Abstract
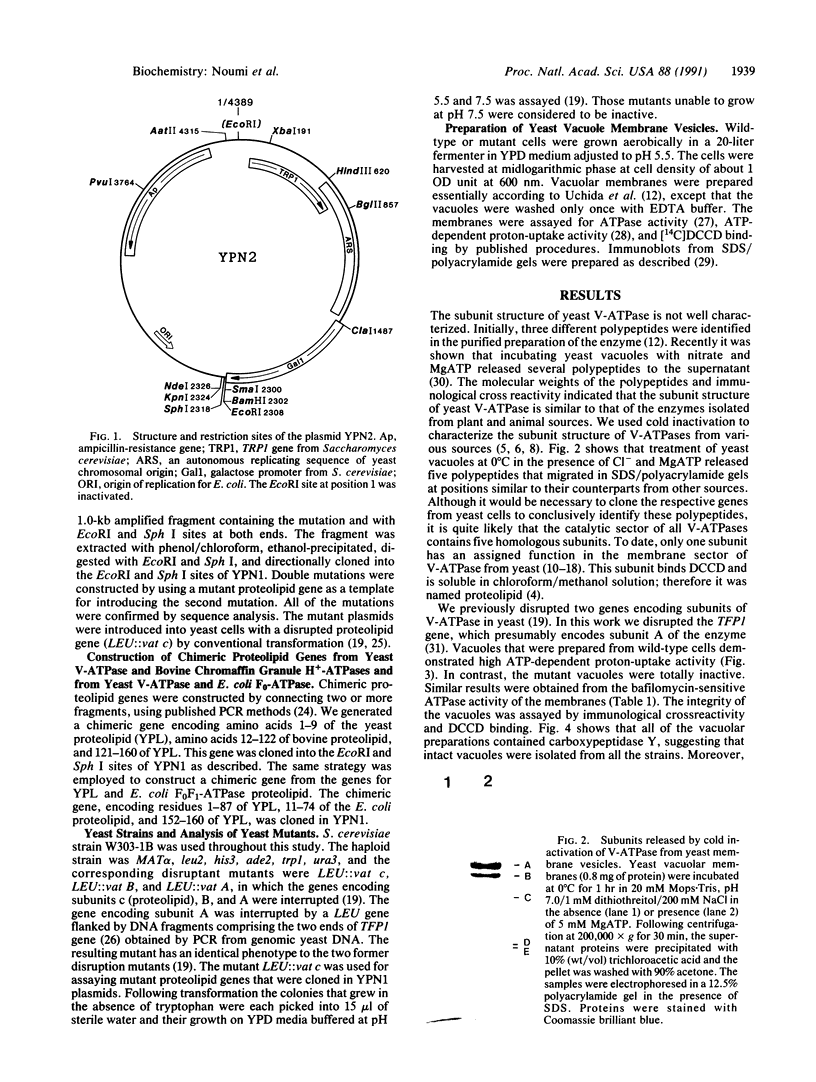
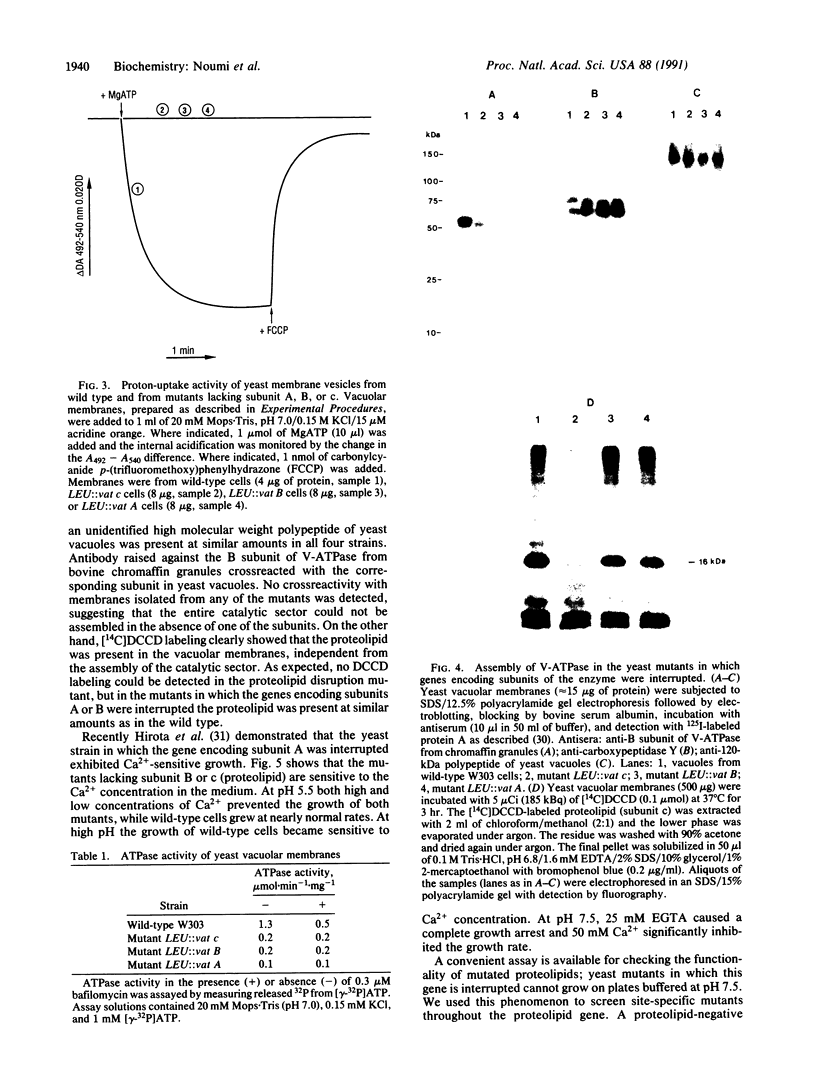
Yeast mutants in which genes encoding subunits of the vacuolar H(+)-ATPase were interrupted were assayed for their vacuolar ATPase and proton-uptake activities. The vacuoles from the mutants lacking subunits A (72 kDa), B (57 kDa), or c (proteolipid, 16 kDa) were completely inactive in these reactions. Immunological studies revealed that in the absence of each one of those subunits the catalytic sector was not assembled. Labeling with N,N'-[14C]dicyclohexylcarbodiimide showed the presence of the proteolipid in vacuoles of mutants in which genes encoding subunits of the catalytic sectors were interrupted. No labeling was detected in the mutant in which the gene encoding the proteolipid was interrupted. We conclude that of all the ATPase subunits only the proteolipid is assembled independently and it serves as a template for the assembly of the other subunits. Site-specific mutations were generated in the gene encoding the proteolipid. All of the drastic changes and replacements gave inactive proteins. About half of the single amino acid replacements gave active proteins. Replacing glutamic acid-137 by any of several amino acids, except for aspartic acid, abolished the activity of the enzyme. Other amino acids that may function in proton conductance were changed. It was found that glycine residues may replace amino acids with exchangeable protons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Berne M., Forgac M. Inhibition of the coated vesicle proton pump and labeling of a 17,000-dalton polypeptide by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1987 Aug 15;262(23):11006–11011. [PubMed] [Google Scholar]
- Arai H., Terres G., Pink S., Forgac M. Topography and subunit stoichiometry of the coated vesicle proton pump. J Biol Chem. 1988 Jun 25;263(18):8796–8802. [PubMed] [Google Scholar]
- Bowman B. J., Bowman E. J. H+-ATPases from mitochondria, plasma membranes, and vacuoles of fungal cells. J Membr Biol. 1986;94(2):83–97. doi: 10.1007/BF01871190. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Dschida W. J., Harris T., Bowman E. J. The vacuolar ATPase of Neurospora crassa contains an F1-like structure. J Biol Chem. 1989 Sep 15;264(26):15606–15612. [PubMed] [Google Scholar]
- Bowman E. J. Comparison of the vacuolar membrane ATPase of Neurospora crassa with the mitochondrial and plasma membrane ATPases. J Biol Chem. 1983 Dec 25;258(24):15238–15244. [PubMed] [Google Scholar]
