Abstract
In patients with acute coronary syndrome, high platelet reactivity (PR) is associated with an increased risk of secondary thrombotic events. However, in patients undergoing elective percutaneous coronary intervention (PCI), no association between high PR and outcome has been demonstrated. At present, the relation of PR and clinical symptoms is unknown.
To examine the association of PR with clinical indication for diagnostic angiography (stable or unstable coronary artery disease [CAD]), taking into account the influence of P2Y12 inhibitors.
A platelet function score (PFS) was determined in 195 patients by quantifying fibrinogen binding and P-selectin expression with flow cytometry. We evaluated the PFS with clinical presentation of stable or unstable CAD, angiographic severity of CAD, and the incidence of cardiovascular events during 2 years of follow-up. All data were analyzed stratified by P2Y12 inhibitor use (long-term and preprocedural versus none).
Surprisingly, among non-P2Y12 inhibitor users, the PFS was lower in patients with unstable CAD compared with stable CAD (5.6 ± 1.8 vs. 7.4 ± 1.6; p = 0.001). Angiographic CAD severity showed no relation with PFS. The SYNTAX score tended to be inversely related with PFS: low PFS, 13.2 (IQR, 11.9–19.1); median PFS, 10.0 (IQR, 5.0–14.0); and high PFS, 8.0 (IQR, 5.0–13.0), without significance (p = 0.304). Patients with low PFSs required more re-PCIs than those with median and high PFSs (11.1 vs. 4.7 vs. 0.0%, p = 0.004). This association was modified for patients using P2Y12 inhibitors.
Among patients without P2Y12 inhibitors undergoing coronary angiography, presentation of unstable CAD is independently associated with lower PR.
Keywords: P2Y12 inhibitor, platelet reactivity testing, coronary artery disease, coronary angiography, high on-treatment platelet reactivity, platelet function testing, flow cytometry
Platelets are crucial for adequate regulation of hemostasis. Low platelet numbers (thrombocytopenia) or platelet dysfunction (thrombocytopathy) will lead to bleeding complications, whereas increased platelet reactivity (PR) leads to thrombosis, mainly in the arteries. Upon vascular endothelial injury, platelets bind to the exposed collagen via glycoprotein (GP)VI and integrin α2β1. This leads to αIIbβ3 activation and granule release.1 Secondary platelet activation is triggered by adenosine diphosphate (ADP) and thromboxane release, which bind to, respectively, P2Y12 and thromboxane receptors on the platelets and thereby reinforce platelet activation.
These interconnected platelet activation pathways give multiple opportunities to inhibit platelet activation with antiplatelet therapy. Currently acetylsalicylic acid, P2Y12 inhibitors, and GPIIb/IIIa inhibitors belong to the standard medical treatment of patients with coronary artery disease (CAD), during coronary interventions, and as secondary prevention after myocardial infarction.2 3 4 The effectiveness of antiplatelet therapy has been measured by several commercially available platelet function tests, including the VerifyNow (Accumetrics®, San Diego, CA), platelet function analyzer, multiplate analyzer, and the vasodilator-stimulated phosphoprotein-phosphorylation assay.5 Although some of these tests may identify patients at risk of atherothrombotic events, no benefit has been observed in adjusting the antiplatelet therapy regimen.5 6
Research on the role of PR in CAD has thus far been dominated by clinical trials evaluating the potential protective effects of antiplatelet therapy, being mainly acetylsalicylic acid7 and P2Y12-receptor inhibitors.8 High on-treatment PR has been associated with an increased risk of secondary cardiovascular events,9 especially among patients with acute coronary syndrome.10 Among patients with stable CAD undergoing elective percutaneous coronary intervention (PCI), this association could not be demonstrated in a recent meta-regression analysis.11 This discrepancy may be because a large proportion of stable CAD patients tolerate high levels of PR without any adverse event. However, what the influence of the P2Y12 inhibitors is on the occurrence of secondary thrombotic events remains unclear in this meta-analysis.
Hence, evidence is lacking on the influence of PR in the development of clinical phenotypes, such as stable angina pectoris or acute myocardial infarction, between non-P2Y12 inhibitor users (NPIUs) and P2Y12 inhibitor users (PIUs). We therefore examined the association of PR with clinical indication for diagnostic angiography (stable or unstable CAD), taking into account the influence of P2Y12 inhibitors. Because high on-treatment PR is a risk factor for secondary thrombotic events, we hypothesized that high PR would be more common in patients with unstable CAD.
Methods
Ethics Statement
This study was approved by the ethics committees of the University Medical Center in Utrecht, the Netherlands, and conforms to the Declaration of Helsinki. All participants provided written informed consent before participation.
Study Population
In this cross-sectional study we analyzed data from the UCORBIO cohort (clinicaltrials.gov identifier: NCT02304744), a biobank of patients undergoing coronary angiography, with or without PCI, in the University Medical Centre in Utrecht. From October 2011 to November 2012. We prospectively and consecutively enrolled 220 patients from the catheterization laboratories in whom PR testing was performed. Valid PR measurements were not obtained in 25 patients, thus leaving 195 valid PR measurements for assessment. Age less than 18 years was the only exclusion criterion. Cardiovascular risk factors data and medical history were collected at baseline.
We grouped the indication for coronary angiography into stable CAD (stable chest pain, dyspnea on exertion, or silent ischemia) and unstable CAD (unstable angina, non–ST-elevation myocardial infarction, and ST-elevation myocardial infarction) according to international guidelines.3 12 Administration of platelet inhibitors before the intervention and the consecutive treatment based on the diagnostic angiography were recorded. Treatment and periprocedural medication with aspirin, P2Y12 inhibitors, and/or GPIIb/IIIa inhibitors were left at the discretion of the operator.
The interventional cardiologists were blinded for the platelet function score (PFS), and those who performed the platelet function tests were blinded for the indication for angiography and angiographic CAD severity.
