Abstract
A fundamental task of sensory systems is to extract relevant social information from a range of environmental stimuli in the face of changing behavioral contexts and reproductive states. Neuromodulatory pathways that interact with such contextual variables are one mechanism for achieving this. In the mouse inferior colliculus (IC), a midbrain auditory region, the neuromodulator serotonin increases in females interacting with courting males, but events downstream of serotonin release have not been investigated. Here, we manipulated serotonin levels in female mice with the serotonin releaser fenfluramine or the serotonin depleter pCPA. Females were then exposed to an empty cage, a male partner, or a playback of courtship vocalizations, and the numbers of neurons in the IC with positive immunoreactivity for the immediate early gene product c-fos were measured. The effects of drug treatments depended on social context and estrous state. Fenfluramine had greater effects in the nonsocial than in the partner social treatments. Females in proestrus or estrus and given fenfluramine had higher densities of c-fos immunoreactive neurons, while females in diestrus had fewer immunoreactive neurons. The drug pCPA had the expected opposite effect of fenfluramine, causing a decreased response in pro/estrus females and an increased response in diestrus females. These findings show that the effects of serotonin on c-fos activity in the IC of females is dependent on both external context and reproductive state, and suggest that these effects occur downstream of serotonin release.
Keywords: sensory modulation, courtship behavior, reproductive state, auditory midbrain, ultrasonic vocalizations
1. Introduction
Vocal signals are an important component of intersexual communication for many vertebrate species (e.g., Bottjer & Johnson, 1997; Holy & Guo, 2005; Arch & Narins, 2009). Reflecting the importance of both external and internal features of behavioral context, the ways in which these signals are processed by receivers may be influenced by interactions among the characteristics of the signals themselves and the physiological state of the receiver. Females may prefer particular types of male calls over others (e.g., Maney et al., 2003; Wilczynski & Lynch et al., 2011; Schubloom & Wooley, 2016; Brenowitz & Remage-Healey, 2016), and this preference behavior may track naturally varying reproductive status, or be influenced by the manipulation of ovarian hormones (Lynch et al., 2005; 2006; Ward et al., 2015). In parallel to this changing behavioral responsiveness, the auditory systems of females show preferential electrophysiological responses, or changes in immediate early gene expression, to male calls of particular structure, and these responses may also interact with variation in the hormonal milieu (Maney et al., 2006; Lynch & Wilczynski, 2008; Miranda & Wilczynski, 2009b; Svec & Wade, 2009; Chakraborty & Burmeister, 2015; Giret et al., 2015; Monbureau et al., 2015; Brenowitz & Remage-Healey, 2016). Peripheral or central changes in hormonal sensitivity may thus result in female auditory systems that are better matched to, more responsive to, or more discriminating of male signals (Forlano, et al., 2005; Sisneros, et al., 2004; Sisneros, 2009; Lynch & Wilczynski, 2008; Zeyl et al., 2013; Caras et al., 2015). In addition to direct effects on auditory neurons (e.g., Krentzel & Remage Healey, 2015), hormones regulate auditory processing in part via centralized neuromodulatory systems. These systems may be engaged by the presence of a social partner, triggering the release of neuromodulators, and their projections into auditory regions are also sensitive to gonadal hormones (Gale & Perkel, 2010; Hall et al., 2011; Matragrano et al., 2011; 2012b; 2013; Maney, 2013; Keesom & Hurley, 2016).
Within the auditory system, serotonin is a neuromodulator with the potential to integrate relatively rapid changes in behavioral context with slower changes in reproductive state. Dense serotonergic projections from the dorsal and median raphe nuclei terminate in multiple auditory regions, including within all subdivisions of the mammalian auditory midbrain nucleus, the inferior colliculus (IC; Klepper & Herbert, 1991; Hurley and Thompson, 2001). Direct measurements of serotonin in the IC of behaving female mice show increases in serotonergic activity during social interaction, including during interaction with courting males (Hall, et al., 2011; Hanson & Hurley, 2014). Interestingly, these increases in serotonergic activity are not significantly different in female mice that are in receptive versus nonreceptive phases of the estrous cycle (Hanson & Hurley, 2014), although in white-throated sparrows multiple aspects of the serotonergic system are altered following treatment with estradiol (Matragrano et al. 2012b). Whether auditory neurons themselves respond differently to serotonin in different phases of the estrous cycle has not been reported. The basis for potential state-dependent changes exists in the different types of receptors that influence the responses of IC neurons, however. Local administration of exogenous serotonin in vivo generally decreases spontaneous and evoked neural firing rates in the IC, but also may increase firing rates, depending on which receptor types are activated (Wang et al., 2008a, Hurley, 2006, Hurley & Sullivan, 2012). In broad terms, serotonin tends to increase the neurophysiological selectivity of IC neurons for behaviorally neutral stimuli and species-specific vocalizations alike (Hurley & Pollak, 2001; 2005). Although these findings define a range of potential actions of serotonin at the cellular level, they do not necessarily translate directly into equivalent actions during behavior. Since cell-level responses to serotonin vary in direction as well as size (Hurley & Sullivan, 2012), the effects of serotonin across large neuron populations are not clear. The influence of serotonin in female mice, an important potential audience for ultrasonic vocalizations, has not been explored, and the effects of estrous state have not been examined. Finally, previous studies have been accomplished in anesthetized mice (e.g., Castellan Baldan Ramsey et al., 2010), so that the influence of social context, involving the production and reception of dynamic suites of natural vocalizations, has not been addressed.
Here, we use a mouse model of vocal communication to address the influence of social context and estrous state on the effects of serotonin on immediate early gene expression in auditory neurons. During opposite-sex encounters, female laboratory mice (Mus musculus) encountering unfamiliar males are exposed to male-produced ultrasonic vocalizations (USVs) for a prolonged period of time (Nyby, 1983). Males begin producing USVs soon after being presented with females or swabs impregnated with female odor cues (Holy & Guo, 2005; Nyby, et at., 1977). Males display courtship behaviors to all females regardless of their estrous state, but modify call structure depending on whether females are in receptive or nonreceptive estrous phases (Barthelemy, et al., 2004; Hanson & Hurley, 2012). Overall production rates of male USVs gradually decrease after the initial investigative phase of interaction, but the proportion of a particular type of USV, the ‘harmonic’ call, increases over time in conjunction with mounting attempts by males (Hanson & Hurley, 2012; White, et al., 1998). Increasing serotonin in females generally coincides with these later events, (Hanson & Hurley, 2014), so that changes in vocalization structure co-occur with increased serotonin in the IC of female mice during later phases of an opposite-sex interaction, as events important to courtship and sexual behavior unfold.
Using this system, we addressed the influence of serotonin on immediate early gene (IEG) activity, which has been used to measure auditory responses to courtship vocalizations in non-rodent model species as well as in the IC of rodents (Burmeister, et al., 2008; Ehret & Fischer, 1991; Maney, et al., 2003; Petersen, et al., 2013; Sadananda, et al., 2008; Woolley & Doupe, 2008). We measured c-fos immunoreactivity in the IC after pharmacologically induced increases or decreases in serotonin levels. During these pharmacological treatments, females were placed in different social treatments, and the natural fluctuations in their estrous cycles were monitored. We made 3 main predictions about the effects of manipulating serotonin levels across these treatments based on previous findings. The first of these was that pharmacologically manipulating serotonin would alter the number of neurons immunopositive for c-fos. Specifically, we predicted that since serotonin decreases the firing rates of many IC neurons (Hurley, 2007; Wang et al., 2008a), c-fos label should decrease following pharmacological treatments that increased serotonin levels. Our second prediction was based on studies indicating that both the activity of neuromodulatory neurons and the effects of neuromodulators on the responses of auditory neurons depend on the salience of auditory stimuli (Gale & Perkel, 2010; Lynch & Ball, 2008). We consequently predicted that social contexts associated with different types of male cues would interact with drug treatment. We expected pronounced differences between groups with different serotonin levels as shown in Table 1. Finally, given that serotonergic pathways within the auditory system are sensitive to estradiol (Mataragrano et al., 2012a), we predicted that estrous state would influence the effects of serotonin on c-fos activity.
Table 1.
Experimental design and expected serotonin levels: six females were used for each drug and behavioral treatment sub-group. Two females were excluded from statistical tests because their estrous stages could not be determined and are shown here as groups of 5. See the Introduction for an explanation of the influence of social context on serotonin levels. See sections 2.3-2.4 for explanations of drug and behavioral treatments.
| Fenfluramine | Saline 1 | pCPA | Saline2 | |
|---|---|---|---|---|
| Non-social | 6, high serotonin | 5, low serotonin | 6, low serotonin | 6, low serotonin |
| Partner | 6, high serotonin | 5, high serotonin | 6, low serotonin | 6, high serotonin |
| Playback | 6, high serotonin | 6, med. serotonin | 6, low serotonin | 6, med. serotonin |
2. Materials and Methods
2.1 Animal use
Female CBA/J mice (The Jackson Laboratory, Maine) of 7 weeks of age were the focus of our behavioral and immunohistochemical measurements. Male mice ranging from 8 to 11 weeks of age were used as stimuli. Each mouse was housed individually from the time of arrival. Males were given sexual experience prior to use in experiments. This consisted of several 5-minute interactions with individual females (not used in this experiment) once per day over 3-5 days and/or use in prior 1-hour experiments. Males were isolated for at least 1 week before an experiment. In our experience this protocol elicited the most reliable sexual behavior. Females were sexually naive. The estrous stage of each female was determined after characterizing cells collected by vaginal lavage. We combined females in proestrus or estrus into a single group called ‘pro/estrus’ because receptive sexual behavior is expressed during both of these stages (Hardy, 1972; Rodgers, 1970). Presence of only cornified epithelial cells or of both cornified and nucleated epithelial cells indicated ‘pro/estrus’ and presence of leukocytes indicated diestrus (Goldman, et al., 2007). The estrous states of two females were unclear.
