Abstract
Dibutyltin (DBT) is used to stabilize polyvinyl chloride (PVC) plastics (including pipes that distribute drinking water) and as a de-worming agent in poultry. DBT is found in human blood, and DBT exposures alter the secretion of tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ) from lymphocytes. Interleukin 1 beta (IL-1β) is a pro-inflammatory cytokine that regulates cellular growth, tissue restoration, and immune response regulation. IL-1β plays a role in increasing invasiveness of certain tumors. This study reveals that exposures to DBT (24 h, 48 h, and 6 day) modify the secretion of IL-1β from increasingly reconstituted preparations of human immune cells (highly enriched human natural killer (NK) cells, monocyte-depleted (MD) peripheral blood mononuclear cells (MD-PBMCs), PBMCs, granulocytes, and a preparation combining both PBMCs and granulocytes (PBMCs+granulocytes)). DBT altered IL-1β secretion from all cell preparations. Higher concentrations of DBT (5 and 2.5 μM) decreased the secretion of IL-1β, while lower concentrations of DBT (0.1 and 0.05 μM) increased the secretion of IL-1β. Selected signaling pathways were examined in MD-PBMCs to determine if they play a role in DBT-induced elevations of IL-1β secretion. Pathways examined were IL-1β converting enzyme (Caspase-1), mitogen-activated protein kinases (MAPKs), and nuclear factor kappa B (NFκB). Caspase 1 and MAPK pathways appear to be utilized by DBT in increasing IL-1β secretion. These results indicate that DBT alters IL-1β secretion from human immune cells in an ex vivo system utilizing several IL-1β regulating signaling pathways. Thus, DBT may have the potential to alter IL-1β secretion in an in vivo system.
Keywords: NK cells, MD-PBMCs, PBMCs, Granulocytes, Dibutyltin, Interleukin 1 beta
Introduction
Dibutyltin (DBT) is an organotin compound that is used as a stabilizer in polyvinyl chloride (PVC) plastics (Roper, 1992). It is found in drinking water and other beverages due to leaching from PVC plastics used in the distribution of drinking water and storage of beverages such as fruit juices (Forsyth et al., 1992, 1997; Sadiki et al., 1996). DBT is also found in some plastic food containers (Nakashima et al., 1990; Yamada et al., 1993) and can leach into food stored in these containers. Use as a de-worming agent in poultry has also lead to its presence in some poultry products (Epstein et al. 1991). Additionally, it is a breakdown product of another organotin, tributyltin (TBT), which also heavily contaminates the environment (Ohhira et al., 2003; Ueno et al., 2003). DBT has been found in human blood samples ranging as high as 0.3 μM (Kannan et al., 1999; Whalen et al., 1999).
Interleukin 1 beta (IL-1β) is a cytokine that stimulates the inflammatory response as well as cellular growth and tissue reconstruction (Apte et al., 2006; Arend, 2002; Dinarello, 1996; 2005; 2009). IL-1β is secreted by a variety of cell types including; monocytes, macrophages, T cells, natural killer (NK) cells, keratinocytes, and fibroblasts (Burger and Dayer, 2002; Dinarello, 2005; Apte et al., 2006; Voronov et al., 2002). It is initially synthesized as a 31-kDa protein (termed pro-IL-1β), which is then processed by Capase-1 to its active form (17-kDa) (Swaan et al., 2001; Dinarello, 1996). Caspase-1 (Kuida et al., 1995; Swaan et al., 2001), is activated by the caspase activating complex, also known as the inflammasome, (Franchi et al., 2009; Lopez-Castejon and Brough, 2011; Martinon et al., 2002). IL-1β can also be secreted by neutrophils; however, the processing may be independent of Caspase-1 (Guma et al., 2009). IL-1β can act as a co-stimulator of the production of interferon gamma (IFNγ), another pro-inflammatory cytokine, in natural killer (NK) cells (Cooper et al., 2001). This multiple-action cytokine contributes to chronic inflammation in diseases such as rheumatoid arthritis and multiple sclerosis (Choy and Panayi, 2001; Lucas and Hohlfeld, 1995). Chronic inflammation also contributes to cancer cell growth. Elevated IL-1β levels occur in breast and pancreatic cancers and melanoma (Arlt et al., 2002; Elaraj et al., 2006; Jin et al., 1997; Lewis and Varghese, 2006; Muerkoster et al., 2006). It has been shown to be required for angiogenesis and invasiveness of both prostate tumors and melanoma in a mouse model (Voronov et al., 2002). Elevation of IL-1β boosts the ability of malignant cells to become adhesive and invade neighboring tissues at the tumor development site (Vidal-Vanaclocha et al., 1996). Poor prognoses in patients with breast, colon, and lung cancers as well as melanomas have been associated with increased IL-1β production (Lewis and Varghese, 2006; Voronov et al., 2002). Conversely, IL-1β is an essential component of the response to infection and thus tight regulation of its levels are required for overall health (Dinarello, 2009). Thus, any agent that causes dysregulation of IL-1β levels has the potential to cause either unwanted inflammation or an inability to mount a competent immune response to infection.
DBT is considered to be an immunotoxin (Seinen et al., 1977; Whalen et al., 1999). It decreases the ability of human NK cells to destroy tumor cells with accompanying decreases in cytotoxic and cell surface protein expression (Dudimah et al., 2007; Catlin et al., 2005; Whalen et al., 2002). In addition, DBT alters the secretion of tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ) from human lymphocytes (Hurt et al., 2013; Lawrence et al., 2015). We recently demonstrated that another organotin, TBT modifies the secretion of IL-1β from human immune cells (Brown and Whalen, 2015). Based on DBT’s effect on TNFα and IFNγ secretion (Hurt et al., 2013; Lawrence et al., 2015) and TBT’s effect on IL-1β (Brown and Whalen, 2015), we hypothesize that exposure to DBT will alter the ability of immune cells to secrete IL-1β.
In this study, an assortment of immune cell preparations was examined for the effects of DBT exposures on the secretion of IL-1β. The preparations studied included: human NK cells, human monocyte-depleted (MD) peripheral blood mononuclear cells (PBMCs) (MD-PBMCs), PBMCs, PBMCs combined with granulocytes (PBMCs+granulocytes), and granulocytes. The use of increasingly reconstituted immune cell preparations allows us to investigate the influence that various immune cell types may have on the ability of DBT to induce alteration in secretion of IL-1β and may more accurately reflect physiological circumstances. An additional goal of this study is to investigate the signaling pathways that may be involved in any DBT-induced increases in IL-1β secretion.
Materials and Methods
Preparation of PBMCs, MD- PBMCs, and Granulocytes
PBMCs were isolated from Leukocyte filters (PALL- RCPL or RC2D) obtained from the Red Cross Blood Bank Facility (Nashville, TN) as described in Meyer et al., 2005. Leukocytes were retrieved from the filters by back-flushing them with an elution medium (sterile phosphate buffered saline (PBS) pH 7.4 containing 5 mM disodium EDTA and 2.5% [w/v] sucrose) and collecting the eluent. The eluent was layered onto Ficoll-Hypaque (1.077 g/mL) and centrifuged at 1200 g for 30–50 min. Granulocytes and red cells pelleted at the bottom of the tube while the PBMCs floated on the Ficoll-Hypaque. Mononuclear cells were collected and washed with PBS (500 g, 10 min). Following washing, the cells were layered on bovine calf serum for platelet removal. The cells were then suspended in RPMI-1640 complete medium which consisted of RPMI-1640 supplemented with 10% heat-inactivated bovine calf serum (BCS), 2 mM L-glutamine and 50 U penicillin G with 50 μg streptomycin/mL. This preparation constituted PBMCs. Monocyte-depleted PBMCs (10–20% CD16+, 10–20 % CD56+, 70–80% CD3+, 3–5% CD19+, 2–20% CD14+) were prepared by incubating the cells in glass Petri dishes (150 × 15 mm) at 37 °C and air/CO2, 19:1 for 1.5 h. This cell preparation is referred to as MD-PBMCs. Granulocytes were isolated with the removal of mononuclear and red blood cells as described in Kuijpers et al., 2013. Granulocytes were collected and washed with PBS (2500 rpm, 15min). Red blood cells were lysed with NH4CL isotonic solution (155mM NH4Cl, 10mM KHCO3, 0.1 mM EDTA) for 10 min. Cells were then washed with PBS and centrifuged at 800g for 10 min. The cells were then suspended in RPMI-1640 complete medium supplemented with 10% heat-inactivated BCS, 2 mM L-glutamine and 50 U penicillin G with 50 μg streptomycin/mL.