Blood Collection
Before coronary angiography (BD, Franklin Lakes, NJ), blood was drawn into a 4.5-mL Vacutainer sodium citrate tube from the arterial sheath that is routinely inserted for the angiography procedure. Blood samples were transported to the laboratory for PR testing and quantification of the platelets with the CELL-DYN Sapphire (Abbott Diagnostics, Wiesbaden, Germany).
Platelet Activation Test
Material: The platelet activation test (PACT) reaction mix was prepared in advance and contained 4.5 µmol/L ADP (01897; Zwijndrecht, the Netherlands), 6 µmol/L SFLLRN (TRAP-6) (H-2936; Bachem, Weil am Rhein, Germany), or 40 ng/mL cross-linked collagen-related peptide (xl-CRP, a generous gift from Professor Richard Farndale) in an HEPES-buffered saline mixture that contains a fixed concentration of R-phycoerythrin (RPE)–conjugated anti–P-selectin (1:25; 55524, BD Pharmingen, Franklin Lakes, NJ) and fluorescein isothiocyanate (FITC)–conjugated antifibrinogen (1:100; F0111, Dako, Glostrup, Denmark).
Methods: The PACT was performed as previously described.13 In short, the agonist wells were filled with a 50-µL assay mixture into which 5 µL whole blood was pipetted. The mix was homogenized and incubated for 8 minutes at room temperature. The reaction was stopped by pipetting 10-µL reaction mix into 190-µL fixative solution (0.2% formaldehyde/0.9% NaCl). Analysis of the samples was performed after a minimum of 30 minutes and maximum of 48 hours on the FACS Canto flow cytometer (BD Biosciences, San Jose, CA).
Single platelets were gated based on forward- and side-scatter properties. Fluorescence intensity in the RPE channel was used to determine P-selectin surface expression, and fluorescence intensity in the FITC channel was used to determine fibrinogen binding, which indicates αIIbβ3 activation. PR was quantified by the maximal expression of P-selectin and αIIbβ3 activation after stimulation. We normalized the maximum fluorescence intensity value per batch per agonist to the overall mean value per agonist (for P-selectin expression and fibrinogen binding separately) to reduce a possible batch effect.
Platelet Function Score
We designed a straightforward PFS based on the maximum fluorescence intensity measurements of the PACT. For each agonist (ADP, TRAP-6, and xl-CRP), we divided the PR measurements into low, medium, and high tertiles, and assigned a score of 1, 2, and 3, respectively (Fig. 1). For each patient, we combined the tertile scores of the three agonists, leading to a PFS of 3 to 9. A score of 3 or 4 represents the lowest platelet reactivity (LPR), 5 to 7 corresponds to medium platelet reactivity (MPR), and a score of 8 or 9 is the highest platelet reactivity (HPR). This score was computed for fibrinogen binding and for P-selectin expression.
Fig. 1.
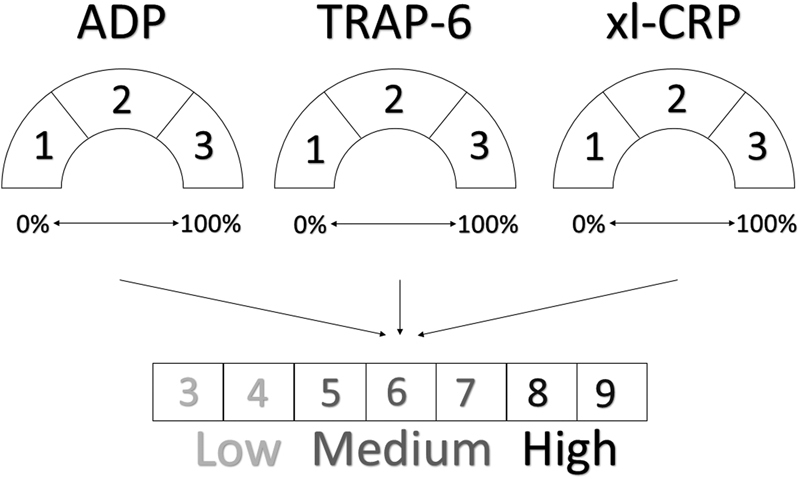
Design of platelet function score (PFS) for fibrinogen. The PFS was based on the maximum fluorescence intensity measurements of the PACT: the results of each agonist (ADP, TRAP-6, and xl-CRP) were divided into tertiles and assigned a score of 1, 2, and 3, respectively. The tertile scores of the three agonists were combined, leading to a PFS of 3 to 9.
Blood Cell Counts
Data from blood cell counts were extracted from the Utrecht Patient Oriented Database (UPOD). UPOD is an infrastructure of relational databases comprising data on patient characteristics, hospital discharge diagnoses, medical procedures, medication orders, and laboratory tests for all patients treated at the UMC Utrecht since 2004. The structure and content of UPOD have been described in more detail elsewhere.14 UPOD data acquisition and data management are in line with current regulations in the Netherlands concerning privacy and ethics. Data used for this study were collected for patient care purposes and were used retrospectively. The automated blood cell analyses were performed with the Abbott Cell-Dyn Sapphire automated hematology analyzer (Abbott Diagnostics, Santa Clara, CA).
Angiographic Coronary Artery Disease Severity
Angiographic data were collected and categorized into two categories: nonsignificant CAD (no stenosis, wall irregularities, < 50% stenosis) and significant CAD (at least one epicardial vessel with > 50% stenosis) based on the standard reporting of the clinical interventional cardiologists.
SYNTAX, Score of CAD Complexity
Two independent observers, using SYNTAX score calculator 2.11 software, measured the SYNTAX scores. The evaluation took place in the central core laboratory facility at the Utrecht University Medical Centre Department of Cardiology. The SYNTAX score allows for the characterization of coronary vasculature with respect to the number of lesions involved and the location and complexity of the lesions. Lesions are scored if they meet the required criteria (> 50% stenosis and vessel diameter > 1.5 mm).15 Higher scores are allocated to more complex lesions. The observers were blinded to the patient's PFS. The two observers had unlimited access to quantitative coronary angiography software (CAAS, Siemens, Maastricht, the Netherlands)16 to measure the percentage of stenosis or the dimension of the vessel if they were unsure about the significance of a lesion by visual estimation. The average of the SYNTAX scores of the two observers was used for the current analysis.
