study of tumor dormancy, since reprogramming of biochemical, biophysical, and metabolic pathways in distant tissues is also implicated in reactivation of tumor cells from dormancy [63–69]. Inhibition of the integrin–FAK–SRC axis induces dormancy in breast cancer and squamous cell carcinoma DTCs [63–67]. Conversely, increased ECM stiffness, as seen in fibrotic lungs, induces metastatic growth of otherwise dormant breast cancer cells [68, 69]. In addition to growth inhibition through reduced mitogenic signaling (i.e., via integrin signaling), the microenvironment may actively regulate DTC quiescence. This has been shown with TSP1 in the perivascular niche [70] and Gas6 in the hematopoietic stem cell/osteoblastic niche [71] (Box 1). Dormant DTCs have been suggested to localize to these niches, indicating that the quiescent phenotype is actively maintained by signals that regulate tissue/stem cell homeostasis. All-trans retinoic acid can reprogram cells into dormancy [72], and tissue-specific expression of bone morphogenetic protein 7 (BMP7) [73] and TGFβ2 [74] have been implicated in dormancy regulation of prostate and breast cancer DTCs in the BM, whereas BMP4 appears to regulate dormancy of breast cancer cells in the lung [75]. As discussed later on, dormant DTCs undergo metabolic adaptions (attenuated mTOR signaling and/or autophagy [4]), which allow their survival under adverse environmental conditions. Besides metabolic and mechanical stresses, recent analyses highlight the importance of the immune system in limiting metastatic spread [76]. Metastatic latency-prone DTCs escape immune surveillance by stochastically entering a quiescent state that they maintain by expressing the Wnt inhibitor Dickkopf 1, which is associated with the downregulation of innate immune receptor ligands [76].
As altered metabolic and mechanical stress and the establishment of an immune-evasive microenvironment were implicated in the development of the metastatic niche, one can conclude that tissue microenvironment not only induces dormancy in cancer cells, but may also promote the formation of a metastatic niche. Clearly, whether and how the pre-metastatic and dormant niches influence each other require further investigation (see Outstanding Questions). The genetic background of tumor cells and the extracellular (stress) signals that rewire intracellular signaling are likely to act in concert to impact the fate of DTCs and MICs. Activation of adaptive pathways is often coupled to transient growth arrest [77], even in the presence of activated oncogenes, suggesting that fully transformed cells can become dormant as a means to survive hostile microenvironments [5].
The Unfolded Protein Response as a Driver of Tumor Cell Plasticity
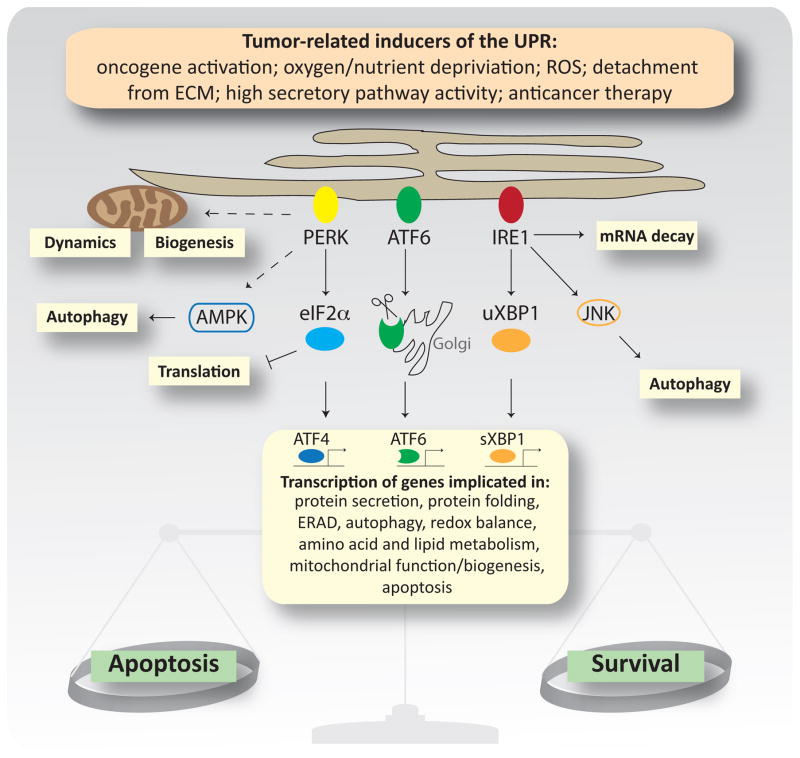
ER stress activates the homeostatic UPR pathway, which orchestrates a number of transcriptional and non-transcriptional events to restore the ER folding capacity or, if unsuccessful, to promote cell death. The major UPR sensors, protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) maintain ER homeostasis by coordinating crosstalk between their downstream effectors (e.g., ATF4 and XBP1) and central signaling pathways (e.g. mTOR and MAPK), which in turn finetunes fundamental cellular functions (e.g., regulation of translation, mRNA decay, autophagy, and mitochondrial function and biogenesis) (Figure 2 and reviewed in [78]). The UPR is often activated in cancer cells [79], where it additionally mediates crosstalk between cancer cells and the stroma (Box 2) to stimulate the pro-tumorigenic properties of endothelial and immune cells.