2.2 Experimental design
Six females were used per treatment group. We used a factorial design with four pharmacological treatments and three social stimulation treatments, for a total of 12 groups and 72 females used (Table 1). Because females freely cycled through estrus, and estrous stages were determined at the end of the experiment, the estrous state groups were not part of this factorial design. Table 1 shows which groups were reduced in size for statistical tests by the two females excluded for inconclusive estrous stages.
2.3 Pharmacology
All injections were administered intraperitoneally. Fenfluramine, which acts as a serotonin releaser/reuptake inhibitor (Rothman & Baumann, 2002; Sigma-Aldrich) and which mimics the effects of serotonin in the IC (Hall & Hurley, 2007), was administered to release endogenous stores of serotonin in 18 females. Fenfluramine was administered in doses of 10 mg/kg (10 ml/kg) 30 minutes prior to the experimental treatment. A control group of 18 females were injected with an equivalent volume of 0.9% saline vehicle in place of fenfluramine.
Serotonin was depleted in 18 females with a repeated treatment of parachlorophenylalaninemethyl ester (pCPA), which depletes serotonin by suppressing the rate-limiting enzyme (tryptophan hydroxolase) for serotonin synthesis (Dailly, et al., 2006; Ruhé, et al., 2007; Sigma-Aldrich). Three doses of 200 mg/kg pCPA were administered at 10 mL/kg at 24 hour intervals. Higher doses were not considered because a previous study exploring specificity and efficacy of brain serotonin depletion using pCPA found that 300 mg/kg depleted dopamine significantly (Dailly, et al., 2006; Ruhé, et al., 2007; Sigma-Aldrich). On the 4th day females were used in experiments. Repeated injections of pCPA are known to alter appetite and weight (Blundell, 1984; Panksepp & Nance, 1974), so we monitored the weight of all animals receiving injections. Animals receiving pCPA on average lost 0.75% (+/− 0.587% SEM) of their pre-injection weight, whereas saline-treated animals gained 1.40% (+/− 0.809% SEM). The change in weight was not significantly different overall between the saline- and pCPA-treated animals (Mann-Whitney U, p = 0.091). A control set of 18 females was injected with an equivalent volume of 0.9% saline vehicle in place of pCPA over 3 days, at the same intervals as pCPA was administered. These females were also tested on the 4th day.
The saline control groups of the fenfluramine and pCPA experiments received different handling protocols and overall injection volumes that differed. We directly compared the average densities of c-fos immunoreactive IC cells between these two groups and found no significant difference: means of 142 and 170 cells/mm2 respectively (Mann-Whitney U, p = 0.088).
2.4 Social and playback stimuli
For all experiments the home cage of each animal was moved into a sound attenuated recording chamber at the time of the experiment and a CCD video camera (30 fps: Q-see 4 channel DVR PCI video capture card; SuperDVR software, Q-See, Digital Peripheral Solutions Inc., Anaheim, CA), a condenser microphone (CM16/CMPA, Avisoft Bioacoustics, Glienicke/Nordbahn, Germany) with a sound card (UltraSoundGate 116 Hb, Avisoft Bioacoustics; 250 kHz sample rate), and an ultrasonic speaker (ScanSpeak, Avisoft Bioacoustics, Glienicke/Nordbahn, Germany) were mounted on the cage. One group (24 females, 6 from each drug treatment group) received direct interaction with a male (‘partner’ treatment). An unfamiliar male was introduced to the home cage of the female during which physical contact, putative olfactory investigation of the anogenital and facial regions, and vocalization occurred. The interaction was monitored for 1 hour. At the end of the interaction the male was returned to his home cage. A second group (24 females, 6 from each drug group) received 1-hour playbacks of courtship interactions (‘playback’ treatment) recorded from an interaction between a male and a drug-matched female. Playbacks of these recordings were intended to isolate the effect of vocal cues alone. The remaining 24 females received no stimulus for an hour (‘non-social’ treatment). All animals were monitored by video camera and ultrasonic microphone during the hour-long experiment. Each female was terminated at the end of the hour by transcardial perfusion while deeply anesthetized.
2.5 Immunohistochemistry
All perfusions were performed following the induction of deep anesthesia with isoflurame fumes, by perfusing Krebs-Heinseleit buffer (pH: 7.2), and then 3.7% paraformaldehyde. The brains were harvested and stored in a solution of 15% sucrose and 3.7% paraformaldehyde in saline for 24 hours, and then moved to a solution of 30% sucrose in saline for 24 hours. The brains were sliced in 50 μm coronal sections through the midbrain area that contained the inferior colliculus. The brain slices of fenfluramine-treated animals and the respective saline-treated controls were processed for c-fos immunoreactivity in batches (usually 3) of brains from females who received the same behavioral and drug treatments. Within each treatment group, individual brains were always processed across a minimum of 2 separate groups. The brain slices of pCPA-treated animals and the respective controls were stored in cryoprotectant (30% sucrose, 1% polyvinylpyrrolidone, 30% ethylene glycol and phosphate buffered saline) at −80 degrees F for 1-2 weeks so that all slices could be processed for c-Fos immunohistochemistry simultaneously regardless of when behavioral trials were completed.
All slices were incubated in 10% goat serum for one hour, and then transferred to wells of 5% goat serum, 0.01% thimerosal (ICN Biomedical, Irvine, CA), and 1:1000 c-fos primary antibody (rabbit, sc-52; Santa Cruz, Dallas, TX) and chilled for 48 hours on an orbital shaker. Following rinses in PBS, the slices from the fenfluramine experiment were incubated in fluorescein secondary antibody (goat anti-rabbit; 10μl/ml; Vector Labs, Burlingame, CA) and 5% goat serum for an hour. The slices from the pCPA experiment were incubated in Alexa Fluor 488 secondary (goat, anti-rabbit; 10μl/ml; Life Technologies, Carlsbad, CA) and 5% goat serum for an hour. Then, all the slices were mounted on chrome-gelatin coated slides and cover-slipped using ProLong Gold antifade hardset mounting medium (Life Technologies, Carlsbad, CA). For the slices from fenfluramine-treated animals and respective saline controls, three consecutive slices from the same rostro-caudal region (ranging from −4.9 to −5.1mm re. Bregma; Franklin & Paxinos, 2007) of each brain were chosen for visualization. For slices from pCPA-treated animals and respective saline-treated controls, 3 alternate slices from the same rostro-caudal region (ranging from −4.9 to −5.2mm re. Bregma; Franklin & Paxinos, 2007) of each brain were chosen for visualization. The slices were visualized with a Nikon (NIE) microscope and images were generated using an Orca-Flash 2.8 sCMOS high resolution camera (Hamamatsu Corporation, Middlesex, NJ; Fig 1). A single researcher blind to treatment group counted the number of cells labeled with c-fos in each slice following previously employed methods (Goodson, Rinaldi, & Kelly, 2009; Ho, Murray, Demas, & Goodson, 2010). In Adobe Photoshop, a layer was generated to mark the location of each labeled cell with a dot. Each dot layer was imported into ImageJ (National Institutes of Health, Bethesda, MD) to obtain a dot count. The area of the IC on each slice was measured by marking the borders in Photoshop and then importing the layer into ImageJ. The density of c-fos labeled cells in each IC was calculated by dividing the number of cells counted in each slice by the IC area of the corresponding slice. Controls with no primary, no secondary, and peptide block were performed to verify labeling of c-fos immunoreactive cells (Fig. 1).
Figure 1.
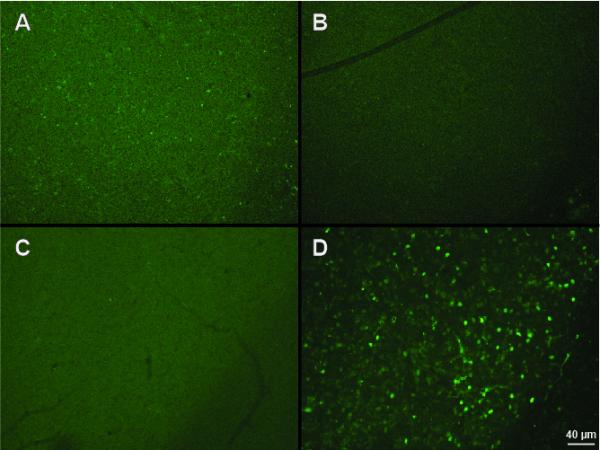
Control treatments prevent nuclear labeling by the c-fos antibody in the IC. A) No primary antibody control. B) No secondary control. C) 1:100 peptide block control. D) Regular c-fos labeling.
