Abstract
Objective
To compare the oncologic outcomes of patients with upper tract urothelial carcinoma (UTUC) undergoing nephroureterectomy (NU) with and without prior ureteroscopy (URS).
Methods
We reviewed records of all patients with no prior history of bladder cancer that underwent NU at our institution (n = 201). We compared patients who underwent URS prior to NU to patients who proceeded directly to NU based on imaging alone. After excluding patients undergoing URS with therapeutic intent, we used multivariable Cox proportional hazards models, adjusting for tumor characteristics with cancer specific survival (CSS), intravesical recurrence free survival (IRFS), metastasis free survival (MFS), and overall survival (OS) as endpoints.
Results
144 (72%) patients underwent URS prior to NU and 57 (28%) patients proceeded directly to NU. The median follow up time for survivors was 5.4 years from diagnosis. The performance of diagnostic URS prior to NU was significantly associated with IR (HR 2.58; 95% CI 1.47, 4.54; p = 0.001), although it was not associated with CSS, MFS, or OS. The adjusted IRFS probability 3 years after diagnosis is 71% and 42% for patients who did not and did receive URS prior to NU, respectively (adjusted risk difference 30%; 95% CI 13%, 47%).
Conclusions
We did not find evidence that URS adversely impacts disease progression and survival in patients with UTUC. Although patients are at higher risk for IR after NU when they have undergone prior diagnostic URS, their CSS, MFS, and OS are not significantly affected.
Keywords: upper tract urothelial carcinoma, nephroureterectomy, ureteroscopy, intravesical recurrence
1. Introduction
Upper tract urothelial carcinoma (UTUC) is a rare and challenging disease to manage, with limited modalities for diagnosis and accurate clinical staging. In the current era, ureteroscopy (URS) with or without biopsy is often utilized for diagnosis and treatment of UTUC, although the oncologic sterility of this procedure has been questioned. Historically, radical nephroureterectomy (NU) was performed for clinical suspicion of UTUC, typically based on imaging findings with or without urinary cytologic evidence.
With the proliferation of endoscopic techniques many urologists today will perform URS prior to NU with either diagnostic or therapeutic intent. As a diagnostic modality, URS with tissue biopsy provides valuable data for risk-stratifying patients, which has proven useful in management decision algorithms (1). Endoscopic tumor ablation has also shown to be effective in highly selected cases, specifically those with low-grade low-volume tumor burden, solitary kidney, bilateral tumors, and/or baseline renal insufficiency (2-6). The procedure is, however, invasive and potentially disturbing to the tumor microenvironment leading some to question the oncologic sterility of this technique. Specifically, there have been reports of disease progression following URS, speculatively as a consequence of tumor manipulation and the increased pyelovenous pressure during the procedure (7-10). Such reports, though anecdotal, have prompted some to advocate against instrumentation of the upper urinary tract prior to surgical resection.
To address the concern for increased risk of disease progression following URS we compared the oncologic outcomes of patients with UTUC treated at our institution that were managed with or without URS prior to NU.
2. Methods
We identified 211 patients with isolated UTUC and no prior history of bladder cancer that underwent definitive NU at a single institution (MSK) between December 1994 and May 2012. Ten patients were excluded due to missing tumor sizes, leaving us with a cohort of 201 UTUC patients. Patients were segregated into one of two groups based upon full review of their prior management including external medical records: those who underwent URS prior to NU (URS+) or those proceeding directly to NU based on imaging alone (URS–).
To determine whether there were differences in demographics and tumor characteristics between URS+ and URS– patients, group comparisons were made using Fisher's exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. We used multivariable Cox proportional hazards regression, adjusting for grade (high vs. low), tumor size, age and hydronephrosis to assess whether URS prior to NU is associated with overall survival (OS). Due to a limited number of events, when assessing whether URS prior to NU is associated with cancer specific survival (CSS), we adjusted for grade and tumor size. As URS can result in a delay to NU, we defined the start of the survival period as date of diagnosis. As a sensitivity analysis, we assessed whether URS prior to NU is associated with CSS and OS with the start of the survival period defined as date of surgery.
