Abstract
Cardiovascular disease is one of the leading causes of death in psoriatic arthritis (PsA). Pathogenesis of accelerated atherosclerosis in PsA remains to be elucidated. Endothelial dysfunction (ED) often precedes manifesting atherosclerosis. This study aims to assess carotid intima-media thickness (CIMT), a marker of atherosclerosis in PsA, in context of markers of inflammation and vascular function. A cross-sectional study was performed in 18 PsA patients who were compared with 18 controls matched for age and sex. Flow-mediated dilatation (FMD) assessed by AngioDefender (Everist Health, Ann Arbor, MI), endothelial progenitor cells (EPCs) quantified by flow cytometry and CIMT measured ultrasonographically. Inflammatory measures included disease activity score of 28 joints count and disease activity index in psoriatic arthritis. We also assayed markers of inflammation, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), proinflammatory cytokines (interleukin [IL]-1, IL-6, and tumor necrosis factor [TNF]-α), and endothelial dysfunction, including lipids, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and EPCs. CIMT is significantly higher in PsA patients compared with controls (0.062 ± 0.18 vs. 0.045 ± 0.10 cm, p < 0.01) whereas FMD%, EPCs%, and high-density lipoproteins (HDL) cholesterol are significantly reduced in PsA compared with controls (p < 0.05). Compared with controls, PsA patients had significantly increased concentrations of ESR, CRP, TNF-α, IL-6, ICAM-1, and VCAM-1. In PsA, CIMT positively correlated with IL-6 and ICAM-1 and inversely correlated with FMD, HDL, and EPCs (p < 0.05). In PsA, FMD and CIMT were impaired, indicating endothelial dysfunction and accelerated atherosclerosis, respectively. PsA-related inflammatory mechanisms (TNF-α, IL-6) and markers of vascular function (CRP, ICAM-1, and EPCs) may all be involved in the development of vascular disease in PsA. Cytokine-triggered inflammation upregulates expression of adhesion molecules, depletes EPCs with endothelial dysfunction, and increased CIMT in PsA.
Keywords: adhesion molecules, atherosclerosis, carotid intima-media thickness, endothelial dysfunction, endothelial progenitor cells, inflammatory cytokines, psoriatic arthritis
Psoriatic arthritis is a chronic and disabling inflammatory arthritis that affects 0.2 to 1% of the population and 6 to 42% of the psoriasis patients.1 Cardiovascular disease (CVD) is one of the leading causes of death in psoriatic arthritis (PsA).2 3 Both PsA and atherosclerosis share similar inflammatory mechanism4 and PsA is an independent risk factor for accelerated CVD.5 PsA is characterized by high prevalence of comorbidities, including metabolic syndrome and its major features (obesity, hypertension, impaired fasting glucose, and hyperlipidemia).6 However, there is no clear evidence that hypertension, diabetes mellitus, dyslipidemia, obesity, and a sedentary lifestyle are directly implicated in accelerated atherosclerosis in PsA. It is possible that immune-mediated inflammatory mechanisms underlying PsA may be crucial for endothelial dysfunction, atherosclerosis, and CVD development. However, the relationship between the cytokines triggering the inflammatory cascade, the upregulated adhesion molecules, depleted endothelial progenitor cells (EPCs) population and carotid intima-media thickness (CIMT) in PsA has not yet been studied. Hence, we assessed CIMT, a marker of atherosclerosis, as well as laboratory markers of inflammation and vascular function in PsA.
Methods
Study Subjects
This was a cross-sectional study of 18 patients with PsA fulfilling the classification criteria for psoriatic arthritis7 and 18 sex- and age-matched healthy subjects recruited from our clinic staff. Detailed patient and healthy control characteristics are depicted in Table 1.
