Abstract
This study aims to evaluate occurrence, size, composition, and clinical significance of embolized debris during superficial femoral artery atherectomy using all commercially available atherectomy devices.
Distal athero/thromboembolic events (DATE) are a universal phenomenon in lower extremity atherectomy procedures (LEAPs) due to the sheer volume of atheroma and the thrombus burden in peripheral arterial disease. Some of these events can be clinically significant.
We prospectively gathered clinical and histopathological data on all commercially available atherectomy devices by using embolic protection devices (EPD) in every case. After intervention, the contents of EPD were examined both microscopically and macroscopically.
Data from 59 consecutive patients undergoing LEAP were analyzed. DATE occurred 100% of the time. The composition of particulate debris varied with the device used. Grossly visible agglomerated debris was captured by the filter in the majority of patients 54/59 (91.5%). Clinically significant debris, defined by the Preventing Lower Extremity Distal Embolization Using Embolic Filter Protection registry as particle diameter > 0.2 cm, was found in 33/59 (56%) patients. The size of captured debris particles ranged from 0.1 to 2.4 mm.
While DATE occurred in all patients, clinically significant DATE occurred in 56% patients undergoing LEAP regardless of the atherectomy device. In spite of a large fraction of the clinically significant debris occurring on our routine dual antiplatelet regimen, no patient suffered an amputation. Although DATE was prevented by the use of EPD in all 59 cases, more data are needed to determine whether the use of EPD translates into a long-term clinical benefit. Use of EPD and optimal thromboprophylaxis should be considered in patients, especially in the setting of compromised distal runoff.
Keywords: cardiac catheterization, peripheral arterial thromboembolism, atherectomy, endovascular procedures
Atherosclerosis starts early and is a diffuse process; angiographically significant disease in one section of a blood vessel does not connote a normal vessel wall in the remainder of the conduit.1 Positive remodeling typically compensates well for early lumen loss often leading to a “normal” appearing vessel on angiography.2 Vascular imaging with intravascular ultrasound and computed tomographic angiography have improved our comprehension of the magnitude of the atheroma burden in patients with vascular disease.2
We hypothesized that given the sizable volume of the atheroma burden in the peripheral vessels of patients with peripheral arterial disease (PAD), all forms of currently available atheroma debulking therapies will often result in clinically significant distal athero/thromboembolic events (DATE). To this end, we prospectively evaluated all available atherectomy devices used in our institution in a sequential manner. Our findings confirm the ubiquitous nature of DATE during lower extremity atherectomy procedures (LEAP), some clinically significant irrespective of the device used.
Methods
Data were collected from prior prospective studies, where we sequentially evaluated five atherectomy devices under the umbrella of four separate institutional review board protocols. Over a 4-year period from 2008 to 2012, where 59 consecutive patients with obstructive superficial femoral artery (SFA) disease, including near-chronic total occlusion who underwent LEAP performed by a single-experienced operator were enrolled in this study. During these 4 years, 1,418 peripheral interventional cases were done in the institution: 409 were LEAP; 1,009 cases did not include lower extremity atherectomies. During this same period, the principal investigator (PI) performed a total of 382 peripheral interventions, or which 248 were lower extremity interventions. Seventy of these 248 had atherectomy and 178 had nonatherectomy interventions. After completing each device evaluation, time was taken to tabulate results. Then a retrospective analysis of the four prospective trials was conducted and the data was pooled to analyze all five devices together. The PI attempted enrolled as many of the 70 patients that underwent atherectomy from 2008 to 2012 into the study and 59 patients were included in the histopathology evaluation of the debris collected from embolic protection devices (EPD).
The study was approved by the institutional review board at our center. Patients were included if they were older than 18 years, able to provide informed consent, and had more than 70% stenosis in the SFA. A uniform protocol for antithrombotic regimen use and general interventional vascular techniques, deploying EPD was applied to all patients. EPD and debris retrieval were achieved in all patients using the Emboshield NAV6 system (Abbott Cardiovascular Systems, Inc., Santa Clara, CA), except for one patient. This one patient, who had atherectomy with the Silverhawk device (EV3, Inc., Plymouth, MN), did not have his EPD successfully deployed, and was excluded from this analysis, leaving 59 patients for this study. Commercially available atherectomy devices were used in a sequential manner: directional atherectomy with the Silverhawk device (n = 14 consecutive patients), Turbohawk supercutter device (Turbohawk) (EV3, Inc., now Medtronics) (n = 4), rotational atherectomy and aspiration with the Pathway Jetstream G3 device (Jetstream) (Pathway Medical Technologies, Inc., Kirkland, WA, now Boston Scientific) (n = 22), excimer laser photoablative atherectomy with the Turbo Elite device (Laser) (Spectranetics Corporation, Colorado Springs, CO) (n = 14), or Orbital atherectomy with CSI Stealth 3600 device (CSI) (Cardiovascular Systems, Inc., St Paul, MN) (n = 5 patients) (see Table 1).
