Abstract
Fanconi Anemia (FA) is a rare autosomal genetic disorder characterized by progressive bone marrow failure (BMF), endocrine dysfunction, cancer, and other clinical features commonly associated with normal aging. The anemia stems directly from an accelerated decline of the hematopoietic stem cell compartment. Although FA is a complex heterogeneous disease linked to mutations in 19 currently identified genes, there has been much progress in understanding the molecular pathology involved. FA is broadly considered a DNA repair disorder and the FA gene products, together with other DNA repair factors, have been implicated in interstrand cross-link (ICL) repair. However, in addition to the defective DNA damage response, altered epigenetic regulation, and telomere defects, FA is also marked by elevated levels of inflammatory mediators in circulation, a hallmark of faster decline in not only other hereditary aging disorders but also normal aging. In this review, we offer a perspective of FA as a monogenic accelerated aging disorder, citing the latest evidence for its multi-factorial deficiencies underlying its unique clinical and cellular features.
Keywords: Fanconi Anemia, aging, DNA repair, bone marrow failure, hematopoietic stem cells, inflammatory response, oxidative stress, epigenetic, telomere
Graphical Abstract
1. Normal aging, accelerated aging, and the DNA repair disorder Fanconi Anemia
The adverse consequences of aging are due to the deterioration of the biological processes that maintain life (Kirkwood and Austad, 2000). Declines in tissue and organs, both structural and functional, are also apparent at the cellular level. For example, tissue degeneration throughout life may be a direct consequence of a reduction in stem cell number or functionality due to failure of self-renewal, impaired response to extrinsic signals, senescence, or apoptosis. Additionally, aberrant differentiation of stem cells resulting in a skewed lineage of differentiated progeny may lead to the alterations in cellular composition seen in aged tissue (Fulop et al., 2015;Jones and Rando, 2011;Pera et al., 2015). In mice, for example, age-related abnormal progenitor differentiation skews the hematopoietic population toward the myeloid lineage, with a correspondingly diminished lymphoid potential (Rossi et al., 2005). As a consequence, the aged mice are compromised in their immune response and predisposed to myeloid leukemia. Evidence suggests that clonal expansion of a sub-population of long-term hematopoietic stem cells to myeloid, as opposed to lymphoid, progeny is characteristic of aging in this compartment (Alter, 2014;Cho et al., 2008).
2. Aging at the cellular level
During aging, the frequency of genomic mutations and rearrangements rises, cell morphology becomes distorted, and mitochondrial function declines (DiLoreto and Murphy, 2015;Labbadia and Morimoto, 2015;Nowotny et al., 2014). Levels of inflammatory cytokines, among them IL-6 and TNF-α, are typically elevated in normal aged individuals, and correlate with risk of morbidity and mortality (Bruunsgaard et al., 2003;Ferrucci et al., 2005;Franceschi and Campisi, 2014;Krabbe et al., 2004;Michaud et al., 2013). TNF-α stimulates many pathways, including apoptosis and necrosis, and can up-regulate its own production as well as that of other inflammatory mediators (Chu, 2013). Franceschi and Campisi have discussed the multiple causes for this pro-inflammatory condition including the release of inducers from damaged mitochondria and the accumulation of senescent cells which release inflammatory cytokines (Franceschi and Campisi, 2014).
Oxidative damage accumulates across lifespan and has long been considered an important effector of normal aging (HARMAN, 1956). While current views substantially modify the original premise (Barja, 2014;Gonzalez-Freire et al., 2015), oxidative damage is clearly linked to inflammation and age-associated disorders (Tatsch et al., 2015). Regardless of the initiating factors, age-associated chronic inflammation, termed inflammaging, contributes to the degeneration of tissues, and is provocative for much of the discomfort and many of the diseases of aging (O'Neill and Hardie, 2013). These include cancer and precancerous conditions (Du et al., 2008;Huang et al., 2005), osteopenia (Franceschi et al., 2000), sarcopenia (Ferrucci et al., 1999;Ferrucci et al., 2002;Pedersen et al., 2003), decline in immune function (Frasca and Blomberg, 2011;Frasca and Blomberg, 2016), and cardiovascular disease (Khaper et al., 2010;Pedersen et al., 2003).
Werner Syndrome (WS), Bloom Syndrome (BS), Cockayne Syndrome (CS), Huntington Guilford Progeria Syndrome (HGPS), and Xeroderma pigmentosum (XP) are among the genetic diseases marked by features of accelerated or premature aging (see accompanying review articles in this special collection of Ageing Research Reviews on monogenic accelerated aging disorders). They are often referred to as segmental progerias because they do not fully recapitulate the aging phenotype, but present a subset of the pathologies of aging at a much earlier age than normal individuals. WS, BS, CS and XP have defects in DNA repair, and a high percentage of all syndromes with elements of premature aging are also characterized by repair deficiencies. HGPS is characterized by altered nuclear morphology and genomic instability; moreover, a number of reports suggest a defect in double-strand break repair which may arise from abnormal recruitment and retention of DNA repair proteins at sites of damage (for review, see (Gonzalo and Kreienkamp, 2015)). Many studies suggest that accumulation of DNA damage and genomic instability are major contributing factors to diminished stem cell homeostasis during normal aging (Behrens et al., 2014;Jones and Rando, 2011;Maslov and Vijg, 2009); this is exaggerated in DNA repair deficient disorders. For example, an Ercc1−/− mouse defective in an endonuclease required for nucleotide excision repair (NER) reveals a collection of premature aging phenotypes (Niedernhofer et al., 2006). Among these is spontaneous bone marrow failure (BMF) occurring within their one-month lifespan (Prasher et al., 2005). For further reading on the topic of tissue-accelerated aging dictated by a deficiency in NER, see (Niedernhofer, 2008). Aging is a major risk factor for cancer, which is also highly prevalent in genetic diseases with a NER deficiency (for review, see (Diderich et al., 2011)). Rossi et al. examined the effect of DNA damage repair deficiency on hematopoietic stem cells by studying mice that were genetically deficient in the DNA repair pathways of NER or non-homologous end-joining, or telomere maintenance (Rossi et al., 2007). Although stem cell reserves were retained in these mouse models of accelerated aging, the functional capacity of hematopoietic stem cells declined significantly with age, and this correlated with an age-dependent accumulation of DNA damage.