Preparation of NK cells
NK cells were prepared from buffy coats (from healthy adult donors) purchased from Key Biologics, LLC (Memphis, TN). NK cells were prepared using a rosetting procedure. RosetteSep human NK cell enrichment antibody cocktail (0.6–0.8 mL) (StemCell Technologies, Vancouver, British Columbia, Canada) was added to 45 mL of buffy coat. The mixture was incubated for 20 min at room temperature (~ 25° C). Approximately 8 mL of the mixture was layered onto 4 mL of Ficoll-Hypaque (1.077 g/mL) (MP Biomedicals, Irvine, CA) and centrifuged at 1200 g for 30–50 min. NK cells were collected and washed twice with PBS and stored in complete media (RPMI-1640 supplemented with 10% heat-inactivated BCS, 2 mM L-glutamine and 50 U penicillin G with 50 μg streptomycin/ml) at 1 million cells/mL at 37 °C and air/CO2, 19:1.
Chemical Preparation
DBT was purchased from Sigma-Aldrich (96%) (St. Louis, MO). A DBT stock solution was prepared by dissolving DBT in dimethylsulfoxide (DMSO). Desired concentrations of DBT were prepared by dilution of the stock into cell culture media. The final concentration of DMSO for DBT exposures did not exceed 0.01%. Appropriate DMSO controls were run.
Inhibitor Preparation
Enzyme inhibitors were purchased from Fisher Scientific (Pittsburgh, PA). The stock solution for each inhibitor was a 50 mM solution in dimethylsulfoxide (DMSO). Capase-1 inhibitor II, p38 inhibitor (SB202190), MEK 1/2 inhibitor (U0126), and NFκB inhibitor (BAY11-7085) were prepared by dilution of the stock solution into cell culture media.
Cell Treatments
NK cells, MD-PBMCs, PBMCs, granulocytes (at a concentration of 1.5 million cells/mL), or PBMCs+granulocytes (at a concentration of 0.75 million cells/mL each) were treated with DBT at concentrations of 0.05–5 μM for 24 h, 48 h, or 6 days. Following the incubations, the cells were pelleted and supernatants were collected and stored at −70° C until assaying for IL-1β. For pathway inhibitor experiments, MD-PBMCs (at a concentration of 1.5 million cells/mL) were treated with pathway inhibitors 1 h before adding DBT at concentrations of 0.05 and 0.1 μM for 24 h. Following the incubations, the cells were pelleted and supernatants were collected and stored at −70° C until assaying for IL-1β.
Cell Viability
Cell viability was assessed at the beginning and end of each exposure period. Viability was determined using the trypan blue exclusion method. Briefly, cells were mixed with trypan blue and counted using a hemocytometer. The total number of cells and the total number of live cells were determined for both control and treated cells to determine the percent viable cells.
IL-1β Secretion Assay
IL-1β levels were measured using the BD OptEIA™ Human IL-1β enzyme-linked immunosorbent assay (ELISA) kit (BD-Pharmingen, San Diego, CA). Briefly, a 96-well micro well plate, designed for ELISA (Fisher, Pittsburgh, PA), was coated with a capture antibody for IL-1β diluted in coating buffer. The plate was incubated with the capture antibody overnight at 4 °C. After incubation, the capture antibody was removed by washing the plate three times with wash buffer (PBS and 0.05% Tween-20). Assay diluent (PBS and bovine calf serum) was added to each well (blocking non-specific binding) and incubated at room temperature for 1h. The assay diluent was removed by washing the plate three times, and the cell supernatants and IL-1β standards were added to the coated plated and incubated for 2 h at room temperature. Following this incubation, the plate was thoroughly washed five times and then incubated for 1h with a detection antibody to IL-1β which was conjugated with horseradish peroxidase. The excess detection antibody was removed by washing seven times and a substrate solution was added for 30 min at room temperature to produce a colored product. The incubation with the substrate was ended by addition of acid and the absorbance was measured at 450 nm on a Thermo Labsystems Multiskan MCC/340 plate reader (Fisher Scientific).
Statistical Analysis
Statistical analysis of the data was performed by using ANOVA and Student’s t test. Data were initially compared within a given experimental setup by ANOVA. A significant ANOVA was followed by pair wise analysis of control versus exposed data using Student’s t test, a p value of less than 0.05 was considered significant.
Results
Viability of NK cells, MD-PBMCs PBMCs, PBMCs+ Granulocytes, and Granulocytes exposed to DBT
Table 1 shows the effects of DBT exposures (0.05–5 μM) on the viability of NK cells, MD-PBMCs, PBMCs, PBMCs+granulocytes, and granulocytes. Exposure of NK cells to 0.05–1 μM DBT for 24 h had no effect on their viability as compared to the control. The higher concentrations of DBT (2.5 and 5 μM) caused some decrease (about 10%) in NK cell viability. Exposure at 48 h showed significant reductions in viability (around 35%) at 2.5 and 5 μM. NK cells exposed to 0.05–1 μM DBT for 6 days showed no change in viability as compared to the control. MD-PBMCs and PBMCs both showed a significant decrease in viability with exposure to 2.5 and 5 μM DBT for 24 h (15% for MD-PBMCs and 25% for PBMCs). The viability of both MD-PBMCs and PBMCs at 48 h was also significantly decreased by exposure to 2.5 (38%) and 5 μM (35%) DBT. After 6 days of exposure, concentrations of 0.5, 1, 2.5 and 5 μM DBT caused decreases in viability of only the MD-PBMCs. Exposure of PBMCs+granulocytes to 0.05–2.5 μM DBT for 24 h had no effect on viability compared to the control; whereas, the viability of granulocytes alone was not affected by any of the DBT exposures after 24 h. Exposure to 5 μM DBT for 48 h significantly diminished the viability of PBMCs+granulocytes as did 6 day exposures to 0.5–5 μM DBT. After 6 days the viability of granulocytes was significantly decreased under all conditions (as would be expected), but the treated cells were no less viable than the control cells.
Table 1.
Percent viability of NK cells, Monocyte-depleted PBMCs, PBMCs, PBMCs combined with Granulocytes, and Granulocytes exposed to DBT for 24 h, 48 h, and 6 days.
| Percent Viable Cells | ||||||
|---|---|---|---|---|---|---|
| 24 h exposure | [DBT] μM | NK | monocyte-depleted PBMCs | PBMCs | PBMCs with Granulocytes | Granulocytes |
| 0 | 92±6 | 94±3 | 94±3 | 92±5 | 90±9 | |
| 0.05 | 97±3 | 98±2 | 96±4 | 94±3 | 90±8 | |
| 0.1 | 98±2 | 98±1 | 97±3 | 93±6 | 92±5 | |
| 0.25 | 98±1 | 97±1 | 96±3 | 93±4 | 95±3 | |
| 0.5 | 95±4 | 91±8 | 95±3 | 94±5 | 94±3 | |
| 1 | 92±5 | 92±4 | 86±7 | 88±6 | 94±2 | |
| 2.5 | 88±1 | 74±15* | 69±10* | 84±8 | 92±3 | |
| 5 | 80±7 | 80±9* | 65±8* | 77±4* | 92±2 | |
| 48 h exposure | 0 | 97±1 | 94±2 | 91±6 | 92±3 | 91±3 |
| 0.05 | 97±0 | 96±2 | 93±3 | 93±5 | 93±4 | |
| 0.1 | 96±1 | 97±1* | 94±4 | 94±2 | 95±3 | |
| 0.25 | 93±0* | 96±2 | 94±4 | 93±5 | 92±5 | |
| 0.5 | 90±1* | 94±3 | 91±8 | 92±2 | 94±3 | |
| 1 | 79±12 | 80±11* | 74±16 | 88±10 | 95±2 | |
| 2.5 | 61±6* | 56±20* | 56±13* | 50±29 | 72±10* | |
| 5 | 64±8* | 55±10* | 56±8* | 49±12* | 73±17 | |
| 6 day exposure | 0 | 70±15 | 87±8 | 70±37 | 77±7 | 68±20 |
| 0.05 | 75±8 | 89±8 | 68±45 | 80±7 | 51±18 | |
| 0.1 | 78±7 | 86±7 | 87±11 | 72±10 | 65±22 | |
| 0.25 | 74±9 | 81±13 | 69±33 | 74±8 | 55±33 | |
| 0.5 | 68±9 | 67±13* | 68±13 | 54±10* | 50±5 | |
| 1 | 69±3 | 52±11* | 43±12 | 50±12* | 57±9 | |
| 2.5 | 58±7 | 47±9* | 36±25 | 32±15* | 34±8* | |
| 5 | 49±19 | 52±8* | 42±15 | 37±10* | 35±20 | |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in viability compared to control cells (cells treated with vehicle alone), p<0.05
Viability of monocyte-depleted PBMCs with pathway inhibitors exposed to DBT
MD-PBMCs were treated with various signaling pathway inhibitors 1 hour prior to adding the appropriate concentrations of DBT (0.05 and 0.1 μM). The viability of all treatments was greater than 90%. (data not shown).