Follow-up
Questionnaires were used to collect follow-up data at 1 and 2 years to assess the occurrence of cardiovascular events (myocardial infarction, coronary revascularization, revascularization of other arteries, cerebrovascular events, hemorrhage, and death). The final occurrence of events was adjudicated by an independent event committee consisting of three cardiologists, two of whom were interventional cardiologists.
Statistical Analysis
Data were analyzed using the R statistical software package.17 We compared patient characteristics at baseline across the tertiles of the PFS. Data were presented as means ± standard deviations, medians with interquartile ranges (IQRs), or as percentages (depending on normality). Categorical data were presented as percentages. Continuous data were compared using ANOVA (analysis of variance) (parametric) or Kruskal-Wallis (nonparametric) testing. Categorical variables were compared with χ2 testing.
We performed multivariable ordinal regression to determine significant predictors of the PFS. Covariates in this analysis were P2Y12 inhibitor use, sex, age, diabetes, hypertension, hypercholesterolemia, smoking, indication for angiography, angiographic CAD severity, and CAD treatment (conservative, PCI, or coronary artery bypass graft [CABG]). All variables were entered in the model. This analysis was performed for the entire cohort and stratified by P2Y12 inhibitor usage.
Follow-up events were collected, but no further analyses were performed because of the low event rate. At baseline, only PFS based on fibrinogen binding showed significant results and not PFS based on P-selectin expression; therefore, all following analyses were only performed for PFS based on fibrinogen binding.
Results
Baseline Characteristics
The joint and stratified baseline patient characteristics are presented in Table 1. Of the 220 patients who were studied, PR measurements were incomplete in 25 due to logistic problems. Thus, complete data were available from 195 patients. The median follow-up time was 662 days, during which 16 deaths and 12 re-PCIs occurred.
Table 1. Baseline clinical and demographic patient characteristics.
| All | No P2Y12 inhibitor | p Value | P2Y12 inhibitor | p Value | p Value no P2Y12 vs. P2Y12 inhibitors | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low PFS | Medium PFS | High PFS | Low PFS | Medium PFS | High PFS | |||||
| N | 220 | 9 | 43 | 63 | 31 | 36 | 13 | |||
| Age (mean ± SD) | 64.9 ± 11.0 | 58.7 ± 9.3 | 67.9 ± 10.2 | 65.2 ± 11.5 | 0.066 | 64.9 ± 9.4 | 62.2 ± 11.7 | 69.4 ± 9.3 | 0.106 | 0.150 |
| Sex (n, % males) | 153 (69.5) | 6 (66.7) | 24 (55.8) | 44 (69.8) | 0.330 | 24 (77.4) | 28 (77.8) | 7 (53.8) | 0.204 | 0.101 |
| Diabetes (n, %) | 51 (23.3) | 1 (11.1) | 11 (25.6) | 16 (25.4) | 0.628 | 6 (20.0) | 10 (27.8) | 3 (23.1) | 0.760 | 0.920 |
| Hypertension (n, %) | 127 (57.7) | 7 (77.8) | 28 (65.1) | 35 (55.6) | 0.341 | 13 (41.9) | 21 (58.3) | 8 (61.5) | 0.316 | 0.470 |
| Hypercholesterolemia (n, %) | 106 (50.0) | 4 (50.0) | 22 (52.4) | 32 (50.8) | 0.984 | 14 (45.2) | 14 (43.8) | 4 (36.4) | 0.877 | 0.675 |
| Smoking (n, %) | 106 (54.6) | 4 (50.0) | 21 (61.8) | 26 (48.1) | 0.452 | 16 (57.1) | 18 (51.4) | 8 (66.7) | 0.649 | 0.569 |
| Kidney failure (n, %) | 7 (3.2) | 0 (0.0) | 2 (4.7) | 2 (3.2) | 0.772 | 1 (3.2) | 1 (2.8) | 0 (0.0) | 0.814 | 1.000 |
| BMI (mean ± SD) | 27.1 ± 4.2 | 27.1 ± 3.3 | 28.5 ± 4.9 | 25.9 ± 4.1 | 0.016 | 26.8 ± 3.6 | 26.7 ± 2.7 | 29.4 ± 5.8 | 0.084 | 0.928 |
| History of ACS (n,%) | 75 (34.1) | 2 (22.2) | 12 (27.9) | 17 (27.0) | 0.941 | 13 (41.9) | 17 (47.2) | 4 (30.8) | 0.587 | 0.040 |
| History of PCI (n,%) | 69 (31.4) | 1 (11.1) | 13 (30.2) | 14 (22.2) | 0.403 | 14 (45.2) | 13 (36.1) | 6 (46.2) | 0.699 | 0.050 |
| History of CABG (n,%) | 35 (15.9) | 2 (22.2) | 5 (11.6) | 12 (19.0) | 0.535 | 5 (16.1) | 3 (8.3) | 2 (15.4) | 0.593 | 0.425 |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; PFS, platelet function score; SD, standard deviation.
Note: Data are shown as number of participants and percentage or mean with SD. When data were incomplete, the percentage of participants that was accessible was calculated. PFS was assessed by stratifying the platelet reactivity per agonist in tertiles (1, 2, and 3) for fibrinogen binding. Significant values (p < 0.05) are given in bold.
Overall the participants (69.5% male) were an average age of 64.9 ± 11.0 years and showed a high prevalence of risk factors, consisting of diabetes (23.3%), hypertension (57.7%), hypercholesterolemia (50.0%), smoking (54.6%), kidney failure (3.2%), and overweight (mean body mass index [BMI], 27.1 ± 4.2 kg/m2). Approximately one-third of the patients had a history of CAD (acute coronary syndrome, 34.1%; previous PCI, 31.4%; and previous CABG, 15.9%).