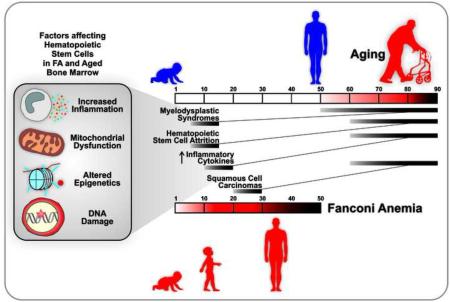
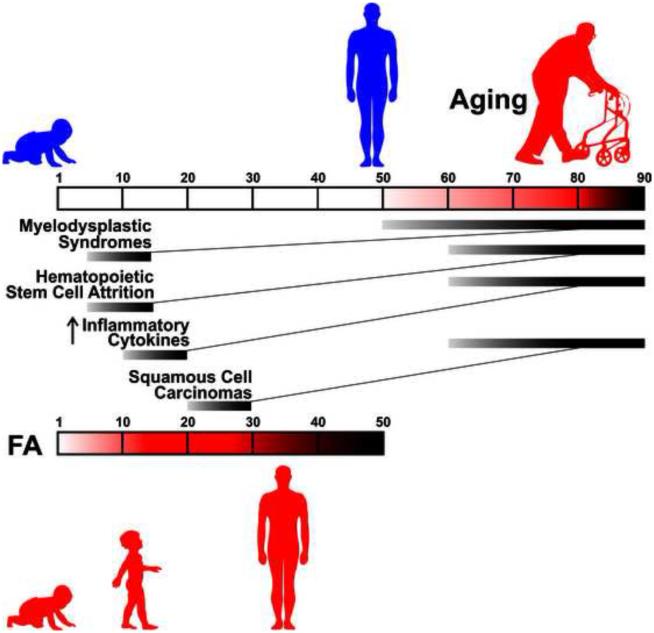
Fanconi Anemia (FA), a disease of BMF, initially presents as macrocytosis/megaloblastic anemia, frequently followed by pancytopenia. Mutations in 19 different genes are causal (Duxin and Walter, 2015). Many of the proteins participate in DNA repair pathways, particularly those involved in the removal of DNA interstrand crosslinks (ICLs). As has been known for many years, cells from FA patients are highly sensitive to oxidative stress (Joenje et al., 1981) and show increased levels of oxidative DNA damage (Pagano et al., 2005). Consequently, in addition to its identity as a BMF syndrome, FA is recognized as a DNA repair disorder (Cantor and Brosh, Jr., 2014). More broadly, FA displays features symptomatic of accelerated aging (Figure 1), which will be the emphasis of this review article.
Figure 1. Clinical symptoms and cellular phenotypes of FA symptomatic of accelerated aging.
FA patients face many health issues in their childhood (teens) and early adulthood (twenties) that also challenge healthy individuals as they age into their fifties and sixties. These include but are not limited to: a dysfunctional bone marrow leading to anemia and immunodeficiency, chronic inflammation, as wells as squamous cell carcinomas and acute myeloid leukemia. Another set of clinical features shared by aging individuals and FA patients are: a decline in endocrine functions, sarcopenia and osteopenia.
3. FA: a premature aging disorder
While FA presents clinically as a disease of BMF, it is also associated with a number of diverse features, including endocrine dysfunction (Giri et al., 2007;Petryk et al., 2015), osteoporosis (Giri et al., 2007), sarcopenia (Neveling et al., 2009), immune deficiency (Fagerlie and Bagby, 2006;Giri et al., 2015), myelodysplastic syndromes (MDS) and cancer (Alter, 2003), among others. In an early study Young and coworkers concluded that many of the characteristics of FA at the cellular level were similar to those of senescent cells (Liu et al., 1994). More recently, Schindler and colleagues perceptively observed that aspects of FA recapitulate normal aging (Neveling et al., 2009). For example, they cited MDS, the development of which is associated with an elevation in TNF-α levels (Calado, 2011). The frequency of MDS begins to rise in affected normal individuals at age 50, with ever increasing probability as a function of age afterwards (Ma, 2012). The median ages of onset of MDS is 10 years in FA patients and 70 years in the general (non-FA) population. Whereas for AML, the median age of onset is also around 70 years for the general population, and 30 years for most FA patients (although it is typically diagnosed by 5 years of age in Fanconi patients carrying mutations in FA-D1 and FA-N) (Alter, 2014;Neveling et al., 2009;Rosenberg et al., 2008). Similarly, in the general population the average age of onset of squamous cell carcinoma of the head and neck is in the early 60’s (Leibovitch et al., 2005), while it occurs 30-40 years earlier in FA patients (Velleuer and Dietrich, 2014). Consequently, FA is proposed to be a segmental premature aging disorder (Neveling et al., 2009), much as WS, BS, CS, and HGPS.
What is the molecular basis for the premature aging phenotype in FA? Production of hydroxyl radicals from FA leukocytes is elevated, and FA cells also display mitochondrial dysfunction and elevated levels of reactive oxygen species (ROS) (Kumari et al., 2014;Pagano et al., 2014). DNA from FA patients shows higher levels of 8-oxo-deoxyguanosine (8-oxo-dG), a marker of base oxidation, than in DNA from aged matched normal controls. Thus FA is characterized by a pro-oxidant state (Degan et al., 1995;Pagano et al., 2004;Pagano et al., 2012). FA cells exposed to oxygen under standard tissue culture conditions incur enhanced mutation frequencies relative to wild type cells (Liebetrau et al., 1997). The increased sensitivity of FA cells to ROS has been interpreted as due to a deficiency in repair of ROS-induced DNA damage (D'Andrea, 2001). In this view FA is a syndrome in which elevated ROS production, combined with repair deficiency, results in higher levels of DNA oxidative lesions, which drive the disorder (see below).
4. Why is ROS production elevated in FA?
Increased levels of circulating inflammatory cytokines, such as TNF-α (Briot et al., 2008;Du et al., 2014;Dufour et al., 2003;Rosselli et al., 1994;Schultz and Shahidi, 1993), Il-6 (Korthof et al., 2013), and IL-1β (Ibanez et al., 2009), are characteristic of FA. Persistent DNA damage, including oxidative damage, is well known to stimulate the production of inflammatory mediators (Chatzinikolaou et al., 2014;Palmai-Pallag and Bachrati, 2014), a dynamic that is exacerbated by DNA repair deficiency (Karakasilioti et al., 2013). Furthermore, Yanagi and co-workers have reported that ubiquitinated FANCD2 inhibits NF-κB transcriptional activity and suppresses transcription of the TNF-α gene (Matsushita et al., 2011). Thus, there are multiple effectors of an inflammatory condition in FA. Consequently, FA patients at an early age phenocopy the inflammaging of normal aging, suggesting that FA should be characterized as a premature inflammaging disorder, from which flow the pathologies that overlap with those of normal aging (see above) (Figure 1). Since DNA damage can induce inflammation and inflammation can provoke DNA damage (Palmai-Pallag and Bachrati, 2014), FA patients are trapped in a downward spiral.
5. Alterations to hematopoietic stem cell function and progenitors as a causative force underlying aging phenotypes
Consistent with the cyclic relationship of DNA damage and inflammation, deficiency in the FA DNA repair pathway was recently demonstrated by several labs to also lead to significant alterations of hematopoietic stem cell function and changes in progeny as a consequence of differentiation (discussed below). Perturbations to stem cell functionality or progeny are likely to both contribute to the accelerated loss of stem cell capacity of the hematopoietic compartment in FA. In addition, DNA damage-induced differentiation checkpoint may limit hematopoietic stem cell self-renewal (Wang et al., 2012). FA mutant mouse models are also characterized by impairment of hematopoietic stem cell function ((Du et al., 2015) and references therein); however, spontaneous BMF is not observed and the mechanism underlying this difference between human and mouse is still not understood.