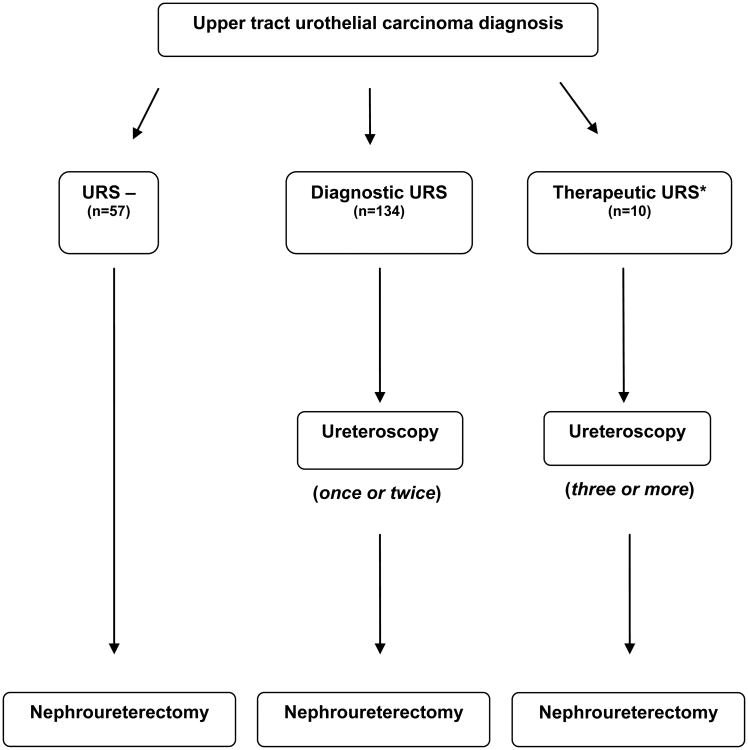
When determining intravesical recurrence-free survival (IRFS) and metastasis-free survival (MFS) rates, we excluded patients who had undergone URS as a means of therapeutic treatment which was defined as >2 URS procedures or documentation of complete tumor eradication (n=10). This was to avoid the heavy impact of selection bias present in these cases and the influence of prolonged intervals of therapeutic URS on the time to event between URS and NU. Figure 1 depicts the different categorization of patients based on treatment schema. We first utilized a univariate Cox proportional hazards model to assess whether URS prior to NU is associated with either outcome. Then, we used multivariable Cox proportional hazards models, adjusting for predictors of oncologic outcomes. We adjusted for grade, tumor size, tumor location, age and hydronephrosis with IRFS as the endpoint. Due to a limited number of events we adjusted for grade, tumor size, and age with MFS as the endpoint. After the exclusion of patients who received therapeutic URS, the median time interval between diagnosis and NU for URS– patients and URS+ was 52 days and 71 days, respectively (p=0.020). Although there was only a slight delay and we did not expect this to have a large impact on our results, we ran a sensitivity analysis with the survival period beginning from date of surgery, rather than date of diagnosis, with IRFS and MFS as the endpoints to account for this delay to surgery.
Figure 1. Schema of treatment for upper tract urothelial carcinoma within our study.
*Therapeutic URS patients were excluded from our analysis of recurrence free survival.
Finally, we evaluated whether any association between URS and any type of recurrence, including metastasis, is lethal by creating a multivariable Cox proportional hazards model adjusting for grade (high vs. low) and tumor size, with CSS from time of recurrence or metastasis as the endpoint. Of the 201 patients, this subanalysis only included the 117 patients who recurred or had metastases after their NU. All statistical analyses were conducted using STATA 12.0 (StataCorp, College Station, TX)
3. Results
We identified 144 (72%) URS+ patients and 57 (28%) URS– patients. Patient characteristics are shown in Table 1. URS– patients had a higher tumor stage (p=0.031), had a majority of renal pelvis tumors (p=0.028), and a larger proportion were female (p=0.012) in comparison to URS+ patients.
Table 1.
Patient Characteristics (n=201). All values are median (IQR) or frequency (proportion).