2.6 Behavioral quantification
Behaviors of subject females were analyzed in order to 1) ascertain whether drug treatment resulted in global behavioral dysfunction, and 2) compare c-fos immunolabel with behavior, both across groups and across individuals. Videos were analyzed using OD Log software (Macropod Software, www.macropodsoftware.com) to measure several behaviors. For the nonsocial, playback, and partner treatments, the duration of time females performed locomotion, rearing, and digging were measured (Table 2). The sum duration of time females spent performing these three behaviors was considered ‘nonsocial behavioral activity.’ For courting pairs, additional behaviors were characterized, including digging performed by males and social interactions between the pair (descriptions in Table 2). All videos were analyzed by a single researcher who was blind to the drug treatment and the estrous state of the subjects. The reliability of behavioral scoring was confirmed at 90%+ using segments of video that were not included in this manuscript. Vocalizations measured by microphone were analyzed by generating spectrographs using software from Avisoft Bioacoustics (SASLab Pro, Glienicke/Nordbahn, Germany). We quantified broadband vocalizations (BBVs) and ultrasonic vocalizations (USVs). BBVs are attributed to females during opposite sex interactions in mice, and occur in conjunction with male-directed kicking or lunging by females (Wang, et al., 2008b). BBVs covered a wide range of frequencies, including frequencies below 20 kHz, and were distinct from ultrasonic vocalizations (USVs), which only contained one or two frequency bands and were entirely above 20 kHz. All vocalizations were counted by a single researcher blind to the drug treatment and estrous state of the subjects. Behaviors scored using videos and spectrographs were analyzed across three 5-minute segments per interaction. The times scored were t= 0-5 minutes, t= 25-30 minutes, and t= 50-55 minutes within each hour-long experiment except in one case in the pCPA experiment where the female was not visible for the 25-30 minute window and the 15-20 minute segment was analyzed for behavior instead.
Table 2.
Ethogram of the behaviors scored during an hour of experimental observation. A few non-social behaviors were scored for females in all stimulus groups, whereas social and male behaviors were scored only for pairs participating in courtship.
| Behavior name | Description of behavior | Context analyzed |
|---|---|---|
| Female locomotion | Lateral displacement of female high quarters | all |
| Female rearing | Female forepaws elevated from the cage floor, either in the air or on the side of the cage, head pointed up, and hind legs extended | all |
| Female digging | Visible movement of any of the limbs of the female that results in scattering of the bedding on the cage floor | all |
| Female anogenital investigation | Female nose makes contact with posterior (roughly half the body mass) of the male | partner |
| Female kicking | Rapid extension of any limb directed towards the male | partner |
| Male anogenital investigation | Male nose makes contact with the posterior (roughly half the body mass) of the female | partner |
| Mounting | Forepaws of the male placed on the back of the female with pelvic orientation over the rear of the female | partner |
| Male digging | Visible movement of any of the limbs of the male that results in scattering of the bedding on the cage floor | partner |
2.7 Statistical Analysis
All statistical tests were performed in SPSS (IBM Corp., Armonk, NY) and Statistica (StatSoft, Inc., Tulsa, OK). Differences in c-fos label among treatment groups were assessed using general linear models with the density of c-fos-positive neurons as the dependent variable and drug treatment, social treatment, and estrous state as fixed factors. Main effects of each fixed factor as well as interactions of drug treatment X social treatment and drug treatment X estrous state were specified in the model. Because fenfluramine and pCPA had different schedules of administration, we considered the effects of each drug, in comparison to saline controls matched to the same schedule of administration, in separate statistical models. For the fenfluramine and respective saline control data, the densities of cells immunoreactive for c-fos were normalized with a log transform. Post-hoc comparisons were made with Fisher's least significant difference (LSD) tests. Kruskal-Wallis tests were used to make comparisons among behaviors, which were not normally distributed, across social treatment groups. Spearman correlations were run to investigate relationships between cell counts and behavioral data and a false discovery rate (FDR) correction for multiple comparisons was used to adjust the significance threshold for these tests (Benjamin & Hochberg, 1995).
3. Results
In order to investigate the effects of serotonin on immediate early gene responses in the IC, we pharmacologically manipulated serotonin while placing females in one of 3 treatments: 1) a treatment in which no male partner was presented, the ‘nonsocial’ treatment, 2) a treatment in which a male partner was presented, the ‘partner’ treatment, and 3) a ‘playback’ treatment in which females were played an audio recording from one of the interactions in the partner treatment. The naturally fluctuating estrous states of females in all treatments were measured after each experiment. Serotonin levels were pharmacologically manipulated across social treatments with fenfluramine, a serotonin releaser and reuptake inhibitor, and pCPA, a drug that depletes serotonin by reducing the activity of an enzyme required for synthesis of serotonin (Dailly et al., 2006; Rothman & Baumann, 2002; Ruhé et al., 2007). As detailed below, we found effects of drug treatment that depended on social treatment and estrous state.
3.1 Pharmacological manipulations influenced c-fos immunoreactivity
We assessed the effects of drug treatment in conjunction with the factors of social treatment and estrous state with univariate general linear models (GLMs), using separate models for each drug and its paired saline control. Each of these models identified significant effects on the densities of neurons immunopositive for c-fos in the IC (Table 3). Contrary to our first prediction, there were no main effects of either fenfluramine or pCPA across social treatments (univariate GLM: results of statistical model in Table 3; immunopositive cell density averages and SEMs given in Table 4). There were also no main effects of social treatment groups on the densities of c-fos immunoreactive cells (Tables 3 and 4).
Table 3.
Results of two univariate general linear models of the densities of IC cells immunopositive for c-fos.
| dataset | term | df | F | p | Partial eta squared |
|---|---|---|---|---|---|
| Fenfluramine/saline | model | 7 | 3.693 | 0.007 | 0.499 |
| intercept | 1 | 989.230 | 0.000 | 0.974 | |
| drug treatment | 1 | 0.168 | 0.685 | 0.006 | |
| social treatment | 2 | 1.535 | 0.234 | 0.106 | |
| estrous state | 1 | 1.345 | 0.257 | 0.049 | |
| drug X social treatment | 2 | 5.663 | 0.009 | 0.303 | |
| drug treatment X estrous state | 1 | 9.316 | 0.005 | 0.264 | |
| pCPA/saline | model | 7 | 2.938 | 0.019 | 0.423 |
| intercept | 1 | 244.068 | 0.000 | 0.897 | |
| drug treatment | 1 | 0.803 | 0.378 | 0.028 | |
| social treatment | 2 | 1.88 | 0.171 | 0.118 | |
| estrous state | 1 | 8.564 | 0.007 | 0.234 | |
| drug X social treatment | 2 | 0.482 | 0.622 | 0.033 | |
| drug treatment X estrous state | 1 | 4.271 | 0.048 | 0.132 |
Table 4.
The average cell densities in cells/mm2 for various groups
| fenfluramine | 141 (+/− 37 SEM) |
| saline (fenfluramine) | 142 (+/− 29 SEM) |
| pCPA | 139 (+/− 15 SEM) |
| saline (pCPA) | 170 (+/− 16 SEM) |
| nonsocial | 134 (+/−13 SEM) |
| playback | 154 (+/−17 SEM) |
| partner | 176 (+/− 15 SEM) |
3.1.1 Social treatment influences effects of fenfluramine
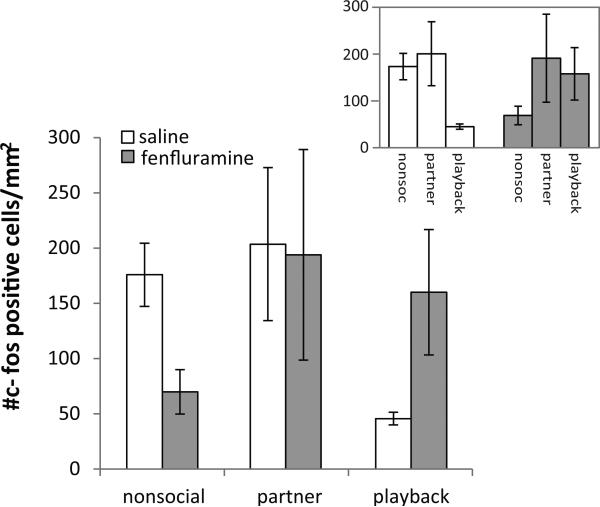
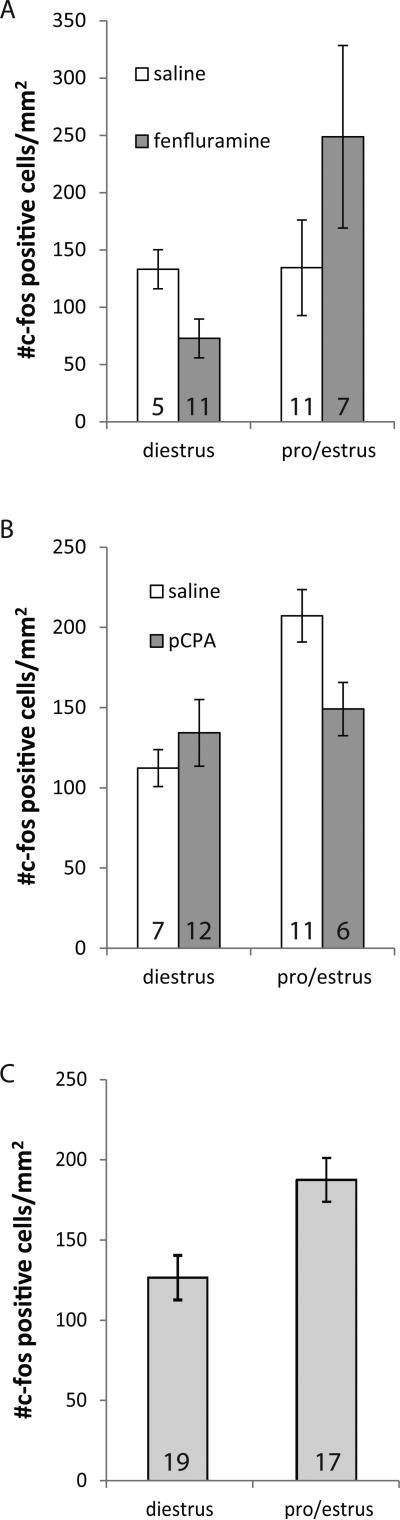
In contrast to the lack of main effect of fenfluramine or social treatment group, these factors interacted to influence c-fos immunoreactivity (univariate GLM: F = 5.663, p = 0.009; Table 3; Fig. 2). Although females in the ‘partner’ and ‘playback’ treatment groups showed no significant difference between the saline and fenfluramine treatments, among females in the nonsocial groups there was reduced c-fos immunoreactivity with fenfluramine treatment (Fisher's LSD between saline and fenfluramine treatments: nonsocial p = 0.014; partner p = 0.98, playback p = 0.077). These effects resulted in patterns of c-fos immunoreactivity that were different across social treatment groups depending on the drug treatment. For the saline groups, the playback treatment was the only group showing low levels of c-fos immunoreactivity (Fig. 2, inset; Fisher's LSD: nonsocial vs. playback p = 0.012, partner vs. playback p = 0.039). In contrast, for the fenfluramine groups, the nonsocial treatment showed the lowest c-fos density and was significantly lower than the partner group (Fig. 2, inset; Fisher's LSD: p = 0.039).