6. Recent discoveries connect FA and accelerated aging with DNA damage accumulation and hematopoietic system failure
While there has been much interest in the relationship between DNA damage and stem cell decline, detailed mechanistic insight has been lacking. It was proposed that pro-inflammatory cytokines mediate FA BMF, but how DNA damage accumulates and plays a role in this pathway is not well understood. In 2012, Ceccaldi et al. determined from an analysis of bone marrow samples of FA patients and healthy donors that FA is characterized by a deficiency in hematopoietic stem cell number appearing even before the onset of clinical BMF (Ceccaldi et al., 2012). The p53/p21 axis of the DNA damage response is hyper-activated in FA patient cells, triggering cell cycle arrest. Significantly, p53 knockdown was able to rescue the hematopoietic stem cell defects, supporting a model in which unresolved DNA damage in FA cells is responsible for p53/p21-mediated checkpoint cell cycle arrest of FA hematopoietic stem cells.
Milsom and colleagues investigated the physiological source of DNA damage that accumulates in hematopoietic stems cells in a FA mutant mouse model under conditions of stress induced by infection or chronic blood loss (Walter et al., 2015). They determined that genomic instability and DNA damage as evidenced by COMET assay and detection of the double-strand markers γH2AX and 53BP1, as well as accumulation of the homologous recombination (HR) intermediates marked by RAD51 foci, accompany the early exit of hematopoietic stem cells from their quiescent state. Furthermore, experimental data indicated that mitochondrial ROS enriched in stress-induced proliferating hematopoietic stem cells compared to quiescent ones led to oxidative base lesions such as 8-oxo-dG. The perturbation in mitochondrial metabolism was found to be exacerbated in Fanca−/− mutant mice that are defective in the FA pathway. In FA mutant mice, DNA damage that accumulates in hematopoietic stem cells which escaped from dormancy is repaired in an error-prone fashion, resulting in their eventual exhaustive depletion from the hematopoietic stem cell pool. Repeated rounds of hematopoietic stem cell activation in Fanca−/− mice led to severe aplastic anemia, recapitulating the progressive BMF observed in FA patients. These observations suggest that stress-induced hematopoietic stem cell activation leads to DNA damage and collapse of the hematopoietic system. This may be relevant in not only genetic disease states such as FA but also to normal aging where pro-inflammatory conditions often persist.
The Passegue group examined aging hematopoietic stems cells in mice and determined that they have elevated replication stress, cell cycle defects and chromosomal instability attributed to reduced expression of mini-chromosome maintenance (MCM) helicase sub-units, resulting in perturbed replication fork dynamics and accumulation of damage in ribosomal DNA (Flach et al., 2014). These observations are consistent with earlier work documenting a role of the FA pathway to respond to replication stress induced by the DNA polymerase inhibitor aphidicolin and its destabilizing effect on chromosome integrity at common fragile sites (Howlett et al., 2005). FA proteins (FANCD2, BRCA1, RAD51) team up to protect stalled replication forks from degradation, in a pathway that is independent of their DNA repair function (Schlacher et al., 2012).
In another study with a mouse model, it was determined that hypomorphic expression of the origin licensing factor MCM3 which limits the response to replication stress by reducing origin licensing negatively affected fetal erythropoiesis and hematopoietic stem cell performance, ultimately causing lethal anemia during embryogenesis (Alvarez et al., 2015). Consistent with the earlier study (Flach et al., 2014), gene expression profiles showed that Mcm2-7 are downregulated in old hematopoietic stem cells compared to young ones, suggesting that replication stress is an underlying cause of hematopoietic stem cell decline in functionality. It was proposed that improvements to the replication stress response may be a means to combat the age-associated impairment of the hematopoietic system observed not only in blood cancers but also aging.
7. Endogenous formaldehyde linked to hematopoietic stem cell attrition in FA
Accumulation of toxins over time is a central theme in theories of aging. Defining precisely what poisons are responsible for cell and tissue decline in structure, integrity and function has been a challenge to researchers. Endogenous biochemical processes, such as oxidative phosphorylation (as mentioned above), or exposure to environmental pollutants over the course of a lifetime may both contribute to the “Wear and Tear” events responsible for carcinogenesis and/or age-related diseases (Wilson, III et al., 2008). Experimental evidence from the Patel laboratory suggests that DNA damage which arises from endogenous formaldehyde is a major factor driving hematopoietic stem cell attrition when the FA pathway is defective (Garaycoechea et al., 2012;Oberbeck et al., 2014;Rosado et al., 2011). Formaldehyde, a byproduct of enzymatic demethylation, acts to covalently cross-link DNA and proteins. Detoxification of formaldehyde by alcohol dehydrogenase ADH5 or the DNA damage inducing agent acetaldehyde by acetaldehyde-catabolizing enzyme ALDH2 are widely believed to preserve genomic stability, and recent evidence points toward the FA pathway to help cells cope with the lesions. An Adh5−/−Fancd2−/− mouse defective in both alcohol dehydrogenase 5 and FANCD2 displayed hematopoietic stem cell attrition and progressive BMF, as well as endogenous DNA damage in hematopoietic stem cells, hepatocytes, and nephrons; consequently, the mice acquired leukemia, kidney dysfunction/karyomegaly, and liver dysfunction/cancer (Pontel et al., 2015). The authors proposed a model in which DNA repair mediated by the FA pathway protects against the damage caused by endogenous formaldehyde, thereby suppressing cancer and organ damage observed in the ADH5-FA mouse model and possibly other human DNA ICL repair defective accelerated aging disorders.
8. New molecular insight to the role of the FA pathway in controlling genomic instability caused by RNA-DNA hybrids
Emerging evidence supports the view that DNA damage caused by free radicals or chemical metabolites such as aldehydes is a major driving force of BMF in FA; however, it is yet unclear what DNA lesions are precisely responsible, and if these same lesions contribute to normal aging. In terms of the FA pathway, recent papers by Schwab et al. (Schwab et al., 2015) and Garcia-Rubio et al. (Garcia-Rubio et al., 2015) argue that the FA pathway suppresses replication stress by helping to stabilize the replication fork when it encounters a transcription-associated DNA-RNA hybrid, the so-called R-loop, and allowing for R-loop resolution. Interestingly, it was shown that formaldehyde exposure can cause R-loops to form in FA-deficient cells (Schwab et al., 2015), suggesting that the compound’s toxicity may be related to the generation of RNA-DNA hybrids; however, this mechanism remains uncharacterized. It was demonstrated, however, that FANCM translocase is able to resolve RNA-DNA hybrids, suggesting a potential contribution to replication restart. Although these findings suggest a direct role of the FA pathway to eliminate a replication-blocking lesion by acting at the interface of replication and transcription, several questions persist: 1) Does accumulation of RNA-DNA hybrids directly contribute to hematopoietic stem cell attrition? 2) How relevant is RNA-DNA hybrid resolution by the FA pathway to normal aging processes? 3) Are other replication-blocking obstacles cleared by the FA pathway, and are these also relevant to disease phenotypes or aging? Abundant interest in R-loop metabolism suggests that answers to these questions are on the horizon. However, given the evidence that endogenous aldehydes cause a spectrum of lesions including ICLs and protein-DNA cross-links (Duxin and Walter, 2015), it remains to be seen how relevant R-loops are to the pathological phenotypes observed in FA and progressive BMF so prevalent in the disorder.