| No URS (N=57) |
URS (N=144) |
p-value | |
|---|---|---|---|
|
| |||
| Age at Diagnosis | 71 (63, 79) | 70 (61, 76) | 0.3 |
|
| |||
| Male | 23 (40%) | 87 (60%) | 0.012 |
|
| |||
| Tumor Size (cm) | 3.5 (2.5, 4.5) | 3.1 (2.0, 4.5) | 0.15 |
|
| |||
| High Grade | 49 (86%) | 112 (78%) | 0.2 |
|
| |||
| Hydronephrosis | 24 (42%) | 65 (45%) | 0.8 |
|
| |||
| Tumor Location | 0.028 | ||
| Pelvis | 44 (77%) | 83 (58%) | |
| Ureter | 7 (12%) | 40 (28%) | |
| Both Pelvis and Ureter | 6 (11%) | 21 (15%) | |
|
| |||
| Pathologic T Stage | 0.031 | ||
| Tis | 0 (0%) | 3 (2.1%) | |
| Ta | 11 (19%) | 49 (34%) | |
| T1 | 9 (16%) | 27 (19%) | |
| T2 | 16 (28%) | 26 (18%) | |
| T3 | 16 (28%) | 37 (26%) | |
| T4 | 5 (8.8%) | 2 (1.4%) | |
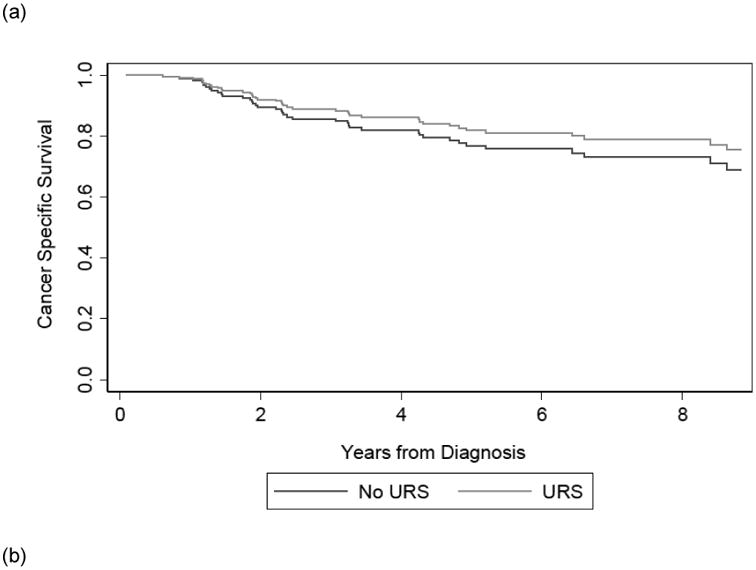
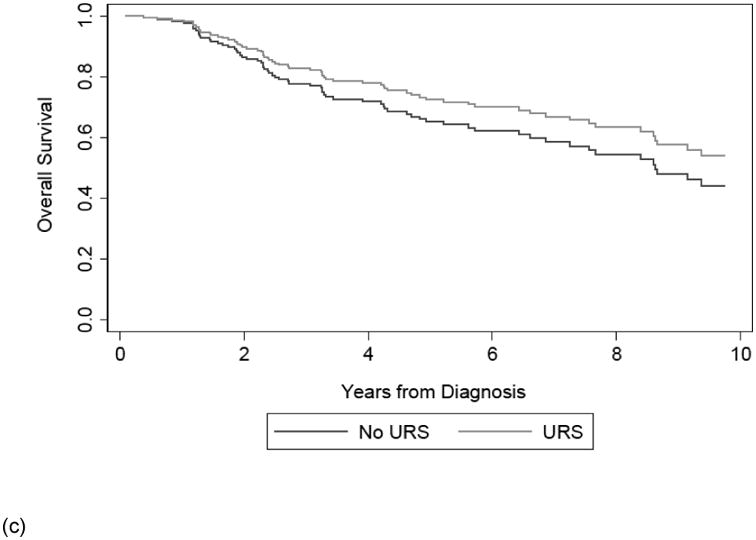
Among the 201 patients, 40 died due to their disease. The median follow up time for survivors was 5.4 years from diagnosis. Table 2 shows that on multivariable analysis, receipt of URS prior to NU was not significantly associated with CSS, with slightly better outcomes in URS+ patients (HR 0.75; 95% CI 0.39, 1.45; p=0.4) or OS (HR 0.73; 95% CI 0.44, 1.21; p=0.2). The confidence interval does not exclude important differences in survival between the two groups. Results were not importantly changed by the exclusion of patients who received therapeutic URS (HR for CSS: 0.78; 95% CI 0.40, 1.50; p=0.4; HR for OS: 0.75; 95%CI 0.45, 1.24; p=0.3). Likewise, results were not importantly changed by setting the beginning of the survival period as date of surgery, rather than date of diagnosis. (HR for CSS: 0.80; 95% CI 0.41, 1.54; p=0.5; HR for OS: 0.77; 95%CI 0.46, 1.27; p=0.3).
Table 2.