Effects of DBT Exposure on Secretion of IL-1β by NK cells
Table 2 shows the effects of exposing NK cells to DBT (0, 0.05, 0.1, 0.25, 0.05, 1, 2.5 and 5 μM) on IL-1β secretion. Cells from 3 different donors were exposed to DBT for 24 h, 48 h, and 6 days (KB=Key Biologic buffy coat). All donors showed significant decreases in IL-1β secretion when exposed to 0.5, 1, 2.5, and 5 μM DBT at all lengths of exposure. Additionally, 2 of the 3 donors showed increased IL-1 β secretion with exposures to 0.05 μM DBT after 24 and/or 48 h exposures.
Table 2.
Effects of 24 h, 48 h, 6 day exposures to DBT on IL-1β secretion from highly purified human NKs.
| 24 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | ||
|---|---|---|---|
| [DBT] μM | KB130 | KB155 | KB169 |
| 0 | 249±20 | 3110±238 | 196±13 |
| 0.05 | 386±41* | 2965±1004 | 1551±25* |
| 0.1 | 116±27* | 2771±70 | 1173±69* |
| 0.25 | 17±20* | 779±25* | 796±137* |
| 0.5 | ND* | 28±23 | 114±21* |
| 1 | ND* | ND* | ND* |
| 2.5 | ND* | ND* | ND* |
| 5 | ND* | ND* | ND* |
| 48 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | ||
|---|---|---|---|
| [DBT] μM | KB130 | KB155 | KB169 |
| 0 | 452±17 | 4916±116 | 186±13 |
| 0.05 | 1372±481 | 5235±531 | 341±10* |
| 0.1 | 298±65* | 4588±130* | 367±53* |
| 0.25 | 139±46* | 1598±450* | 262±14* |
| 0.5 | ND* | 130±39* | ND* |
| 1 | ND* | ND* | ND* |
| 2.5 | ND* | ND* | ND* |
| 5 | ND* | ND* | ND* |
| 6day | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | ||
|---|---|---|---|
| [DBT] μM | KB130 | KB155 | KB169 |
| 0 | 600±97 | 1472±182 | 615±93 |
| 0.05 | 782±35 | 1625±164 | 453±91 |
| 0.1 | 550±21 | 1572±245 | 602±257 |
| 0.25 | 142±27* | 960±152* | 511±227 |
| 0.5 | 46±53* | 138±43* | 157±61* |
| 1 | ND* | ND* | ND* |
| 2.5 | ND* | ND* | ND* |
| 5 | ND* | ND* | ND* |
Values are mean±S.D. of triplicate determinations.
Indicates a significant change in secretion compared to control cells (cells treated with vehicle alone), p<0.05.
ND = not detectable. The limit of detection on the assay is 3 pg/mL
Effects of DBT Exposure on Secretion of IL-1β by MD-PBMCs
The effects of exposures to DBT on secretion of IL-1β from MD-PBMCs from 5 donors (F=filter obtained from the Red Cross) are shown in Table 3. Exposure conditions were the same as for NK cells. This preparation mainly consists of NK cells and T cells. When MD-PBMCs were exposed to DBT for 24 h there were statistically significant increases in IL-1β secretion induced by DBT (all donors) at the 0.05 μM concentration with variation in the fold increase among different donors. F142 showed a 1.5 fold increase while F166 showed a 3.0 fold increase. Some donors showed increases in IL-1β at additional concentrations of DBT. Significant decreases in IL-1β secretion were seen at 1, 2.5, and 5 μM DBT (cells from all donors) after a 24 h exposure. The results seen after 48 h and 6 day exposures to DBT were similar to those seen after 24 h.
Table 3.
Effects of 24 h, 48 h, 6 day exposures to DBT on IL-1β secretion from monocyte-depleted PBMCs.
| 24 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | ||||
|---|---|---|---|---|---|
| [DBT] μM | F142 | F145 | F152 | F163 | F166 |
| 0 | 7687±241 | 2235±114 | 360±34 | 379±81 | 2266±205 |
| 0.05 | 11284±763* | 3333±164* | 721±36* | 613±57* | 6772±386* |
| 0.1 | 12495±3130 | 2390±497 | 516±26* | 370±13 | 5569±527* |
| 0.25 | 4456±561* | 700±8* | 406±29 | 309±15 | 2788±126* |
| 0.5 | 2637±1438* | 47±59* | 340±57 | 310±16 | 158±43* |
| 1 | 77±24* | ND* | 224±9* | 130±5* | ND* |
| 2.5 | ND* | ND* | 83±22* | 70±25* | ND* |
| 5 | ND* | ND* | 19±12* | 86±44* | ND* |
| 48 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | ||||
|---|---|---|---|---|---|
| [DBT] μM | F142 | F145 | F152 | F163 | F166 |
| 0 | 10707±8 | 2447±162 | 279±114 | 455±58 | 5258±599 |
| 0.05 | 14932±420* | 780±323* | 563±54* | 607±86 | 14662±1044* |
| 0.1 | 15275±650* | 2232±1035 | 495±72 | 342±34 | 12941±423* |
| 0.25 | 6455±123* | 337±347* | 607±71* | 547±68 | 5307±349 |
| 0.5 | 2604±133* | ND* | 703±34* | 677±378 | 924±101* |
| 1 | 851±54* | ND* | 296±56 | 701±111* | 1075±230* |
| 2.5 | 6±27* | ND* | 35±38* | ND* | 1981±3260 |
| 5 | ND* | ND* | 86±37 | ND* | ND* |
| 6day | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | ||||
|---|---|---|---|---|---|
| [DBT] μM | F142 | F145 | F152 | F163 | F166 |
| 0 | 3136±148 | 829±160 | 107±19 | 176±36 | 7622±484 |
| 0.05 | 2303±313* | 983±248 | 81±21 | 715±87* | 6655±930 |
| 0.1 | 1606±808 | 987±577 | 71±6 | 497±75* | 4959±11* |
| 0.25 | 560±127* | 535±16* | 118±11 | 305±36* | 2424±95* |
| 0.5 | 270±197* | 147±55* | 161±62 | 664±70* | 2118±698* |
| 1 | 90±100* | ND* | 41±7* | 175±3 | ND* |
| 2.5 | ND* | ND* | 20±18* | ND* | ND* |
| 5 | ND* | ND* | 14±1* | ND* | ND* |
Values are mean±S.D. of triplicate determinations.
Indicates a significant change in secretion compared to control cells (cells treated with vehicle alone), p<0.05.
ND = not detectable.