Coronary Artery Disease
Antiplatelet therapy included aspirin in 77.4% of the participants, clopidogrel in 33.8%, prasugrel in 1.5%, and ticagrelor in 5.1% (Table 2). P2Y12 inhibitors were used by 74.4% of the patients with unstable CAD and by 33.1% of the patients with stable CAD.
Table 2. Patient characteristics: antiplatelet therapy and coronary artery disease severity.
| All | Low PFS | No P2Y12 inhibitor | High PFS | p Value low-medium-high | Low PFS | P2Y12 inhibitor | High PFS | p Value low-medium-high | p Value no P2Y12 vs. P2Y12 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Medium PFS | Medium PFS | |||||||||
| N | 195 | 9 | 43 | 63 | 31 | 36 | 13 | |||
| Medical therapy | ||||||||||
| Aspirin (n, %) | 151 (77.4) | 6 (66.7) | 33 (76.7) | 39 (61.9) | 0.275 | 28 (90.3) | 32 (88.9) | 13 (100.0) | 0.465 | < 0.001 |
| Clopidogrel (n, %) | 66 (33.8) | – | – | – | 23 (74.2) | 33 (91.7) | 10 (76.9) | 0.145 | < 0.001 | |
| Prasugrel (n, %) | 3 (1.5) | – | – | – | 2 (6.5) | 1 (2.8) | 0 (0.0) | 0.541 | 0.142 | |
| Ticagrelor (n, %) | 10 (5.1) | – | – | – | 7 (22.6) | 2 (5.6) | 1 (7.7) | 0.093 | < 0.001 | |
| Vitamin K antagonists (n, %) | 28 | 1 (11.1) | 6 (14.0) | 8 (12.7) | 0.960 | (9.7) | 9 (25.0) | 1 (7.7) | 0.157 | 0.870 |
| Indication for angiography | ||||||||||
| Stable CAD (n, %) | 145 (74.4) | 5 (62.5) | 37 (88.1) | 55 (96.5) | 0.006 | 16 (53.3) | 22 (64.7) | 10 (76.9) | 0.317 | <0.001 |
| Unstable CAD (n, %) | 39 (20.0) | 3 (37.5) | 5 (11.9) | 2 (3.5) | 14 (46.7) | 12 (35.3) | 3 (23.1) | |||
| Angiographic CAD severity | ||||||||||
| Nonsignificant (n, %) | 54 (27.7) | 3 (33.3) | 12 (27.9) | 30 (47.6) | 0.116 | 3 (9.7) | 4 (11.1) | 2 (15.4) | 0.861 | <0.001 |
| Significant (n, %) | 141 (72.3) | 6 (66.7) | 31 (72.1) | 33 (52.4) | 28 (90.3) | 32 (88.9) | 11 (84.6) | |||
| SYNTAX score | 13.2 | 10.0 | 8.0 | 0.304 | 13.5 | 7.0 | 0.032 | 0.261 | ||
| (median, IQR) | 10.0 (5.0,16.0) |
(11.9, 19.1) | (5.0, 14.0) | (5.0, 13.0) | 8.5 (5.1, 13.1) |
(9.6, 20.0) | (5.0, 11.5) | |||
| Treatment CAD | ||||||||||
| Conservative (n, %) | 74 (37.9) | 3 (33.3) | 18 (42.9) | 38 (61.3) | 0.205 | 6 (20.0) | 6 (16.7) | 3 (23.1) | 0.893 | <0.001 |
| PCI (n, %) | 103 (52.8) | 4 (44.4) | 20 (47.6) | 19 (30.6) | 22 (73.3) | 28 (77.8) | 10 (76.9) | |||
| CABG (n, %) | 15 (7.7) | 2 (22.2) | 4 (9.5) | 5 (8.1) | 2 (6.7) | 2 (5.6) | 0 (0.0) | |||
Abbreviations: CAD, coronary artery disease; CABG, coronary artery bypass graft; IQR, interquartile range; PCI, percutaneous coronary intervention; PFS, platelet function score.
Note: Data are shown as percentage, or median with IQR. Significant values (p < 0.05) are given in bold.
Surprisingly, among NPIU, the PFS was significantly lower in patients with unstable CAD (5.6 ± 1.8) than in those with stable CAD (7.4 ± 1.6; p < 0.001; Fig. 2). Consequently for NPIU, the presentation of unstable CAD was more common in patients with LPR (37.5%) compared with MPR (11.9%) or HPR (3.5%; p = 0.006). The difference in the PFS was not significant among PIU, at 5.6 ± 2.1 for stable versus 5.0 ± 1.9 for unstable (p = 0.192). Also, angiographic CAD severity showed no significant differences in the PFS.
Fig. 2.
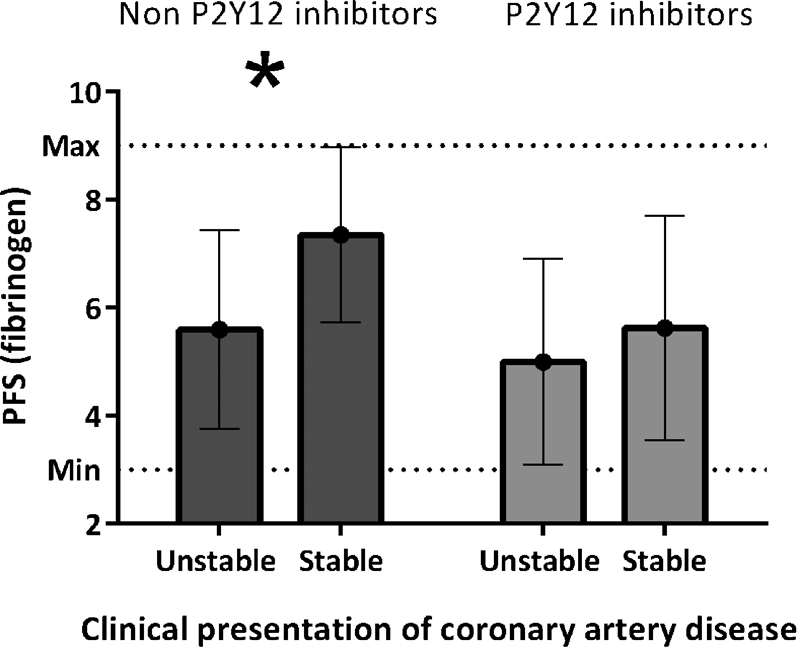
Platelet function score (fibrinogen) by clinical presentation of coronary artery disease. Means and standard deviation platelet function score (fibrinogen) stratified by indication for coronary angiography and P2Y12 inhibitor usage. The p values for the difference between stable and unstable CAD was p < 0.001 among non-P2Y12 inhibitor users and p = 0.192 for P2Y12 inhibitor users.