Figure 2. Molecular Mechanisms of the Unfolded Protein Response.
Cancer cells are exposed to diverse stress stimuli that activate the unfolded protein response (UPR), a homeostatic pathway that aims to restore ER folding capacity under ER stress conditions, but can induce cell death when restoration fails. Accumulation of unfolded/misfolded proteins in the ER activates three proteins that orchestrate the UPR: protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6). PERK inhibits cap-dependent translation (via eIF2α phosphorylation) and activates transcription via translation of specific mRNAs, such as the transcription factor ATF4. PERK also regulates cellular bioenergetics by influencing mitochondrial dynamics and biogenesis through crosstalk with components of metabolic pathways, such as AMPK. IRE1α activation results in splicing of the transcription factor uXBP-1 to its active form sXBP-1, which translocates to the nucleus and induces gene expression. IRE1α also splices other mRNAs to reduce the protein load in the ER, and modulates survival and autophagy by crosstalk with other pathway components such as JNK. Activation of ATF6 induces its translocation to the Golgi where it is cleaved to its active form. The transcriptional output of the UPR depends on the relative activity of each UPR arm and the degree of stress. All major transcription factors activated by the UPR regulate genes involved in restoring ER proteostasis, but specialized transcriptional programs are also activated. For example, the PERK–ATF4 arm regulates genes involved in autophagy and induces expression of the transcription factor CHOP, a major regulator of UPR-mediated apoptosis. A fine balance between homeostatic or apoptotic signals determines cellular outcome and thus, activation of the UPR can display pro or anti-metastatic effects, dependent on their degree of activation.
Box 2. The UPR in Tumor Angiogenesis and Immunoediting.
UPR in angiogenesis
Expanding tumor masses, whether at the primary site or during metastatic colonization, depend on angiogenesis to ensure sufficient oxygen and nutrient supply. Since tumor blood vessels differ from the normal vasculature in terms of endothelial barrier function, angiogenesis may increase invasion [107]. The UPR can promote an angiogenic switch via transcriptional and translational mechanisms (reviewed in [108]). In brief, transcriptional regulation of VEGF expression occurs through stabilization of HIFs, cooperation between HIF and XBP-1, or HIF-independent modulation of ATF4 or XBP-1. Translational control of VEGF occurs in an AMPK-dependent manner under ER stress conditions [108]. Additionally, UPR activation in stromal cells contributes to increased angiogenesis. GRP78 localization to the plasma membrane is required for VEGF-stimulated endothelial cell proliferation and angiogenic signaling [109]. VEGF treatment of endothelial cells also potently induces the UPR via PLCγ–mTORC1 signaling, leading to AKT-dependent survival [108, 110]. UPR activation supports the survival and angiogenic signaling of both endothelial and cancer cells, making it a potential target for antiangiogenic cancer therapy.