Effects of DBT Exposure on Secretion of IL-1β by PBMCs
Table 4 summarizes the effects of exposing PBMCs from 4 individual donors to DBT on IL-1β secretion (F=filter obtained from the Red Cross). A 24 h exposure to 2.5 and 5 μM DBT caused a significant decrease (P<0.05) in IL-1β secretion in cells from all donors and at 1 μM DBT for 3 of the 4 donors. Significant increases in IL-1β secretion were seen (all donors) at 0.1 μM and in 3 of the 4 donors at 0.05 μM. Again, the magnitude of DBT-induced increases in IL-1β secretion was donor dependent. For instance, cells from donor F159 treated with 0.05, 0.1, and 0.25 μM DBT showed significant increases of 1.7, 3.8, and 3.4 fold, respectively. 48 h exposures of PBMCs to 0.5, 1, 2.5 and 5 μM DBT caused significant decreases in IL-1β for donor F154. Significant increases were also seen at some of these same concentrations for donors F159 and F173. After 6 days of exposure, significant decreases in IL-1β secretion were seen in cells from all donors when exposed to 2.5 and 5 μM DBT. Significant increases in IL-1β secretion were seen inall donors at 0.25 μM DBT.
Table 4.
Effects of 24 h, 48 h, 6 day exposures to DBT on IL-1β secretion from PBMCs.
| 24 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [DBT] μM | F148 | F154 | F159 | F173 |
| 0 | 65733±3402 | 1611±98 | 1692±204 | 5331±216 |
| 0.05 | 120533±8211* | 5056±273* | 2812±150* | 7072±935 |
| 0.1 | 107467±4636* | 4568±168* | 6376±233* | 8100±822* |
| 0.25 | 137333±3926* | 1383±123 | 5803±150* | 4443±405* |
| 0.5 | 32400±1833* | 765±84* | 1667±112 | 20063±1077* |
| 1 | 11600±11085* | 380±13* | 812±65* | 9989±155* |
| 2.5 | 12133±462* | 111±67* | ND* | 3813±58* |
| 5 | 11867±924* | ND* | ND* | 2517±73* |
| 48 h | Interleukin 1 beta secreted in ng/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [DBT] μM | F148 | F154 | F159 | F173 |
| 0 | 10041±316 | 1944±56 | 346±36 | 436±15 |
| 0.05 | 15851±836* | 4249±294* | 1034±299 | 614±63* |
| 0.1 | 17620±257* | 4193±556* | 1211±71* | 500±41 |
| 0.25 | 18728±597* | 1687±42* | 1243±192* | 425±20 |
| 0.5 | 5493±409* | 1036±128* | 1099±93* | 745±36* |
| 1 | 2224±329* | 1325±72* | 1246±9* | 2064±194* |
| 2.5 | ND* | 378±61 | 532±101 | 591±32* |
| 5 | 483±358* | 193±0* | 565±15* | 478±32 |
| 6day | Interleukin 1 beta secreted in ng/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [DBT] μM | F148 | F154 | F159 | F173 |
| 0 | 21±0 | 1589±24 | 91±2 | 376±18 |
| 0.05 | ND* | 3507±155* | 210±62 | 465±151 |
| 0.1 | 25±0* | 3300±114* | 236±10* | 398±28 |
| 0.25 | 25±1* | 3816±464* | 507±21* | 453±16* |
| 0.5 | 6±0* | 2056±114* | 229±7* | 932±8* |
| 1 | ND* | 2531±464 | 256±12* | 690±15* |
| 2.5 | ND* | 586±179* | 52±1* | 181±8* |
| 5 | ND* | 118±146* | 51±9* | 145±2* |
Values are mean±S.D. of triplicate determinations.
Indicates a significant change in secretion compared to control cells (cells treated with vehicle alone), p<0.05.
ND = not detectable.
Effects of DBT Exposure on Secretion of IL-1β by PBMCs+Granulocytes
Effects of exposing PBMCs+granulocytes (prepared from the same 4 donors as PBMCs) to DBT on IL-1β secretion are shown in Table 5 (F=filter obtained from the Red Cross). Like the previous cell types discussed, the concentrations that caused increases and decreases in IL-1β secretion varied from one donor to the next at all lengths of exposures. After 24 h exposures to 2.5 and 5 μM DBT, there were significant decreases in IL-1β secretion in cell from 3 of the 4 donors. There were also significant increases in IL-1β secretion over the range of 0.05–2.5 μM DBT (all donors). The specific concentration(s) at which the increase(s) were seen varied among donors. For example, cells from donor F159 treated with 0.25, 0.5, and 1 μM DBT showed fold increases at 1.9, 1.8, and 1.8, respectively at 24 h and 35, 56, and 77 fold, respectively at 48 h. After 6 days, control cells no longer demonstrated any meaningful secretion of IL-1β (detection limit of the assay was 3 pg/mL). However, the cells from donors F154 and F159 did show secretion when exposed to certain concentrations of DBT.
Table 5.
Effects of 24 h, 48 h, 6 day exposures to DBT on IL-1β secretion from PBMCs plus Granulocytes
| 24 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [DBT] μM | F148 | F154 | F159 | F173 |
| 0 | 2972±267 | 900±38 | 329±26 | 143±7 |
| 0.05 | 4217±397* | 1364±181* | 438±18* | 150±26 |
| 0.1 | 3060±191 | 1059±141 | 375±28 | 168±34 |
| 0.25 | 3344±212 | 835±38 | 648±18* | 146±12 |
| 0.5 | 7275±2061 | 538±33* | 620±40* | 165±13 |
| 1 | 2011±148* | 386±157* | 617±74* | 632±19* |
| 2.5 | 736±552* | ND* | 87±12* | 287±22* |
| 5 | 197±78* | ND* | ND* | 140±10 |
| 48 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [DBT] μM | F148 | F154 | F159 | F173 |
| 0 | 492±25 | 320±10 | 4±3 | 26±2 |
| 0.05 | 646±25* | 358±88 | 109±93 | 57±35 |
| 0.1 | 864±11* | 346±40 | 48±8* | 17±2* |
| 0.25 | 1017±76* | 372±47 | 141±16* | 42±6* |
| 0.5 | 1191±200* | 372±24* | 226±16* | 32±1* |
| 1 | 491±26 | 322±11 | 311±39* | 160±2* |
| 2.5 | ND* | 321±35 | 505±54* | 124±3* |
| 5 | ND* | 321±20 | 192±15* | 63±1* |
| 6day | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [DBT] μM | F148 | F154 | F159 | F173 |
| 0 | ND | 3±0 | ND | ND |
| 0.05 | ND | 65±34 | 3±2 | ND |
| 0.1 | ND | 31±16 | 3±2 | ND |
| 0.25 | ND | 8±1* | ND | ND |
| 0.5 | 3±1 | 11±0* | 5±1* | ND |
| 1 | ND | 10±0* | 5±1* | ND |
| 2.5 | ND | 3±0 | 7±1* | ND |
| 5 | ND | ND | ND | ND |
Values are mean±S.D. of triplicate determinations.
Indicates a significant change in secretion compared to control cells (cells treated with vehicle alone), p<0.05.
ND = not detectable.
Effects of DBT Exposure on Secretion of IL-1β by Granulocytes
The effects of exposures to DBT on secretion of IL-1β were examined in granulocytes prepared from the same 4 donors as were PBMCs. Secretion of IL-1β was not above the limit of detection (3 pg/mL) under any of the conditions tested after 24 h. However after 48 h, cells from 3 of the 4 donors showed DBT-induced increases in IL-β secretion. The DBT-stimulated increases in secretion occurred over the concentration range of 0.25–5 μM. The data from a representative donor (F173) are shown in Figure 1. Although IL-1β secretion was lower for granulocytes than all other cell types tested, there were still significant increases in IL-1β secretion induced by DBT in two of the three donors.
Figure 1.