Table 1. Demographic, clinical, and laboratory features of healthy controls and patients with psoriatic arthritis.
| Variables | Healthy controls (n = 18) |
Psoriatic arthritis (n = 18) |
p Value |
|---|---|---|---|
| Sex (F:M) | 8:10 | 7:11 | – |
| Age (y) | 42.93 ± 10.16 | 43.81 ± 8.33 | 0.27 |
| Height (cm) | 170 ± 8.5 | 159 ± 4.9 | 0.08 |
| Body weight (kg) | 65.13 ± 12.5 | 63.07 ± 12.58 | 0.12 |
| BMI (kg/m2) | 22.45 ± 3.05 | 23.95 ± 5.63 | 0.23 |
| Disease duration (y) | – | 8.87 ± 5.85 | – |
| DAS28 | – | 4.08 ± 0.56 | – |
| DAPSA | – | 23.08 ± 8.76 | – |
| ESR (mm 1st h) | 16.68 ± 4.54 | 26.04 ± 10.36 | 0.03a |
| CRP (mg/dL) | 3.93 ± 1.10 | 12.76 ± 15.18 | 0.01a |
| Total cholesterol (mg/dL) | 187.2 ± 24.3 | 193.6 ± 32.4 | 0.26 |
| HDL cholesterol (mg/dL) | 53.9 ± 11.6 | 45.5 ± 10.7 | 0.03a |
| LDL cholesterol (mg/dL) | 114.2 ± 22.3 | 115.7 ± 26.5 | 0.48 |
| TG (mg/dL) | 99.1 ± 9.2 | 101.4 ± 13.4 | 0.43 |
| IL-1 (pg/mL) | 145 ± 8.3 | 150 ± 9.4 | 0.18 |
| IL-6 (pg/mL) | 6.5 ± 5.3 | 18.5 ± 6.8 | 0.03a |
| TNF-α (pg/mL) | 3.8 ± 1.8 | 6.4 ± 2.6 | 0.02a |
| ICAM-1 (ng/mL) | 120 ± 5.3 | 175 ± 6.8 | 0.01a |
| VCAM-1 (ng/mL) | 920 ± 2.6 | 940 ± 4.8 | 0.01a |
| FMD% | 9.3 ± 1.4 | 6.3 ± 0.95 | 0.01a |
| EPCs (%) | 0.041 ± 0.001 | 0.020 ± 0.001 | 0.01a |
| CIMT (cm) | 0.045 ± 0.10 | 0.062 ± 0.018 | 0.01a |
Abbreviations: BMI, body mass index; CIMT, carotid intima-media thickness; CRP, C-reactive protein; DAPSA, disease activity in psoriatic arthritis; DAS28, disease activity score of 28 joints; EPCs, endothelial progenitor cells; ESR, erythrocyte sedimentation rate; F, female; FMD, flow-mediated dilation; HDL, high-density lipoprotein; ICAM-1, intracellular adhesion molecule; IL-1, interleukin-1; IL-6, interleukin-6; LDL, low-density lipoprotein; M, male; TG, triglycerides; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule.
Note: Data are presented as mean ± SD.
p < 0.05 statistically significant.
All subjects enrolled in the study signed an informed consent document approved by the institutional ethics committee of the Punjabi University, Patiala, India. The study complies with the Declaration of Helsinki.
Patients with diabetes mellitus (glycated hemoglobin A1c ≥ 6.5), hypertension (systolic blood pressure > 140 mm Hg or diastolic blood pressure > 90 mm Hg), a history of cardiovascular or cerebrovascular disease, renal failure (serum creatinine values > 1.3 mg/dL), thyroid dysfunction, multiple sclerosis, human immunodeficiency virus, rheumatic diseases other than PsA, smokers, alcoholic, and age younger than 18 years were excluded from the study. Subjects who were receiving lipid-lowering therapy, antihypertensive or antiaggregant drugs, nitrates, peroxisome proliferator-activated receptor α agonists (clofibrate, gemfibrozil, ciprofibrate, bezafibrate, and fenofibrate), or systemic steroids were also excluded. Any previous use of anti-tumor necrosis factor (TNF)-α medications was also excluded.
Blood Sampling
Venous blood samples were taken between 8 and 10 am after overnight fasting to heparinized tubes to measure EPCs and tubes containing EDTA or without anticoagulant for biochemical determinations. The latter were centrifuged immediately. After centrifugation, serum and plasma were separated and analyzed for routine analyses.
Biochemical Assessment
Biochemical analysis included a complete blood count, liver function tests, renal function test, vitamin B12, thyroid stimulating hormone, fasting blood sugar, glycated hemoglobin, lipid profile, and urine analysis to detect proteinuria, hematuria, or cellular casts.