9. Is FA exclusively a deficiency of DNA repair?
The designation of FA as a DNA repair disorder, and the recent literature cited above, leads naturally to the inclination to interpret all pathologies as the result of this deficiency. However, the groups of Grover Bagby and Qishen Pang, among others, have argued that the BMF observed in FA children is a consequence of multiple adverse effects provoked by the pro-inflammatory state of FA patients (Bagby, Jr., 2003;Du et al., 2014). This view is extended in recent work in which it was shown that DNA damage and repair deficiencies do not explain the over-production of inflammatory cytokines by FA cells (Garbati et al., 2015). Pang and Bagby have called attention to the cytotoxicity of TNF-α to FA hematopoietic cells (Koh et al., 1999;Pang et al., 2001). While the toxicity of TNF-α could be indirect, due to DNA damage mediated by ROS, it is also known to be a direct inducer, via receptor-mediated pathways, of both apoptosis and necrosis (Chu, 2013).
In addition to their role in resolving DNA damage, individual FA gene products have functions that are non-nuclear and appear to be quite distinct from participation in DNA repair pathways. FANCC directly binds and inhibits a subset of microsomal NADPH cytochrome-P450 reductase (Kruyt et al., 1998;Kruyt and Youssoufian, 2000). This enzyme reduces cytochrome P450, which is involved in ROS-producing redox reactions with a broad range of exogenous and endogenous substrates. Similarly, FANCG associates with cytochrome P4502E1, which generates ROS. The activity of CYP2E1 is elevated in FANCG-deficient cells and lowered on complementation with wild type FANCG (Futaki et al., 2002). Mitochondrial peroxidase peroxiredoxin-3 (PRDX3), which reduces H2O2, is bound by FANCG. The activity of PRDX3 is substantially reduced in FANCG-deficient cells. Additionally, FANCG cells show aberrant mitochondrial morphology (Mukhopadhyay et al., 2006). Thus, as either positive or negative effectors, in normal individuals these FA proteins act to lower ROS. Collectively, the published results suggest FA patients suffer BMF because of their failure to respond effectively to DNA damage, and also because of the chronic exposure of the FA marrow to inflammatory factors, to which they are particularly sensitive.
10. Other consequences of defective FA functions
The preceding discussion has focused on the view of FA as a disorder of accelerated inflammaging. However, there are reports pointing to other contributors to BMF. In another example of a non-repair related function of an FA protein, FANCL (the E3 ubiquitin ligase of the FA pathway) ubiquitinates β-catenin. β-catenin ubiquitination stimulates the activity of this multifunctional protein, which is involved in cell adhesion as well as binding and regulating the activity of transcription factors. β-catenin plays a critical role in development, and is important for hematopoietic stem cell development, the maintenance of pluripotency, and the hematopoietic stem cell pool (Fleming et al., 2008). In the absence of FANCL, these functions of β-catenin are reduced with negative implications for the hematopoietic stem cell pool (Dao et al., 2012).
11. Abnormal epigenetic regulation may underlie defective hematopoiesis characteristic of FA
A recent review by Beerman and Rossi summarizes a series of papers which make a strong case for the importance of epigenetic control of stem cell potential to preserve normal cellular homeostasis and suppression of aging-related and disease phenotypes (Beerman and Rossi, 2015). They argued that abnormal epigenetic control in stem cells perturbs the heritable transmittance of genetic information and programs passed on to differentiated cell progeny as well as disrupting normal homeostasis of the stem cell pool that renews itself in subsequent divisions. Epigenetic dysregulation in FA mutant stem cells may underlie the poor hematopoietic potential characteristic of FA. A key mechanism of epigenetic regulation is DNA methylation and demethylation, which is well documented to regulate the processes of stem cell self-renewal versus differentiation in a variety of tissue-specific cell types. From an FA standpoint, abnormal methylation patterns may perturb the self-renewal and differentiation of hematopoietic stem cells, potentially leading to the progressive BMF characteristic of the disorder. Thus, FA may be thought of as a disease displaying an accelerated decline of hematopoietic potential that occurs at least in part from aberrant epigenetic controls of gene expression that maintain the hematopoietic stem cell compartment. In addition to abnormal DNA methylation, unusual histone modifications may contribute to defective hematopoietic stem cell homeostasis in FA. A variety of histone modifications (methylation, acetylation, phosphorylation, sumoylation, ubiquitination, etc) influence chromatin state and accessibility which may alter gene expression in stem cells. Further characterization of DNA methylation patterns (hypo- or hyper-methylation) or histone modifications in FA and how these profiles may relate to known aging signatures may be informative for understanding the pathological basis of FA progressive BMF and perhaps other clinical symptoms characteristic of the disorder.
What experimental evidence supports the tenet that epigenetic alterations are perturbed in FA? A recent study assessed if epigenetic modifications uniquely found in clinical samples of FA patients affected cancer-related cellular phenotypes (Belo et al., 2015). The researchers found that peripheral blood mononuclear cells from blood samples of FA patients displayed hypo-methylation in tumor suppressor gene promoters, and that exposure to a histone deacetylase inhibitor (Vorinostat) altered gene expression patterns in a FA-specific manner; moreover, Vorinostat exposure to FA mutant cells suppressed DNA cross-linker induced chromosomal breaks. It was suggested that the protective effect of the histone deacetylase inhibitor may be a potential approach to alleviating the known toxicity of chemotherapy DNA damaging drugs for FA patients. Given that hematological malignancy is highly prevalent in FA, the development of novel anti-cancer strategies such as modulation of epigenetic alterations is a valuable area to explore.
Aside from regulation of transcription, it is possible that epigenetic modifications may affect DNA repair dependent on the FA pathway. This concept was investigated by the Moses lab in which the biological significance of the interaction between FANCD2 and the acetyltransferase TIP60 was explored (Renaud et al., 2015). Histone H4K16 acetylation by TIP60 was previously shown to be instrumental for HR repair by suppressing 53BP1 accumulation at DNA ends that arise from double strand breaks (Tang et al., 2013). Renaud et al. determined that FA pathway dysfunction resulted in accumulation of 53BP1 and RAP80 at chromatinized double-strand breaks thereby suppressing HR, allowing the lower fidelity nonhomologous end-joining to persist (Renaud et al., 2015). This effect could be suppressed by deacetylase inhibition. Whether H4K16 acetylation is relevant to double strand break repair and genome integrity by this mechanism in hematopoietic stem cells remains to be determined. Nonetheless, it seems probable that epigenetic modifications such as acetylation or methylation are likely to have broad effects on gene regulation and DNA repair that would likely affect tissues and cells of various types, including those of the hematopoietic stem cell compartment.
12. Telomere defects and FA
Telomeres are specialized structures consisting of tandem repeats (TTAGGG in mammals) together with telomere associated proteins to form caps at the ends of linear chromosomes. Telomere maintenance is involved in two inter-related, sequential cellular processes that determine cell fate in ageing: cellular senescence/apoptosis and inappropriate survival/immortalization. Critically short telomeres can trigger DNA damage responses that subsequently lead to cellular senescence and apoptosis, which commonly limits cell survival and malignant transformation. Cellular immortalization can be achieved by maintaining critically short telomeres through either activation of telomerase or HR-based alternative lengthening of telomere (ALT) pathway (Cesare and Reddel, 2008;Shay, 1997).