After diagnostic coronary angiography, 52.8% of patients underwent subsequent PCI. There was no association of the subsequent procedure and the PFS; however, more PCIs were performed within the PIU group than in the NPIU group (p < 0.001).
Blood Cell Counts
Among NPIU, patients with low PFS displayed a significant lower platelet count compared with median PFS and high PFS (respectively, 247.3 [IQR, 201.9–279.9] vs. 223.9 [IQR, 186.2–264.7] vs. 193.7 [IQR, 161.0–248.8]; p = 0.042; Table 3). No differences were seen in mean platelet volume.
Table 3. Blood cell count in patients without P2Y12 inhibitor, stratified by platelet reactivity score (fibrinogen).
| Low PFS | Medium PFS | High PFS | p Value | |
|---|---|---|---|---|
| 9 | 43 | 63 | ||
| Leukocyte count (109 cells/L) | 9.3 (8.3–12.5) | 6.6 (5.7–8.1) | 6.0 (5.0–7.2) | 0.001 |
| Neutrophil count (109 cells/L) | 5.7 (5.4–9.4) | 4.1 (3.1–5.2) | 3.5 (3.0–4.3) | 0.001 |
| Monocyte count (109 cells/L) | 0.75 (0.66–0.94) | 0.56 (0.42–0.72) | 0.50 (0.42–0.63) | 0.021 |
| Lymphocyte count (109 cells/L) | 2.1 (1.6–2.7) | 1.6 (1.2–2.1) | 1.5 (1.3–1.9) | 0.314 |
| Eosinophil count (109 cells/L) | 0.08 (0.06–0.10) | 0.13 (0.08–0.22) | 0.13 (0.08–0.21) | 0.224 |
| Basophil count (109 cells/L) | 0.03 (0.03–0.04) | 0.03 (0.02–0.05) | 0.03 (0.01–0.04) | 0.667 |
| Platelet count (109 cells/L) | 247.3 (201.9–279.9) | 223.9 (186.2–264.7) | 193.7 (161.0–248.8) | 0.042 |
| Mean platelet volume (fL) | 8.2 (7.8–8.9) | 7.7 (7.2–8.4) | 8.2 (7.6–8.8) | 0.096 |
Abbreviation: PFS, platelet function.
Note: All presented values are medians with interquartile ranges. Only values for non-P2Y12 users are shown. Significant values (p < 0.05) are given in bold.
Within the same patient group, the white blood cell, neutrophil, and monocyte counts were increased in patients with LPR compared with median and high PFS. This resulted in, respectively, a white blood cell count of 9.3 × 109/L (IQR, 8.3–12.5 × 109/L), 6.6 × 109/L (IQR, 5.7–8.109/L), and 6.0 × 109/L (IQR, 5.0–7.2 × 109/L; p = 0.001); a neutrophil count of 5.7 × 109/L (IQR, 5.4–9.4 × 109/L), 4.109/L (IQR, 3.1–5.2 × 109/L), and 3.5 × 109/L (IQR, 3.0–4.3 × 109/L; p = 0.001); and a monocyte count of 0.75 × 109/L (IQR 0.66–0.94 × 109/L), 0.56 × 109/L (IQR 0.42–0.72 × 109/L), and 0.50 × 109/L (IQR 0.42–0.63 × 109/L; p = 0.021). All medians were within the normal range of blood cell counts. The differences were not observed among PIU.
SYNTAX Score
The SYNTAX score tended to be inversely related with PFS (fibrinogen) for NPIU: low PFS, 13.2 (IQR, 11.9–19.1); median PFS, 10.0 (IQR, 5.0–14.0); and high PFS, 8.0 (IQR, 5.0–13.0), although this did not reach significance (p = 0.304; Table 2 and supporting information Fig. A1). The SYNTAX score for PIU was significantly higher in the medium PFS group (p = 0.032).
Fig. A1.
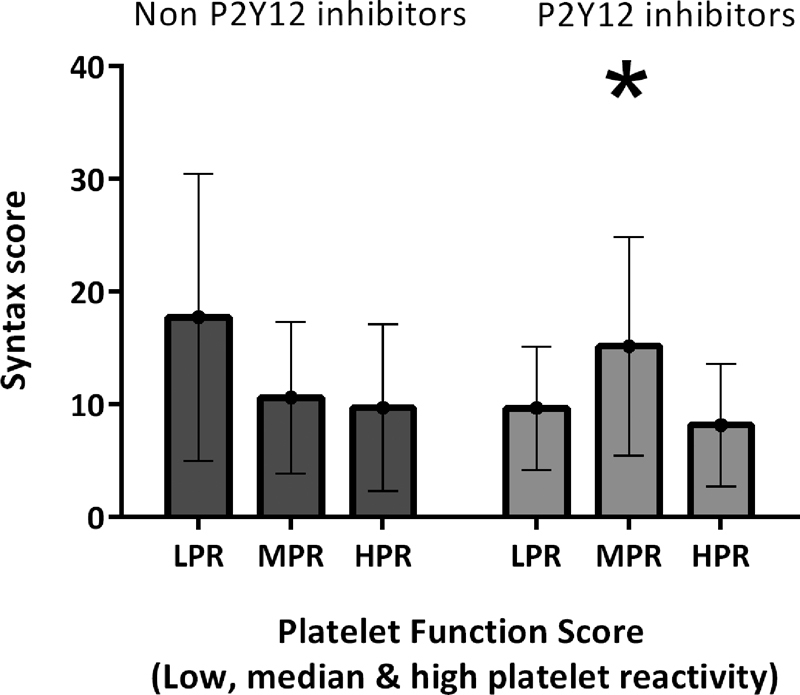
SYNTAX score in patients with and without P2Y12 inhibitor according to tertiles of Platelet function score (low, medium, and high). Medians and interquartile ranges of the SYNTAX score stratified by platelet function score and P2Y12 inhibitor usage. The difference in SYNTAX score among non-P2Y12 inhibitors was not significant (p = 0.304) but was significant among patients with P2Y12 inhibitor (p = 0.032).