Multivariable* analysis of the association of URS prior to NU with cancer specific survival (CSS) and overall survival (OS).
| Entire Cohort1 (n=201) | Excluding Therapeutic Cohort (n=191) | Excluding Therapeutic Cohort (n=191)3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| CSS* | 0.75 | 0.78 | |||||||
| URS | 0.39, 1.45 | 0.4 | 0.40, 1.50 | 0.4 | 0.80 | 0.41, 1.54 | 0.5 | ||
| OS2 | 0.73 | 0.75 | |||||||
| URS | 0.44, 1.21 | 0.2 | 0.45, 1.24 | 0.3 | 0.77 | 0.46, 1.27 | 0.3 | ||
Entire cohort used to compare No URS vs URS (included for both therapeutic and diagnostic purposes)
Adjusted for high grade disease, tumor size (cm)
Additionally adjusted for age at diagnosis and hydronephrosis (binary)
Survival Period from date of surgery
HR: Hazard Ratio; CI: Confidence Interval
Of the 191 patients who either had diagnostic URS or no URS prior to surgery, 89 experienced intravesical recurrence and 43 experienced metastases. The difference in median time interval between diagnosis and NU in URS– and URS+ patients was 20 (95% CI 2, 35; p=0.020) days.
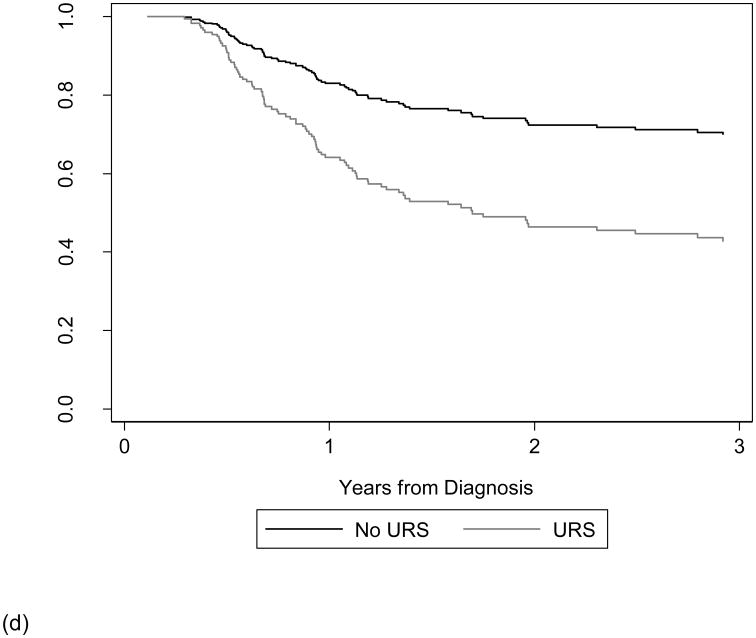
Receipt of diagnostic URS prior to NU was not significantly associated with MFS (HR 0.90; 95% CI 0.47, 1.73; p=0.8). In contrast, receipt of diagnostic URS prior to NU was significantly associated with worse IRFS on univariate (HR 2.51; 95% CI 1.44, 4.38; p=0.001) and multivariable analysis (HR 2.37; 95% CI 1.34, 4.20; p = 0.003) when compared to patients who did not receive a URS prior to NU. The adjusted risk difference at three years for patients experiencing intravesical recurrence from this multivariable Cox proportional hazards model is 27% (95% CI 9%, 44%).
The results of our supplemental analysis show that accounting for the delay to surgery by changing the beginning of our survival time period from time of surgery, rather than time of diagnosis did not alter our conclusion. Receipt of URS prior to NU remained significantly associated with IRFS (HR 2.43; 95% CI 1.37, 4.30; p = 0.002) and receipt of URS prior to NU was again not significantly associated with MFS (HR 0.93; 95% CI 0.49, 1.77; p=0.8). Figure 2 displays the survival curves estimated from our multivariable Cox model of CSS, OS, IRFS, and MFS from time of diagnosis based on receipt of URS prior to NU.
Figure 2. Adjusted survival curve of CSS (a), OS (b), IRFS (c), and MFS (d) based on receipt of URS prior to NU.