Telomerase replenishes telomere loss due to incomplete DNA replication and is essential in telomere length maintenance (Greider, 1998). Telomerase deficiency leads to progressive telomere shortening in mice, accompanied by cell proliferation defect and apoptosis in highly proliferating organs, e.g. hematopoietic lineage (Liu and Harrington, 2012). Mutations in genes involved in telomere biology have been linked to accelerated telomere shortening and the development of rare human disorders with inherited BMF as seen in FA, e.g. Dyskeratosis congenita (DC) and acquired aplastic anemia (Savage and Alter, 2008). Unlike DC, telomere shortening in FA patient leukocytes is relatively subtle (Gadalla et al., 2010), and the involvement of telomere abnormalities in the pathogenesis of FA needs further investigation. Laboratory models, however, implicate FA proteins (FANCC, D2, and P) in maintenance of telomeres in hematopoietic and ALT cancer cells (Fan et al., 2009;Lyakhovich et al., 2011;Rhee et al., 2010;Sarkar et al., 2015;Wan et al., 2013;Wilson et al., 2013).
It has been shown that FANCC does not play a direct role in regulating telomere length and telomere capping in mice, but Fancc null murine bone marrow cells display excessive telomere attrition after serial transplantation, supporting the role of FANCC in telomere length maintenance during high hematopoietic cell turnover. In addition, ablation of FANCC function promotes HR-based telomere length maintenance of critically short telomeres to take place (Rhee et al., 2010). It remains unclear if FANCC limits hematopoietic cell death or immortalization via suppressing either telomere attrition or ALT pathway. Although currently there is lack of direct evidence of FA proteins in initiation and/or maintenance of ALT cells, both FANCD2 and FANCP (or SLX4) localize to ALT telomeres and regulate telomere length maintenance and telomere HR events, e.g. telomere sister chromatid exchanges and extrachromosomal telomere fragment or circle formation (Fan et al., 2009;Lyakhovich et al., 2011;Sarkar et al., 2015;Wan et al., 2013;Wilson et al., 2013).
Telomeres are difficult-to-replicate regions (resembling genomic common fragile sites) (Sfeir et al., 2009). Because of high cell turnover, hematopoietic cells may face high replicative stress. For tumorigenesis of ALT cells, telomeres must be maintained by a HR-dependent process. Given that FA proteins are critical in safeguarding genome stability and FA patients without functional FA proteins are predisposed to BMF and malignancies (D'Andrea and Grompe, 2003), we speculate that FA proteins may participate in telomere maintenance by relieving telomere replication stress and regulating telomere HR in hematopoietic cells and ALT cancers.
13. Model for hematopoietic stem cell defects and bone marrow failure in FA
More work is required to fully comprehend the complex heterogeneity of FA and its associated accelerated aging phenotypes. While FA is now generally accepted as a DNA repair and chromosomal instability disorder, multiple lines of evidence indicate that the genetic disorder is characterized by elevated oxidative stress and an aberrant inflammatory response. We believe these deficiencies are intertwined with one another and act synergistically to cause not only hematopoietic stem cell defects and progressive BMF punctuated by blood cancers at an early age but also problems in endocrine function, immune response, osteoporosis, and sarcopenia. The basis for these clinical features may derive from defects in the classic FA pathway, i.e., FANCD2 ubiquitination-dependent DNA transactions, epigenetic abnormalities in transcription regulation, inflammation coupled to DNA damage, and sensitivity of the stem cell compartment to the toxic effects of TNF-α (Figure 2). Although complex in its origin, the cellular and tissue-specific defects of FA resemble features often observed in normal aging, only at an accelerated pace. Therefore, understanding the causes of FA may not only benefit efforts to treat and cure patients but expand to promote healthy aging throughout the population.
Figure 2. Dual problems in DNA transactions and inflammatory response are responsible for hematopoietic stem cell defects and bone marrow failure in FA.
Reactive aldehydes and ROS generated during cellular metabolism and as a consequence of an inflammatory response will induce DNA damage, triggering the DNA damage response (DDR). This will halt cell cycle progression and trigger repair pathways that will attempt to repair the damage. A persistent DDR will eventually lead to either apoptosis or senescence. Senescent cells are characterized by the senescence associated secretory phenotype (SASP) that will induce inflammatory responses in neighboring cells. In FA cells, the inflammation/senescence cycle is fueled at several levels: (a) deficient oxidative stress signaling induces a pro-oxidant state, (b) inefficient repair results in a persistent DDR, and (c) an increase in the transcription of NF-κB induces the SASP, generating more inflammation (d) and perpetuating the vicious cycle.
Highlights.
Fanconi Anemia displays features commonly associated with normal aging
Accelerated decline of hematopoietic stem cells is characteristic of Fanconi Anemia
Oxidative stress and inflammation underlie defects observed in Fanconi Anemia
Understanding pathology of Fanconi Anemia may provide insights for healthy aging
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging (Z01AG000752-08; Z01AG000746-08) and the Fanconi Anemia Research Fund (RMB, MMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter BP. Cancer in Fanconi anemia, 1927-2001. Cancer. 2003;97:425–440. doi: 10.1002/cncr.11046. [DOI] [PubMed] [Google Scholar]
- Alter BP. Fanconi anemia and the development of leukemia. Best Pract. Res. Clin. Haematol. 2014;27:214–221. doi: 10.1016/j.beha.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez S, Diaz M, Flach J, Rodriguez-Acebes S, Lopez-Contreras AJ, Martinez D, Canamero M, Fernandez-Capetillo O, Isern J, Passegue E, Mendez J. Replication stress caused by low MCM expression limits fetal erythropoiesis and hematopoietic stem cell functionality. Nat. Commun. 2015;6:8548. doi: 10.1038/ncomms9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby GC., Jr. Genetic basis of Fanconi anemia. Curr. Opin. Hematol. 2003;10:68–76. doi: 10.1097/00062752-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Barja G. The mitochondrial free radical theory of aging. Prog. Mol. Biol. Transl. Sci. 2014;127:1–27. doi: 10.1016/B978-0-12-394625-6.00001-5. [DOI] [PubMed] [Google Scholar]