Prediction of PFS
A multivariable ordinal regression analysis was performed for the outcome PFS for fibrinogen (range: 3–9) with cardiovascular risk factors and coronary angiography characteristics as covariates (Table 4). As expected, P2Y12 inhibitor usage was a strong predictor of lower PR (odds ratio [OR]: 0.22 95% confidence interval [CI]: 0.11–0.44, p < 0.001). Again, the association of unstable CAD and PFS was confirmed among NPIU: the multivariable adjusted OR of unstable CAD was 0.23 (95% CI: 0.06–0.83; p = 0.026) among NPIU. In all patients, the multivariable OR of unstable CAD for PFS was 0.34 (95% CI: 0.15–0.074; p = 0.007). No association was found among PIU. These results may indicate an independent association of clinical presentation of CAD with decreased PR.
Table 4. Predictors of platelet function score (fibrinogen).
| All patients | No P2Y12 inhibitor | P2Y12 inhibitor | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| P2Y12 usage (yes vs. no) Aspirin usage (yes vs. no) |
0.22 (0.11–0.44) 1.17 (0.58–2.36) |
< 0.001
0.666 |
0.91 (0.39–2.12) | 0.829 | 3.37 (0.71–17.62) | 0.133 |
| Sex (male vs. female) | 1.01 (0.53–1.92) | 0.969 | 1.34 (0.58–3.09) | 0.492 | 0.55 (0.17–1.71) | 0.302 |
| Age (per year increase) | 1.01 (0.98–1.04) | 0.464 | 1.02 (0.98–1.06) | 0.357 | 1.00 (0.95–1.04) | 0.914 |
| Diabetes (yes vs. no) | 1.10 (0.55–2.22) | 0.791 | 0.85 (0.33–2.24) | 0.744 | 1.45 (0.48–4.32) | 0.507 |
| Hypertension (yes vs. no) | 0.87 (0.46–1.64) | 0.677 | 0.57 (0.23–1.38) | 0.211 | 1.63 (0.61–4.38) | 0.332 |
| Hypercholesterolemia (yes vs. no) | 1.21 (0.67–2.21) | 0.528 | 1.67 (0.73–3.87) | 0.223 | 0.66 (0.25–1.77) | 0.412 |
| Smoking (yes vs. no) | 1.08 (0.60–1.96) | 0.790 | 1.02 (0.45–2.35) | 0.955 | 0.94 (0.38–2.30) | 0.887 |
| Indication (unstable CAD vs. stable CAD) | 0.34 (0.15–0.74) | 0.007 | 0.23 (0.06–0.83) | 0.026 | 0.40 (0.14–1.07) | 0.071 |
| Angiographic CAD (significant vs. nonsignificant) | 0.89 (0.31–2.62) | 0.836 | 1.26 (0.35–4.71) | 0.723 | 0.42 (0.05–3.45) | 0.418 |
| PCI (vs. conservative) | 0.85 (0.30–2.36) | 0.761 | 0.62 (0.16–2.22) | 0.461 | 1.29 (0.23–7.29) | 0.768 |
| CABG (vs. conservative) | 0.69 (0.18–2.58) | 0.576 | 0.51 (0.09–2.71) | 0.426 | 1.12 (0.10–2.71) | 0.927 |
Abbreviations: CAD, coronary artery disease; CABG, coronary artery bypass graft; CI, confidence interval; OR, odds ratio; PCI, percutaneous coronary intervention.
Note: The presented OR with 95% CI resulting from a multivariable ordinal regression analysis for the outcome platelet function score (ranging from 3 to 9). All reported variables were entered in the model. Significant values (p < 0.05) are given in bold.
Follow-up
In total, 16 patients died during a median follow-up duration of 662 days; causes of death were cardiovascular in 8 patients and noncardiovascular in 8 patients. This number was too low to perform reliable analyses.
Also, other end points, such as myocardial infarction (n = 6), cerebrovascular accident or transient ischemic attack (n = 5), vascular intervention (n = 8), and bleeding (n = 4) were rare, and thus we could not examine the relationship of PFS and clinical outcome in the 2-year follow-up interval.
Repeat PCI was performed in 12 patients in the presence of residual symptoms and angiographic significant restenosis in the initially placed stent (n = 3) or other location in the coronary arteries (n = 4), both in-stent restenosis and stenosis at another location (n = 4), and dissection after initial PCI (n = 1). Within NPIU, significantly more repeat PCIs were performed in the low PFS than in median and high PFS (11.1 vs. 4.7 vs. 0.0%, respectively; p = 0.004).
Discussion
Our study demonstrates that patients with unstable CAD had a lower PFS than those with stable CAD, but that this association was modified by the use of P2Y12 inhibitors. Among NPIU, unstable CAD is more prevalent in patients with a low PFS than with a median or high PFS. The relationship between unstable CAD and PFS (fibrinogen) was independent of other baseline differences. No relationship was found between prevalence of unstable CAD and PFS in patients using P2Y12 inhibitors. P2Y12 inhibitor usage was a strong predictor for lower PR.