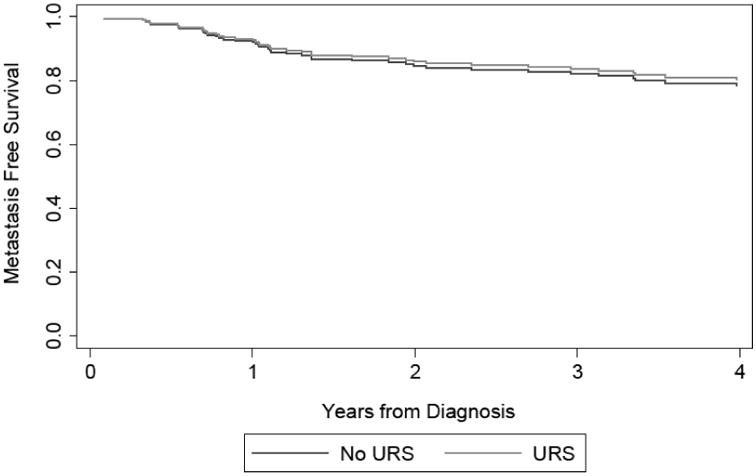
Lastly, we created a model assessing the association between URS prior to NU and CSS after recurrence. Receipt of URS was significantly associated with improved cancer specific survival in patients who recurred at any site (0.47; 95% CI 0.24, 0.90; p=0.024).
4. Discussion
Urothelial tumors are difficult to diagnose and stage accurately prior to definitive treatment. Up to 40% of UTUC's are upgraded and/or upstaged following surgical extirpation (11), a rate that is similar to bladder tumors (12, 13). The propensity for understaging of UTUC patients reinforces the necessity for frequent and thorough ureteroscopic evaluation in order to accurately risk stratify patients and select patients for NU.
The notion that URS of UTUC predisposes the patient to tumor seeding is based mostly on speculation and lacks sufficient empiric evidence. Many have theorized that high intra-pelvic irrigation pressures promote pollination of friable cancer cells through pyelovenous networks. Irrigation during URS can raise intrapelvic pressure to greater than 50 cm H20 (14). With this potentially dangerous pressure in mind, many urologists will make attempts to reduce intrapelvic pressure, such as limiting irrigation pressures to gravity and/or the use of a nephrostomy tube as a pop-off valve. One of the initial investigations discrediting the hypothesis of friable cancer cell pollination was a study performed by Kulp and Bagley in 1994 (10). They systematically examined kidney specimens removed after NU with prior URS. Close inspection of these renal units failed to demonstrate any free floating tumor cells in the vascular or lymphatic spaces of the submucosa or surrounding renal parenchyma. However, advancements in endoscopic technology and improved techniques in URS have shifted management of some UTUC patients to endoscopy and reserved radical surgery for patients with disease progression or high grade tumors. Therefore, the safety of URS requires validation in a contemporary cohort.
Another potential criticism of ureteroscopic management prior to NU for UTUC is the time delay of definitive treatment. To address this notion and examine its potential effect on outcomes, Boorjian et al reviewed the cases of 121 patients undergoing NU with or without prior URS at New York Presbyterian Hospital (15). They further subdivided the URS group to those undergoing solely tissue biopsy and those undergoing therapeutic laser ablation of their tumors. While time of delay between the biopsy group and the ablation group differed considerably (mean time of delay was 28 days and 196 days, respectively) there was no difference in tumor pathology, followup duration, and disease status between the groups. This is in contrast to our results, in which we found the time delay, albeit small, after receipt of URS to be a median of 20 days, and prior URS was associated with an increased risk of intravesical recurrence after NU (HR 2.58; 95% CI 1.47, 4.54; p = 0.001).
The study with the largest cohort to date looking into the potential oncologic consequences of URS for UTUC was a multi-institutional study conducted by Gurbuz et al in 2010 (16). This international collaborative review focused on 1268 patients undergoing NU for UTUC. Of this group, 175 patients (13%) underwent prior ureteroscopic tumor ablation. With a median followup of 52 months, the authors found no difference in disease recurrence and cancer specific mortality between the URS and non-URS group (the 5-year disease free survival and cancer specific survival rates were 72% and 77% in the URS group versus 69% and 73% in the non-URS group (p=0.17 and p=0.4, respectively)). This study does not address the risk of bladder recurrence and lacks initial tumor pathology data and time interval to NU.