Measurement of Intima-Media Thickness of Carotid Artery
CIMT was measured ultrasonographically. All subjects were examined using a high-resolution Doppler ultrasound (HD 11 XE ultrasound machine, Philips Medical System, Sorrento, Italy) using a 13–5-MHz linear array transducer in the supine position. The common carotid artery (CCA) intima-media thickness (IMT) was defined as the average of the maximum IMT of the near and far wall measurements in the distal CCA (1 cm proximal to the carotid bulb). All images of the carotid arteries were recorded for subsequent analysis and evaluated by a well-experienced radiologist who was blinded to the clinical characteristics of the participants.8
Assessment of Inflammatory Disease Activity Measures
Inflammatory disease activity measures include: disease activity score of 28 joints count (DAS28) and disease activity in psoriatic arthritis (DAPSA). Other measures of inflammation include: Erythrocyte sedimentation rate (ESR), measured by Westergren method and C-reactive protein (CRP) determined using standard commercial kits.
Assessment of Proinflammatory Cytokines
Interleukin-1 (IL-1), interleukin-6 (IL-6), and TNF-α were measured using enzyme-linked immunosorbent assays (Diaclone Diagnostics, France).
Assessment of Endothelial Function
Flow-mediated dilation (FMD) was assessed by using AngioDefender (Everist Health, Ann Arbor, MI).9 The AngioDefender procedure is noninvasive and employs neither ultrasound nor Doppler flow analysis. Results are expressed as a percentage of FMD (FMD%).
Flow Cytometry Analysis
EPCs were quantified by flow cytometric analysis (Canto II; BD Biosciences, San Jose, CA).10 11
Flow cytometric analysis was performed by using three markers: Fluorescein isothiocyanate anti-CD45 (BD Biosciences), phycoerythrin anti-CD34 (BD Biosciences), allophycocyanin anti-CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany). Results are expressed as percent cells gated.10 11
Assessment of Adhesion Molecules
Intracellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) were measured using enzyme-linked immunosorbent assays (Diaclone Diagnostics, France).
Statistical Analysis
Continuous data are expressed as mean ± standard deviation. Comparison between the two groups (patients with PsA vs. controls) was performed using unpaired Student t-tests. Spearman correlation coefficients were calculated in the PsA group, to study the relationship between CIMT and other disease variables. Statistical significance was assumed at p < 0.05. Statistical analysis was performed using Sigmastat 3.5 (Systat Software, San Jose, CA) for Windows 7.
Results
The demographic and clinical characteristics of PsA patients and healthy controls are presented in Table 1. Patients and healthy controls were not significantly different regarding demographic characteristics (age, sex, and body mass index). DAS28 and DAPSA were significantly higher in PsA patients (4.08 ± 0.56 and 23.08 ± 8.76), respectively.
There was a statistically significant difference (p < 0.05) between PsA patients and controls regarding mean values of CIMT, FMD, and EPCs and serum levels of ESR, CRP, IL-6, TNF-α, and ICAM-1 (Table 1).
Association of CIMT with Markers of Inflammation and Vascular Function
Spearman correlation analysis was done to find the relationship between CIMT and other disease variables. In PsA patients, CIMT positively correlated with IL-6 (r = 0.41, p = 0.04) and ICAM-1 (r = 0.48, p = 0.01) and inversely correlated with FMD (r = − 0.51, p = 0.01), EPC (r = − 0.44, p = 0.02), and HDL cholesterol (r = − 0.50, p = 0.01) (Fig. 1; Table 2).
Fig. 1.
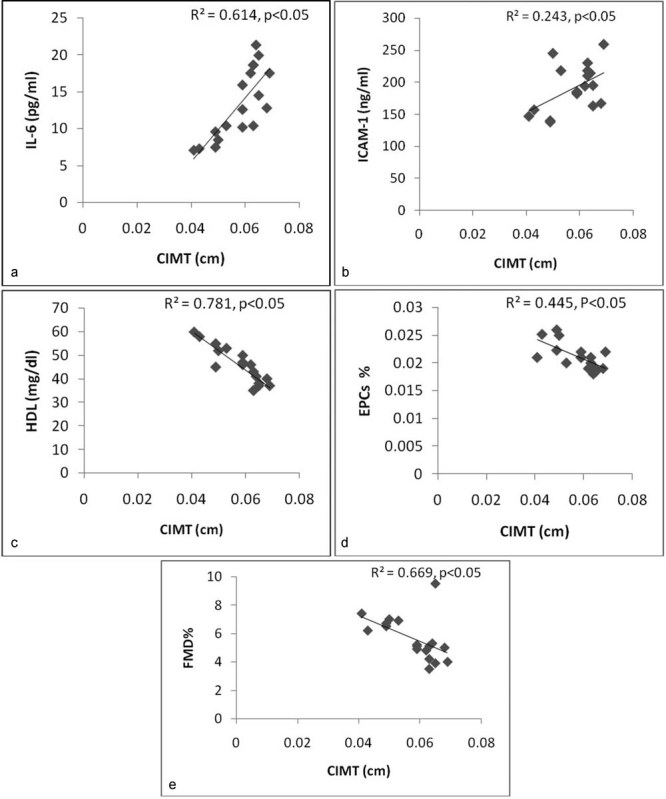
Correlation between CIMT and (a) IL-6, (b) ICAM-1, (c) HDL, (d) EPCs%, and (e) FMD% in PsA patients. CIMT, carotid intima-media thickness; EPCs, endothelial progenitor cells; FMD, flow-mediated dilatation; HDL, high-density lipoprotein; ICAM-1, intracellular adhesion molecule-1; IL-6, interleukin-6; PsA, psoriatic arthritis.