Although this correlation was contrary to our hypothesis, several previous studies have found similar results. In patients with stable or unstable AP, mostly using P2Y12 inhibitors, higher PR was observed in response to mental stress. However, in patients with cardiac syndrome X, not using P2Y12 inhibitors, a decrease in PR was found after mental stress, suggesting potential myocardial release of adenosine, a powerful antiplatelet agent.18 Milovanovic et al found an inverse relationship between PR and severity of coronary blood flow obstruction in patients with stable angina pectoris after stimulation of the platelets with TRAP-6 and ADP.19 Another study in patients with critical limb ischemia and tissue loss showed lower PR after in vitro stimulation compared with in those with intermittent calf claudication.20 A possible explanation for a relationship between LPR and a high prevalence of unstable CAD could be that platelets have a protective role in development of CAD. Unstable atherosclerotic plaques are characterized by the increased formation of neo-microvessels. These neo-microvessels have weak integrity and are leaky, which leads to recurrent intraplaque bleeding.21 This intraplaque bleeding could enable inflammatory cells (mostly monocytes and macrophages) to infiltrate the adventitia and to secrete proteases and inflammatory proteins, weakening the fibrous cap of the atherosclerotic plaque and hence increasing infarction risk. Platelets might stabilize this intraplaque bleeding and simultaneously prevent further atherosclerotic plaque development. Our finding that a low PFS is associated with unstable CAD in patients without P2Y12 inhibitors could be explained by increased intraplaque bleeding in patients with low PFS.
Reduced PR by ischemic preconditioning has also been reported. Brief episodes of myocardial ischemia paradoxically provide resistance of cardiomyocytes to a later sustained ischemic insult.22 23 The resistance is presumably due to preconditioning-induced attenuation of platelet-mediated thrombosis because it is accompanied by a significant decline in platelet-fibrinogen binding, a decrease in the formation of neutrophil-platelet aggregates, and a trend toward a reduction in platelet P-selectin expression.24 The mechanisms responsible for this attenuation in platelet activation and aggregation are still unknown. Ischemic preconditioning supports our finding of lower PR in patients with unstable CAD, assuming that these patients were previously more often exposed to brief episodes of myocardial ischemia than those with stable CAD.
The current study found that a lower PFS was also related to a lower platelet count, potentially caused by platelet exhaustion and a high turnover of activated platelets. A low PFS in patients with unstable CAD could be caused by overstimulation of the platelets due to the irregularities of the atherosclerotic plaque, causing extinguishment of remaining PR. Highly reactive platelets that have formed platelet-platelet and platelet-leukocyte aggregates are rapidly removed from the circulation, leaving the less responsive and the preactivated platelets that are no longer susceptible to further stimulation behind. This phenomenon has been described previously in different subsets of patients.25 26 27 Low PR would not be the cause of more severe CAD in this case but rather the result of platelet prestimulation by more severe atherosclerosis.
It can be debated whether high PR is a reflection of the patients overall cardiovascular risk rather than representing an independent modifiable parameter associated with clinical prognosis. PR in patients on P2Y12 inhibitors undergoing PCI was strongly influenced by clinical risk variables such as age, BMI, diabetes mellitus, serum creatinine, and left ventricular function.28 An inverse trend for the correlation between PR and markers for renal function, creatinine, and urea was also found in patients with critical limb ischemia.29 Even more, increased PR was associated with impaired arterial stiffness in patients undergoing PCI and loading dose clopidogrel, suggesting a potential predicting clinical factor for high PR despite antiplatelet therapy.30 Risk factor assessment together with platelet function tests might improve future personalized antiplatelet therapy.
Finally, most studies investigating PR in patients with CAD are performed in patients who have been prescribed extensive antiplatelet therapy, such as P2Y12 inhibitors. Currently there is little knowledge about “unaffected PR” in patients with CAD. In our opinion, the current consensus that high PR is a prognostic risk factor for cardiovascular events in P2Y12 users is therefore not directly extractable to patients who do not use P2Y12 inhibitors. A clear distinction should be made between the terms “high on-treatment PR” and “high PR.”
Limitations
An important limitation of our study was that it was underpowered, foremost in the follow-up events. Although there seems to be a difference in the occurrence of re-PCI in the low PFS compared with the median and high PFS, we could not thoroughly examine confounding factors because only 12 repeat PCIs occurred. Therefore, the follow-up results should be interpreted with care due to the limitation in statistical power.
Furthermore, an unstable indication was more prevalent in patients with LPR, which could also be the cause of a higher number of re-PCIs in this group. However, more indicators of CAD severity, such as the SYNTAX score and angiographic CAD severity, tended toward the same results (the association of lower PFS in patients with more severe CAD), but no significant differences could be demonstrated (Appendix 1, SYNTAX score) because of the low numbers of participants.
Conclusion
Among patients undergoing coronary angiography, without P2Y12 inhibitors, presentation of unstable CAD is independently associated with lower PR. These findings are perpendicular to results of studies reporting outcomes of patients with P2Y12 inhibitors.
Note
Clinicaltrials.gov. Identifier: NCT02304744.
Footnotes
Both the authors contributed equally to this manuscript.
