Abstract
Early and delayed afterdepolarizations (EAD/DAD) cause triggered ventricular ectopy. Because ranolazine (RAN) suppresses EAD/DAD, we postulated that RAN might be effective in reducing premature ventricular contractions (PVCs).
To assess the effect of RAN in patients with symptomatic PVCs due to triggered ectopy and its safety and tolerability.
A total of 59 patients with symptomatic PVCs were identified from full-disclosure Holters. Doses of 500 and 1,000 mg offlabel RAN, daily, were given to 34 and 66% patients, respectively, and repeat Holters were performed prospectively during mean followup of 3.1 months. The two Holters were retrospectively compared. Congestive heart failure (CHF) was defined as symptoms including dyspnea, orthopnea, paroxysmal nocturnal dyspnea, and fatigue, with a brain natriuretic peptide > 400. Systolic (heart failure with reduced ejection fraction) versus diastolic (heart failure with preserved ejection fraction, HFpEF) CHF depended upon an echocardiographic left ventricular ejection fraction (LVEF) at least 50% by apical two- and four-chamber Simpson's method (HFpEF).
The mean age of the patients was 63 years, 60% were males, mean left ventricular ejection fraction was 60%, with 34% having coronary artery disease, 73% were hypertensive, 24% had type 2 diabetic, and 34% were on beta blockers. Upon repeat Holters at a mean of 3.1 months after initiating RAN, 95% (56/59) of the patients had their PVC count reduced as follows: 24% (14/59) had more than 90% decrease, 34% (20/59) had 71 to 90% decrease, and 17% (10/59) had 50 to 70% decrease. In the entire group, RAN reduced PVCs by 71% (mean: 13,329 to 3,837; p < 0.001). Ventricular bigeminy was reduced by 80% (4,168 to 851; p < 0.001), ventricular coupletswere reduced by 78% (374 to 81; p < 0.001), and ventricular tachycardiawas reduced by 91% (56 to 5; p < 0.001). The PVC reduction was dose dependent.
Off-label RAN offers an effective and safe pharmacologic treatment for symptomatic triggered PVCs. A large, prospective randomized study is needed.
Keywords: ranolazine, premature ventricular contractions, early and delayed afterdepolarizations
Ranolazine (RAN) is a novel antianginal agent with antiarrhythmic properties. In the therapeutic concentration range of 2 to 6 μM, RAN inhibits the late sodium current (INa), resulting in suppression of early and delayed afterdepolarizations (EAD/DAD), thereby reducing triggered ventricular ectopy.1 An increase of the late INa induces EAD/DAD resulting in triggered activity.2 The diastolic transient inward current in the long QT syndrome3 is caused by calcium overload and is inhibited by RAN.3 Because RAN has no known proarrhythmic effects and, to the contrary, protects against torsades de pointes,4 we hypothesized that RAN could be an effective and safe pharmacologic treatment for symptomatic premature ventricular contractions (PVCs).
Methods
Using full-disclosure 24-hour Holters (Burlick), 59 adult patients with highly symptomatic, frequent PVCs (examples in Fig. 1) were identified during routine outpatient clinic visits (Table 1). The PVCs met criteria for “ventricular parasystole” (VP): nonfixed coupling, fusion, interpolation, and a mathematical relationship with R-R intervals. Doses of 500 and 1,000 mg RAN, daily,were given to 34 and 66% patients, respectively, depending on tolerability, without the side effects of headache, dizziness, nausea, or constipation, or the patients' symptomatic improvement. Holters were repeated at 1 week and up to 2 years (mean: 3.1 months) and were retrospectively compared. Response was defined as at least 50% reduction in PVC count and/or at least 70% reduction in complex PVCs. All statistics, including means, standard deviations, and Student's t-tests, were performed under SPSS v 14.1 (IBM). Student's t-tests were performed as two-tailed tests with equal variance. Significant values were determined on the null hypothesis that the pre- and posttreatment values were equal. All patients were informed that RAN administration for PVCs was not approved by the U.S. Food and Drug Administration, hence it was off-label use, and gave appropriate informed consent.
Fig. 1.