Table 1. Patient demographics.
| CSI (n = 5) | Jetstream (n = 22) | Silverhawk (n = 14) | Excimer laser (n = 14) | Turbohawk (n = 4) | Overall (n = 59) | |
|---|---|---|---|---|---|---|
| Age (mean), y | 71.8 | 71.5 | 68.5 | 70.7 | 63 | 69.1 |
| Gender (male) | 60% | 50% | 40% | 57.10% | 60% | 51.80% |
| CKD (GFR < 60 mL/min) | 20% | 18% | 13% | 21% | 25% | 19.40% |
| DM | 60% | 64% | 53% | 43% | 75% | 59% |
| Multiple risk factors for PAD (2) | 100% | 100% | 100% | 100% | 100% | 100% |
| Procedural success (< 20% residual stenosis) |
5 (100%) | 20 (91%) | 14 (100%) | 13 (92.8%) | 4 (100%) | 56 (95%) |
| Grossly visible debris agglomerate | 4 (80%) | 21 (95.4%) | 14 (100%) | 12 (85.7%) | 3 (75%) | 54 (91.5%) |
| Clinically significant debris (d > 0.2 cm) |
3 (60%) | 16 (72.7%) | 7 (50%) | 4 (28.6%) | 3 (75%) | 33 (56%) |
Abbreviations: CKD, chronic kidney disease; DM, diabetes mellitus; GFR, glomerular filteration rate; PAD, peripheral arterial disease.
Interventions
All patients were fully loaded with aspirin 325 mg and clopidogrel 600 mg orally immediately before the procedure. Intravenous unfractionated heparin was utilized to achieve an activated clotting time of 250 to 300 seconds. One Emboshield NAV6 system was used per patient for distal protection and retrieval of embolized material. The Emboshield NAV6 system was successfully delivered distal to the lesion and retrieved successfully in all 59 patients in this study. All interventions were performed by a single operator experienced in peripheral interventions.
The first intervention in all of these study patients was always an atherectomy. The Emboshield NAV6 filter with its contents was then removed after the atherectomy. All patients had a subsequent adjunctive balloon angioplasty postatherectomy after the filter had been removed. Therefore, there are no data among our study patients regarding debris after plain old balloon angioplasty (POBA) even though all patients had a subsequent adjunctive balloon angioplasty.
Debris and Histological Analysis
The basket of the Emboshield NAV6 system was placed in a sterile container with saline and immediately hand delivered to the pathology laboratory for analysis. The saline solutions were then centrifuged, prepared for cytology evaluation, and stained with Papanicolaou stain (ThinPrep, Cytyc Corporation, Boxborough, MA). The grossly visible material was fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin for light microscopy.
Statistical Analysis
All data are presented as mean ± standard deviation or percentages.
Results
A total of 59 consecutive patients were enrolled in this atherectomy protocol, utilizing the five devices in a sequential manner. The mean age was 69.1 years, 51.8% were males and 59% were diabetic. All patients had multiple risk factors for PAD. Procedural success, defined as less than or equal to 20% residual arterial stenosis, was achieved in 56/59 (95%) of the patients. All lesions; prestenosis ranged from 70 to 95% occlusion and lesion length ranged from 20 to 330 mm with a mean range of 118.2 mm.
Grossly visible agglomerated debris was collected by the filter in the majority of patients 54/59 (91.5%). However, clinically significant debris, (defined as greatest particle diameter > 0.2 cm using the Preventing Lower Extremity Distal Embolization Using Embolic Filter Protection [PROTECT] registry's definition),3 was found in 33/59 (56%) patients (Table 1).