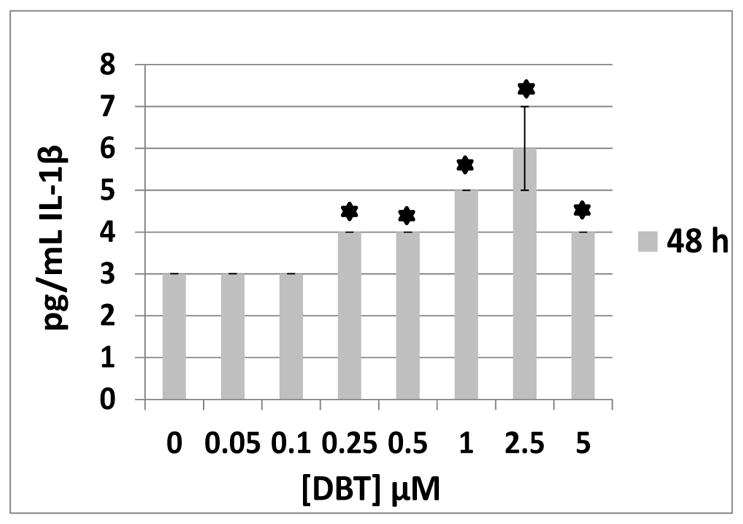
Granulocytes exposed to 0–5 μM DBT at 48 h exposure (donor F173), asterisk indicates a significant difference from control
Effects of DBT Exposure on Secretion of IL-1β by MD-PBMCs treated with Selective Signaling Pathway Inhibitors
IL-1β Cleavage Inhibitor (Caspase-1 Inhibitor II)
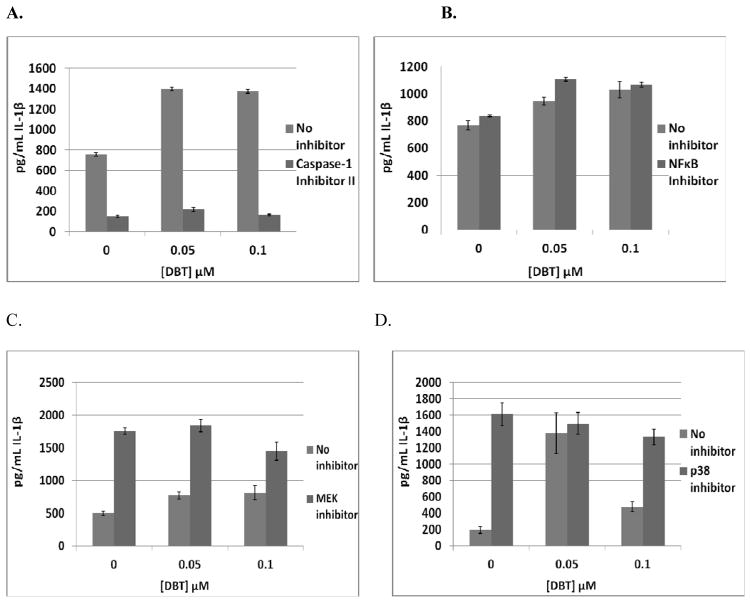
The effects of exposures to 0.05 and 0.1 μM DBT on secretion of IL-1β from MD-PBMCs where Caspase-1 had been inhibited with Caspase-1 inhibitor II (50 μM) are shown in Table 6. Since this enzyme is required for IL-1β processing, Caspase-1 inhibitor II significantly decreased (p<0.05) baseline IL-1β production in cells from each donor. Figure 2A shows the fold increases from a representative experiment (F245). There were 1.8 fold increases when MD-PBMCs were exposed to 0.05 and 0.1 μM DBT in the absence of the Caspase-1 inhibitor. When the inhibitor was present those same DBT exposures caused 1.4 fold and no increase in IL-1β secretion. Thus, DBT showed diminished ability to increase IL-1β secretion in 4 of the 5 donors. However, in cells from 1 of the donors DBT-induced increases in Il-1β secretion were unaffected by the Caspase I inhibitor. This indicates that Caspase-1 is likely needed for DBT to cause increased IL-1β secretion. However, this requirement varies among donors.
Table 6.
Effects of 24 h exposure to DBT +/- Pathway inhibitors on IL-1β secretion from MD-PBMCs
| IL-1β Cleavage Inhibitor (Caspase-1 Inhibitor II (CI))
| |||||
|---|---|---|---|---|---|
| 24 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | ||||
| [DBT] μM | F240 | F242 | F244 | F245 | F254 |
| 0 | 626±35 | 1698±77 | 845±26 | 755±18 | 251±13 |
| 0 + Caspase | 237±19 | 460±15 | 233±11 | 150±8 | 39±4 |
| 0.05 | 757±17* | 2752±49* | 1291±34* | 1399±20* | 521±62* |
| 0.05 + Caspase | 337±7** | 495±25 | 334±11** | 216±19** | 41±9 |
| 0.1 | 916±67* | 2874±77* | 1444±43* | 1375±18* | 206±2* |
| 0.1 + Caspase | 467±12** | 354±43** | 302±3** | 163±10 | 17±16 |
| NFκB Inhibitor (BAY 11-7085)
| ||||
|---|---|---|---|---|
| 24 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | |||
| [DBT] μM | F244 | F245 | F333 | F334 |
| 0 | 768±34 | 1101±60 | 3269±45 | 1600±109 |
| 0 + BAY | 836±8 | 536±51 | 2039±62 | 703±70 |
| 0.05 | 947±29* | 883±66* | 3995±161* | 2281±53* |
| 0.05 + BAY | 1107±14* | 630±60 | 2518±60* | 1035±19* |
| 0.1 | 1029±59* | 1206±91 | 2755±84* | 1468±94 |
| 0.1 + BAY | 1067±19* | 514±80 | 1960±102 | 702±82 |
| p44/42 Pathway Inhibitor (U0126)
| ||||
|---|---|---|---|---|
| 24 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | |||
| [DBT] μM | F240 | F242 | F244 | F245 |
| 0 | 498±30 | 1548±73 | 1161±73 | 793±33 |
| 0 + U | 1756±54 | 479±29 | 119±28 | 255±25 |
| 0.05 | 772±54* | 2230±89* | 1764±62* | 1174±49* |
| 0.05 + U | 1840±95 | 759±10** | 61±23** | 277±8 |
| 0.1 | 812±109* | 2172±25* | 2001±184* | 1246±65* |
| 0.1 + U | 1447±142** | 312±27** | 143±50 | 231±14 |
| p38 Inhibitor (SB202190)
| ||||
|---|---|---|---|---|
| 24 h | Interleukin 1 beta secreted in pg/mL (mean±S.D.) | |||
| [DBT] μM | F247 | F262 | F372 | F385 |
| 0 | 191±41 | 853±59 | 1040±63 | 959±47 |
| 0 + SB | 1613±141* | 149±41* | 36±3* | 357±61* |
| 0.05 | 1378±246* | 1100±58* | 2388±322* | 1863±83* |
| 0.05 + SB | 1495±134 | 146±8 | 348±198 | 65±24* |
| 0.1 | 477±60* | 1021±61* | 1985±53* | 1126±29* |
| 0.1 + SB | 1329±99* | 134±183 | 70±14* | ND* |
Values are mean±S.D. of triplicate determinations.
indicates a significant increase compared to control without inhibitor (0), p<0.05;
indicates a significant increase compared to control+inhibitor (0+I; I=CI, BAY, U, SB) p<0.05,
ND = not detectable
Figure 2.
Effects of 24 h exposure to 0.05 and 0.1 μM DBT on IL-1β secretion from monocyte-depleted PBMCs treated with selective pathway component inhibitors in individual donors. A) IL-1β Cleavage Inhibitor (Caspase-1 Inhibitor II) (donor F245). B) NFκB Inhibitor (BAY 11-7085) (donor F244). C) MEK 1/2 (p44/42) Inhibitor (U0126) (donor F240). D) p38 Inhibitor (SB202190) (donor F247).
Nuclear Factor kappa B (NFκB) Inhibitor (BAY 11-7085)
Table 6 also shows the effects of exposures to 0.05 and 0.1 μM DBT on secretion of IL-1β from MD-PBMCs where NFκB function had been inhibited with BAY 11-7085. BAY 11-7085 had varying effects on baseline IL-1β secretion. Cells exposed to DBT when the inhibitor was present showed no substantial change in DBT-stimulated secretion of IL-1β. For instance, when cells from donor F244 were exposed to 0.05 and 0.1 μM DBT in the absence of NFκB inhibitor there were 1.2, and 1.3 fold increases in IL-1β secretion and when the inhibitor was present those same DBT exposures caused 1.3 and 1.3 fold increases in IL-1β secretion (Figure 2B). Thus, unlike the Caspase-1, these results suggest that NFκB is not utilized by DBT to increase the secretion of IL-1β from the cells.