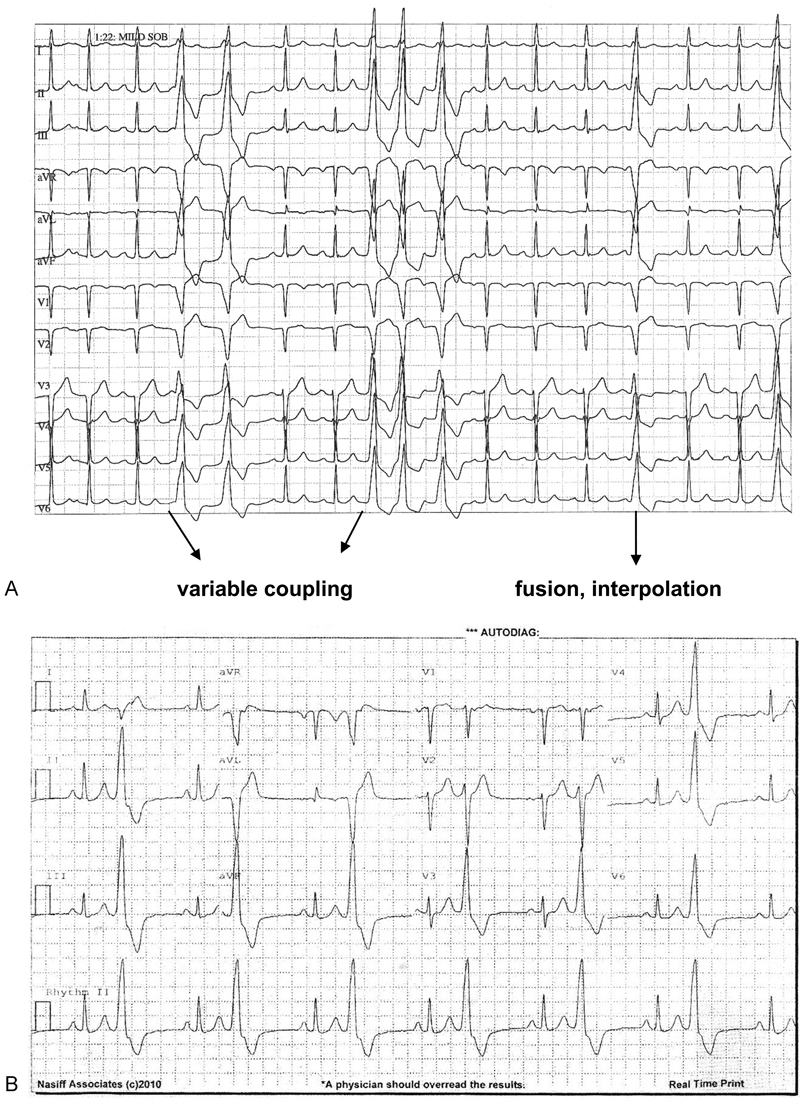
Representative electrocardiographic findings. (A) A 72-year-old male with 28,466 PVCs per 24 hours, 149 bigeminal cycles, 4,662 couplets, and 862 runs ventricular tachycardia. (B) 83-year-old male with 47,211 PVCs per 24 hours, 29,573 bigeminal cycles, and 24 couplets. Post-RAN, only 13 isolated PVCs per 24 hours
Table 1. Patient demographics.
| Gender | 34 males, 25 females |
|---|---|
| Age (mean) | 63 |
| LVEF (mean) | 0.60 |
| HTN | 73% |
| CAD | 34% |
| DM | 24% |
| BB | 34% |
| PHx of CHF | 8% (three diastolic, two systolic patients) |
| RAN dose (mean) | 866 mg daily |
| Time in between Holters (mean) | 3.10 mo |
| Symptoms | |
| Palpitations | 100% |
| Dizziness | 65% |
| Fatigue | 33% |
Abbreviations: BB, beta blocker; CAD, coronary artery disease; CHF, congestive heart failure; DM, type 2 diabetes; HTN, hypertension; LVEF, left ventricular ejection fraction; PHx, past history; RAN, ranolazine.
Results
Patient demographics are summarized in Table 1. Mean age was 63 years, 58% were males, mean left ventricular ejection fraction (LVEF) was 0.60 with only 8% having a history of congestive heart failure (CHF; two systolic, three diastolic),73% were hypertensive, 34% had coronary artery disease (CAD; all revascularized), 34% were taking a beta blocker, and the mean RAN dose was 866 mg per day. All patients experienced palpitations, 65% had dizziness, and 33% complained of fatigue. These symptoms improved in proportion to PVC reduction: 100% of responders reported fewer palpitations, 90% were less fatigued, and dizziness improved in 73%.