UPR in immunity
Anti-tumor immunity has been suggested to be a potent defense against metastasis [76, 111], and overcoming immune surveillance is a prerequisite for macrometastatic growth [76]. Although it is well established that UPR activation in cancer or immune cells regulates cancer-associated inflammation, and that UPR-signaling is necessary for immune cell differentiation and MHC-I maturation and pepitide processing [79, 95], it remains largely unexplored whether and how UPR is implicated in immunoediting at the metastatic site.
Dendritic cells in the tumor vicinity but also in the spleen or the lymph node show increased XBP-1 splicing [112], indicating that the UPR is activated in these cells. Although the source of UPR activation is unknown, byproducts of lipid peroxidation could activate XBP1, which in turn upregulates lipid biosynthetic genes. The accumulation of lipids in DCs reportedly inhibits T cell-mediated anti-tumor immunity. Further, intraperitoneal injection of ovarian cancer cells decreased tumor burden when injected into DC-specific XBP-1 knockout mice compared to their WT mice [112], suggesting UPR effect on immunosurveillance at secondary sites. Further along these observations, treatment of myeloid cells with conditioned media from cancer cells exposed to ER stress activates the UPR and enhances secretion of inflammatory cytokines while decreasing antigen presentation [113], suggesting that the UPR can be transmitted from cancer cells to immune cells.
Cells that have undergone the EMT show increased secretion of ECM proteins such as PAI1 and FN1 and activate the PERK–eIF2α axis of the UPR to accommodate increased secretory pathway activation [80]. PERK inhibition diminishes the migratory and sphere-forming capacity of breast cancer cells and decreases lung metastasis in xenograft models. Interestingly, activation of the EMT program also renders cells more susceptible to pharmacological ER stress-inducers, which attenuate lung metastasis even more effectively than does PERK inhibition [80]. ROS-dependent PERK activation has been implicated in anoikis resistance [46]. Mechanistically, ATF4 upregulates autophagy genes (ATG5, ATG7, and ULK1), and together with NRF2, ATF4 increases expression of the antioxidant gene HO-1. [46]. Activation of autophagy accompanied by reduced oxidative stress results in enhanced lung metastasis in fibrosarcoma [46]. In addition to conferring stress tolerance, PERK signaling actively enhances migration via hypoxia-induced ATF4-dependent transcriptional upregulation of the metastasis-associated gene lysosomal-associated membrane protein 3 (LAMP3) [81, 82].
The IRE1α–XBP1 branch of the UPR reportedly regulates the progression of triple-negative breast cancer [83]. The spliced (active) isoform of XBP-1 is enriched in stem-like cells with increased tumor-initiating potential and cooperates with HIF to transactivate known HIF targets [83]. In xenotransplantation experiments, silencing of XBP-1 in breast cancer cell lines decreased tumor growth and metastasis, and abrogated or delayed relapse following doxorubicin treatment. The latter was associated with decreased angiogenesis and a reduction in stem-like cells [83].
Several UPR-regulated genes (e.g., Grp78, Grp94, PDI, HSP47, and cyclophilin B) are upregulated in dormant cells from experimental models, patient-derived dormant DTCs, and DTC-derived cell lines [84–87], supporting a role for the UPR in metastatic dormancy. Mechanistic analysis revealed that increased PERK–eIF2α phosphorylation, XBP-1 splicing [85], and ATF6 activation [88] contribute to the survival and increased stress tolerance of dormant DTCs. PERK signaling was also suggested to contribute to the quiescence phenotype, and inhibition of PERK signaling reverses the dormant phenotype and renders cells more susceptible to therapy [85]. Activation of the UPR in the BM possibly occurs in response to varying oxygen and nutrient availability, and is thought to confer a survival advantage to DTC within this niche [87]. Indeed, in vitro analysis of cell lines derived from BM DTCs revealed a heightened resistance to glucose and oxygen deprivation, which was reversed by Grp78 knockdown [87]. This suggests that DTCs in the hypoxic BM environment may be especially likely to upregulate the cellular stress response. It will be interesting to determine whether there is a relationship between the degree of UPR activation and the organ/niche oxygenation state. Such analyses may reveal a role for the UPR in metastatic tropism.