Mitogen activated protein kinase kinase (MAP2K), MEK, Inhibitor (U0126)
The effects of inhibiting the p44/42 mitogen activated protein kinase (MAPK) pathway with U0126 (50 μM) on DBT-induced increases in IL-1β secretion are shown in Table 6. When this pathway was inhibited the ability of DBT to increase IL-1β secretion was markedly diminished or blocked completely. For example, cells from donor F240 showed 1.6 fold increases in IL-1β when exposed to 0.05 and 0.1 μM DBT in the absence of MEK inhibitor and no DBT-induced increases in IL-1β secretion when the inhibitor was present (Figure 2C). This same effect was seen in the cells from each of the 4 donors tested. This indicates that the p44/42 MAPK pathway is being utilized by DBT to lead to the increase in IL-1β secretion.
p38 Inhibitor (SB202190)
The effects of p38 MAPK pathway inhibition on DBT-induced increases in IL-1β secretion from MD-PBMCs are shown in Table 6. In 3 of the 4 donors the p38 inhibitor significantly decreased baseline secretion, and in 1 donor it greatly increased secretion. In Figure 2D we see there were 7.2 and 2.5 fold increases when MD-PBMCs (donor F247) were exposed to 0.05 and 0.1 μM DBT in the absence of p38 inhibitor. When the inhibitor was present those same DBT exposures caused no increases in IL-1β secretion. This effect was consistent in cells from all donors (Table 6). This indicates that the p38 pathway is being utilized by DBT to stimulate IL-1β secretion.
Discussion
DBT is mainly used as a stabilizer in PVC plastics (Forsyth et al., 1992, 1997; Sadiki et al., 1996) and levels as high as 0.3 μM have been found in human blood samples (Kannan et al., 1999; Whalen et al., 1999). DBT decreases the ability of human NK cells to destroy tumor cells (Dudimah et al., 2007; Catlin et al., 2005; Whalen et al., 2002). It also alters the secretion of tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ) from human lymphocytes (Hurt et al., 2013; Lawrence et al., 2015). IL-1β is an inflammatory cytokine that contributes to normal immune responsiveness (Dinarello, 2009). However, when its levels are inappropriately elevated it causes chronic inflammation which can increase tumor invasiveness (Voronov et al., 2003). Thus, tight regulation of IL-1β levels is required for maintaining health. We investigated whether DBT alters the secretion of IL-β from immune cells in an ex vivo human model. Increasingly reconstituted human immune cell preparations were used (NK cells, MD-PBMCs, PBMCs, PBMC+granulocytes, and granulocytes) to determine if the complexity of the cell preparation influences the effects of DBT. Furthermore, experiments to identify signaling pathways involved in DBT-induced increases of IL-1β levels were also performed.
DBT induced both increases and decreases in IL-1β secretion from immune cells. Higher concentrations of DBT (5 and 2.5 μM) tended to decrease IL-1 β secretion from all immune cell preparations and effects varied somewhat in a donor dependent manner. Lower concentrations stimulated IL-1β secretion in the cell preparations. Higher concentrations of DBT will be able to bind to a wider range of cellular targets than will lower concentrations, based on DBT’s affinity for specific cellular components. Thus, far fewer target molecules will be bound by lower concentrations of DBT and this may account for the opposite effects of higher concentrations of DBT versus lower concentrations on IL-1β secretion. For instance at 5 μM, DBT may be able to bind cellular components that result in blocking either IL-1β synthesis and/or secretion and these effects may overwhelm the effects of binding to any cellular components that increase IL-1β synthesis/secretion. However, at 0.05 μM the DBT may be binding predominantly to components that stimulate synthesis/secretion. The concentration at which DBT caused the maximum fold increase in IL-β secretion from each of the different cell types (after 24 h of exposure) varied among the donors. The average of the maximum fold increase was similar for all cell preparations: NK cells 3.5-fold (ranging from 1.0- to 8.0-fold); MD-PBMCs 2.0-fold (ranging from 1.5- to 3.0-fold); PBMCs 3.2 fold (ranging from 2.1- to 3.8-fold); PBMCs+granulocytes 3.8-fold (range of 1.5- to 9.2-fold); and granulocytes 3.7-fold (ranging from 1.3- to 12-fold). Granulocytes alone showed very low or no baseline secretion of IL-1β (depending on the donor). Granulocytes have been shown by others to be capable of secreting IL-1β (Guma et al., 2009).
The viability of the NK cells, MD-PBMCs, PBMCs, and PBMCs+granulocytes was decreased significantly after all lengths of exposure at the higher concentrations of DBT. For instance after a 24 h exposure to 5 μM DBT there were average decreases in the viability of NK, MD-PBMC, PBMC, and PBMC+granulocytes of 13%, 15%, 31%, 16%, respectively. However, the decreases in viability seen at those concentrations were not sufficient to account for the very large decreases in IL-1β secretion seen in the vast majority of donors. Additionally, it has been shown that cell death can act as a stimulator of IL-1β secretion (Lopez-Castejon and Brough, 2011) and would not necessarily be expected to decrease secretion. Thus, it appears that the decreases in IL-1β secretion seen after 24 h are not due to cell death but to some inhibitory effect of DBT on the ability of the immune cells to secrete IL-1β.
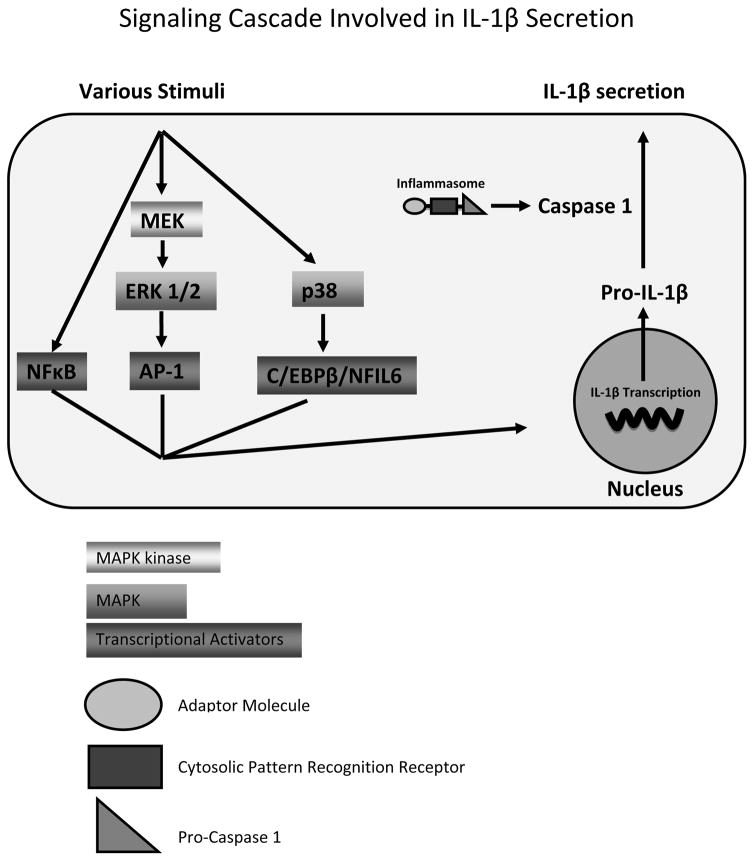
Many signaling pathways are involved in the production and secretion of IL-1β from human immune cells. These pathways include Caspase 1, NFκB, and MAPKs (p44/42 and p38). Our results indicate that three of these pathways appear to be involved in DBT-induced increases in IL-1β secretion. Inhibition of Caspase-1, the enzyme that is needed for secretion of IL-1β from human immune cells, tended to diminish DBT-induced secretion. Thus, Caspase-1 appears to be one of the cellular components/pathways that DBT interacts with in causing increased IL-1β secretion. NFκB is needed as a transcription factor for several cytokine genes including IL-1β (Cogswell et al., 1994; Perez et al., 1999; Scheibel et al., 2010). However, the data indicated that DBT-induced IL-1β secretion did not require the NFκB pathway. The p44/42 MAPK (ERK 1 and ERK 2) is an important regulator of cell growth (Cowley et al., 1994; Hunter, 1995; Marshall, 1995). Once activated, p44/42 leads to activation of the transcription regulator AP-1 (comprised of fos and jun), and activation of AP-1 leads to increased transcription of IL-1β (Glauser and Schlegel, 2007). When the p44/42 pathway was inhibited by an MEK inhibitor, DBT-induced increases in IL-1β secretion were diminished or blocked. Thus, DBT may be activating the p44/42 pathway which then leads to an increase in IL-1β secretion. The MAPK, p38, has also been shown to regulate cytokine production (Davis, 1995; Young et al., 1997). This includes activation of IL-1β gene transcription by way of C/EBPβ/NFIL-6 transcription activator (Baldassare et al., 1999). Inhibition of the p38 pathway diminished the ability of at least one concentration of DBT to stimulate IL-1β secretion in all donors tested. Thus it appears that the p38 pathway is likely being utilized by DBT to stimulate IL-1β secretion. Figure 3 summarizes the pathways that may possibly be involved in the regulation of secretion and synthesis relating to IL-1β.