The Holter results of the responders (95% of patients) to RAN are listed in Table 2. Over 40% of patients had at least 10,000 PVCs, and over 25% had greater than 20,000 PVCs. In the entire group, RAN reduced PVCs by 71% (mean: 13,329 to 3,837; p < 0.001). Approximately 24% (14/59) of patients had more than 90% decrease in PVCs, 34% (20/59) had 71 to 90% decrease, and 17% (10/59) had 50 to 70% decrease. Ventricular bigeminy was reduced by 80% (4,168 to 851; p < 0.001), couplets were reduced by 78% (374 to 81; p < 0.001), and ventricular tachycardia (VT) reduced by 91% (56 to 5; p < 0.001).The maximum reduction in PVCs was from 47,211 with 29,573, ventricular bigeminy to 13 PVCs per 24 hour, and no bigeminy, accompanied by a robust resolution of the patient's incapacitating fatigue. This patient stated: “My life has been returned to me. I can return to work”. No proarrhythmia was observed, and there were no significant side effects of treatment. Approximately 6% of patients reported one or more of the following side effects: constipation, dizziness, nausea, or headache. One of the initial three nonresponders had response 1.5 years later with 16,890 PVCs and 10,114 ventricular bigeminy reduced to only 3 PVCs per 24 hours.
Table 2. Holter results of patients responding to ranolazinea .
| Pre-RAN | Post-RAN | p-Value | |
|---|---|---|---|
| Total QRS | 102,667 | 99,826 | p = NS |
| Isolated PVCs | 13,329 | 3,837 (−71%) | p < 0.001 |
| Ventricular bigeminy | 4,168 | 851 (−80%) | p < 0.001 |
| Ventricular couplets | 374 | 81 (−78%) | p < 0.001 |
| Runs VT | 56 | 5 (−91%) | p < 0.001 |
Abbreviations: PVCs, premature ventricular contractions; RAN, ranolazine; VT, ventricular tachycardia.
*95% (56/59) of patients had their ventricular ectopy reduced by RAN.
Discussion
RAN has several electrophysiological effects with no known proarrhythmia.1 2 IKr and late INa are inhibited at concentrations within therapeutic range. In addition, RAN has been shown to inhibit the diastolic transient inward current,3 resulting in suppression of afterdepolarizations. Although the QT interval is prolonged by approximately 6 ms due to IKr inhibition, there is no transmural dispersion of repolarization, and RAN is protective against torsades de pointes.4 EAD/DAD are causes of triggered ventricular ectopy5 6 and can be induced by late INa that RAN inhibits.1 2 DAD are due to spontaneous release of Ca++ from the sarcoplasmic reticulum, and EAD are directly due to Ca++ entry through the Ca++ window current, except in Purkinje fibers where EAD are due to late INa window current.2 7
Some clinical scenarios of EAD/DAD-mediated ventricular arrhythmias include CHF,8 catecholaminergic polymorphic VT,9 hypokalemia,10 left ventricular hypertrophy (LVH),11 long QT syndrome,12 and cocaine use.13 Our patients met criteria for VP.14 15 This is the second study reporting effects of RAN on PVCs in humans,16 but the first focusing exclusively on triggered ventricular ectopy.
VP (PVCs with variable coupling, fusion, interpolation, and a mathematical relationship with R-R intervals) occurs in 1 of 1,300 patients and can be a highly symptomatic arrhythmia, which is thought to be caused by EAD/DAD.17 Prognosis depends upon any coexisting cardiac disease. Rarely does ventricular fibrillation or syncope occur, and VT is slower than reentrant VT. Several drugs have been tried as treatment for VP. Verapamil produced a satisfactory response in 18% of treated patients.18 A report of two patients responding to adenosine has been published.19 Dilantin was successful in one patient.20 Cardiac pacing succeeded in two patients.21 Amiodarone produced good results in nine patients.22 Only 33% of patients with VP responded to the usual sodium channel blockers.
Activation of late INa (for example, by phosphoralization by Ca + +/calmodulin kinase ll), may be a common myocardial response to stress. Therefore, RAN may have a therapeutic role in treating many cardiac conditions, including unstable ischemic patients with PVCs and patients with atrial fibrillation.23
RAN was very well tolerated, with only 6% of patients experiencing headache, dizziness (not BP-related, but a direct CNS effect), nausea, or constipation, with no known organ toxicity. Patients' symptoms improved proportionally to PVC reduction.