References
- 1.Nuyttens B P, Thijs T, Deckmyn H, Broos K. Platelet adhesion to collagen. Thromb Res. 2011;127 02:S26–S29. doi: 10.1016/S0049-3848(10)70151-1. [DOI] [PubMed] [Google Scholar]
- 2.Montalescot G, Sechtem U, Achenbach S. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 3.Hamm C W, Bassand J P, Agewall S. et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(23):2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 4.Steg P G, James S K, Atar D. et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 5.Janssen P W, ten Berg J M, Hackeng C M. The use of platelet function testing in PCI and CABG patients. Blood Rev. 2014;28(3):109–121. doi: 10.1016/j.blre.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Breet N J, van Werkum J W, Bouman H J. et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303(8):754–762. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 7.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative group of the primary prevention project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 8.Montalescot G, Wiviott S D, Braunwald E. et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373(9665):723–731. doi: 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 9.Wisman P P, Roest M, Asselbergs F W. et al. Platelet-reactivity tests identify patients at risk of secondary cardiovascular events: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(5):736–747. doi: 10.1111/jth.12538. [DOI] [PubMed] [Google Scholar]
- 10.Tantry U S, Bonello L, Aradi D. et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 11.Piccolo R, Galasso G, De Luca G. et al. Relationship between changes in platelet reactivity and ischemic events following percutaneous coronary intervention: a meta-regression analysis of 30 randomized trials. Atherosclerosis. 2014;234(1):176–184. doi: 10.1016/j.atherosclerosis.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert J S, Jaffe A S. et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 13.Roest M, van Holten T C, Fleurke G J, Remijn J A. Platelet activation test in unprocessed blood (pac-t-ub) to monitor platelet concentrates and whole blood of thrombocytopenic patients. Transfus Med Hemother. 2013;40(2):117–125. doi: 10.1159/000350688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ten Berg M J, Huisman A, van den Bemt P M, Schobben A F, Egberts A C, van Solinge W W. Linking laboratory and medication data: new opportunities for pharmacoepidemiological research. Clin Chem Lab Med. 2007;45(1):13–19. doi: 10.1515/CCLM.2007.009. [DOI] [PubMed] [Google Scholar]
- 15.Sianos G, Morel M A, Kappetein A P. et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219–227. [PubMed] [Google Scholar]
- 16.Généreux P, Palmerini T, Caixeta A. et al. SYNTAX score reproducibility and variability between interventional cardiologists, core laboratory technicians, and quantitative coronary measurements. Circ Cardiovasc Interv. 2011;4(6):553–561. doi: 10.1161/CIRCINTERVENTIONS.111.961862. [DOI] [PubMed] [Google Scholar]
- 17.Team R DC. Vienna, Austria: Foundation for Statistical Computing; 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 18.Sestito A, Maccallini A, Sgueglia G A. et al. Platelet reactivity in response to mental stress in syndrome X and in stable or unstable coronary artery disease. Thromb Res. 2005;116(1):25–31. doi: 10.1016/j.thromres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Milovanovic M, Fransson S G, Richter A, Järemo P. Inverse relationships between coronary blood flow obstruction and platelet reactivity in stable angina pectoris. Platelets. 2005;16(3–4):211–213. doi: 10.1080/09537100400016813. [DOI] [PubMed] [Google Scholar]
- 20.Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J. Platelet activation is increased in peripheral arterial disease. J Vasc Surg. 2003;38(1):99–103. doi: 10.1016/s0741-5214(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 21.Chistiakov D A, Orekhov A N, Bobryshev Y V. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol (Oxf) 2015;213(3):539–553. doi: 10.1111/apha.12438. [DOI] [PubMed] [Google Scholar]
- 22.Przyklenk K, Whittaker P. Brief antecedent ischemia enhances recombinant tissue plasminogen activator-induced coronary thrombolysis by adenosine-mediated mechanism. Circulation. 2000;102(1):88–95. doi: 10.1161/01.cir.102.1.88. [DOI] [PubMed] [Google Scholar]
- 23.Muller D W Topol E J Califf R M et al. Relationship between antecedent angina pectoris and short-term prognosis after thrombolytic therapy for acute myocardial infarction. Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group Am Heart J 1990119(2 Pt 1):224–231. [DOI] [PubMed] [Google Scholar]
- 24.Linden M D, Whittaker P, Frelinger A L III, Barnard M R, Michelson A D, Przyklenk K. Preconditioning ischemia attenuates molecular indices of platelet activation-aggregation. J Thromb Haemost. 2006;4(12):2670–2677. doi: 10.1111/j.1538-7836.2006.02228.x. [DOI] [PubMed] [Google Scholar]
- 25.Nenci G G, Berrettini M, Todisco T, Costantini V, Grasselli S. Exhausted platelets in chronic obstructive pulmonary disease. Respiration. 1983;44(1):71–76. doi: 10.1159/000194530. [DOI] [PubMed] [Google Scholar]
- 26.Mannucci P M, Cattaneo M, Canciani M T, Maniezzo M, Vaglini M, Cascinelli N. Early presence of activated (‘exhausted’) platelets in malignant tumors (breast adenocarcinoma and malignant melanoma) Eur J Cancer Clin Oncol. 1989;25(10):1413–1417. doi: 10.1016/0277-5379(89)90098-9. [DOI] [PubMed] [Google Scholar]
- 27.Jurk K, Jahn U R, Van Aken H. et al. Platelets in patients with acute ischemic stroke are exhausted and refractory to thrombin, due to cleavage of the seven-transmembrane thrombin receptor (PAR-1) Thromb Haemost. 2004;91(2):334–344. doi: 10.1160/TH03-01-0044. [DOI] [PubMed] [Google Scholar]
- 28.Droppa M, Tschernow D, Müller K A. et al. Evaluation of clinical risk factors to predict high on-treatment platelet reactivity and outcome in patients with stable coronary artery disease (PREDICT-STABLE) PLoS One. 2015;10(3):e0121620. doi: 10.1371/journal.pone.0121620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisman P P, Teraa M, de Borst G J, Verhaar M C, Roest M, Moll F L. Baseline platelet activation and reactivity in patients with critical limb ischemia. PLoS One. 2015;10(7):e0131356. doi: 10.1371/journal.pone.0131356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siasos G, Oikonomou E, Zaromitidou M. et al. High platelet reactivity is associated with vascular function in patients after percutaneous coronary intervention receiving clopidogrel. Int J Cardiol. 2014;177(1):192–196. doi: 10.1016/j.ijcard.2014.09.030. [DOI] [PubMed] [Google Scholar]