Our current knowledge suggests that the UPR promotes metastasis by (i) conferring a survival advantage under adverse environmental conditions; (ii) directly enhancing the migratory capacity of cancer cells; (iii) maintaining stem-like properties; and (iv) remodeling the stroma (Box 2). Whether strategies aimed at fine-tuning the UPR could offer new therapeutic modalities is among the key questions currently being addressed.
Stress-Induced Survival Pathways as Therapeutic Targets
As outlined above, isogenic tumor cells can rapidly adjust their cellular needs in response to environmental pressure, including anti-cancer therapies, and adaptive stress pathways are therefore thought to contribute significantly to therapy resistance. Resistance to PI3K/AKT-targeting therapy, for example, has been attributed to a global reprogramming of transcription that not only increases resistance but also increases metastatic potential [37]. AKT is among the most hyperactivated kinases in cancer cells, and the AKT pathway has been suggested to be a major survival pathway under anchorage-independent growth conditions [49] and during metastatic seeding of target organs [89, 90]. Nonetheless, AKT signaling is often downregulated in dormant DTCs [4, 88, 91], suggesting that, despite its importance for the survival of proliferating cells, AKT signaling may be dispensable for quiescent cells, thereby rendering them resistant to AKT inhibitors. Cancer cells may even benefit from transient downregulation of AKT because the concomitant inhibition of mTOR induces autophagy. Autophagy plays a protective role in the cellular response to a broad spectrum of stresses [78, 92]. Although AKT inhibition is not always coupled to attenuated mTOR signaling [88], several reports suggest that autophagy plays a role in the survival of dormant DTCs [4]. Consequently, autophagy has been suggested as a therapeutic target to prevent the emergence of resistance and to sensitize intrinsically resistant cells (including stem-like cells, MICs, and dormant DTCs) to cytotoxic drugs.
Another pro-survival mechanism activated as part of the adaptive response to stress is the attenuation of translation. Diminished mTOR signaling and AMPK activation inhibits translation initiation and elongation and promotes cell survival under metabolic stress [93]. Inhibition of translation via PERK-mediated eIF2α phosphorylation is an integral component of the UPR (Figure 2). However, at least three additional kinases have been reported to phosphorylate eIF2α under stress conditions; namely, PKR (in response to viral infection, inflammation, and oxidative stress), GCN2 (amino acid or glucose starvation), and HRI (iron deficiency, oxidative stress, heat shock) [94]. Although overall translation is inhibited under these conditions, certain key mRNAs, such as ATF4 and CHOP, continue to be translated, and other mRNAs (heat shock, UPR, or inflammatory proteins) are translated when a specific type of stress is encountered (thermal, ER, or inflammatory stress) [95]. Interfering with the ability of cells to downregulate translation leads to cell death [93], highlighting the importance of this process. Interestingly, the same signals that activate the protective UPR can induce an apoptotic switch under more severe stress conditions. ATF4 and CHOP have been shown to enhance protein synthesis in stressed cells, resulting in oxidative stress, ATP depletion, and ultimately, cell death [96, 97]. Therefore, restoring translation under adverse environmental conditions by increasing the stress level may offer a novel therapeutic approach to targeting heterogeneous tumor subpopulations. For example, increased activation of the UPR effectively diminishes lung metastasis in breast cancer xenograft models [80]. In this regard, direct activators of the PERK–eIF2α–ATF4–CHOP axis have been identified and await preclinical testing [98].