In canine ventricular wedge preparations, RAN did not induce torsades de pointes, reduced the action potential duration of M cells, and suppressed EAD induced by d-sotalol.24 These are potential explanations of why RAN administration caused no proarrhythmia in this study.
RAN is metabolized by CYP 3A so that inhibitors of this enzyme, such as ketoconazole, diltiazem, verapamil, macrolide antibiotics, HIV protease inhibitors, and grapefruit juice, increase RAN levels. Inhibitors of g-glycoprotein increase plasma levels two- to threefold. RAN increases digoxin concentrations 1.4- to 1.6-fold, and simvastatin Cmax is doubled.
The patient population herein reported (Table 1) seems reasonably typical of adults who would be referred to a cardiology practice primarily for ventricular arrhythmia evaluation and therapy. Patients were essentially Medicare-age with multiple comorbidities, but well-preserved LVEF and highly symptomatic with palpitations, dizziness, and fatigue (Table 1). Syncope and cardiac arrest were not methods of presentation.
In summary, RAN was found to be highly effective in suppressing triggered VPC. Isolated PVCs were reduced from 13,329 to 3,837, ventricular bigeminy reduced from 4,168 to 851, ventricular couplets reduced from 374 to 81, and VT was reduced from 56 to 5, representing reductions of 71, 80, 78, and 91%, respectively. One of the initial three nonresponders demonstrated a remarkable response 1.5 years later with 16,890 PVCs reduced to only 3 PVCs per 24 hours (99% reduction). The presenting symptoms were improved in proportion to PVC reduction (marked decrease in palpitations, fatigue, and dizziness).
Limitations
This is a single-center open-label study. A larger, randomized prospective study might be useful in confirming these results. Furthermore, RAN can suppress the more common reentrant PVCs.25 Reentrant patients weren't studied, but if RAN were a successful therapy because of its safety, then RAN could be the first drug choice to treat the majority of patients with symptomatic PVCs.
Conclusion
RAN offers a safe, effective pharmacologic therapy for symptomatic VP patients whose PVCs are due to triggered activity, with no known proarrhythmia or significant organ toxicity. It may have a role to play in treating symptomatic PVCs in patients with LVH, CHF, hypokalemia, acute hypoxia,26 oxidative stress,27 28 long QT syndrome,12 especially long QT3,3 catecholaminergic polymorphic VT,9 cocaine-related PVCs,13 and drug-induced torsades de pointes.4 It is the pharmacologic treatment of choice for VP.
Acknowledgements
The author appreciates the statistical analysis provided by R. Kabra, MD, and advice of Luiz Belardinelli, MD.
Conflict of Interest The authors report no conflicts of interest.
Disclosure
This is the only article ever written on this subject.
References
- 1.Antzelevitch C, Belardinelli L, Zygmunt A C. et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110(8):904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belardinelli L, Giles W, Rajamani S. et al. Cardiac Late Na+ current: proarrhythmic effects, roles in long QT syndromes, and pathological relationship to CaMKII and oxidative stress. Heart Rhythm. 2015;12(2):440–448. doi: 10.1016/j.hrthm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Lindegger N, Hagen B M, Marks A R, Lederer W J, Kass R S. Diastolic transient inward current in long QT syndrome type 3 is caused by Ca2+ overload and inhibited by ranolazine. J Mol Cell Cardiol. 2009;47(2):326–334. doi: 10.1016/j.yjmcc.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoons G, Oros A, Beekman J D. et al. Late Na(+) current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. J Am Coll Cardiol. 2010;55(8):801–809. doi: 10.1016/j.jacc.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Fozzard H A. Afterdepolarizations and triggered activity. Basic Res Cardiol. 1992;87 02:105–113. doi: 10.1007/978-3-642-72477-0_10. [DOI] [PubMed] [Google Scholar]