Table 2. Correlation of CIMT with demographic and clinical disease variables in psoriatic arthritis patients.
| Variables | r | p Value |
|---|---|---|
| Age | −0.321 | 0.21 |
| Disease duration | 0.234 | 0.35 |
| DAS 28 | 0.381 | 0.18 |
| DAPSA | 0.345 | 0.16 |
| ESR (mm 1st h) | 0.228 | 0.20 |
| CRP (mg/dL) | 0.304 | 0.10 |
| TC (mg/dL) | 0.210 | 0.23 |
| HDL (mg/dL) | -0.502 | 0.01a |
| LDL (mg/dL) | 0.251 | 0.14 |
| IL-1 (pg/mL) | 0.184 | 0.28 |
| IL-6 (pg/mL) | 0.417 | 0.04a |
| TNF-α (pg/mL) | 0.374 | 0.11 |
| ICAM-1 (ng/mL) | 0.481 | 0.01a |
| VCAM-1 (ng/mL) | 0.284 | 0.17 |
| EPC% | 0.443 | 0.02a |
| FMD% | 0.512 | 0.01a |
Abbreviations: CIMT, carotid intima-media thickness; CRP, C-reactive protein; DAS28, disease activity score of 28 joints; DAPSA, disease activity in psoriatic arthritis; EPCs, endothelial progenitor cells; ESR, erythrocyte sedimentation rate; FMD, flow-mediated dilation; HDL, high-density lipoprotein; ICAM-1, intracellular adhesion molecule 1; IL-1, interleukin-1; IL-6, interleukin-6; LDL, low-density lipoprotein; TC, total cholesterol; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule 1.
Statiscally significant p < 0.05.
Discussion
The present study is novel for several reasons. This study demonstrates that patients with active PsA have an increased CIMT, decreased FMD, reduced level of EPCs in the absence of cardiovascular risk factors or manifest CVD. It has also suggested that impaired levels of inflammatory and vascular markers in PsA compared with healthy subjects are possibly a part of the disease process. To our knowledge, this is the first study to report a plausible association between CIMT and various biomarkers responsible for subclinical atherosclerosis in patients with PsA without clinically evident cardiovascular disease. Although CIMT and FMD as well as some laboratory factors have previously been assessed by other investigators in PsA-associated atherosclerosis, our results regarding the possible involvement of proinflammatory cytokines, adhesion molecules, and EPCs level are novel and suggest a possible mechanism of atherosclerosis in PsA.
Recognition of the importance of inflammation in atherogenesis has led to the hypothesis that subclinical atherosclerosis is increased in patients with chronic inflammatory diseases.12 13 We and others have shown that this is indeed the case in patients with systemic lupus erythematosus, rheumatoid arthritis (RA), and ankylosing spondylitis (AS).14 15 However, specific predictors of subclinical or coronary atherosclerosis in PsA remain largely speculative. Therefore, we studied markers of inflammation and vascular function that have been implicated in the process of atherogenesis in PsA.
The prevalence of increased subclinical atherosclerosis has been studied previously in PsA through the assessment of CIMT and FMD, but the partial exclusion of traditional cardiovascular risk factors, cardiovascular events, or concomitant medications have led to ambiguous results.5 16 17 Hence, we excluded PsA patients with traditional cardiovascular risk factors, manifest CVD or on medications likely to influence vascular function (TNF-α inhibitors, steroids, β blockers, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, statins, aldosterone antagonist, and peroxisome proliferator-activated receptor).