- Beerman I, Rossi DJ. Epigenetic control of stem cell potential during homeostasis, aging, and disease. Cell Stem Cell. 2015;16:613–625. doi: 10.1016/j.stem.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat. Cell. Biol. 2014;16:201–207. doi: 10.1038/ncb2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo H, Silva G, Cardoso BA, Porto B, Minguillon J, Barbot J, Coutinho J, Casado JA, Benedito M, Saturnino H, Costa E, Bueren JA, Surralles J, Almeida A. Epigenetic alterations in Fanconi Anaemia: role in pathophysiology and therapeutic potential. PLoS ONE. 2015;10:e0139740. doi: 10.1371/journal.pone.0139740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briot D, Mace-Aime G, Subra F, Rosselli F. Aberrant activation of stress-response pathways leads to TNF-alpha oversecretion in Fanconi anemia. Blood. 2008;111:1913–1923. doi: 10.1182/blood-2007-07-099218. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 2003;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- Calado RT. Immunologic aspects of hypoplastic myelodysplastic syndrome. Semin. Oncol. 2011;38:667–672. doi: 10.1053/j.seminoncol.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor SB, Brosh RM., Jr. What is wrong with Fanconi anemia cells? Cell Cycle. 2014;13:3823–3827. doi: 10.4161/15384101.2014.980633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R, Parmar K, Mouly E, Delord M, Kim JM, Regairaz M, Pla M, Vasquez N, Zhang QS, Pondarre C, de Peffault LR, Gluckman E, Cavazzana-Calvo M, Leblanc T, Larghero J, Grompe M, Socie G, D'Andrea AD, Soulier J. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. 2012;11:36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare AJ, Reddel RR. Telomere uncapping and alternative lengthening of telomeres. Mech. Ageing Dev. 2008;129:99–108. doi: 10.1016/j.mad.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Chatzinikolaou G, Karakasilioti I, Garinis GA. DNA damage and innate immunity: links and trade-offs. Trends Immunol. 2014;35:429–435. doi: 10.1016/j.it.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea AD. Cellular function of the Fanconi anemia pathway. Nat. Med. 2001;7:1259–1260. doi: 10.1038/nm1201-1259a. [DOI] [PubMed] [Google Scholar]
- D'Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat. Rev. Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- Dao KH, Rotelli MD, Petersen CL, Kaech S, Nelson WD, Yates JE, Hanlon Newell AE, Olson SB, Druker BJ, Bagby GC. FANCL ubiquitinates beta-catenin and enhances its nuclear function. Blood. 2012;120:323–334. doi: 10.1182/blood-2011-11-388355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degan P, Bonassi S, De CM, Korkina LG, Pinto L, Scopacasa F, Zatterale A, Calzone R, Pagano G. In vivo accumulation of 8-hydroxy-2'-deoxyguanosine in DNA correlates with release of reactive oxygen species in Fanconi's anaemia families. Carcinogenesis. 1995;16:735–741. doi: 10.1093/carcin/16.4.735. [DOI] [PubMed] [Google Scholar]
- Diderich K, Alanazi M, Hoeijmakers JH. Premature aging and cancer in nucleotide excision repair-disorders. DNA Repair (Amst) 2011;10:772–780. doi: 10.1016/j.dnarep.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLoreto R, Murphy CT. The cell biology of aging. Mol. Biol. Cell. 2015;26:4524–4531. doi: 10.1091/mbc.E14-06-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Adam Z, Rani R, Zhang X, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid. Redox Signal. 2008;10:1909–1921. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Amarachintha S, Erden O, Wilson A, Meetei AR, Andreassen PR, Namekawa SH, Pang Q. Fancb deficiency impairs hematopoietic stem cell function. Sci. Rep. 2015;5:18127. doi: 10.1038/srep18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Erden O, Pang Q. TNF-alpha signaling in Fanconi anemia. Blood Cells Mol. Dis. 2014;52:2–11. doi: 10.1016/j.bcmd.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour C, Corcione A, Svahn J, Haupt R, Poggi V, Beka'ssy AN, Scime R, Pistorio A, Pistoia V. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 2003;102:2053–2059. doi: 10.1182/blood-2003-01-0114. [DOI] [PubMed] [Google Scholar]
- Duxin JP, Walter JC. What is the DNA repair defect underlying Fanconi anemia? Curr. Opin. Cell Biol. 2015;37:49–60. doi: 10.1016/j.ceb.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlie SR, Bagby GC. Immune defects in Fanconi anemia. Crit. Rev. Immunol. 2006;26:81–96. doi: 10.1615/critrevimmunol.v26.i1.40. [DOI] [PubMed] [Google Scholar]
- Fan Q, Zhang F, Barrett B, Ren K, Andreassen PR. A role for monoubiquitinated FANCD2 at telomeres in ALT cells. Nucleic Acids Res. 2009;37:1740–1754. doi: 10.1093/nar/gkn995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, Ferrucci L, Cavazzini C, Corsi AM, Bartali B, Russo CR, Lauretani F, Bandinelli S, Bandinelli S, Guralnik JM. Biomarkers of frailty in older persons. J. Endocrinol. Invest. 2002;25:10–15. [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, Alvarez S, Diolaiti ME, Ugarte F, Forsberg EC, Le Beau MM, Stohr BA, Mendez J, Morrison CG, Passegue E. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512:198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo CC, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De BG. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A. Biol. Sci. Med. Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Aging affects human B cell responses. J. Clin. Immunol. 2011;31:430–435. doi: 10.1007/s10875-010-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17:7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Dupuis G, Baehl S, Le Page A, Bourgade K, Frost E, Witkowski JM, Pawelec G, Larbi A, Cunnane S. From inflamm-aging to immune-paralysis: a slippery slope during aging for immune-adaptation. Biogerontology. 2015 doi: 10.1007/s10522-015-9615-7. In press. [DOI] [PubMed] [Google Scholar]
- Futaki M, Igarashi T, Watanabe S, Kajigaya S, Tatsuguchi A, Wang J, Liu JM. The FANCG Fanconi anemia protein interacts with CYP2E1: possible role in protection against oxidative DNA damage. Carcinogenesis. 2002;23:67–72. doi: 10.1093/carcin/23.1.67. [DOI] [PubMed] [Google Scholar]
- Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Aging. Vol. 2. Albany NY: 2010. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes; pp. 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KJ. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- Garbati MR, Hays LE, Rathbun RK, Jillette N, Chin K, Al-Dhalimy M, Agarwal A, Newell AE, Olson SB, Bagby GC., Jr. Cytokine overproduction and crosslinker hypersensitivity are unlinked in Fanconi anemia macrophages. J. Leukoc. Biol. 2015;99:455–465. doi: 10.1189/jlb.3A0515-201R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rubio ML, Perez-Calero C, Barroso SI, Tumini E, Herrera-Moyano E, Rosado IV, Aguilera A. The Fanconi Anemia pathway protects genome integrity from R-loops. PLoS Genet. 2015;11:e1005674. doi: 10.1371/journal.pgen.1005674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri N, Alter BP, Penrose K, Falk RT, Pan Y, Savage SA, Williams M, Kemp TJ, Pinto LA. Immune status of patients with inherited bone marrow failure syndromes. Am. J. Hematol. 2015;90:702–708. doi: 10.1002/ajh.24046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with Fanconi anemia. J. Clin. Endocrinol. Metab. 2007;92:2624–2631. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Freire M, de C,R, Bernier M, Sollott S,J, Fabbri E, Navas P, Ferrucci L. Reconsidering the role of mitochondria in aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2015;70:1334–1342. doi: 10.1093/gerona/glv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S, Kreienkamp R. DNA repair defects and genome instability in Hutchinson-Gilford Progeria Syndrome. Curr. Opin. Cell Biol. 2015;34:75–83. doi: 10.1016/j.ceb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW. Telomerase activity, cell proliferation, and cancer. Proc. Natl. Acad. Sci. U.S.A. 1998;95:90–92. doi: 10.1073/pnas.95.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Howlett NG, Taniguchi T, Durkin SG, D'Andrea AD, Glover TW. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Hum. Mol. Genet. 2005;14:693–701. doi: 10.1093/hmg/ddi065. [DOI] [PubMed] [Google Scholar]