- 6.Xie L H, Weiss J N. Arrhythmogenic consequences of intracellular calcium waves. Am J Physiol Heart Circ Physiol. 2009;297(3):H997–H1002. doi: 10.1152/ajpheart.00390.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P, Rudy Y. A model of canine Purkinje cell electrophysiology and Ca(2+) cycling: rate dependence, triggered activity, and comparison to ventricular myocytes. Circ Res. 2011;109(1):71–79. doi: 10.1161/CIRCRESAHA.111.246512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janse M J. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61(2):208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Kujala K, Paavola J, Lahti A. et al. Cell model of catecholaminergic polymorphic ventricular tachycardia reveals early and delayed afterdepolarizations. PLoS ONE. 2012;7(9):e44660. doi: 10.1371/journal.pone.0044660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace. 2000;2(3):216–223. doi: 10.1053/eupc.2000.0110. [DOI] [PubMed] [Google Scholar]
- 11.Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace. 2000;2(3):216–223. doi: 10.1053/eupc.2000.0110. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu W, Ohe T, Kurita T. et al. Effects of verapamil and propranolol on early afterdepolarizations and ventricular arrhythmias induced by epinephrine in congenital long QT syndrome. J Am Coll Cardiol. 1995;26(5):1299–1309. doi: 10.1016/0735-1097(95)00313-4. [DOI] [PubMed] [Google Scholar]
- 13.Kimura S, Bassett A L, Xi H, Myerburg R J. Early afterdepolarizations and triggered activity induced by cocaine. A possible mechanism of cocaine arrhythmogenesis. Circulation. 1992;85(6):2227–2235. doi: 10.1161/01.cir.85.6.2227. [DOI] [PubMed] [Google Scholar]
- 14.Katz L, Pick A. Philadelphia, PA: Lea & Feibinger; 1956. Clinical Electrophysiology, Part 1: The Arrhythmias; pp. 224–226. [Google Scholar]
- 15.Chung E. Sodertaje, Sweden: AB Astra; 1970. Diagnosis and clinical significance of parasystole; pp. 271–294. [Google Scholar]
- 16.Scirica B M, Braunwald E, Belardinelli L. et al. Relationship between nonsustained ventricular tachycardia after non-ST-elevation acute coronary syndrome and sudden cardiac death: observations from the metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndrome-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2010;122(5):455–462. doi: 10.1161/CIRCULATIONAHA.110.937136. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos A, Sandudi N, Myerberg R. Philadelphia, PA: WB Saunders; 2000. Parasystole; pp. 690–695. [Google Scholar]
- 18.Lipnitskiĭ T N, Denisiuk V I, Kolesnik P F, Sizova M P, Ivanov V P, Stoliarchuk V A. The clinical efficacy of verapamil in ventricular extrasystolic arrhythmia and parasystole [in Russian] Ter Arkh. 1993;65(12):42–44. [PubMed] [Google Scholar]
- 19.Tomcsányi J, Tenczer J, Horváth L. Effect of adenosine on ventricular parasystole. J Electrocardiol. 1996;29(1):61–63. doi: 10.1016/s0022-0736(96)80114-2. [DOI] [PubMed] [Google Scholar]
- 20.Zanini S, Rossi R. Ventricular parasystole: successful treatment with diphenylhydantoin [in Italian] G Ital Cardiol. 1972;2(4):575–578. [PubMed] [Google Scholar]
- 21.Furuse A, Shindo G, Makuuchi H. et al. Apparent suppression of ventricular parasystole by cardiac pacing. Jpn Heart J. 1979;20(6):843–851. doi: 10.1536/ihj.20.843. [DOI] [PubMed] [Google Scholar]
- 22.Paleev N R, Kel'man I M, Kovaleva L I, Nikiforova T B, Gurevich M A. Cordarone treatment of parasystole [in Russian] Kardiologiia. 1980;20(4):19–21. [PubMed] [Google Scholar]
- 23.Saad M, Mahmoud A, Elgendy I Y, Richard Conti C. Ranolazine in cardiac arrhythmia. Clin Cardiol. 2016;39(3):170–178. doi: 10.1002/clc.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaitman B R. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113(20):2462–2472. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 25.Morita N, Lee J H, Xie Y. et al. Suppression of re-entrant and multifocal ventricular fibrillation by the late sodium current blocker ranolazine. J Am Coll Cardiol. 2011;57(3):366–375. doi: 10.1016/j.jacc.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaur N, Rudy Y, Hool L. Contributions of ion channel currents to ventricular action potential changes and induction of early afterdepolarizations during acute hypoxia. Circ Res. 2009;105(12):1196–1203. doi: 10.1161/CIRCRESAHA.109.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L H, Chen F, Karagueuzian H S, Weiss J N. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104(1):79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y, Shryock J C, Wagner S, Maier L S, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318(1):214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]