Figure 2.
The average density of c-fos positive cells in females treated with either fenfluramine or saline (control). There was a significant interaction between drug and social treatment (univariate GLM: F = 5.663, p = 0.009). Inset shows the same data re-arranged to highlight differences among social groups. Bars represent standard error of the mean.
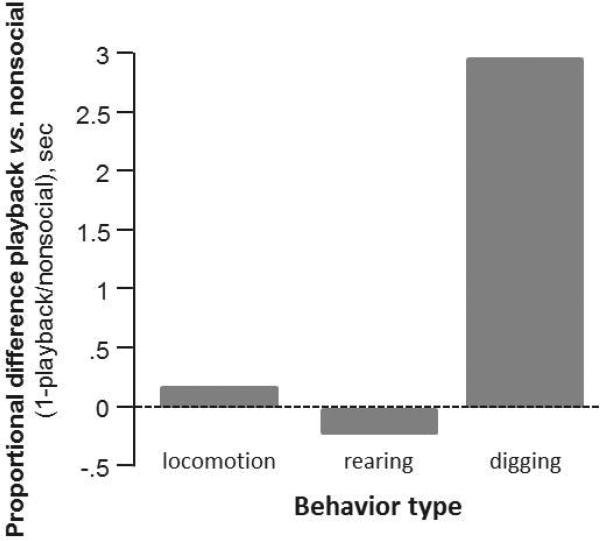
Because the differences in drug effects that we observed among social treatment groups was partly due to the low density of c-fos immunoreactive neurons in the ‘playback’ group relative to the ‘social’ group following saline treatment, we assessed whether these differences among social groups following saline treatment were paralleled by behavioral differences. Since the actual presence of a male partner would introduce a separate class of social behaviors unique to the ‘partner’ group, we compared the ‘nonsocial’ versus ‘playback’ treatments in three nonsocial female behaviors: rearing, digging, and locomotion. Of these behaviors, females showed a three-fold increase in digging behavior in the playback relative to the nonsocial group, but relatively little difference in rearing or locomotion (Fig. 3).
Figure 3.
The proportional difference in the time spent performing locomotion, rearing, and digging in females in the playback vs. nonsocial groups. Females in the playback group displayed a large (3-fold) increase in digging behavior relative to the nonsocial group.
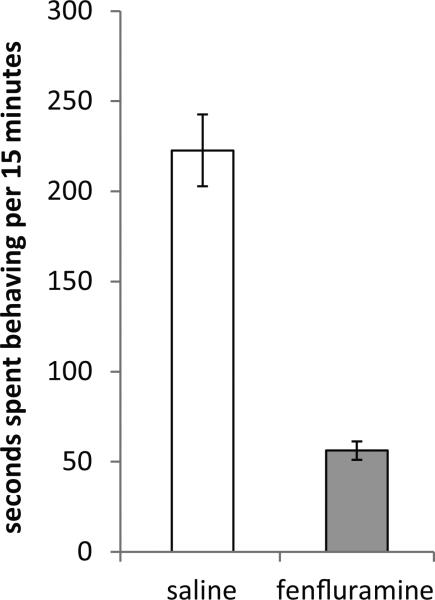
Fenfluramine altered non-social female behavior relative to saline controls, such that animals receiving the drug were less active (assessed by the sum duration of time spent performing locomotion, digging, and rearing; Fig. 4; Mann-Whitney U, p < 0.001). Despite this effect on general behavioral activity, fenfluramine did not affect either male or female vocal or other social behaviors in the partner group (Table 2; Mann-Whitney U, p > 0.05 for all tests). Thus, females received similar courtship signals regardless of their pharmacological state. There were no significant correlations across individuals between the density of c-fos positive cells and duration of any non-vocal behaviors displayed (social or nonsocial) or vocalizations produced (Spearman's ρ range: −0.371 - 0.037, p > 0.05 with FDR correction).
Figure 4.
The average of the total duration of time females given either fenfluramine or a corresponding saline treatment spent performing locomotion, digging, and rearing. Fenfluramine significantly reduced activity (Mann-Whitney U, p < 0.001). Bars represent standard error of the mean.
3.1.2 pCPA treatment
Repeated injections of pCPA were used to deplete serotonin, in order to test whether preventing the normal release that occurs during courtship influences c-fos immunoreactivity in the IC. Although the overall model was significant, there were no main effects of drug treatment or social group, similar to fenfluramine (Table 3). In contrast to fenfluramine, pCPA did not interact with social treatment to influence the density of c-fos positive IC cells.
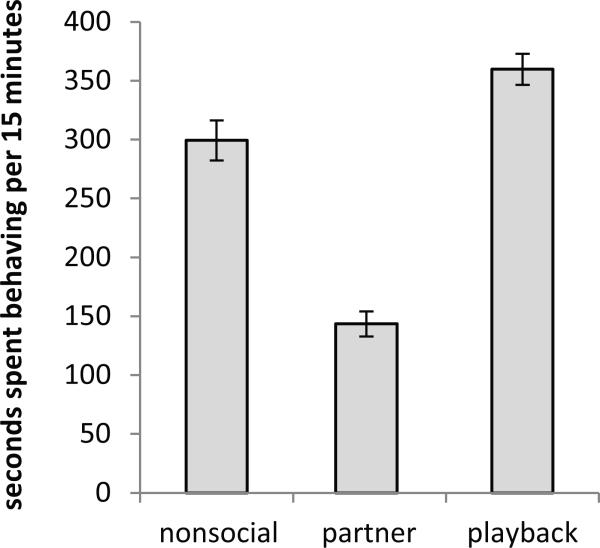
Administration of pCPA had no overall influence on any behaviors measured (Mann-Whitney U, nonsocial behavioral activity p = 0.628, female anogenital investigation p = 0.522, female kicking p = 0.749, male anogenital investigation p = 0.631, mounting p = 0.631, BBVs p = 0.522, USVs p = 0.749). The behavior of pCPA-treated females did vary by stimulus treatment group. As expected, partnered females displayed less nonsocial behavior (Fig. 5; Kruskal-Wallis p < 0.001), likely because social behaviors consumed time that would have otherwise have been spent performing nonsocial behaviors.
Figure 5.
The average of the total duration of time females given pCPA or a corresponding saline treatment spent performing locomotion, digging, and rearing. Females in the partner treatment group displayed lower levels of these behaviors (Kruskal-Wallis, p < 0.001). Bars represent standard error of the mean.
Within the partner group, USVs were observed in every partner interaction and BBVs were observed during 11/12 interactions. The densities of c-fos- positive neurons and the production of BBVs by females were negatively correlated (Spearman's ρ = −0.779, p = 0.002 [a p-vale of < 0.007 was required for significance at the 0.05 level after FDR correction]; Fig. 6A). In contrast, there was no correlation between c-fos densities and the number of BBVs females were exposed to in the playback groups (Spearman's ρ = −0.007, p = 0.983; Fig. 6B).
Figure 6.