- Huang H, Patel DD, Manton KG. The immune system in aging: roles of cytokines, T cells and NK cells. Front. Biosci. 2005;10:192–215. doi: 10.2741/1521. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Rio P, Casado JA, Bueren JA, Fernandez-Luna JL, Pipaon C. Elevated levels of IL-1beta in Fanconi anaemia group A patients due to a constitutively active phosphoinositide 3-kinase-Akt pathway are capable of promoting tumour cell proliferation. Biochem. J. 2009;422:161–170. doi: 10.1042/BJ20082118. [DOI] [PubMed] [Google Scholar]
- Joenje H, Arwert F, Eriksson AW, de Koning H, Oostra AB. Oxygen-dependence of chromosomal aberrations in Fanconi's anaemia. Nature. 1981;290:142–143. doi: 10.1038/290142a0. [DOI] [PubMed] [Google Scholar]
- Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat. Cell. Biol. 2011;13:506–512. doi: 10.1038/ncb0511-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakasilioti I, Kamileri I, Chatzinikolaou G, Kosteas T, Vergadi E, Robinson AR, Tsamardinos I, Rozgaja TA, Siakouli S, Tsatsanis C, Niedernhofer LJ, Garinis GA. DNA damage triggers a chronic autoinflammatory response, leading to fat depletion in NER progeria. Cell Metab. 2013;18:403–415. doi: 10.1016/j.cmet.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, Singal PK. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid. Redox Signal. 2010;13:1033–1049. doi: 10.1089/ars.2009.2930. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Koh PS, Hughes GC, Faulkner GR, Keeble WW, Bagby GC. The Fanconi anemia group C gene product modulates apoptotic responses to tumor necrosis factor-alpha and Fas ligand but does not suppress expression of receptors of the tumor necrosis factor receptor superfamily. Exp. Hematol. 1999;27:1–8. doi: 10.1016/s0301-472x(98)00064-2. [DOI] [PubMed] [Google Scholar]
- Korthof ET, Svahn J, de Peffault LR, Terranova P, Moins-Teisserenc H, Socie G, Soulier J, Kok M, Bredius RG, van TM, Jol-van der Zijde EC, Pistorio A, Corsolini F, Parodi A, Battaglia F, Pistoia V, Dufour C, Cappelli E. Immunological profile of Fanconi anemia: a multicentric retrospective analysis of 61 patients. Am. J. Hematol. 2013;88:472–476. doi: 10.1002/ajh.23435. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kruyt FA, Hoshino T, Liu JM, Joseph P, Jaiswal AK, Youssoufian H. Abnormal microsomal detoxification implicated in Fanconi anemia group C by interaction of the FAC protein with NADPH cytochrome P450 reductase. Blood. 1998;92:3050–3056. [PubMed] [Google Scholar]
- Kruyt FA, Youssoufian H. Do Fanconi anemia genes control cell response to cross-linking agents by modulating cytochrome P-450 reductase activity? Drug Resist. Updat. 2000;3:211–215. doi: 10.1054/drup.2000.0159. [DOI] [PubMed] [Google Scholar]
- Kumari U, Ya JW, Huat BB, Lyakhovich A. Evidence of mitochondrial dysfunction and impaired ROS detoxifying machinery in Fanconi anemia cells. Oncogene. 2014;33:165–172. doi: 10.1038/onc.2012.583. [DOI] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J. Am. Acad. Dermatol. 2005;53:253–260. doi: 10.1016/j.jaad.2005.02.059. [DOI] [PubMed] [Google Scholar]
- Liebetrau W, Runger TM, Mehling BE, Poot M, Hoehn H. Mutagenic activity of ambient oxygen and mitomycin C in Fanconi's anaemia cells. Mutagenesis. 1997;12:69–77. doi: 10.1093/mutage/12.2.69. [DOI] [PubMed] [Google Scholar]
- Liu JM, Buchwald M, Walsh CE, Young NS. Fanconi anemia and novel strategies for therapy. Blood. 1994;84:3995–4007. [PubMed] [Google Scholar]
- Liu Y, Harrington L. Telomerase: chemistry, biology and clinical application. John Wiley & Sons, Inc.; 2012. [Google Scholar]
- Lyakhovich A, Ramirez MJ, Castellanos A, Castella M, Simons AM, Parvin JD, Surralles J. Fanconi anemia protein FANCD2 inhibits TRF1 polyADP-ribosylation through tankyrase1-dependent manner. Genome Integr. 2011;2:4. doi: 10.1186/2041-9414-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. Epidemiology of myelodysplastic syndromes. Am. J. Med. 2012;125:S2–S5. doi: 10.1016/j.amjmed.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Vijg J. Genome instability, cancer and aging. Biochim. Biophys. Acta. 2009;1790:963–969. doi: 10.1016/j.bbagen.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita N, Endo Y, Sato K, Kurumizaka H, Yamashita T, Takata M, Yanagi S. Direct inhibition of TNF-alpha promoter activity by Fanconi anemia protein FANCD2. PLoS ONE. 2011;6:e23324. doi: 10.1371/journal.pone.0023324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay SS, Leung KS, Hicks MJ, Hastings PJ, Youssoufian H, Plon SE. Defective mitochondrial peroxiredoxin-3 results in sensitivity to oxidative stress in Fanconi anemia. J. Cell Biol. 2006;175:225–235. doi: 10.1083/jcb.200607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveling K, Endt D, Hoehn H, Schindler D. Genotype-phenotype correlations in Fanconi anemia. Mutat. Res. 2009;668:73–91. doi: 10.1016/j.mrfmmm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ. Tissue-specific accelerated aging in nucleotide excision repair deficiency. Mech. Ageing Dev. 2008;129:408–415. doi: 10.1016/j.mad.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van LW, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- Nowotny K, Jung T, Grune T, Hohn A. Accumulation of modified proteins and aggregate formation in aging. Exp. Gerontol. 2014;59:3–12. doi: 10.1016/j.exger.2014.10.001. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- Oberbeck N, Langevin F, King G, de Wind N, Crossan GP, Patel KJ. Maternal aldehyde elimination during pregnancy preserves the fetal genome. Mol Cell. 2014;55:807–817. doi: 10.1016/j.molcel.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G, Degan P, d'Ischia M, Kelly FJ, Pallardo FV, Zatterale A, Anak SS, Akisik EE, Beneduce G, Calzone R, De Nicola E, Dunster C, Lloret A, Manini P, Nobili B, Saviano A, Vuttariello E, Warnau M. Gender- and age-related distinctions for the in vivo prooxidant state in Fanconi anaemia patients. Carcinogenesis. 2004;25:1899–1909. doi: 10.1093/carcin/bgh194. [DOI] [PubMed] [Google Scholar]