Figure 3.
Signaling pathways regulating IL-1β secretion.
In a previous study, we showed that TBT increased IL1β secretion from immune cells at certain concentrations in a donor dependent manner (Brown and Whalen; 2015). We were able to carry out the study of both TBT and DBT in NK cells prepared from a single donor (KB169). IL-1β increased 7.7-, and 6.3-fold at 50 and 100 nM TBT (Brown and Whalen, 2015). These same cells showed increased IL-1β secretion of 7.9 and, 6.0 fold at 0.05 μM (50 nM) and 0.1 μM (100 nM) DBT. These results suggest that TBT and DBT at the same concentrations caused roughly similar stimulation of IL-1β secretion in this one donor. However, when we looked at both chemicals in MD-PBMCs for 3 common donors (F142, F145, and F152), 24 h exposures to TBT, caused significant increases of 2.1 and 1.8 fold at the 50 and 100 nM concentrations in cells from donor F142 (Brown and Whalen, 2015) while DBT at these same concentrations caused increases of 1.5 and 1.6 fold. In cells from donor F145, 50 and 100 nM TBT caused increases of 1.8 and 1.5 fold while DBT caused an increase of 1.5 fold at 50 nM and no increase at 100 nM. The cells from the third common donor showed increases of 11 and 33 fold with 50 and 100 nM TBT and increases of 2.1 and 1.4 fold with these same concentrations of DBT. Taken together, these comparisons indicate that DBT may be generally less effective at inducing an increase in IL-1β secretion than is TBT. This may be due to the fact that DBT is less hydrophobic than TBT due to the loss of the third butyl group (Laughlin et al., 1986; Harper, 2005) and is thus less able to enter the cell to bind to intracellular targets (such as MAPKs) that appear to be involved in the compound-induced increases in IL-1β secretion. It may also mean that DBT simply has a lower affinity for those intracellular components and thus is somewhat less effective at inducing the effect.
Previously we found that, MAPKs (p44/42 and p38) were needed for TBT-induced secretion of IL-1β in MD-PBMCs (Brown and Whalen; 2015). The present study indicates that Caspase 1 and MAPKs (p44/42 and p38) are being utilized in DBT-induced secretion of IL-1β in MD-PBMCs. This suggests that DBT is able to interact with the Caspase 1 pathway while TBT does not. The ability to compare the effects of TBT and DBT gives a clearer understanding of the similarities and differences of their effects on immune cells. Beyond the common effects that we have noted on cytokine secretion, they have also both been shown to induce apoptosis of thymocytes in vitro (Gennari et al., 1997). Furthermore they have been shown to cause degeneration of the thymus (maturation site of T-cells) in rats (Snoeij et al., 1988).
In summary, the current study demonstrates that DBT alters IL-1β secretion from increasingly reconstituted preparations of human immune cells. Higher concentrations of DBT (5 and 2.5 μM) decreased the secretion of IL-1β, while lower concentrations (0.1 and 0.05 μM) increased the secretion of IL-1β. These results suggest that DBT-induced alterations of IL-1β secretion from immune cells may potentially affect immune function and cancer invasiveness. The levels of DBT found in human blood samples (0.3 μM and below) fall in the range of DBT concentrations that elevated IL-1β levels in this study and thus DBT may be more of problem in terms of causing chronic inflammation rather than loss of immune competence in exposed individuals. This study also indicates that components of Caspase 1 and MAPKs (p44/42 and p38) pathways are targets of DBT. The MAPK pathways are known to lead to increased transcription of the IL-1β gene (Baldassare et al., 1999; Cogswell et al., 1995; Glauser and Schlegel, 2007; Guma et al., 2009; Perez et al., 1999; Scheibel et al., 2010), suggesting that DBT is increasing IL-1β transcription as one of its effects on immune cells.
Acknowledgments
Grant U54CA163066 from the National Institutes of Health
References
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer and Metastasis Reviews. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine and Growth Factor Reviews. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Arlt A, Vorndamm J, Müerköster S, Yu H, Schmidt WE, Fölsch UR, Schäfer H. Autocrine Production of Interleukin 1β Confers Constitutive Nuclear Factor κB Activity and Chemoresistance in Pancreatic Carcinoma Cell Lines. Cancer Research. 2002;62:910–916. [PubMed] [Google Scholar]
- Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. The Journal of Immunology. 1999;162:5367–73. [PubMed] [Google Scholar]
- Brown S, Whalen M. Tributyltin alters secretion of interleukin 1 beta from human immune cells. Journal of Applied Toxicology. 2015;35:895–908. doi: 10.1002/jat.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D, Dayer J. Cytokines, Acute-Phase Proteins, and Hormones. Annals of the New York Academy of Science. 2002;473:464–473. doi: 10.1111/j.1749-6632.2002.tb04248.x. [DOI] [PubMed] [Google Scholar]
- Catlin R, Shah H, Bankhurst AD, Whalen MM. Dibutyltin exposure decreases granzyme B and perforin in human natural killer cells. Environmetal Toxicolology and Pharmacology. 2005;20:395–403. doi: 10.1016/j.etap.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Choy EHS, Panayi GS. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. New England Journal of Medicine. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Cogswell JP, Codlevski MM, Wisely CB, Leesnitzer LM, Ways JP, Gray JG, Clay C. NF-kB Regulates IL-1B Transcription Through a Consensus NF-kB Binding Site and a Nonconsensus CRE-Like Site. The Journal of Immunology. 1994;153:712–723. [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Ponnappan A, Mehta V, Wewers MD, Caligiun MA. Interleukin-1β costimulates interferon-γ production by human natural killer cells. European Journal of Immunology. 2001;31:792–801. doi: 10.1002/1521-4141(200103)31:3<792::aid-immu792>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Transcriptional regulation by MAP kinases. Molecular Reproduction and Development. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic Basis for IL-1 in Disease. The Journal of The American Society of Hematology. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dinarello CA. Blocking IL-1 in systemic inflammation. The Journal of Experimental Medicine. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annual Review of Immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Gibson C, Whalen MM. Effect of dibutyltin on ATP levels in human natural killer cells. Environmental Toxicology. 2007;22:117–123. doi: 10.1002/tox.20252. [DOI] [PubMed] [Google Scholar]
- Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM, Alexander HR. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clinical Cancer Research. 2006;12:1088–1096. doi: 10.1158/1078-0432.CCR-05-1603. [DOI] [PubMed] [Google Scholar]
- Epstein RL, Phillippo ET, Harr R, Koscinski W, Vosco G. Organotin residue determination in poultry and turkey sample survey in the United States. J Agric Food Chem. 1991;39:917–921. [Google Scholar]
- Forsyth DS, Weber D, Cldroux C. Determination of butyltin, cyclohexyltin and phenyltin compounds in beers and wines. Food Addit Contam. 1992;9:161–169. doi: 10.1080/02652039209374058. [DOI] [PubMed] [Google Scholar]
