Abstract
TH17 lymphocytes appear to be essential in the pathogenesis of numerous inflammatory diseases. We demonstrate here the expression of IL-17 and IL-22 receptors on blood-brain barrier endothelial cells (BBB-ECs) in multiple sclerosis lesions, and show that IL-17 and IL-22 disrupt BBB tight junctions in vitro and in vivo. Furthermore, TH17 lymphocytes transmigrate efficiently across BBB-ECs, highly express granzyme B, kill human neurons and promote central nervous system inflammation through CD4+ lymphocyte recruitment.
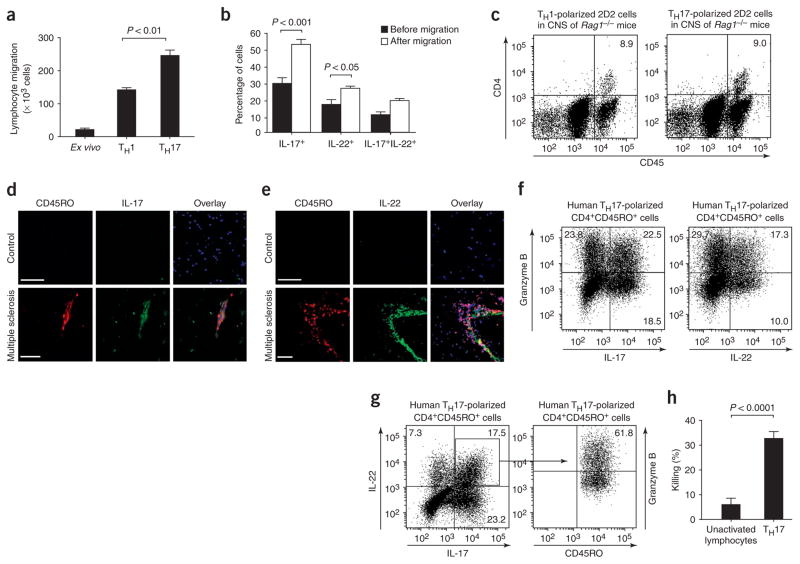
T-helper type 1 (TH1)1,2 and type 17 (TH17) lymphocytes contribute to autoimmune inflammatory diseases3 including multiple sclerosis and its mouse model, experimental autoimmune encephalomyelitis (EAE)4,5. Disruption of the BBB and trafficking of autoreactive T cells from the systemic compartment into the central nervous system (CNS) are important, early events in the development of multiple sclerosis lesions6. In support of TH1 lymphocytes have been shown to migrate efficiently across the human BBB7,8. To evaluate TH17 lymphocyte migration to the brain relative to TH1 cells, we employed an in vitro model of the human BBB using human brain-derived microvascular endothelial cells. We generated human TH1 and TH17 lymphocytes in vitro using peripheral blood CD4+ lymphocytes cultured with IL-12 and IL-23, respectively (Supplementary Methods online). Human TH17 lymphocytes migrated more avidly across the BBB than did TH1 or freshly isolated (ex vivo) CD4+ lymphocytes (Fig. 1a, P < 0.01). To ensure that the selective accumulation of TH17 lymphocytes indeed reflects the preferential transmigration ability of TH17 cells, we analyzed the intracellular cytokine profile of the cell population before and after migration across BBB-ECs, looking specifically at IL-17 and at IL-22, a recently identified cytokine product of TH17 cells9–11. We noted a significant enrichment in the number of IL-17– and IL-22–expressing CD4+CD45RO+ memory lymphocytes upon migration across the BBB (Fig. 1b, P < 0.001 for IL-17+ and P <0.05 for IL-22+ cells, n = 3), confirming the ability of TH17 lymphocytes to cross the BBB in vitro. To further substantiate these observations, we generated myelin oligodendrocyte glycoprotein (MOG)-specific TH1 and TH17 lymphocytes from 2D2 mice in vitro and transferred these separately into T and B lymphocyte–deficient Rag1−/− mice. Equal numbers of TH cells were found in the CNS of Rag1−/− mice 7 d after transfer, regardless of whether donor cells were polarized into TH1 or TH17 cells, confirming that TH1 and TH17 cells primed and expanded in the periphery access the CNS in vivo (Fig. 1c). To validate these human in vitro and mouse in vivo observations, brain sections from humans with multiple sclerosis and from unaffected controls were immunostained for CD45RO and IL-17 or IL-22. Numerous CD45RO+ cells immunopositive for IL-17 or IL-22 were detected in highly infiltrated multiple sclerosis lesions, but not in normal-appearing white matter or non-inflamed brain specimens (Fig. 1d,e). Taken together, these results emphasize the potential importance of TH17 lymphocyte infiltration into the CNS and these lymphocytes’ consequent involvement in lesion formation in multiple sclerosis and EAE.