Measurement of CIMT is considered a strong biomarker of cardiovascular risk.18 In the present study, a high CIMT was associated with an impaired vascular function. Other studies have also shown a similar increase in CIMT in PsA patients in the absence or presence of cardiovascular risk factors. The mean CIMT (0.62 mm) observed in the present study is comparable to that in the reported studies.19 20 This assumes great significance when viewed in context of the fact that the median age of our patient population was only 43 years, a group traditionally thought to have a lesser burden of atherosclerotic CVD as compared with others with higher age. Endothelial dysfunction indicated by FMD% may precede manifest atherosclerosis indicated by CIMT. Thus, more pronounced endothelial dysfunction may lead to more accelerated atherosclerosis in PsA.
EPC depletion represents a pathogenic step in atherogenesis and a biomarker of cardiovascular risk in rheumatic diseases.21 22 23 In the present study, EPC population was estimated to study its role in accelerated atherosclerosis associated with PsA. The novel finding of the present study is that depleted EPC population in PsA inversely correlates with CIMT. This result is consistent with two recent observations indicating that level of EPCs independently predicts cardiovascular events and atherosclerosis progression in patients with coronary artery disease.24 25 These results indicate that the integrative regenerative capacity of circulating EPC may be relevant for this important state of vascular dysfunction and subsequent development of atherosclerosis.
To date, any relationship between PsA variables, inflammatory cytokines, and atherosclerosis has been merely speculative. Increased DAS28 and DAPSA in PsA patients support the hypothesis that disease activity may influence atherosclerosis in PsA. However, we did not find any correlation between CIMT and DAS 28 and DAPSA scores. These results are consistent with previous reports demonstrating no correlation between disease activity and CIMT scores.26 27 In addition, we also found that increased CIMT significantly correlated with CRP, suggesting that systemic inflammation in PsA may accelerate the atherosclerotic process, while previous studies did not find a significant correlation between CIMT and CRP in PsA.12 26 An elevated CRP, one of the best surrogate markers of systemic inflammation, is known to promote endothelial cell activation and atherosclerotic processes.28 29 Our study is consistent with this observation that higher levels of CRP (12.76 mg/dL) may contribute to accelerated atherosclerosis in PsA. However, no correlation has been observed between CIMT and CRP in PsA studies with relatively lower CRP levels (8.4 and 8.5 mg/dL).5 12
We also found that increased CIMT significantly correlated with IL-6 and ICAM-1 in the present study. IL-6 is an important proinflammatory cytokine that may also be involved in the pathogenesis of PsA and a potential risk factor of CV risk.30 High levels of circulating cytokines, TNF-α, IL-6, and IL-1, alter the function of distant tissues, including adipose, skeletal muscle, liver and vascular endothelium, to generate a spectrum of proatherogenic changes that includes insulin resistance, a characteristic dyslipidemia, prothrombotic effects, prooxidative stress, and endothelial dysfunction.31 Previously, proinflammatory cytokines (TNF-α, IL-6, and IL-1) have not been measured in PsA in the context of accelerated atherosclerosis.5 12 19 20 However, Alenius et al and Sattar et al estimated the serum level of IL-6 and demonstrated higher levels of IL-6 in PsA patients.30 32 To the best of our knowledge, the impact of IL-6 blockade on CIMT has not been investigated in PsA. A recent case study also demonstrated that IL-6 blockade with tocilizumab significantly reduced ESR and CRP in a PsA patient who failed anti-TNF therapy.33
As in RA, the synovial membrane of PsA has also demonstrated the presence of ICAM-1 and VCAM-1.34 ICAM-1 has been shown to be present in atherosclerotic lesions and also involved in the progression of atherosclerotic lesions.35 We found that increased CIMT significantly correlated with ICAM-1 suggesting a contribution to accelerated atherosclerosis in PsA. In a previous study, conducted by Dessein et al in RA patients, VCAM-1 was found to be related to ultrasonographically detected CIMT.36 Therefore, increased levels of IL-6 and ICAM-1 may be risk factors for subclinical atherosclerosis and accelerated progression of atherosclerosis in PsA. This provides support for the idea that specific mediators, rather than a nonspecific inflammatory response, are important in the pathogenesis of atherosclerosis in PsA. Important in this context is that high adhesion molecule levels may not only reflect synovial inflammation, but also indicate exposure of the systemic vascular endothelium to high circulating cytokine concentrations. These data are consistent with the concept that inflammation promotes atherogenesis, as suggested by recent studies in AS and RA.15 37
It has been previously reported that, psoriasis patients had higher triglyceride levels and lower HDL levels.38 We also observed lower HDL levels in PsA patients. Our study shows a negative correlation of CIMT with HDL in PsA patients. Thus, lower HDL-cholesterol in PsA may also accelerate the progression of atherosclerosis and increase the risk of CV events.