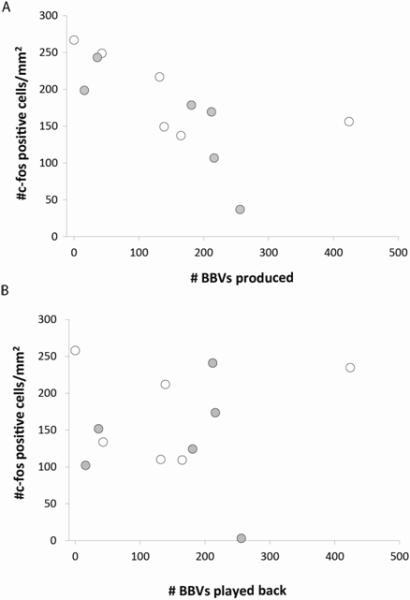
Relationship between the number of broadband vocalizations (BBVs) per interaction and c-fos immunoreactivity in the IC. A) The production of BBVs by females was negatively correlated with the density of c-fos positive cells in the IC cells (Spearman's ρ = −0.779, p = 0.002). B) Females listening to playbacks did not exhibit a correlation between BBVs and c-fos density (Spearman's ρ = −0.007, p = 0.983). Open circles represent saline treatment and closed circles represent pCPA treatment.
3.2 Estrous state influences effects of drugs
For both fenfluramine and pCPA, drug treatment interacted significantly with estrous state (Table 3). Fenfluramine-treated females in pro/estrus had qualitatively elevated c-fos immunoreactivity, while fenfluramine-treated females in diestrus had decreased c-fos immunoreactivity relative to saline controls, although posthoc tests for each pairwise comparison were not individually significant (Fig. 7A). For pCPA-treated females, the interaction between drug treatment and estrous state was in the opposite direction to the fenfluramine experiment. Females treated with pCPA that were in pro/estrus had lower densities of c-fos immunoreactive neurons than saline controls (Fig. 7B; Fisher's LSD: pro/estrus p = 0.048). In addition to interacting with pCPA treatment, estrous state had a main effect in the pCPA model (Table 3), such that females in estrus or proestrus had higher densities of c-fos-positive neurons than did females in diestrus (Fig. 7C).
Figure 7.
The average density of c-fos immunoreactive neurons in females either in diestrus or pro/estrus. A) Fenfluramine decreased c-fos immunoreactivity in diestrous females and increased c-fos immunoreactivity in estrous females (univariate GLM: Table 3). B) The treatment of pCPA increased c-fos immunoreactivity in diestrous females and decreased c-fos immunoreactivity in estrous females (univariate GLM: Table 3) Numbers of females are listed at the bottom of each bar. C) Among females represented in Fig. 7b there was a main effect of estrous state (univariate GLM: Table 3). Bars represent standard error of the mean.
4. Discussion
A proposed function of neuromodulation within sensory systems is to emphasize the representation of behaviorally salient stimuli (Castelino & Schmidt, 2010; Devilbiss, et al., 2006; Hurley et al., 2004). At the cellular level, neuromodulators like norepinephrine may improve the signal-to-noise ratio or increase selectivity for sensory signals (Devilbiss et al., 2006; Remage-Healey, et al., 2008). At the systems level, they may emphasize neural responses to socially salient stimuli over controls (Cardin & Schmidt, 2004; Lynch & Ball, 2008; Velho et al., 2012). In the current study, we assessed the influence of serotonin on responses to social and nonsocial contexts in the inferior colliculus, an auditory midbrain nucleus in which high levels of selectivity for species-specific vocalizations have been reported (Klug et al., 2002). Neural responses were measured as the number of neurons immunoreactive for c-fos, an immediate early gene product that is both activity-dependent, and regulates experience-dependent plasticity (Lyons & West, 2011). We found that the effects of pharmacologically manipulating serotonin depended upon two components of context—on the social environment, and on the estrous states of female subjects.
4.1 Social context and its interaction with the serotonergic system
To test whether the effects of serotonin were dependent on the social environment, we placed female mice in one of three different treatments, while comparing saline injections with injections of fenfluramine or pCPA. The treatments included 1) females with no social stimulus (the ‘nonsocial’ group), and 2) females who were given a male partner (the ‘partner’ group). In all partner pairs, males and females interacted through physical contact, putative olfactory investigation of the anogenital and facial regions, and vocalization. An additional treatment was created by 3) playback of soundtracks recorded during social interactions from the partner group (1:1) to otherwise lone females (the ‘playback’ group). This group was intended to isolate the effects of acoustic cues alone, given that we measured c-fos immunoreactivity in the auditory midbrain.
In the saline controls, there were differences in the densities of c-fos-positive neurons among social treatment groups, but these differences did not occur in a systematic fashion. In comparison to the ‘nonsocial’ and ‘partner’ groups, the females that received playback alone in the fenfluramine control group showed reduced c-fos immunoreactivity. The cause of this difference is unclear. Although playbacks of vocalizations are used as stimuli for testing the social responsiveness of many species (e.g., Christie, et al., 2010; Gess, et al., 2011; Hernandez, et al., 2008; Musolf et al. 2015; Wilczynski & Lynch et al. 2011; Brenowitz & Remage-Healey, 2016), mice also rely heavily on other types of sensory cues, notably olfactory cues, to elicit or regulate social behaviors (Grimsley, et al., 2011; Holy & Guo, 2005; Nyby et al., 1977). Thus, playbacks in the absence of other cues from a partner could create a very different behavioral context than actual social interaction. An increase in digging, sometimes interpreted as indicating anxious or repetitive behavior in mice (e.g., Deacon, 2006; McPhie-Lalmansingh, et al., 2008; Thomas et al., 2009), in the playback group relative to the nonsocial group is consistent with this hypothesis. Some of our other results also supported a link between low c-fos immunoreactivity and negative valence for females. In social treatments in the pCPA experiment, c-fos densities were negatively correlated with the production of BBVs, which are part of a suite of ‘rejection’ behaviors towards males (Lupanova & Egorova, 2015; Wang, et al., 2008b). Simply hearing BBVs in the playback groups did not create a similar relationship. This finding makes an interesting comparison to a study showing a positive rather than a negative correlation between immediate early gene immunoreactivity in one auditory forebrain region and pro-receptive calls made by female white-crowned sparrows in response to male song playback (Maney et al. 2003). The authors of this study speculated that c-fos immunoreactivity corresponded to self-stimulation by female-produced calls consistent with the playback versus partner groups in our study.
Comparing the less ambiguous nonsocial and partner groups, fenfluramine decreased the density of c-fos-positive neurons in the nonsocial treatment, but made no change in the partner treatment. Mechanistically, this pattern is consistent with direct measurements of serotonergic dynamics within the IC in these two conditions. Serotonergic activity measured directly in the IC with carbon fiber voltammetry increases in females placed with males, but does not increase for lone females (Hanson & Hurley, 2014). The ‘partnered’ females in the current study would therefore be expected to have relatively high endogenous levels of serotonin, consistent with this group showing few added effects of fenfluramine, whereas ‘nonsocial’ females would experience an increase in serotonin after fenfluramine injection relative to saline. Direct application of serotonin has varied effects on the responses of IC neurons but most often decreases spike rates, such that the responses of IC neurons for tonal stimuli and vocalizations are more selective (Hurley & Pollak, 1999; 2001; 2005). The decreased c-fos immunoreactivity in the groups of females for which serotonin was experimentally elevated (e.g., fenfluramine) is consistent with suppressive effects of serotonin in relation to injections of saline. However, because fenfluramine was injected intraperitoneally in the current study rather than being locally applied as in past studies, we cannot assert that the effects of fenfluramine were direct. The effects we observed could have been due to fenfluramine acting in regions synaptically upstream of the IC.
Several methodological issues could have influenced these outcomes. One of these is that the sample sizes within each treatment group were relatively low, reducing our power to detect differences among treatments. It seems unlikely that main effects of drug treatments would have emerged with moderately increased sample sizes, however. Because the effects of fenfluramine were the opposite in different social treatment groups, and the effects of pCPA were minimal across treatment groups, these differences could potentially require very large sample sizes to detect. It is possible that sample size precluded us from observing main effects of our social treatment groups in statistical models for both drugs, or of main effects of estrous state in the fenfluramine model. Our finding that there were effects of interactions between factors but no main effects must therefore be interpreted as a conservative one. A second issue is that although auditory stimuli in the ‘playback’ group were recorded from the ‘social’ group and so were matched between these treatments, there was still substantial variation in the different interactions among the ‘social’ pairs. Although particular features of male calls induce preferential behavioral or neural responses in female birds (e.g. Gentner et al., 2001; Leitner et al., 2005; Dunning et al., 2014), this type of information is not as well-understood for the vocalizations of male mice. Female mice show attraction to male calls in general (Hammerschmidt et al., 2009), and may even prefer the calls of their own subspecies over those of a different subspecies (Musolf et al., 2015), but the acoustic parameters that gate these responses are unclear. Therefore, despite similarity in the number of vocalizations produced across social interactions, the social and playback groups could have incorporated auditory stimuli of widely varying salience to females.
4.2 Does serotonin emphasize socially salient stimuli?
Our data suggest that serotonin creates different levels of c-fos activation in socially salient versus non-salient contexts. This can be illustrated by comparing nonsocial versus partner contexts under conditions of low serotonin and high serotonin (Fig. 2 inset). For saline treatments, there were no differences in the densities of IC neurons showing c-fos immunoreactivity following a nonsocial treatment versus following interaction with a social partner. In contrast, for female mice with high serotonin levels following fenfluramine treatment, a distinction emerged, such that more neurons were c-fos-positive in the partner group than in the nonsocial group.