The size of captured debris particles ranged from 0.1 to 2.4 mm. Microscopic analysis revealed the following: 79% of samples consisted predominantly of collagen and fibrous tissue; no more than half, 52% of samples contained fibrin; 41% macrophages; 36% calcium; and 25% of the samples contained cholesterol (Fig. 1).
Fig. 1.
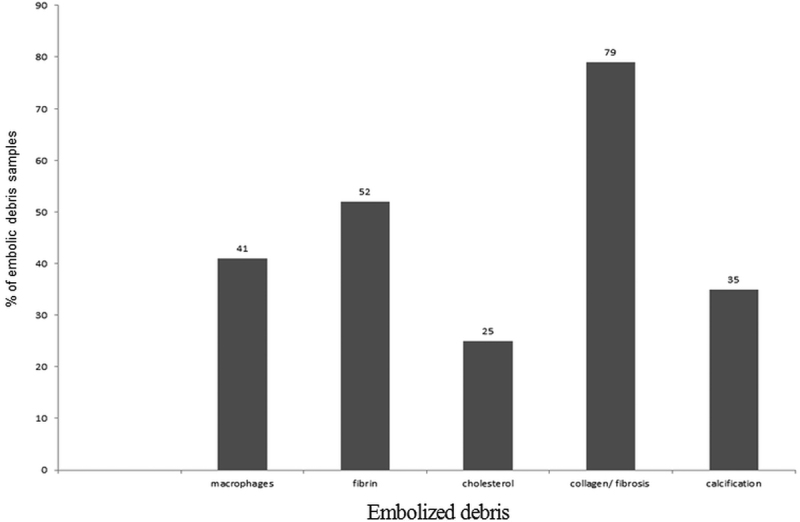
Overall histology of debris using atherectomy with different devices.
The histological analysis also demonstrated variability in the composition of the retrieved debris in relation to the atherectomy device used. Macrophages were most commonly found in the debris after atherectomy with Jetstream, 71%, versus 40% with CSI, 36% with Silverhawk, 33% with laser, and 26% with Turbohawk. Fibrin was most frequently retrieved after laser atherectomy; 92 versus 71% with Jetstream, 40% with CSI, 28.6% with Silverhawk, and 26% with Turbohawk. We could not tell if the fibrin was deposited before the laser therapy or immediately after. Cholesterol-rich particles were most commonly retrieved after Silverhawk; 50 versus 33% with Jetstream, 20% with CSI, 13% with Turbohawk, and 8% with laser. As the cholesterol-rich particles reside deep within atherosclerotic plaque, our findings may be lending testimony to the deeper cuts made by Silverhawk. It has been shown recently that deeper cuts leading to medial/adventitial injury results in higher rates of restenosis.4 Collagen/fibrotic tissue was found in almost all samples, irrespective of the atherectomy method used (95% with Jetstream, 86% with Silverhawk, 83% with laser, 80% with CSI, and 52% with Turbohawk). Finally, calcium was least frequent in the Turbohawk debris (8%) versus Silverhawk debris (29%) and almost six times as frequent with Jetstream (52%), Laser (50%) and CSI (40%) (Table 2 and Fig. 2).
Table 2. Comparative composition of distally embolized debris during atherectomy with different devices.
| Device | Macrophages | Fibrin | Cholesterol | Collagen/fibrous tissue | Calcium |
|---|---|---|---|---|---|
| CSI (OAS) (n = 5) | 40% | 40% | 20% | 80% | 40% |
| Jetstream (RAA-SFA) (n = 22) | 71% | 71% | 33% | 95% | 52% |
| Silverhawk (D-SFA-S) (n = 14) | 36% | 29% | 50% | 86% | 29% |
| Excimer laser (ELP-SFA) (n = 14) | 33% | 92% | 8% | 83% | 50% |
| Turbohawk (D-SFA-T) (n = 4) | 26% | 26% | 13% | 52% | 8% |
| Total = 60 | 41% | 52% | 25% | 79% | 36% |
Abbreviations: D-S, directional atherectomy-Silverhawk; D-T, directional atherectomy-Turbohawk; ELP, excimer laser photoablative atherectomy; OAS, orbital atherectomy system; RAA, rotational atherectomy and aspirations; SFA, superficial femoral artery.
Fig. 2.