This study design had several strengths, analyses of a well-characterized patient population, and measurement of inflammatory mediators and markers of vascular function reported to be associated with atherosclerosis. A potential limitation of the current study was the small sample size.
In conclusion, markers related to inflammation and vascular function, including CRP, IL-6, ICAM-1, and EPCs may all be involved in the progression of atherosclerosis in PsA. These markers would possibly serve as therapeutic targets to prevent premature atherosclerosis and cardiovascular disease in PsA.
Footnotes
Conflicts of Interest None to declare.
References
- 1.Gladman D D, Antoni C, Mease P, Clegg D O, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64 02:ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong K, Gladman D D, Husted J, Long J A, Farewell V T. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 1997;40(10):1868–1872. doi: 10.1002/art.1780401021. [DOI] [PubMed] [Google Scholar]
- 3.Peters M J, van der Horst-Bruinsma I E, Dijkmans B A, Nurmohamed M T. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum. 2004;34(3):585–592. doi: 10.1016/j.semarthrit.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Kölliker Frers R A, Bisoendial R J, Montoya S F. et al. Psoriasis and cardiovascular risk: Immune-mediated crosstalk between metabolic, vascular and autoimmune inflammation. IJC Metab Endocr. 2015;6:43–54. [Google Scholar]
- 5.Kimhi O, Caspi D, Bornstein N M. et al. Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum. 2007;36(4):203–209. doi: 10.1016/j.semarthrit.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Di Minno M ND, Ambrosino P, Lupoli R. et al. Cardiovascular risk markers in patients with psoriatic arthritis: A meta-analysis of literature studies. Ann Med. 2015;47(4):346–353. doi: 10.3109/07853890.2015.1031822. [DOI] [PubMed] [Google Scholar]
- 7.Taylor W Gladman D Helliwell P Marchesoni A Mease P Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study Arthritis Rheum 20065482665–2673. [DOI] [PubMed] [Google Scholar]
- 8.Huang K, Zou C C, Yang X Z, Chen X Q, Liang L. Carotid intima-media thickness and serum endothelial marker levels in obese children with metabolic syndrome. Arch Pediatr Adolesc Med. 2010;164(9):846–851. doi: 10.1001/archpediatrics.2010.160. [DOI] [PubMed] [Google Scholar]
- 9.Garg N, Krishan P, Syngle A. Rosuvastatin improves endothelial dysfunction in ankylosing spondylitis. Clin Rheumatol. 2015;34(6):1065–1071. doi: 10.1007/s10067-015-2912-3. [DOI] [PubMed] [Google Scholar]
- 10.Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25(1):60–66. [PubMed] [Google Scholar]
- 11.Herbrig K, Haensel S, Oelschlaegel U, Pistrosch F, Foerster S, Passauer J. Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis. 2006;65(2):157–163. doi: 10.1136/ard.2005.035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Juanatey C, Llorca J, Amigo-Diaz E, Dierssen T, Martin J, Gonzalez-Gay M A. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57(6):1074–1080. doi: 10.1002/art.22884. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Juanatey C, Llorca J, Testa A, Revuelta J, Garcia-Porrua C, Gonzalez-Gay M A. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine (Baltimore) 2003;82(6):407–413. doi: 10.1097/01.md.0000101572.76273.60. [DOI] [PubMed] [Google Scholar]
- 14.Salmon J E, Roman M J. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am J Med. 2008;121(10) 01:S3–S8. doi: 10.1016/j.amjmed.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma I, Krishan P, Syngle A. Predictors of Atherosclerosis in Ankylosing Spondylitis. Rheumatol Ther. 2015;2:173–182. doi: 10.1007/s40744-015-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puato M, Ramonda R, Doria A. et al. Impact of hypertension on vascular remodeling in patients with psoriatic arthritis. J Hum Hypertens. 2014;28(2):105–110. doi: 10.1038/jhh.2013.62. [DOI] [PubMed] [Google Scholar]