- Cidon S., Nelson N. Purification of N-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J Biol Chem. 1986 Jul 15;261(20):9222–9227. [PubMed] [Google Scholar]
- Citron B. A., Donelson J. E. Sequence of the Saccharomyces GAL region and its transcription in vivo. J Bacteriol. 1984 Apr;158(1):269–278. doi: 10.1128/jb.158.1.269-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N., Nelson N., Fox T. D., Claudio T., Lindstrom J., Riezman H., Hess G. P. Biosynthesis of the Torpedo californica acetylcholine receptor alpha subunit in yeast. Science. 1986 Mar 14;231(4743):1284–1287. doi: 10.1126/science.3511531. [DOI] [PubMed] [Google Scholar]
- Hirata R., Ohsumk Y., Nakano A., Kawasaki H., Suzuki K., Anraku Y. Molecular structure of a gene, VMA1, encoding the catalytic subunit of H(+)-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1990 Apr 25;265(12):6726–6733. [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane P. M., Yamashiro C. T., Stevens T. H. Biochemical characterization of the yeast vacuolar H(+)-ATPase. J Biol Chem. 1989 Nov 15;264(32):19236–19244. [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Moriyama Y., Hulmes J. D., Pan Y. C., Nelson H., Nelson N. cDNA sequence encoding the 16-kDa proteolipid of chromaffin granules implies gene duplication in the evolution of H+-ATPases. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5521–5524. doi: 10.1073/pnas.85.15.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Rea P. A., Poole R. J. Identification of 3-O-(4-benzoyl)benzoyladenosine 5'-triphosphate- and N,N'-dicyclohexylcarbodiimide-binding subunits of a higher plant H+-translocating tonoplast ATPase. J Biol Chem. 1985 Oct 5;260(22):12273–12279. [PubMed] [Google Scholar]
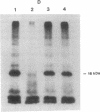
- Moriyama Y., Nelson N. Cold inactivation of vacuolar proton-ATPases. J Biol Chem. 1989 Feb 25;264(6):3577–3582. [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. H+-translocating ATPase in Golgi apparatus. Characterization as vacuolar H+-ATPase and its subunit structures. J Biol Chem. 1989 Nov 5;264(31):18445–18450. [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. Lysosomal H+-translocating ATPase has a similar subunit structure to chromaffin granule H+-ATPase complex. Biochim Biophys Acta. 1989 Apr 14;980(2):241–247. doi: 10.1016/0005-2736(89)90405-7. [DOI] [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. The purified ATPase from chromaffin granule membranes is an anion-dependent proton pump. J Biol Chem. 1987 Jul 5;262(19):9175–9180. [PubMed] [Google Scholar]