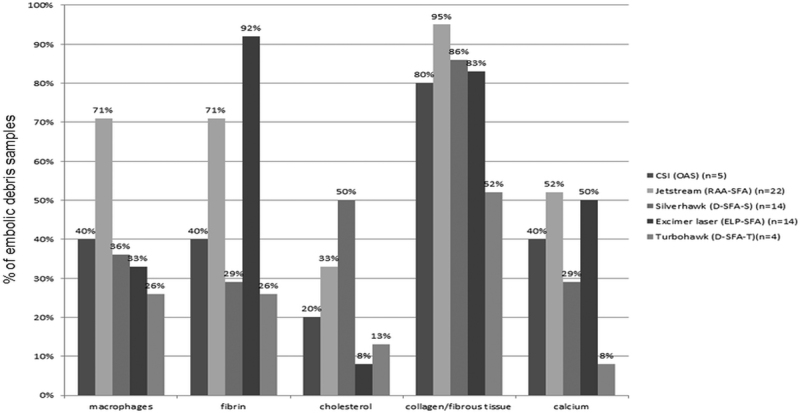
Comparative histology of embolized debris using different atherectomy devices.
Discussion
PAD affects up to 20% of patients over 65 years,5 and is associated with a higher risk of cardiovascular events, and mortality risk.6 7 Endovascular therapy of PAD is now a standard treatment modality. A variety of atherectomy devices are commercially available for the purpose of debulking plaque. DATE frequently complicates otherwise successful interventions and is reported to occur in the wake of catheter-based therapies in a variety of vascular beds.8 9 10 11 The use of EPD has become the standard of care in both carotid and saphenous vein coronary bypass graft interventions, and has improved outcomes in these vascular territories.3 12 13 14 15 Up to 5% of all endovascular treatments of the SFA are complicated by clinically significant distal embolization.10 13 Moreover, certain peripheral interventions, particularly directional atherectomy, have been associated with a higher risk of embolic complications.13 16 17 abThe role of EPD during SFA interventions has not been clearly established. Furthermore, little information is available not only about the incidence of DATE but also about the composition of embolized material during LEAPS with different atherectomy devices available on the market. Unfortunately, all of them cause DATE. Atherectomy being a strong predictor of DATE, other factors such as long lesions, chronic total occlusion, thrombotic disease, and in-stent re-stenosis are also independent predictors of distal embolization.18 However, all lesions we encountered were mostly de novo, nonthrombotic, nonchronic total occlusion, were long, calcified, and had critical stenosis.
Previously, Lam et al studied the incidence and clinical significance of distal embolization during percutaneous interventions involving the SFA in 60 patients. They used continuous Doppler ultrasound monitoring and found embolic signals in all phases of SFA interventions. The highest embolic signals were found when utilizing the Silverhawk atherectomy device and during stent deployment.16 In contrast, Kaid et al provided information regarding the composition of debris captured within EPD by comparing it with debris captured in the nose cone of the Silverhawk atherectomy device during SFA interventions. They found that the embolized debris differed significantly from nose cone debris and mainly contained macrophages, cholesterol, collagen.19 They also showed that macroscopic debris was captured in 93% of interventions and clinically significant debris (particle size > 0.2 cm) large enough to potentially cause clinically significant embolization, no-reflow, and/or ischemia occurred in nearly 50% of the cases.
In the PROTECT registry, Shammas et al3 studied the safety and effectiveness of EPD in reducing DATE. They found that Silverhawk atherectomy was an independent predictor of macroembolization (particle size > 0.2 cm.) and it was shown to have a 31-fold increased chance of DATE.
Shammas et al in comparing Silverhawk with adjunctive POBA versus POBA alone found that there was a decreased need for bailout stent in the atherectomy arm of the study and distal macroembolization occurred in 64.7% treated with atherectomy versus none of 10 in the PTA group, similar findings to our study.20 In studying directional atherectomy Roberts et al found that Silverhawk and Turbohawk can be used safely in moderate-to-severe calcified lesions and 88.5% of the patients experienced improvement in one or more Rutherford categories, while EPD caught embolic material in 97.5% of the cases, grossly visible in 88.4%.21
DEEP EMBOLI registry looking at embolic debris following laser atherectomy found that macrodebris occurred in 66.7% of cases, 22.2% of which were clinically significant. Two EPDs were used, one prior to laser therapy and one before final adjunctive POBA. The rates of clinically significant debris were found to be similar around 20%.22
However, Lam et al,16 Roberts et al21 only evaluated two atherectomy devices, (Silverhawk and Excimer laser), while Shammas et al3 20 only evaluated the Silverhawk and DEEP EMBOLI studied laser. Moreover, histopathological evaluation of DATE particulate debris was never conducted. In our present study, we not only studied the frequency of DATE but also examined the histopathology of the material retrieved during LEAPS with all five different commercially available devices.