Figure 1.
TH17 lymphocytes migrate efficiently across the BBB in vitro and in vivo and kill human neurons. (a) Human CD4+CD45RO+ TH17, CD4+ TH1 (both generated in vitro, see Supplementary Methods) and ex vivo CD4+ lymphocytes were allowed to migrate across human BBB-ECs in a modified Boyden chamber assay (ref. 14 and Supplementary Methods) for 18 h. Significantly more TH17 lymphocytes migrated than either TH1 or ex vivo CD4+ lymphocytes. (b) CD4+CD45RO+ TH17 lymphocytes were allowed to migrate across human BBB-ECs for 18 h. Cells were stained for IL-17 and IL-22 before and after migration. The cytokine profile revealed the preferential migration of IL-17+ and IL-22+ lymphocytes. (c) Immune cells from lymph nodes and spleen of MOG35–55-immunized 2D2 mice were polarized toward TH1 or TH17 and transferred to Rag1−/− mice, and CD45hiCD4+ lymphocytes were isolated from the CNS 7 d after transfer. Shown is a representative flow cytometry dot plot of CNS cell content from Rag1−/− mice injected with either TH1- (left) or TH17-polarized (right) 2D2 lymphocytes (n = 4 mice per group). (d) Human CNS postmortem material from unaffected individuals (control, non-inflamed; above) and heavily infiltrated CNS material from individuals with multiple sclerosis (below) were immunostained for CD45RO (red), IL-17 (green) and nuclear stain TO-PRO3 (blue). Confocal microscopy imaging confirmed the presence of IL-17+CD45RO+ cells (yellow) in infiltrated multiple sclerosis lesions but not in control CNS. Bar, 75 μm. (e) Similarly, IL-22+CD45RO+ staining was observed in multiple sclerosis lesions, but not in control CNS material. (f) Human CD4+CD45RO+ TH17–polarized lymphocytes were stained for CD45RO, IL-17, IL-22 and granzyme B. Both IL-17– and IL-22–producing lymphocytes expressed granzyme B (22.5% and 17.3%, respectively). (g) More than 60% of IL-17+IL-22+ lymphocytes highly expressed granzyme B. Granzyme A and perforin were not detected in TH17 cells, whether or not these cells produced IL-22 (data not shown). (h) The cytotoxic activity of TH17 lymphocytes was assessed using neuron-enriched cultures obtained from human fetal CNS material and compared to that of unactivated T lymphocytes. All data shown are representative of the mean ± s.e.m. of three independent experiments.
So far, the encephalitogenic activity of TH17 cells has been attributed to IL-17 (refs. 12,13). To investigate whether the action of TH17 cells extends beyond the proinflammatory influence of IL-17, we explored the possibility that TH17 cells might express cytolytic molecules and therefore analyzed TH17 cells for the expression of perforin, granzyme A and B. Notably, whereas virtually no ex vivo CD4+CD45RO+ cells produced cytolytic enzymes (data not shown), granzyme B was expressed in as many as 22.5% of IL-17–producing CD4+CD45RO+ cells and 17.3% of IL-22+ lymphocytes after 6 d of culture with IL-23 (Fig. 1f). Even more striking is that 60% of cells coexpressing IL-17 and IL-22 also expressed granzyme B (Fig. 1g). We therefore tested the capacity of granzyme B+ TH17 cells to kill human fetal neuron–enriched cultures and found that they showed considerable cytolytic activity (32.83 ± 2.54%) as compared to unactivated T lymphocytes (6.15 ± 2.37%) (Fig. 1h, P < 0.0001, n = 3).