These results are compatible with models describing the effects of another neuromodulator, norepinephrine, in emphasizing responses to salient vocal signals in songbird auditory forebrain. Blocking noradrenergic receptors in female zebra finches greatly reduces immediate early gene responses to song playback in auditory forebrain areas but has little effect on the relatively low level of activity that occurs with no auditory stimulus (Velho et al., 2012). Likewise, chemical lesion of the noradrenergic system reduces selectivity of immediate early gene responses for conspecific over heterospecific song in the auditory forebrain of female canaries (Lynch & Ball, 2008). In both studies, a decrease in noradrenergic activation was associated with reduced immediate early gene response overall. Thus, both norepinephrine and serotonin may create immediate early gene responses that are more pronounced for salient stimuli, but potentially by different mechanisms. Whereas norepinephrine increases responses for socially salient stimuli, our results are consistent with serotonin decreasing immediate early gene responses in contexts that are not socially salient.
There are several inconsistencies in our data with this interpretation. One of these is that there was no difference in c-fos activation between the ‘nonsocial’ and ‘partner’ groups in the saline treatments. This finding is unexpected, since c-fos activation in the IC is influenced by auditory stimulation (D'Alessandro & Harrison, 2014; Saint Marie, et al., 1999). A second inconsistency is that pCPA did not increase the density of c-fos-positive neurons relative to saline in the ‘partner’ treatment. We expected to see this increase based on differences in serotonin levels resulting from both drug and social treatments (see Table 1). Since pCPA decreases serotonin levels, serotonin is predicted to be at low levels in the ‘partner’ social group after pCPA treatment, as indicated in Table 1. In contrast, endogenous levels of serotonin should be relatively high in the saline-treated mice placed with a partner, since social interaction triggers an increase in serotonin (Hall et al. 2011; Hanson & Hurley, 2014). Because serotonin often decreases the firing rates of IC neurons (Hurley & Pollak 1999), we would therefore predict that the count of c-fos-positive neurons should be higher in the ‘partner’ social treatment for pCPA-treated mice (which should have lower serotonin) than for saline-treated mice (which should have higher serotonin). We observed no such difference. Several considerations relevant to both c-fos activity and to neural responses in the IC could have contributed to these unexpected patterns. The first of these is that the relationship between neural activity and c-fos expression is nonlinear; c-fos immunoreactivity may be a marker for neurons in which stimulus-evoked activity has triggered synaptic plasticity (Knapska & Kaczmarek, 2004; Mello et al., 2004; Lyons & West, 2011). In the auditory midbrain itself, different types of immediate early genes show different patterns of expression following auditory playback, demonstrating that immediate early gene activation does not mirror neurophysiological activity (Burmeister et al., 2008). Neuromodulators are also capable of altering immediate early gene activation without influencing auditory-evoked spiking activity, potentially by intracellular pathways in parallel to spiking activity (Velho et al., 2012). As noted above, the effects of our systemically injected pharmacological agents could be occurring at neural loci upstream of the IC, and so reflect processes that we did not measure. Finally, even assuming that our pharmacological agents directly influenced the activity of IC neurons, auditory stimuli trigger massive inhibitory as well as excitatory input to IC neurons (Pollak, 2013; Pollak, et al., 2011). The relative pattern of c-fos label across the IC following tonal stimulation is thought to represent excitatory as well as inhibitory response areas, with a higher density of c-fos immunoreactive neurons in frequency laminae representing the tone, and a reduced density of c-fos immunoreactive neurons relative to background in adjacent ‘sidebands’ corresponding to inhibited regions (D'Alessandro & Harrison, 2014). Each of these factors could have caused our results to fail to conform to predictions based solely on the vocalizations present in a given social context in combination with the predicted levels of serotonin.
4.3 Modulation across reproductive states
One of our statistical models (pCPA) showed a main effect of estrous state on the densities of c-fos immunoreactive neurons in the IC, such that females in pro/estrus had higher densities of cells immunopositive for c-fos. Our results fit with other research showing that auditory activity in general is influenced by reproductive state in females across a range of vertebrate species. The auditory systems of female midshipman fish are most sensitive to the frequency range of male calls while they are gravid, but treatment with estrogens can induce gravid-like responses in the auditory system of non-gravid females, an effect which is likely mediated by estrogen receptors (Forlano, et al., 2005; Sisneros & Bass, 2003; Sisneros, et al., 2004). In frogs, activity in the auditory midbrain (torus semicircularis) varies across female reproductive and hormonal state in a way that closely matches phonotaxis in response to male calls (Chakraborty & Burmeister, 2009; Lynch & Wilczynski, 2008; Miranda & Wilczynski, 2009b). Because of this correlation between behavior and neurophysiology combined with the presence of estrogen and progesterone receptors in the torus semicircularis, the auditory midbrain is likely an important site for modulation of behavioral responses across reproductive states in frogs (Hoke & Pitts, 2012; Lynch & Wilczynski, 2008). A variety of songbirds show seasonal changes in auditory brainstem responses, and estrogens modulate both the behavioral preferences for, and the auditory processing of male courtship vocalizations (reviewed in Maney & Pinaud, 2011). The mammalian auditory system is known to be sensitive to reproductive hormones as well. In the rat ovariectomy induces increases in auditory brainstem responses (ABRs) latencies, which are reversed by estrogen treatment (Coleman, et al., 1994).
4.4 Reproductive state and serotonergic effects
The effects of pharmacological manipulation of serotonin levels also depended on estrous state. We chose drugs with opposing effects on serotonin levels and we found, as predicted, that the interaction between estrous state and drug manipulation was opposite for the two drugs. Fenfluramine decreased c-fos immunoreactivity in diestrous females and increased it in pro/estrous females, while pCPA increased c-fos immunoreactivity during diestrus, and decreased it during pro/estrus (Fig. 7). Alteration of neuromodulatory pathways may be a relatively common route for reproductive hormones to affect auditory processing. In female white throated sparrows, estrogen treatment increases the densities of catecholaminergic and serotonergic fibers in auditory midbrain or forebrain, and correspondingly can increase measures of neuromodulatory activity, such as the levels of neuromodulators or their metabolites (Matragrano, et al., 2012b; Matragrano, et al., 2011) Increased neuromodulatory activity following estrogen priming is seen even after a brief exposure to social cues like the playback of song, indicating that reproductive hormones can alter the short-term dynamics of neuromodulatory activity within the auditory system (Matragrano, et al. 2012a).
In past studies of female mice presented with males, there was no significant difference in IC serotonin release between females in estrus or proestrus versus diestrus (Hanson & Hurley, 2014). This finding is consistent with the effects of estrus in the current study occurring downstream of serotonin release, potentially at the level of serotonin receptor expression. Although the effect of serotonin measured at the level of single IC neurons is generally suppressive, different receptor types have opposite effects on the firing rates of IC neurons (Hurley, 2006). If the balance of receptor types changed in accordance with the estrous cycle, a phenomenon observed in other brain regions (Biegon, Bercovitz, & Samuel, 1980), this could account for the effects of fenfluramine and pCPA on c-fos activity varying from inhibitory to excitatory at different estrous phases.
4.5 Summary
Our results overall suggest that the influence of serotonin on immediate early gene activity in the auditory midbrain of female mice depends on both social context and estrous state. Increased serotonin levels decreased c-fos labeling more in a non-socially relevant context than during interaction with a male. Increased serotonin levels led to higher c-fos immunoreactivity during proestrus and estrus, but lower levels of labeling during diestrus. These findings suggest that serotonergic filters on the activity of auditory neurons are matched to both external and internal features of social context. In the birdsong model of noradrenergic modulation disrupting neuromodulatory activity influences behavioral preferences for socially appropriate song (Appeltants, et al., 2002; Pawlisch, et al., 2011). Whether the changes in immediate early gene activity that we observed correspond to the sexual behaviors or preferences of female mice is unknown. However, our findings provide a framework for addressing additional functional and mechanistic questions regarding a major neuromodulatory pathway in an increasingly important mouse model of signal reception.
Acknowledgements
We would like to thank Zita Erbowor-Becksen for the analysis of c-fos labeling and vocalizations, Nicole Jansing for the analysis of behavior recorded in videos, and members of the Hurley lab, especially Sarah Keesom and Chris Peterson for valuable input in the assembly of this manuscript. We would also like to thank Jim Goodson, Marcy Kingsbury, Aubrey Kelly, and Sara Schrock for training in imunohistochemical techniques and sharing reagents. This work was supported in part by the National Science Foundation through a Graduate Research Fellowship to JLH. Support was also provided by the Center for the Integrative Study of Animal Behavior at Indiana University and NIDCD grant DC-008963. We would also like to thank the two anonymous reviewers for their useful input on the manuscript.