- Nelson H., Nelson N. Disruption of genes encoding subunits of yeast vacuolar H(+)-ATPase causes conditional lethality. Proc Natl Acad Sci U S A. 1990 May;87(9):3503–3507. doi: 10.1073/pnas.87.9.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H., Nelson N. The progenitor of ATP synthases was closely related to the current vacuolar H+-ATPase. FEBS Lett. 1989 Apr 10;247(1):147–153. doi: 10.1016/0014-5793(89)81259-1. [DOI] [PubMed] [Google Scholar]
- Nelson N. Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr. 1989 Oct;21(5):553–571. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- Parry R. V., Turner J. C., Rea P. A. High purity preparations of higher plant vacuolar H+-ATPase reveal additional subunits. Revised subunit composition. J Biol Chem. 1989 Nov 25;264(33):20025–20032. [PubMed] [Google Scholar]
- Randall S. K., Sze H. Properties of the partially purified tonoplast H+-pumping ATPase from oat roots. J Biol Chem. 1986 Jan 25;261(3):1364–1371. [PubMed] [Google Scholar]
- Reilly P., Hulmes J. D., Pan Y. C., Nelson N. Molecular cloning and sequencing of the psaD gene encoding subunit II of photosystem I from the cyanobacterium, Synechocystis sp. PCC 6803. J Biol Chem. 1988 Nov 25;263(33):17658–17662. [PubMed] [Google Scholar]
- Rott R., Nelson N. Purification and immunological properties of proton-ATPase complexes from yeast and rat liver mitochondria. J Biol Chem. 1981 Sep 10;256(17):9224–9228. [PubMed] [Google Scholar]
- Shih C. K., Wagner R., Feinstein S., Kanik-Ennulat C., Neff N. A dominant trifluoperazine resistance gene from Saccharomyces cerevisiae has homology with F0F1 ATP synthase and confers calcium-sensitive growth. Mol Cell Biol. 1988 Aug;8(8):3094–3103. doi: 10.1128/mcb.8.8.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. Z., Xie X. S., Stone D. K. Isolation and reconstitution of the dicyclohexylcarbodiimide-sensitive proton pore of the clathrin-coated vesicle proton translocating complex. J Biol Chem. 1987 Oct 25;262(30):14790–14794. [PubMed] [Google Scholar]
- Sutton R., Apps D. K. Isolation of a DCCD-binding protein from bovine chromaffin-granule membranes. FEBS Lett. 1981 Jul 20;130(1):103–106. doi: 10.1016/0014-5793(81)80675-8. [DOI] [PubMed] [Google Scholar]
- Uchida E., Ohsumi Y., Anraku Y. Purification and properties of H+-translocating, Mg2+-adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1985 Jan 25;260(2):1090–1095. [PubMed] [Google Scholar]