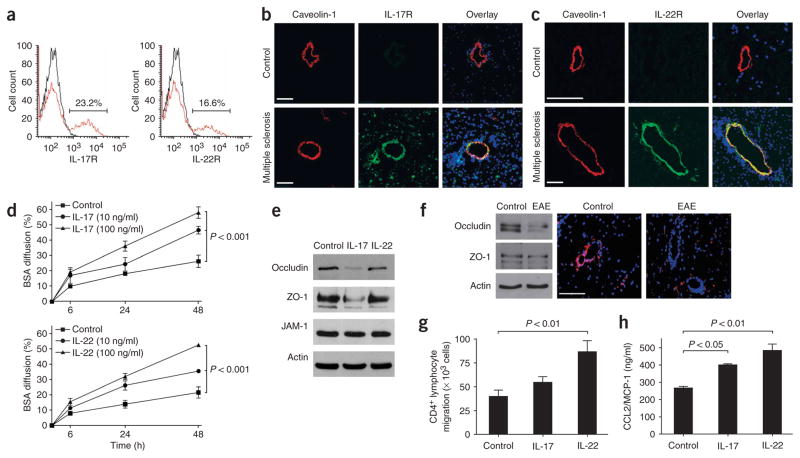
We next analyzed IL-17 receptor (IL-17R) and IL-22 receptor (IL-22R) expression on human BBB-ECs, and investigated whether IL-17 and IL-22 influence BBB integrity. IL-17R and IL-22R were detected on the surface of a subset of human BBB-ECs in primary culture (Fig. 2a; 23% of IL-17R+ and 16% of IL-22R+). In situ, however, IL-17R and IL-22R were undetectable in CNS material from subjects without multiple sclerosis. However, both receptors were strongly expressed on CNS vessels within heavily infiltrated multiple sclerosis lesions, colocalized with caveolin-1, a marker of brain endothelial cells (Fig. 2b,c).
Figure 2.
IL-17 and IL-22 receptors are expressed on human brain endothelium, and their activation permeabilizes the BBB. (a) Unactivated human BBB-ECs grown in primary culture were stained for IL-17R and IL-22R, revealing their expression on the surface of 23.2% and 16.6% of BBB-ECs, respectively. (b) Human CNS postmortem material from unaffected individuals (control, non-inflamed; above) and heavily infiltrated CNS material from individuals with multiple sclerosis (below) were immunostained for IL-17R (green), caveolin-1 (red) and nuclear stain TO-PRO3 (blue). Confocal microscopy imaging confirmed the expression of IL-17R on caveolin-1+ endothelium in inflamed CNS material. IL-17R expression was undetectable in control CNS material. Bar, 75 μm. (c) Similarly, IL-22R staining was observed on endothelial cells in multiple sclerosis lesions, but not in controls. (d) Human BBB-ECs were grown in Boyden chambers and treated with IL-17 (top) or IL-22 (bottom). Permeability of the monolayers was monitored with fluorescent BSA, showing that BBB-EC monolayer permeability increased after treatment with either IL-17 or IL-22. (e) Western blot for the tight-junction proteins occludin, ZO-1 and junction adhesion molecule (JAM)-1 from human BBB-ECs revealed disruption of occludin and ZO-1 by IL-17 (100 ng/ml, 18 h). (f) Western blot for tight-junction proteins in spinal cord homogenates of MOG35–55-immunized EAE mice revealed a similar reduction in occludin and ZO-1. In situ immunostaining for ZO-1 (red) and nuclear stain TO-PRO3 (blue) in normal-appearing cerebellar white matter (control) and in infiltrated and demyelinated cerebellar lesions from C57BL/6 mice immunized with MOG35–55 (EAE, grade 4). Confocal microscopy imaging confirmed disruption of ZO-1 around infiltrated vessels. Bar, 75 μm. (g) Freshly isolated peripheral blood human CD4+ lymphocytes were allowed to migrate for 18 h across IL-17– (100 ng/ml) or IL-22–treated (100 ng/ml) human BBB-ECs. Both cytokines promoted migration of human ex vivo CD4+ lymphocytes across human BBB-ECs, as compared to control. (h) CCL2 (or MCP-1) secretion by human BBB-ECs was assessed by ELISA in untreated and IL-17– or IL-22–treated cultures (100 ng/ml, 18 h). Both IL-17 and IL-22 upregulate CCL2 secretion by human BBB-ECs. All data shown represent the mean ± s.e.m. from three independent experiments performed in triplicate.
We further investigated whether brain endothelial IL-17R and IL-22R were functional, and whether IL-17 and IL-22 could affect BBB permeability. Addition of 10 ng/ml of IL-17 or IL-22 to monolayers of human BBB-ECs induced a marked and sustained increase in the diffusion of fluorescence-labeled BSA (Fig. 2d). This effect was dose dependent, reached a plateau at 100 ng/ml and coincided, for IL-17, with a decrease in the expression of occludin and zonula occludens (ZO)-1, two important tight junction–associated molecules (Fig. 2e). A similar reduction of occludin, and to a lesser extent ZO-1, expression was demonstrated by western blotting in spinal cord homogenates from EAE mice (Fig. 2f). In situ staining further confirmed a decrease in ZO-1 immunoreactivity in cerebellar lesions of MOG-immunized mice (Fig. 2f) These results are in line with our recent data showing a disruption of tight-junction proteins in highly infiltrated vessels of multiple sclerosis lesions14. The exact mechanism mediating IL-22–induced BBB permeability remains uncertain, however.