Our study's principal finding is that particulate debris is “always” liberated during LEAPS regardless of the device type. While this particulate matter may play a role in the pathogenesis of no-flow phenomena and ischemia following LEAPS, clinically significant adverse events, and/or acute thrombotic occlusion are never-the-less, rare. Analyses of the debris captured in the EPD, in our study, showed mostly large, visible atherothrombotic material composed of cholesterol, fibrin, collagen, macrophages, and calcium. While the specific composition of the debris varied between atherectomy devices, the most common constituent of the debris across all devices was collagen/fibrotic material. In one-third of the particulate debris samples, there was calcium. The importance of this finding is uncertain, but it might be hypothesized that calcium-laden emboli are less likely to be readily resorbed and therefore may be more likely to cause no-flow phenomena. Distal embolization and possibly debris types are likely to be related to lesion characteristics, use of atherectomy devices, and operators' experience.
Study Limitations
This single-center study is limited by its small size, short clinical follow-up, and the lack of randomization. A larger trial is needed to determine the long-term benefits of distal EPD use during LEAPS.
Second, it is possible that the different design of the atherectomy devices can influence the makeup of the debris available for analysis. Presuming that the atherosclerotic lesions are similar, then why the differences in the debris composition. Each of the five atherectomy devices, dealt with debris in drastically different ways, and that could have affected the “makeup” of the debris: (1) Silverhawk and Turbohawk: The atheroma was sliced and packed in the nose cone. (2) Pathway: The atheroma is pulverized and extracted by forceful vacuum. (c) Laser: The atheroma is vaporized into CO2 and H2O with supposedly very little solid left behind. (d) CSI: The atheroma is pulverized into small-sized, microparticles.
Conclusions
Our study demonstrates that DATE is ubiquitous during LEAPS, regardless of the atherectomy device employed. Furthermore, debris large enough to cause clinically significant DATE following LEAPS occurred in nearly one out of two (56%) of our cases. In spite of the high prevalence clinically significant debris in our study, none of the 59 patients suffered an amputation while on a standard dual antiplatelet regimen utilized in these patients, which is may be attributable to use of EPD in all cases, but more data and long-term follow-up are needed to confirm clinical benefit. Based on these findings, we recommend the use of EPD be seriously considered when performing LEAPS, especially in situations with compromised (one-vessel) distal runoff.
References
- 1.Viles-Gonzalez J F, Fuster V, Badimon J J. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. Eur Heart J. 2004;25(14):1197–1207. doi: 10.1016/j.ehj.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Schoenhagen P, Ziada K M, Vince D G, Nissen S E, Tuzcu E M. Arterial remodeling and coronary artery disease: the concept of “dilated” versus “obstructive” coronary atherosclerosis. J Am Coll Cardiol. 2001;38(2):297–306. doi: 10.1016/s0735-1097(01)01374-2. [DOI] [PubMed] [Google Scholar]
- 3.Shammas N W, Dippel E J, Coiner D, Shammas G A, Jerin M, Kumar A. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J Endovasc Ther. 2008;15(3):270–276. doi: 10.1583/08-2397.1. [DOI] [PubMed] [Google Scholar]
- 4.Tarricone A, Ali Z, Rajamanickam A. et al. Histopathological Evidence of Adventitial or Medial Injury Is a Strong Predictor of Restenosis During Directional Atherectomy for Peripheral Artery Disease. J Endovasc Ther. 2015;22(5):712–715. doi: 10.1177/1526602815597683. [DOI] [PubMed] [Google Scholar]
- 5.Becker G J, McClenny T E, Kovacs M E, Raabe R D, Katzen B T. The importance of increasing public and physician awareness of peripheral arterial disease. J Vasc Interv Radiol. 2002;13(1):7–11. doi: 10.1016/s1051-0443(07)60002-5. [DOI] [PubMed] [Google Scholar]