References
- Arch VS, Narins PM. Sexual hearing: The influence of sex hormones on acoustic communication in frogs. Hearing research. 2009;252(1):15–20. doi: 10.1016/j.heares.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Del Negro C, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behavioural Brain Research. 2002;133(2):221–235. doi: 10.1016/s0166-4328(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Barthelemy M, Gourbal BEF, Gabrion C, Petit G. Influence of the female sexual cycle on BALB/c mouse calling behaviour during mating. Naturwissenschaften. 2004;91(3):135–138. doi: 10.1007/s00114-004-0501-4. doi: 10.1007/s00114-004-0501-4. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Biegon A, Bercovitz H, Samuel D. Serotonin receptor concentration during the estrous-cycle of the rat. Brain Research. 1980;187(1):221–225. doi: 10.1016/0006-8993(80)90509-0. [DOI] [PubMed] [Google Scholar]
- Blundell JE. Serotonin and appetite. Neuropharmacology. 1984;23(12, Part 2):1537–1551. doi: 10.1016/0028-3908(84)90098-4. doi: http://dx.doi.org/10.1016/0028-3908(84)90098-4. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. Journal of neurobiology. 1997;33(5):602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Remage-Healey L. It takes a seasoned bird to be a good listener: communication between the sexes. Current opinion in neurobiology. 2016;38:12–17. doi: 10.1016/j.conb.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Mangiamele LA, Lebonville CL. Acoustic modulation of immediate early gene expression in the auditory midbrain of female tungara frogs. Brain Research. 2008;1190:105–114. doi: 10.1016/j.brainres.2007.11.008. doi: 10.1016/j.brainres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Caras ML, Sen K, Rubel EW, Brenowitz EA. Seasonal Plasticity of Precise Spike Timing in the Avian Auditory System. The Journal of Neuroscience. 2015;35(8):3431–3445. doi: 10.1523/JNEUROSCI.3407-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. Journal of Neurophysiology. 2004;91(5):2148–2163. doi: 10.1152/jn.00918.2003. doi: 10.1152/jn.00918.2003. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Schmidt MF. What birdsong can teach us about the central noradrenergic system. Journal of chemical neuroanatomy. 2010;39(2):96–111. doi: 10.1016/j.jchemneu.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan Baldan Ramsey L, Sinha SR, Hurley LM. 5-HT1A and 5-HT1B receptors differentially modulation rate and timing of auditory responses in the mouse inferior colliculus. European Journal of Neuroscience. 2010;32(3):368–379. doi: 10.1111/j.1460-9568.2010.07299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, Burmeister SS. Estradiol induces sexual behavior in female tungara frogs. Hormones and Behavior. 2009;55(1):106–112. doi: 10.1016/j.yhbeh.2008.09.001. doi: 10.1016/j.yhbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Chakraborty M, Burmeister SS. Effects of estradiol on neural responses to social signals in female túngara frogs. Journal of Experimental Biology. 2015;218(22):3671–3677. doi: 10.1242/jeb.127738. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Canlon B. Estradiol treatment and hormonal fluctuations during the estrous cycle modulate the expression of estrogen receptors in the auditory system and the prepulse inhibition of acoustic startle response. Endocrinology. 2012;153(9):4412–4421. doi: 10.1210/en.2012-1416. [DOI] [PubMed] [Google Scholar]
- Christie K, Schul J, Feng AS. Phonotaxis to male's calls embedded within a chorus by female gray treefrogs, Hyla versicolor. Journal of Comparative Physiology A. 2010;196(8):569–579. doi: 10.1007/s00359-010-0544-2. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hearing Research. 1994;80(2):209–215. doi: 10.1016/0378-5955(94)90112-0. doi: http://dx.doi.org/10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- D'Alessandro LM, Harrison RV. Excitatory and inhibitory tonotopic bands in chinchilla inferior colliculus revealed by c-fos immuno-labeling. Hearing Research. 2014;316:122–128. doi: 10.1016/j.heares.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Dailly E, Chenu F, Petit-Demoulière B, Bourin M. Specificity and efficacy of noradrenaline, serotonin depletion in discrete brain areas of Swiss mice by neurotoxins. Journal of Neuroscience Methods. 2006;150(1):111–115. doi: 10.1016/j.jneumeth.2005.06.008. doi: http://dx.doi.org/10.1016/j.jneumeth.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Davis AG, Leary CJ. Elevated stress hormone diminishes the strength of female preferences for acoustic signals in the green treefrog. Hormones and Behavior. 2015;69:119–122. doi: 10.1016/j.yhbeh.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Deacon R. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nature protocols-electronic edition. 2006;1(1):122. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Page ME, Waterhouse BD. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. The Journal of Neuroscience. 2006;26(39):9860–9872. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning JL, Pant S, Bass A, Coburn Z, Prather JF. Mate choice in adult female Bengalese finches: females express consistent preferences for individual males and prefer female-directed song performances. PloS One. 2014;9(2):e89438. doi: 10.1371/journal.pone.0089438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Fischer R. Neuronal activity and tonotopy in the auditory system visualized by c-fos gene expression. Brain Research. 1991;567(2):350–354. doi: 10.1016/0006-8993(91)90819-h. doi: http://dx.doi.org/10.1016/0006-8993(91)90819-H. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. Journal of Comparative Neurology. 2005;483(1):91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. Third Edition Elsevier Inc.; 2007. [Google Scholar]
- Gale SD, Perkel DJ. A basal ganglia pathway drives selective auditory responses in songbird dopaminergic neurons via disinhibition. The Journal of Neuroscience. 2010;30(3):1027–1037. doi: 10.1523/JNEUROSCI.3585-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall MD. Effects of species, sex and season on auditory processing in songbirds. 2012 [Google Scholar]
- Gess A, Schneider DM, Vyas A, Woolley SM. Automated auditory recognition training and testing. Animal Behaviour. 2011;82(2):285–293. doi: 10.1016/j.anbehav.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giret N, Menardy F, Del Negro C. Sex differences in the representation of call stimuli in a songbird secondary auditory area. Frontiers in Behavioral Neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Hormones and Behavior. 2009;55(1):197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley JMS, Monaghan JJM, Wenstrup JJ. Development of Social Vocalizations in Mice. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0017460. doi: 10.1371/journal.pone.0017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Hurley LM. The serotonin releaser fenfluramine alters the auditory responses of inferior colliculus neurons. Hearing research. 2007;228(1):82–94. doi: 10.1016/j.heares.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Sell GL, Hurley LM. Social regulation of serotonin in the auditory midbrain. Behavioral Neuroscience. 2011;125(4):501–511. doi: 10.1037/a0024426. doi: 10.1037/a0024426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biology letters. 2009:rsbl20090317. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One. 2012;7(7):e40782. doi: 10.1371/journal.pone.0040782. doi: 10.1371/journal.pone.0040782 PONE-D-12-09866 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM. Context-dependent fluctuation of serotonin in the auditory midbrain: the influence of sex, reproductive state and experience. The Journal of Experimental Biology. 2014;217(4):526–535. doi: 10.1242/jeb.087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DF. Sexual behavior in continuously cycling rats. Behaviour. 1972;41(3):288–297. doi: 10.1163/156853972x00068. [DOI] [PubMed] [Google Scholar]
- Hernandez AM, Phillmore LS, MacDougall-Shackleton SA. Effects of learning on song preferences and Zenk expression in female songbirds. Behavioural processes. 2008;77(2):278–284. doi: 10.1016/j.beproc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Ho JM, Murray JH, Demas GE, Goodson JL. Vasopressin cell groups exhibit strongly divergent responses to copulation and male–male interactions in mice. Hormones and Behavior. 2010;58(3):368–377. doi: 10.1016/j.yhbeh.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Pitts NL. Modulation of sensory–motor integration as a general mechanism for context dependence of behavior. General and Comparative Endocrinology. 2012;176(3):465–471. doi: 10.1016/j.ygcen.2012.02.014. doi: http://dx.doi.org/10.1016/j.ygcen.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo ZS. Ultrasonic songs of male mice. Plos Biology. 2005;3(12):2177–2186. doi: 10.1371/journal.pbio.0030386. doi: ARTN e386 DOI 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. Journal of Neurophysiology. 2006;96(5):2177–2188. doi: 10.1152/jn.00046.2006. doi: 10.1152/jn.00046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain research. 2007;1181:21–29. doi: 10.1016/j.brainres.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Current Opinion in Neurobiology. 2004;14(4):488–495. doi: 10.1016/j.conb.2004.06.007. doi: http://dx.doi.org/10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. Journal of Neuroscience. 1999;19(18):8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin effects on frequency tuning of inferior colliculus neurons. Journal of Neurophysiology. 2001;85(2):828–842. doi: 10.1152/jn.2001.85.2.828. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. Journal of Comparative Physiology A. 2005;191(6):535–546. doi: 10.1007/s00359-005-0623-y. doi: 10.1007/s00359-005-0623-y. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Sullivan MR. From behavioral context to receptors: serotonergic modulatory pathways in the IC. Frontiers in Neural Circuits. 2012;6 doi: 10.3389/fncir.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed bat, Tadarida brasiliensis. Journal of Comparative Neurology. 2001;435(1):78–88. doi: 10.1002/cne.1194. [DOI] [PubMed] [Google Scholar]