There are conflicting data in the literature on the association between URS and intravesical recurrence after NU. Similar to our findings, a recent study by Luo et al. found that patients (n=115) undergoing URS prior to definitive NU had an increased risk of intravesical recurrence compared to those who had not undergone prior URS (n=281) (HR 1.44, p=0.05) (17). In contrast, however, a study by Ishikawa et al. found no association between URS and intravesical tumor recurrence rate (18). This group looked at 208 patients undergoing NU for UTUC, of which 53 had undergone previous URS. They found no difference in intravesical recurrence rates (60% in the URS cohort versus 59% in the non-URS cohort, p=0.9) at two years between the two groups. Moreover, they found that the only factor that predisposes one to intravesical tumor recurrence is a distal location of primary ureteral tumor.
The results of our study show that receipt of URS prior to definitive NU is associated with increased intravesical tumor recurrence. The exact mechanism underlying this phenomenon is not completely understood. One possible explanation may be that viable upper tract tumor cells slough off during ureteroscopy, drift downstream and re-implant in bladder mucosa. Another explanation may be that patients with distal ureteral tumors, a site that is associated with increased risk of intravesical recurrence, are more likely to undergo URS prior to NU.
Recent clinical trials have studied the utility of administering adjuvant intravesical chemotherapy after NU in preventing intravesical recurrence. A recent meta analysis of the pooled data of these clinical trials found that peri-operative administration of intravesical chemotherapy reduced the likelihood of a bladder tumor recurrence with an odds ratio of 0.45 (19). It is unclear if this finding is applicable to the post-URS setting.
It may be a worthwhile endeavor to prospectively study the use of adjuvant intravesical chemotherapy after URS.
The observed increased risk of intravesical tumor recurrence in the URS+ group should be interpreted with caution. This is especially true when considering there is no evidence of association of URS prior to NU with cancer specific mortality, despite the observed increased risk of intravesical recurrence. Additionally, we observed that time to death from disease after recurrence at any site was longer in patients undergoing URS prior to NU. This is likely explained by a higher proportion of recurrences in the URS+ group being intravesical, which are commonly detected at an early state when tumors are superficial and effectively controlled with local treatment. Lastly, we did not find any evidence to suggest that URS prior to NU is associated with a higher rate of metastasis or a higher overall mortality rate. These findings contrast with previous anecdotal reports of URS leading to poor outcomes for UTUC patients.
Some limitations of this study should be addressed. Retrospective studies are inherently susceptible to selection bias. Specifically, since our subjects were not randomized into treatment arms, the criteria for performing pre-NU URS were subject to variability. Additionally, as there is a lack of absolute indication for URS, surgeon preference is the primary motivation for this procedure. For example, in our study patients receiving URS had lower tumor stages (p=0.031) with fewer renal pelvis tumors (p=0.028) when compared to patients who did not receive URS. Additionally, we included patients treated by multiple surgeons, which could lead to inconsistency in treatment paradigms.
URS has a role in diagnostic and therapeutic management prior to definitive extirpation of UTUC and based on our study, we did not find any evidence that it increases the risk of disease progression or death from disease. However, there is conflicting results in literature assessing the association between URS and IRFS—with most studies, like ours, consisting of small cohorts and adjusting for different covariates Future studies should be designed to prospectively analyze the effects of URS, ideally by randomizing subjects to treatment and control arms and analyzing the tumor recurrence and survival rates.
5. Conclusion
We did not find evidence that URS adversely impacts cancer-specific survival in patients prior to NU. Patients are at higher risk for IR after NU when they have undergone prior diagnostic URS; however, recurrence in these patients is not associated with mortality. A multi-institutional study would clarify whether a significant association between URS and IRFS still exists once more measured covariates are adjusted for, and whether treating physicians must weigh the benefits derived from pre–NU URS, including more accurate staging and possibility of endoscopic ablation, with the increased risk of post–NU IR.
Acknowledgments
This work has been supported by the Sidney Kimmel Center for Prostate and Urologic Cancers, the National Cancer Institute T32 CA082088-14 training grant (Sankin), and the Stephen P Hanson Family Fund Fellowship in Kidney Cancer (Sankin).
Footnotes
Study received institutional review board approval.