We next explored the capacity of IL-17 and IL-22 to modulate lymphocyte migration across human BBB-ECs and found that IL-17 and IL-22 promote transmigration of human ex vivo CD4+ lymphocytes (Fig. 2g), most likely through enhanced BBB-EC–mediated secretion of CCL2 (or MCP-1) (Fig. 2h). IL-17 also induced IL-6 and CXCL8 (or IL-8) expression by BBB-ECs, whereas expression of TGF-β, ICAM-1, VCAM-1, CCL5 (or RANTES) and CXCL10 (or IP-10) remained unaffected (data not shown). Taken together, these results strongly suggest that TH17 cells, through the action of IL-17 and IL-22, play a unique role in permeabilizing the human BBB both to soluble molecules and to circulating CD4+ lymphocytes.
Our study further refines the phenotype of human TH17 lymphocytes as cells coexpressing IL-17, IL-22 and granzyme B, and provides strong evidence that IL-17 and IL-22 induce a breach in the BBB and promote the recruitment of additional CD4+ lymphocytes. Although IL-22 has the potential to influence the ability of TH17 lymphocytes to gain access to the CNS, in vivo evidence indicates that IL-22 does not directly affect their encephalitogenicity (B.B., personal communication). We postulate that TH17 cells produce multiple mediators contributing to their highly encephalitogenic potential, among them cytolytic enzymes such as granzyme B.
Supplementary Material
Acknowledgments
This study was supported by funding from the Multiple Sclerosis Society of Canada (MSSC) and from the Canadian Fund for Innovation to A.P. The animal studies were supported through grants from the US National MS Society and the Swiss National Science Foundation (B.B.). H.K., I.I., A.D.-D. and R.C. hold studentships from the MSSC and the Canadian Institutes of Health Research (CIHR)/Strategic Training Initiative in Health Research Neuroinflammation Training Program. K.K. has a fellowship from the Center for Neurosciences in Zurich. N.A. holds a CIHR Senior Research Fellowship Phase 2. B.B. is a Neuroscience Scholar of the US National MS Society. A.P. is a Research Scholar from the Fonds de la Recherche en Santédu Québec, and holds the Donald Paty Career Award of the MSSC. We thank I. Gutcher, S. Haak, D. Pasichnyk and J. Laganiére for their excellent technical assistance. We are grateful to V.K. Kuchroo (Harvard Medical School), who kindly provided the 2D2 mice, and to J.P. Antel (McGill University) for providing assistance and human tissue.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
H.K. conducted most of the experiments; K.K. performed and analyzed animal studies; I.I. and A.D.-D. contributed to immunostaining and in vitro protocols; R.C. assisted with confocal microscopy and performed some EAE experiments; M.B. assisted with BBB-EC isolation and culture; F.G. performed the killing assay; N.A. provided critical input on data analysis; B.B. designed and supervised the animal studies; H.K. and A.P. designed the study, analyzed the data and wrote the manuscript; A.P. secured the funding.
References
- 1.Renno T, et al. Int Immunol. 1994;6:347–354. doi: 10.1093/intimm/6.3.347. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E, et al. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman L. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 4.Cua DJ, et al. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 5.Langrish CL, et al. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sospedra M, Martin R. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 7.Biernacki K, Prat A, Blain M, Antel JP. J Neuropathol Exp Neurol. 2001;60:1127–1136. doi: 10.1093/jnen/60.12.1127. [DOI] [PubMed] [Google Scholar]
- 8.Prat A, Biernacki K, Antel JP. J Autoimmun. 2005;24:119–124. doi: 10.1016/j.jaut.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Liang SC, et al. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung Y, et al. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y, et al. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 12.Komiyama Y, et al. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 13.Uyttenhove C, Van SJ. Eur J Immunol. 2006;36:2868–2874. doi: 10.1002/eji.200636662. [DOI] [PubMed] [Google Scholar]
- 14.Wosik K, et al. J Neurosci. 2007;27:9032–9042. doi: 10.1523/JNEUROSCI.2088-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.