- Pagano G, Shyamsunder P, Verma RS, Lyakhovich A. Damaged mitochondria in Fanconi anemia - an isolated event or a general phenomenon? Oncoscience. 2014;1:287–295. doi: 10.18632/oncoscience.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G, Talamanca AA, Castello G, Pallardo FV, Zatterale A, Degan P. Oxidative stress in Fanconi anaemia: from cells and molecules towards prospects in clinical management. Biol Chem. 2012;393:11–21. doi: 10.1515/BC-2011-227. [DOI] [PubMed] [Google Scholar]
- Pagano G, Zatterale A, Degan P, d'Ischia M, Kelly FJ, Pallardo FV, Calzone R, Castello G, Dunster C, Giudice A, Kilinc Y, Lloret A, Manini P, Masella R, Vuttariello E, Warnau M. In vivo prooxidant state in Werner syndrome (WS): results from three WS patients and two WS heterozygotes. Free Radic. Res. 2005;39:529–533. doi: 10.1080/10715760500092683. [DOI] [PubMed] [Google Scholar]
- Palmai-Pallag T, Bachrati CZ. Inflammation-induced DNA damage and damage-induced inflammation: a vicious cycle. Microbes Infect. 2014;16:822–832. doi: 10.1016/j.micinf.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Pang Q, Keeble W, Christianson TA, Faulkner GR, Bagby GC. FANCC interacts with Hsp70 to protect hematopoietic cells from IFN-gamma/TNF-alpha-mediated cytotoxicity. EMBO J. 2001;20:4478–4489. doi: 10.1093/emboj/20.16.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, Dela F, Pedersen BK. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech. Ageing Dev. 2003;124:495–502. doi: 10.1016/s0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- Pera A, Campos C, Lopez N, Hassouneh F, Alonso C, Tarazona R, Solana R. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas. 2015;82:50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Petryk A, Kanakatti SR, Giri N, Hollenberg AN, Rutter MM, Nathan B, Lodish M, Alter BP, Stratakis CA, Rose SR. Endocrine disorders in Fanconi anemia: recommendations for screening and treatment. J. Clin. Endocrinol. Metab. 2015;100:803–811. doi: 10.1210/jc.2014-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, Chandrasekaran G, Broecker V, Wei W, Liu L, Swenberg JA, Crossan GP, Patel KJ. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol. Cell. 2015;60:177–188. doi: 10.1016/j.molcel.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher JM, Lalai AS, Heijmans-Antonissen C, Ploemacher RE, Hoeijmakers JH, Touw IP, Niedernhofer LJ. Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1−/−mice. EMBO J. 2005;24:861–871. doi: 10.1038/sj.emboj.7600542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud E, Barascu A, Rosselli F. Impaired TIP60-mediated H4K16 acetylation accounts for the aberrant chromatin accumulation of 53BP1 and RAP80 in Fanconi anemia pathway-deficient cells. Nucleic Acids Res. 2016;44:648–656. doi: 10.1093/nar/gkv1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee DB, Wang Y, Mizesko M, Zhou F, Haneline L, Liu Y. FANCC suppresses short telomere-initiated telomere sister chromatid exchange. Hum. Mol. Genet. 2010;19:879–887. doi: 10.1093/hmg/ddp556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado I, Langevin F, Crossan GP, Takata M, Patel KJ. Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway. Nat. Struct. Mol. Biol. 2011;18:1432–1434. doi: 10.1038/nsmb.2173. [DOI] [PubMed] [Google Scholar]
- Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93:511–517. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- Rosselli F, Sanceau J, Gluckman E, Wietzerbin J, Moustacchi E. Abnormal lymphokine production: a novel feature of the genetic disease Fanconi anemia. II. In vitro and in vivo spontaneous overproduction of tumor necrosis factor alpha. Blood. 1994;83:1216–1225. [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Wan B, Yin J, Vallabhaneni H, Horvath K, Kulikowicz T, Bohr VA, Zhang Y, Lei M, Liu Y. SLX4 contributes to telomere preservation and regulated processing of telomeric joint molecule intermediates. Nucleic Acids Res. 2015;43:5912–5923. doi: 10.1093/nar/gkv522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Alter BP. The role of telomere biology in bone marrow failure and other disorders. Mech. Ageing Dev. 2008;129:35–47. doi: 10.1016/j.mad.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi Anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JC, Shahidi NT. Tumor necrosis factor-alpha overproduction in Fanconi's anemia. Am. J. Hematol. 1993;42:196–201. doi: 10.1002/ajh.2830420211. [DOI] [PubMed] [Google Scholar]
- Schwab RA, Nieminuszczy J, Shah F, Langton J, Lopez MD, Liang CC, Cohn MA, Gibbons RJ, Deans AJ, Niedzwiedz W. The Fanconi Anemia pathway maintains genome stability by coordinating replication and transcription. Mol. Cell. 2015;60:351–361. doi: 10.1016/j.molcel.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW. Telomerase in human development and cancer. J. Cell. Physiol. 1997;173:266–270. doi: 10.1002/(SICI)1097-4652(199711)173:2<266::AID-JCP33>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Tang J, Cho NW, Cui G, Manion EM, Shanbhag NM, Botuyan MV, Mer G, Greenberg RA. Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nat. Struct. Mol. Biol. 2013;20:317–325. doi: 10.1038/nsmb.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsch E, Carvalho JA, Hausen BS, Bollick YS, Torbitz VD, Duarte T, Scolari R, Duarte MM, Londero SW, Vaucher RA, Premaor MO, Comim FV, Moresco RN. Oxidative DNA damage is associated with inflammatory response, insulin resistance and microvascular complications in type 2 diabetes. Mutat. Res. 2015;782:17–22. doi: 10.1016/j.mrfmmm.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Velleuer E, Dietrich R. Fanconi anemia: young patients at high risk for squamous cell carcinoma. Mol. Cell. Pediatr. 2014;1:9. doi: 10.1186/s40348-014-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, Moehrle B, Brocks D, Bayindir I, Kaschutnig P, Muedder K, Klein C, Jauch A, Schroeder T, Geiger H, Dick TP, Holland-Letz T, Schmezer P, Lane SW, Rieger MA, Essers MA, Williams DA, Trumpp A, Milsom MD. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- Wan B, Yin J, Horvath K, Sarkar J, Chen Y, Wu J, Wan K, Lu J, Gu P, Yu E,Y, Lue NF, Chang S, Liu Y, Lei M. SLX4 assembles a telomere maintenance toolkit by bridging multiple endonucleases with telomeres. Cell Rep. 2013;4:861–869. doi: 10.1016/j.celrep.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, Hildner K, Guachalla LM, Gompf A, Hartmann D, Schambach A, Wuestefeld T, Dauch D, Schrezenmeier H, Hofmann WK, Nakauchi H, Ju Z, Kestler HA, Zender L, Rudolph KL. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–1014. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Wilson DM, III, Bohr VA, McKinnon PJ. DNA damage, DNA repair, ageing and age-related disease. Mech. Ageing Dev. 2008;129:349–352. doi: 10.1016/j.mad.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JS, Tejera AM, Castor D, Toth R, Blasco MA, Rouse J. Localization-dependent and -independent roles of SLX4 in regulating telomeres. Cell Rep. 2013;4:853–860. doi: 10.1016/j.celrep.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]