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Favaretto RL, Shariat SF, Savage C, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU international. 2012;109(1):77–82. doi: 10.1111/j.1464-410X.2011.10288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen GL, Bagley DH. Ureteroscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. The Journal of urology. 2000;164(4):1173–6. [PubMed] [Google Scholar]
- 3.Elliott DS, Segura JW, Lightner D, et al. Is nephroureterectomy necessary in all cases of upper tract transitional cell carcinoma? Long-term results of conservative endourologic management of upper tract transitional cell carcinoma in individuals with a normal contralateral kidney. Urology. 2001;58(2):174–8. doi: 10.1016/s0090-4295(01)01109-8. [DOI] [PubMed] [Google Scholar]
- 4.Grasso M, Fishman AI, Cohen J, Alexander B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU international. 2012;110(11):1618–26. doi: 10.1111/j.1464-410X.2012.11066.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee BR, Jabbour ME, Marshall FF, et al. 13-year survival comparison of percutaneous and open nephroureterectomy approaches for management of transitional cell carcinoma of renal collecting system: equivalent outcomes. Journal of endourology / Endourological Society. 1999;13(4):289–94. doi: 10.1089/end.1999.13.289. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RH, Krambeck AE, Lohse CM, et al. Endoscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. Urology. 2008;71(4):713–7. doi: 10.1016/j.urology.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Grasso M, McCue P, Bagley DH. Multiple urothelial recurrences of renal cell carcinoma after initial diagnostic ureteroscopy. The Journal of urology. 1992;147(5):1358–60. doi: 10.1016/s0022-5347(17)37565-1. [DOI] [PubMed] [Google Scholar]
- 8.Kulp DA, Bagley DH. Does flexible ureteropyeloscopy promote local recurrence of transitional cell carcinoma? Journal of endourology / Endourological Society. 1994;8(2):111–3. doi: 10.1089/end.1994.8.111. [DOI] [PubMed] [Google Scholar]
- 9.Lim DJ, Shattuck MC, Cook WA. Pyelovenous lymphatic migration of transitional cell carcinoma following flexible ureterorenoscopy. The Journal of urology. 1993;149(1):109–11. doi: 10.1016/s0022-5347(17)36014-7. [DOI] [PubMed] [Google Scholar]
- 10.Tomera KM, Leary FJ, Zincke H. Pyeloscopy in urothelial tumors. The Journal of urology. 1982;127(6):1088–9. doi: 10.1016/s0022-5347(17)54240-8. [DOI] [PubMed] [Google Scholar]
- 11.Smith AK, Stephenson AJ, Lane BR, et al. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology. 2011;78(1):82–6. doi: 10.1016/j.urology.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Amling CL, Thrasher JB, Frazier HA, et al. Radical cystectomy for stages Ta, Tis and T1 transitional cell carcinoma of the bladder. The Journal of urology. 1994;151(1):31–5. doi: 10.1016/s0022-5347(17)34865-6. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 13.Dutta SC, Smith JA, Jr, Shappell SB, et al. Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. The Journal of urology. 2001;166(2):490–3. [PubMed] [Google Scholar]
- 14.Rehman J, Monga M, Landman J, et al. Characterization of intrapelvic pressure during ureteropyeloscopy with ureteral access sheaths. Urology. 2003;61(4):713–8. doi: 10.1016/s0090-4295(02)02440-8. [DOI] [PubMed] [Google Scholar]
- 15.Boorjian S, Ng C, Munver R, et al. Impact of delay to nephroureterectomy for patients undergoing ureteroscopic biopsy and laser tumor ablation of upper tract transitional cell carcinoma. Urology. 2005;66(2):283–7. doi: 10.1016/j.urology.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Gurbuz C, Youssef RF, Shariat SF, et al. The impact of previous ureteroscopic tumor ablation on oncologic outcomes after radical nephrouretectomy for upper urinary tract urothelial carcinoma. Journal of endourology / Endourological Society. 2011;25(5):775–9. doi: 10.1089/end.2010.0396. [DOI] [PubMed] [Google Scholar]
- 17.Luo HL, Kang CH, Chen YT, et al. Diagnostic ureteroscopy independently correlates with intravesical recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma. Annals of surgical oncology. 2013;20(9):3121–6. doi: 10.1245/s10434-013-3000-z. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa S, Abe T, Shinohara N, et al. Impact of diagnostic ureteroscopy on intravesical recurrence and survival in patients with urothelial carcinoma of the upper urinary tract. The Journal of urology. 2010;184(3):883–7. doi: 10.1016/j.juro.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Wu P, Zhu G, Wei D, et al. Prophylactic intravesical chemotherapy decreases bladder tumor recurrence after nephroureterectomy for primary upper tract urothelial carcinoma: a systemic review and meta-analysis. J BUON. 2015;20(5):1229–38. [PubMed] [Google Scholar]