- Forsyth DS, Jay B. Organotin leachates in drinking water from chlorinated poly (vinyl chloride) (CPVC) pipe. Appl Organomet Chem. 1997;11:551–558. [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The Inflammasome: A Caspase-1 Activation Platform Regulating Immune Responses and Disease Pathogenesis. Nature Immunology. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari A, Potters M, Seinen W, Pieters R. Organotin-Induced Apoptosis as Observed in Vitro Is Not Relevant for Induction of Thymus Atrophy at Antiproliferative Doses. Toxicology and Applied Pharmacology. 1997;147:259–266. doi: 10.1006/taap.1997.8265. [DOI] [PubMed] [Google Scholar]
- Glauser DA, Schlegel W. Sequential actions of ERK1/2 on the AP-1 transcription factor allow temporal integration of metabolic signals in pancreatic beta cells. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2007;21:3240–3249. doi: 10.1096/fj.06-7798com. [DOI] [PubMed] [Google Scholar]
- Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M. Caspase 1- Independent Activation of Interleukin-1 beta in Neutrophil-Predominant Inflammation. Arthritis and Rheumatism. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. Toxicological profile for tin and tin compounds. United States Agency for Toxic Substances and Disease Registry: US EPA; 2005. [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: The Yin and Yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Hurt K, Hurd-Brown T, Whalen M. Tributyltin and dibutyltin alter secretion of tumor necrosis factor alpha from human natural killer cells and a mixture of T cells and natural killer cells. Journal of Applied Toxicology. 2013;33:503–510. doi: 10.1002/jat.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guidae A, Hastings HM, Andres J, Turkel G, Polverini PJ, Goldberg ID, Rosen EM. Expression of interleukin-1β in human breast carcinoma. Cancer. 1997;80:421–434. doi: 10.1002/(sici)1097-0142(19970801)80:3<421::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP. Occurrence of butyltin compounds in human blood. Environmental Science and Technology. 1999;33:1776–1779. [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Tool ATJ, van der Schoot CE, Ginsel LA, Onderwater JJM, Roos D, Verhoeven AJ. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 2013;78:1105–1111. [PubMed] [Google Scholar]
- Laughlin RB, Guard HE, Coleman WM., III Tributyltin in seawater: Speciation and octanol-water coefficient. Environ Sci Technol. 1986;20:201–204. doi: 10.1021/es00144a016. [DOI] [PubMed] [Google Scholar]
- Lawrence S, Reid J, Whalen MM. Secretion of interferon gamma from human immune cells is altered by exposure to tributyltin and dibutyltin. Environmental Toxicology. 2015;30:559–571. doi: 10.1002/tox.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A, Varghese S. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. Journal of Translational Medicine. 2006;12:1–12. doi: 10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine and Growth Factor Reviews. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K, Hohlfeld R. Different aspects of cytokines in the immunopathology of multiple sclerosis. Neurology. 1995;45:S4–S5. doi: 10.1212/wnl.45.6_suppl_6.s4. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of prolIL-β. Molecular Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, Weinauer F, Schmitz J, Illert WE. Filter Buffy Coats (FBC): A source of peripheral blood leukocytes recovered from leukocyte depletion filters. Journal of Immunological Methods. 2005;307:150–166. doi: 10.1016/j.jim.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Muerkoster SS, Lust J, Arlt A, Hasler R, Witt M, Sebens T, Schreiber S, Folsch UR, Schafer H. Acquired chemoresistance in pancreatic carcinoma cells: induced secretion of IL-1beta and NO lead to inactivation of caspases. Oncogene. 2006;25:3973–3981. doi: 10.1038/sj.onc.1209423. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Hori S, Nakazawa H. Determination of dibutyltin and dioctyltin compounds in PVC food containers, wrappage and clothes by reversed phase HPLC with column switching. Eisei Kagaku. 1990;36:155–220. [Google Scholar]
- Ohhira S, Watanabe M, Matsui H. Metabolism of tributyltin and triphenyltin by rat, hamster, and human hepatic microsomes. Archives of Toxicology. 2003;77:138–144. doi: 10.1007/s00204-002-0428-5. [DOI] [PubMed] [Google Scholar]
- Perez R, Ritzenthaler J, Roman J. Transcriptional regulation of the interleukin-1 β promoter via fibrinogen engagement of the CD18 integrin receptor. American Journal of Respiratory Cell and Molecular Biology. 1999;20:1059–1066. doi: 10.1165/ajrcmb.20.5.3281. [DOI] [PubMed] [Google Scholar]
- Roper WL. Toxicological Profile for Tin. U.S Department of Health and Human Services. Agency for Toxic Substances and Disease Registry; 1992. [Google Scholar]
- Sadiki A-I, Williams DT, Carrier R, Thomas B. Pilot study on the contamination of drinking water by organotin compounds from PVC materials. Chemosphere. 1996;32:2389–2398. doi: 10.1016/0045-6535(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Scheibel M, Klein B, Merkle H, Schulz M, Fritsch R, Greten FR, Arkan MC, Schneider G, Schmid RM. IkappaBbeta is an essential co-activator for LPS-induced IL-1beta transcription in vivo. The Journal of Experimental Medicine. 2010;207:2621–30. doi: 10.1084/jem.20100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinen W, Vos JG, Spanje I, Snoek M, Brands R, Hooykaas H. Toxicity of organotin compounds. II. Comparative in vivo and in vitro studies with various organotin and organolead compounds in different animal species with special emphasis on lymphocyte cytotoxicity. Toxicology and Applied Pharmacology. 1977;42:197–212. doi: 10.1016/0041-008x(77)90210-1. [DOI] [PubMed] [Google Scholar]
- Snoeij NJ, Penninks AH, Seinen W. Dibutyltin and tributyltin compounds induce thymus atrophy in rats due to selective action on thymic lymphoblasts. International Journal of Immunopharmacology. 1988;10:891–899. doi: 10.1016/0192-0561(88)90014-8. [DOI] [PubMed] [Google Scholar]
- Swaan P, Knoell D, Helsper F, Wewers M. Sequential Processing of Human ProIL-1β by Caspase-1 and Subsequent Folding Determined by a Combined In Vitro and In Silico Approach. Pharmaceutical Research. 2001;18:1083–1090. doi: 10.1023/a:1010958406364. [DOI] [PubMed] [Google Scholar]
- Ueno S, Kashimoto T, Susa N, Ishii M, Chiba Mutoh K, Hoshi F, Suzuki T, Sugiyama M. Comparison of heopatotoxicity and metabolism of butyltin compounds in the liver of mice, rats, and guinea pigs. Archives of Toxicology. 2003;77:173–181. doi: 10.1007/s00204-002-0429-4. [DOI] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello CA. Interleukin 1 (IL-1)-Dependent Melanoma Hepatic Metastasis In Vivo; Increased Endothelial Adherence by IL-1-Induced Mannose Receptors and Growth Factor Production In Vitro. Journal of the National Cancer Institute. 1996;88:198–205. doi: 10.1093/jnci/88.3-4.198. [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of Environmentally Relevant Concentrations of Butyltins on Human Natural Killer Cells in Vitro. Environmental Research. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Ghazi S, Loganathan BG, Hatcher F. Expression of CD16, CD18, and CD56 in Tributyltin-Exposed Human Natural Killer Cells. Chemico-Biological Interactions. 2002;139:159–176. doi: 10.1016/s0009-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Yamada S, Fuji Y, Mikami E, Kawamura N, Hayakawa J. Small-scale survey of organotin compounds in household commodities. J AOAC Int. 1993;76:436–441. [Google Scholar]
- Young PR, McLaughlin MM, Kumar S, Kassis S, Doyle ML, McNulty D, Gallagher TF, Fisher S, McDonnell PC, Carr SA, Huddleston MJ, Seibel G, Porter TG, Livi GP, Adams JL, Lee JC. Pyridinyl Imidazole Inhibitors of p38 Mitogen-activated Protein Kinase Bind in the ATP Site. Journal of Biological Chemistry. 1997;272:12116–12121. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]