Partial inhibition of translation (e.g., by fine-tuning ribosome biogenesis) has also been identified as an efficient anti-cancer strategy in MYC-driven hematopoietic malignancies [99]. Given that oncogenic signaling culminates in increased translation, and that partial inhibition of the translation machinery elicits a more focused therapeutic advantage than inhibition of upstream signaling pathways (e.g., PI3K–AKT, MAPK, mTOR), targeted inhibition of aberrant translation may also hold promise as a therapy for solid tumors. Indeed, inhibition of translation initiation attenuates metastatic outgrowth to the lung in experimental melanoma and breast cancer metastasis models [100, 101]. Combined targeting of ribosome biogenesis and mTOR-dependent mRNA translation in a Eμ-Myc B lymphoma model not only showed a strong synergistic effect on progression-free survival but decreased the tumor cell count in the bone marrow [102], raising the possibility that (if transferable to solid tumors) interfering with protein translation might enable targeting of dormant BM DTCs. The synergistic anti-tumor response of ribosome and mTOR inhibition indicates that enhanced translation is beneficial to cancer cells, and targeting different components of the translation machinery may be necessary to achieve maximal inhibition and a potent therapeutic response. Targeting dormant cells may require a focus on specialized components of the translational machinery, since attenuated translation is likely to be integral to the quiescence program. In this regard, targeting alternative initiation factors like eIF2α, which was recently suggested to affect the expression of Grp78 and to shape the translational output of the integrated stress response [95] may offer a valuable therapeutic modality for interfering with the survival of dormant DTCs.
Concluding Remarks
Here, we have highlighted the ability of the microenvironment to influence a cancer cell’s phenotype and delineated how plasticity aids cancer cells in acquiring or selecting for metastatic traits under adverse environmental conditions. The metabolic features of cancer cells at different stages of the metastatic cascade reveal differences, attributable to distinct lineages and mutational backgrounds, enables alternative adaptive mechanisms that in turn serve to accommodate bioenergetic and biosynthetic needs and avert oxidative stress (see Outstanding Questions). Therefore, rather than making specific metabolic adaptions (such as a preference for oxidative phosphorylation over glycolysis), common among MICs is the propensity to switch between these states and to utilize alternative energy sources in a spatial and temporal manner (i.e. plasticity). Another example of plasticity is the ability of MICs to adapt their proliferative rates either transiently or enduringly for prolonged periods of time (dormancy) despite constitutive activation of oncogenic signaling. The UPR serves as an example of a fundamental mechanism that links the cellular stress response to the persistence of MICs during different stages of the metastatic cascade. Given that particular microenvironments, such as hypoxia, link the plasticity of cells to their survival advantage, altering specific components of the cellular response to stress may enable the targeting of tumor cell plasticity. Anti-cancer therapies, which are aimed at interfering between the tumor-microenvironment crosstalk, such as anti-angiogenic therapy or immune-checkpoint therapy, significantly improve progression-free survival of cancer patients without impacting pro-survival signals. Thus, these types of therapies can be considered for combination with therapies that target pro-survival signals.
Trends Box.
Metastasis is an inefficient process in which only very few cells survive the dissemination phase and are capable of resuming malignant growth at distant sites.
Tumor cell plasticity enables the rapid adjustment to adverse environmental conditions encountered during the dissemination process.