- 17.Eder L, Zisman D, Barzilai M. et al. Subclinical atherosclerosis in psoriatic arthritis: a case-control study. J Rheumatol. 2008;35(5):877–882. [PubMed] [Google Scholar]
- 18.Bots M L, Grobbee D E. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc Drugs Ther. 2002;16(4):341–351. doi: 10.1023/a:1021738111273. [DOI] [PubMed] [Google Scholar]
- 19.Atzeni F, Sarzi-Puttini P, Sitia S. et al. Coronary flow reserve and asymmetric dimethylarginine levels: new measurements for identifying subclinical atherosclerosis in patients with psoriatic arthritis. J Rheumatol. 2011;38(8):1661–1664. doi: 10.3899/jrheum.100893. [DOI] [PubMed] [Google Scholar]
- 20.Magro-Checa C, Orgaz-Molina J, Rosales-Alexander J L. et al. Comparison of subclinical carotid atherosclerosis in patients with psoriatic arthritis, psoriasis alone and controls. Ann Rheum Dis. 2013;71:572. [Google Scholar]
- 21.Grisar J, Aletaha D, Steiner C W. et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111(2):204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 22.Del Papa N, Quirici N, Soligo D. et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006;54(8):2605–2615. doi: 10.1002/art.22035. [DOI] [PubMed] [Google Scholar]
- 23.Verma I, Syngle A, Krishan P. Endothelial dysfunction in ankylosing spondylitis associated with reduced endothelial progenitor cell population. IJRCI. 2015;3(1):OA3. [Google Scholar]
- 24.Vasa M, Fichtlscherer S, Aicher A. et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Lucke C, Rössig L, Fichtlscherer S. et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Juanatey C, Llorca J, Miranda-Filloy J A. et al. Endothelial dysfunction in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57(2):287–293. doi: 10.1002/art.22530. [DOI] [PubMed] [Google Scholar]
- 27.Altekin E R, Koç S, Karakaş M S. et al. Determination of subclinical atherosclerosis in plaque type psoriasis patients without traditional risk factors for atherosclerosis. Turk Kardiyol Dern Ars. 2012;40(7):574–580. doi: 10.5543/tkda.2012.54920. [DOI] [PubMed] [Google Scholar]
- 28.Verma S, Wang C H, Li S H. et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 29.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 30.Alenius G M, Eriksson C, Rantapää Dahlqvist S. Interleukin-6 and soluble interleukin-2 receptor alpha-markers of inflammation in patients with psoriatic arthritis? Clin Exp Rheumatol. 2009;27(1):120–123. [PubMed] [Google Scholar]
- 31.Sattar N, McCarey D W, Capell H, McInnes I B. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108(24):2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 32.Sattar N, Crompton P, Cherry L, Kane D, Lowe G, McInnes I B. Effects of tumor necrosis factor blockade on cardiovascular risk factors in psoriatic arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2007;56(3):831–839. doi: 10.1002/art.22447. [DOI] [PubMed] [Google Scholar]
- 33.Hughes M, Chinoy H. Successful use of tocilizumab in a patient with psoriatic arthritis. Rheumatology (Oxford) 2013;52(9):1728–1729. doi: 10.1093/rheumatology/kes432. [DOI] [PubMed] [Google Scholar]
- 34.Riccieri V, Spadaro A, Taccari E. et al. Adhesion molecule expression in the synovial membrane of psoriatic arthritis. Ann Rheum Dis. 2002;61(6):569–570. doi: 10.1136/ard.61.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poston R N, Haskard D O, Coucher J R, Gall N P, Johnson-Tidey R R. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol. 1992;140(3):665–673. [PMC free article] [PubMed] [Google Scholar]
- 36.Dessein P H, Joffe B I, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther. 2005;7(3):R634–R643. doi: 10.1186/ar1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rho Y H, Chung C P, Oeser A. et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1580–1585. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam L S, Tomlinson B, Chu T T. et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—the role of inflammation. Rheumatology (Oxford) 2008;47(5):718–723. doi: 10.1093/rheumatology/ken090. [DOI] [PubMed] [Google Scholar]