- Keesom SM, Hurley LM. Socially induced serotonergic fluctuations in the male auditory midbrain correlate with female behavior during courtship. Journal of Neurophysiology. 2016:jn–00742. doi: 10.1152/jn.00742.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Research. 1991;557(1–2):190–201. doi: 10.1016/0006-8993(91)90134-h. doi: http://dx.doi.org/10.1016/0006-8993(91)90134-H. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. Journal of Neurophysiology. 2002;88(4):1941–1954. doi: 10.1152/jn.2002.88.4.1941. [DOI] [PubMed] [Google Scholar]
- Krentzel AA, Remage-Healey L. Sex differences and rapid estrogen signaling: a look at songbird audition. Frontiers in neuroendocrinology. 2015;38:37–49. doi: 10.1016/j.yfrne.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. Journal of neurobiology. 2005;64(3):275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- Lupanova A, Egorova M. Vocalization of sex partners in the house mouse (Mus Musculus). Journal of Evolutionary Biochemistry and Physiology. 2015;51(4):324–331. [PubMed] [Google Scholar]
- Lynch KS, Ball GF. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain, Behavior and Evolution. 2008;72(3):207–214. doi: 10.1159/000157357. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Rand AS, Ryan MJ, Wilczynski W. Plasticity in female mate choice associated with changing reproductive states. Animal Behaviour. 2005;69(3):689–699. [Google Scholar]
- Lynch KS, Wilczynski W. Reproductive Hormones Modify Reception of Species-Typical Communication Signals in a Female Anuran. Brain, Behavior and Evolution. 2008;71(2):143–150. doi: 10.1159/000111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Progress in neurobiology. 2011;94(3):259–295. doi: 10.1016/j.pneurobio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. European Journal of Neuroscience. 2006;23(6):1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL. The incentive salience of courtship vocalizations: hormone-mediated ‘wanting’in the auditory system. Hearing research. 2013;305:19–30. doi: 10.1016/j.heares.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Maney D, MacDougall-Shackleton E, MacDougall-Shackleton S, Ball G, Hahn T. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. Journal of Comparative Physiology A. 2003;189(9):667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- Maney D, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Frontiers in Neuroendocrinology. 2011;32(3):287–302. doi: 10.1016/j.yfrne.2010.12.002. doi: http://dx.doi.org/10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Beaulieu M. l., Phillip JO, Rae AI, Sanford SE, Sockman KW, Maney DL. Rapid effects of hearing song on catecholaminergic activity in the songbird auditory pathway. Plos One. 2012a:e39388. doi: 10.1371/journal.pone.0039388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, LeBlanc MM, Chitrapu A, Blanton ZE, Maney DL. Testosterone alters genomic responses to song and monoaminergic innervation of auditory areas in a seasonally breeding songbird. Developmental neurobiology. 2013;73(6):455–468. doi: 10.1002/dneu.22072. [DOI] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Beaulieu M, Sockman KW, Maney DL. Estradiol-Dependent Modulation of Serotonergic Markers in Auditory Areas of a Seasonally Breeding Songbird. Behavioral Neuroscience. 2012b;126(1):110–122. doi: 10.1037/a0025586. doi: 10.1037/a0025586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Sockman KW, Maney DL. Estradiol-dependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird. European Journal of Neuroscience. 2011;34(3):416–425. doi: 10.1111/j.1460-9568.2011.07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF. Sex chromosome complement affects social interactions in mice. Hormones and Behavior. 2008;54(4):565–570. doi: 10.1016/j.yhbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Female reproductive state influences the auditory midbrain response. Journal of Comparative Physiology A. 2009a;195(4):341–349. doi: 10.1007/s00359-008-0410-7. doi: 10.1007/s00359-008-0410-7. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Sex differences and androgen influences on midbrain auditory thresholds in the green treefrog, Hyla cinerea. Hearing Research. 2009b;252(1-2):79–88. doi: 10.1016/j.heares.2009.04.004. doi: 10.1016/j.heares.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monbureau M, Barker JM, Leboucher G, Balthazart J. Male song quality modulates c-Fos expression in the auditory forebrain of the female canary. Physiology & behavior. 2015;147:7–15. doi: 10.1016/j.physbeh.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolf K, Meindl S, Larsen AL, Kalcounis-Rueppell MC, Penn DJ. Ultrasonic vocalizations of male mice differ among species and females show assortative preferences for male calls. PloS One. 2015;10(8):e0134123. doi: 10.1371/journal.pone.0134123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyby J. Ultrasonic Vocalizations during Sex Behavior of Male House Mice (Mus-Musculus) - a Description. Behavioral and Neural Biology. 1983;39(1):128–134. doi: 10.1016/s0163-1047(83)90722-7. [DOI] [PubMed] [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G. Pheromonal Regulation of Male Mouse Ultrasonic Courtship (Mus-Musculus). Animal Behaviour. 1977;25(May):333–341. doi: 10.1016/0003-3472(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nance D. Effects of para-chlorophenylalanine on food intake in rats. Physiological Psychology. 1974;2(3):360–364. doi: 10.3758/bf03333040. [Google Scholar]
- Pawlisch BA, Stevenson SA, Riters LV. α 1-Noradrenegic receptor antagonism disrupts female songbird responses to male song. Neuroscience Letters. 2011;496(1):20–24. doi: 10.1016/j.neulet.2011.03.078. [DOI] [PubMed] [Google Scholar]
- Petersen CL, Timothy M, Kim DS, Bhandiwad AA, Mohr RA, Sisneros JA, Forlano PM. Exposure to advertisement calls of reproductive competitors activates vocal-acoustic and catecholaminergic neurons in the plainfin midshipman fish, Porichthys notatus. PloS One. 2013;8(8):e70474. doi: 10.1371/journal.pone.0070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak GD. The dominant role of inhibition in creating response selectivities for communication calls in the brainstem auditory system. Hearing Research. 2013;(0) doi: 10.1016/j.heares.2013.03.001. doi: http://dx.doi.org/10.1016/j.heares.2013.03.001. [DOI] [PMC free article] [PubMed]
- Pollak GD, Xie R, Gittelman JX, Andoni S, Li N. The dominance of inhibition in the inferior colliculus. Hearing Research. 2011;274(1–2):27–39. doi: 10.1016/j.heares.2010.05.010. doi: http://dx.doi.org/10.1016/j.heares.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nature Neuroscience. 2008;11(11):1327–1334. doi: 10.1038/nn.2200. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers CH. Timing of sexual behavior in the female rat. Endocrinology. 1970;86(5):1181–1183. doi: 10.1210/endo-86-5-1181. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Serotonin releasing agents: neurochemical, therapeutic and adverse effects. Pharmacology Biochemistry and Behavior. 2002;71(4):825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Molecular psychiatry. 2007;12(4):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Wöhr M, Schwarting RKW. Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neuroscience Letters. 2008;435(1):17–23. doi: 10.1016/j.neulet.2008.02.002. doi: http://dx.doi.org/10.1016/j.neulet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Luo L, Ryan AF. Effects of stimulus frequency and intensity on c-fos mRNA expression in the adult rat auditory brainstem. Journal of Comparative Neurology. 1999;404(2):258–270. doi: 10.1002/(sici)1096-9861(19990208)404:2<258::aid-cne9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schubloom HE, Woolley SC. Variation in social relationships relates to song preferences and EGR1 expression in a female songbird. Developmental neurobiology. 2016 doi: 10.1002/dneu.22373. [DOI] [PubMed] [Google Scholar]
- Sisneros JA. Seasonal Plasticity of Auditory Saccular Sensitivity in the Vocal Plainfin Midshipman Fish, Porichthys notatus. Journal of Neurophysiology. 2009;102(2):1121–1131. doi: 10.1152/jn.00236.2009. doi: 10.1152/jn.00236.2009. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. Journal of Neuroscience. 2003;23(3):1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305(5682):404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Complementary neural systems for the experience dependent integration of mate-choice cues in European starlings. Journal of neurobiology. 2005;62(1):72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- Svec LA, Wade J. Estradiol induces region-specific inhibition of ZENK but does not affect the behavioral preference for tutored song in adult female zebra finches. Behavioural Brain Research. 2009;199(2):298–306. doi: 10.1016/j.bbr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho T, Lu K, Ribeiro S, Pinaud R, Vicario D, Mello CV. Noradrenergic control of gene expression and long-term neuronal adaptation evoked by learned vocalizations in songbirds. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hearing research. 2008;236(1):42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Wang HR, Liang SY, Burgdorf J, Wess J, Yeomans J. Ultrasonic Vocalizations Induced by Sex and Amphetamine in M2, M4, M5 Muscarinic and D2 Dopamine Receptor Knockout Mice. PLoS One. 2008;3(4) doi: 10.1371/journal.pone.0001893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JL, Love EK, Baugh AT, Gordon NM, Tanner JC, Bee MA. Progesterone and prostaglandin F2α induce species-typical female preferences for male sexual displays in Cope's gray treefrog (Hyla chrysoscelis). Physiology & behavior. 2015;152:280–287. doi: 10.1016/j.physbeh.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiology & Behavior. 1998;63(4):467–473. doi: 10.1016/s0031-9384(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Lynch KS. Female sexual arousal in amphibians. Hormones and behavior. 2011;59(5):630–636. doi: 10.1016/j.yhbeh.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context - induced song variation affects female behavior and gene expression. Plos Biology. 2008;6(3):525–537. doi: 10.1371/journal.pbio.0060062. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyl JN, Love OP, Higgs DM. Condition-dependent auditory processing in the round goby (Neogobius melanostomus): links to sex, reproductive condition and female estrogen levels. Journal of Experimental Biology. 2013;216(6):1075–1084. doi: 10.1242/jeb.076935. [DOI] [PubMed] [Google Scholar]