Spatial and temporal changes in cellular homeostasis pathways contribute to intra-tumoral heterogeneity and confer a survival advantage for tumor-initiating, metastasis-initiating and dormant cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luzzi KJ, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. 2011;21:42–49. doi: 10.1016/j.gde.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sosa MS, et al. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 7.Lawson DA, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieter SM, et al. Distinct types of tumor-initiating cells form human colon cancer tumors and metastases. Cell Stem Cell. 2011;9:357–365. doi: 10.1016/j.stem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Oskarsson T, et al. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 11.Gilkes DM, et al. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32:4057–4063. doi: 10.1038/onc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seftor RE, et al. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181:1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagenblast E, et al. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015;520:358–362. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruf W, et al. Differential role of tissue factor pathway inhibitors 1 and 2 in melanoma vasculogenic mimicry. Cancer Res. 2003;63:5381–5389. [PubMed] [Google Scholar]
- 16.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 17.Thiery JP, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Labelle M, et al. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBleu VS, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. 1001–1015. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X, et al. Isolation and molecular characterization of circulating melanoma cells. Cell Rep. 2014;7:645–653. doi: 10.1016/j.celrep.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting DT, et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu M, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brabletz T, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stankic M, et al. TGF-beta-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep. 2013;5:1228–1242. doi: 10.1016/j.celrep.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer KR, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng X, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maddipati R, Stanger BZ. Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer Discov. 2015;5:1086–1097. doi: 10.1158/2159-8290.CD-15-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung KJ, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A. 2016;113:E854–863. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Au SH, et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc Natl Acad Sci U S A. 2016;113:4947–4952. doi: 10.1073/pnas.1524448113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson SM, et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab. 2016;23:517–528. doi: 10.1016/j.cmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hensley CT, et al. Metabolic Heterogeneity in Human Lung Tumors. Cell. 2016 doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viale A, et al. Tumors and mitochondrial respiration: a neglected connection. Cancer Res. 2015;75:3685–3686. doi: 10.1158/0008-5472.CAN-15-0491. [DOI] [PubMed] [Google Scholar]
- 36.Aguilar E, et al. Metabolic Reprogramming and Dependencies Associated with Epithelial Cancer Stem Cells Independent of the Epithelial-Mesenchymal Transition Program. Stem Cells. 2016;34:1163–1176. doi: 10.1002/stem.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caino MC, et al. PI3K therapy reprograms mitochondrial trafficking to fuel tumor cell invasion. Proc Natl Acad Sci U S A. 2015;112:8638–8643. doi: 10.1073/pnas.1500722112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivadeneira DB, et al. Survivin promotes oxidative phosphorylation, subcellular mitochondrial repositioning, and tumor cell invasion. Sci Signal. 2015;8:ra80. doi: 10.1126/scisignal.aab1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torrano V, et al. The metabolic co-regulator PGC1alpha suppresses prostate cancer metastasis. Nat Cell Biol. 2016;18:645–656. doi: 10.1038/ncb3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorrini C, et al. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 41.Harris IS, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Xu IM, et al. Transketolase counteracts oxidative stress to drive cancer development. Proc Natl Acad Sci U S A. 2016;113:E725–734. doi: 10.1073/pnas.1508779113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piskounova E, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015 doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa K, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 45.Porporato PE, et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8:754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 46.Dey S, et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J Clin Invest. 2015;125:2592–2608. doi: 10.1172/JCI78031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qu Y, et al. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-kappaB signaling. J Clin Invest. 2011;121:212–225. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Gal K, et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7:308re308. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 49.Schafer ZT, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dupuy F, et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015;22:577–589. doi: 10.1016/j.cmet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Loo JM, et al. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160:393–406. doi: 10.1016/j.cell.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen A, et al. PKLR promotes colorectal cancer liver colonization through induction of glutathione synthesis. J Clin Invest. 2016;126:681–694. doi: 10.1172/JCI83587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 54.Mashimo T, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong CC, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci U S A. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox TR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 62.Fong MY, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aguirre Ghiso JA. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21:2513–2524. doi: 10.1038/sj.onc.1205342. [DOI] [PubMed] [Google Scholar]