- 6.Criqui M H, Langer R D, Fronek A. et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 7.Newman A B, Sutton-Tyrrell K, Vogt M T, Kuller L H. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270(4):487–489. [PubMed] [Google Scholar]
- 8.Becker G J, Katzen B T, Dake M D. Noncoronary angioplasty. Radiology. 1989;170(3 Pt 2):921–940. doi: 10.1148/radiology.170.3.2521745. [DOI] [PubMed] [Google Scholar]
- 9.McDermott J C, Crummy A B. Complications of angioplasty. Semin Intervent Radiol. 1994;11:145–149. [Google Scholar]
- 10.Freeman H J Rundback J H Embolic protection in femoro-popliteal artery intervention Endovascular Today, 2006. Available at: http://evtoday.com/pdfs/EVT1006_06.pdf. Accessed October 2006
- 11.Casserly I P, Abou-Chebl A, Fathi R B. et al. Slow-flow phenomenon during carotid artery intervention with embolic protection devices: predictors and clinical outcome. J Am Coll Cardiol. 2005;46(8):1466–1472. doi: 10.1016/j.jacc.2005.05.082. [DOI] [PubMed] [Google Scholar]
- 12.Bartorelli A L, Koh T H, Di Pede F. et al. Distal embolic protection during percutaneous coronary intervention in patients with acute coronary syndromes: the RUBY study. Acute Card Care. 2006;8(3):148–154. doi: 10.1080/17482940600931966. [DOI] [PubMed] [Google Scholar]
- 13.Suri R, Wholey M H, Postoak D, Hagino R T, Toursarkissian B. Distal embolic protection during femoropopliteal atherectomy. Catheter Cardiovasc Interv. 2006;67(3):417–422. doi: 10.1002/ccd.20634. [DOI] [PubMed] [Google Scholar]
- 14.Kastrup A, Nägele T, Gröschel K. et al. Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke. 2006;37(9):2312–2316. doi: 10.1161/01.STR.0000236492.86303.85. [DOI] [PubMed] [Google Scholar]
- 15.van Gaal W J, Choudhury R P, Porto I. et al. Prediction of distal embolization during percutaneous coronary intervention in saphenous vein grafts. Am J Cardiol. 2007;99(5):603–606. doi: 10.1016/j.amjcard.2006.09.106. [DOI] [PubMed] [Google Scholar]
- 16.Lam R C, Shah S, Faries P L, McKinsey J F, Kent K C, Morrissey N J. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46(6):1155–1159. doi: 10.1016/j.jvs.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 17.McKinsey J F Zeller T Rocha-Singh K J Jaff M R Garcia L A; DEFINITIVE LE Investigators. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study JACC Cardiovasc Interv 201478923–933. [DOI] [PubMed] [Google Scholar]
- 18.Shammas N W, Shammas G A, Dippel E J, Jerin M, Shammas W J. Predictors of distal embolization in peripheral percutaneous interventions: a report from a large peripheral vascular registry. J Invasive Cardiol. 2009;21(12):628–631. [PubMed] [Google Scholar]
- 19.Kaid K A, Gopinathapillai R, Qian F, Salvaji M, Wasty N, Cohen M. Analysis of particulate debris after superficial femoral artery atherectomy. J Invasive Cardiol. 2009;21(1):7–10. [PubMed] [Google Scholar]
- 20.Shammas N W, Coiner D, Shammas G A, Dippel E J, Christensen L, Jerin M. Percutaneous lower-extremity arterial interventions with primary balloon angioplasty versus Silverhawk atherectomy and adjunctive balloon angioplasty: randomized trial. J Vasc Interv Radiol. 2011;22(9):1223–1228. doi: 10.1016/j.jvir.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Roberts D, Niazi K, Miller W. et al. Effective endovascular treatment of calcified femoropopliteal disease with directional atherectomy and distal embolic protection: final results of the DEFINITIVE Ca++ trial. Catheter Cardiovasc Interv. 2014;84(2):236–244. doi: 10.1002/ccd.25384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shammas N W, Coiner D, Shammas G A, Christensen L, Dippel E J, Jerin M. Distal embolic event protection using excimer laser ablation in peripheral vascular interventions: results of the DEEP EMBOLI registry. J Endovasc Ther. 2009;16(2):197–202. doi: 10.1583/08-2642.1. [DOI] [PubMed] [Google Scholar]