- 64.Barkan D, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lahlou H, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu D, et al. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 68.Barkan D, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cox TR, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghajar CM, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taichman RS, et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One. 2013;8:e61873. doi: 10.1371/journal.pone.0061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sosa MS, et al. NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat Commun. 2015;6:6170. doi: 10.1038/ncomms7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobayashi A, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bragado P, et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao H, et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malladi S, et al. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lopez-Maury L, et al. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet. 2008;9:583–593. doi: 10.1038/nrg2398. [DOI] [PubMed] [Google Scholar]
- 78.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 80.Feng YX, et al. Epithelial-to-mesenchymal transition activates PERK-eIF2alpha and sensitizes cells to endoplasmic reticulum stress. Cancer Discov. 2014;4:702–715. doi: 10.1158/2159-8290.CD-13-0945. [DOI] [PubMed] [Google Scholar]
- 81.Mujcic H, et al. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19:6126–6137. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- 82.Nagelkerke A, et al. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. doi: 10.1186/bcr3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen X, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508:103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartkowiak K, et al. Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J Proteome Res. 2010;9:3158–3168. doi: 10.1021/pr100039d. [DOI] [PubMed] [Google Scholar]
- 85.Ranganathan AC, et al. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–1711. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chery L, et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget. 2014;5:9939–9951. doi: 10.18632/oncotarget.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bartkowiak K, et al. Disseminated Tumor Cells Persist in the Bone Marrow of Breast Cancer Patients through Sustained Activation of the Unfolded Protein Response. Cancer Res. 2015;75:5367–5377. doi: 10.1158/0008-5472.CAN-14-3728. [DOI] [PubMed] [Google Scholar]
- 88.Schewe DM, Aguirre-Ghiso JA. ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci U S A. 2008;105:10519–10524. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang XH, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Q, et al. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Correa RJ, et al. Modulation of AKT activity is associated with reversible dormancy in ascites-derived epithelial ovarian cancer spheroids. Carcinogenesis. 2012;33:49–58. doi: 10.1093/carcin/bgr241. [DOI] [PubMed] [Google Scholar]
- 92.Kroemer G, et al. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leprivier G, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell. 2013;153:1064–1079. doi: 10.1016/j.cell.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holcik M. Could the eIF2alpha-Independent Translation Be the Achilles Heel of Cancer? Front Oncol. 2015;5:264. doi: 10.3389/fonc.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Starck SR, et al. Translation from the 5′ untranslated region shapes the integrated stress response. Science. 2016;351:aad3867. doi: 10.1126/science.aad3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flaherty DP, et al. Discovery of Sulfonamidebenzamides as Selective Apoptotic CHOP Pathway Activators of the Unfolded Protein Response. ACS Med Chem Lett. 2014;5:1278–1283. doi: 10.1021/ml5003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bywater MJ, et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51–65. doi: 10.1016/j.ccr.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Konicek BW, et al. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 2011;71:1849–1857. doi: 10.1158/0008-5472.CAN-10-3298. [DOI] [PubMed] [Google Scholar]
- 101.Pettersson F, et al. Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis. Cancer Res. 2015;75:1102–1112. doi: 10.1158/0008-5472.CAN-14-1996. [DOI] [PubMed] [Google Scholar]
- 102.Devlin JR, et al. Combination Therapy Targeting Ribosome Biogenesis and mRNA Translation Synergistically Extends Survival in MYC-Driven Lymphoma. Cancer Discov. 2016;6:59–70. doi: 10.1158/2159-8290.CD-14-0673. [DOI] [PubMed] [Google Scholar]
- 103.Holmgren L, et al. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 104.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 105.Muller-Hermelink N, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 106.Shiozawa Y, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zervantonakis IK, et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Binet F, Sapieha P. ER Stress and Angiogenesis. Cell Metab. 2015;22:560–575. doi: 10.1016/j.cmet.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 109.Katanasaka Y, et al. Cancer antineovascular therapy with liposome drug delivery systems targeted to BiP/GRP78. Int J Cancer. 2010;127:2685–2698. doi: 10.1002/ijc.25276. [DOI] [PubMed] [Google Scholar]
- 110.Karali E, et al. VEGF Signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol Cell. 2014;54:559–572. doi: 10.1016/j.molcel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 111.Eyles J, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120:2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cubillos-Ruiz JR, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mahadevan NR, et al. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108:6561–6566. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]