Abstract
Intracranial hemangiopericytoma (HPC) is a rare brain tumor with aggressive biologic behavior associated with high recurrence rate and often with extracranial metastasis. The most common sites of extracranial metastasis of the intracranial HPC are the long bones, lung, liver and abdominal cavity in the order of frequencies. Extracranial metastases usually occur long after the initial diagnosis of the primary tumor. Metastatic intracranial HPC to the vertebra has been rarely reported. We present a case of intracranial HPC metastasized to the L2 vertebral body 13 years after multiple surgical resections and radiotherapy of the primary intracranial HPC.
Keywords: Hemangiopericytoma, Metastasis, Surgery, Lumbar vertebrae
INTRODUCTION
Intracranial hemangiopericytoma (HPC) is a rare tumor with malignant features [1], of which incidence accounts for 0.5% of all primary central nervous system tumors [2]. Known to arise from Zimmermann pericytes of the meninges [3,4], HPC can occur in many locations of the central nervous system. Extracranial metastases have been reported in delayed fashion long after the primary lesions are diagnosed [5,6]. Long bone, liver, lung, central nervous system and abdominal cavity are the most commonly reported sites of metastasis [7]. However, metastasis to the vertebral bones from the intracranial HPC have been reported very rarely. In this report, we present the unique case of intracranial HPC metastasized to the lumbar spine 13 years after the initial diagnosis of the primary tumor, which has been managed successfully by repeated surgeries and radiosurgeries.
CASE REPORT
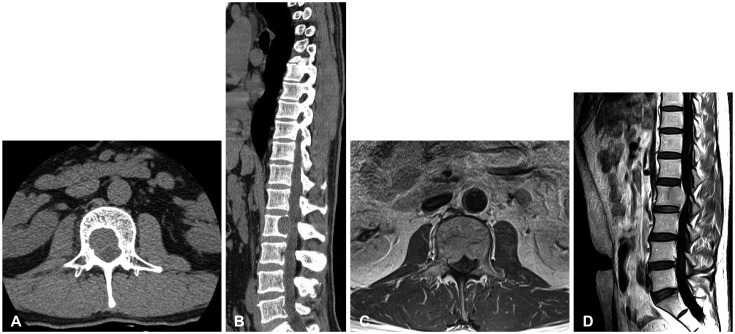
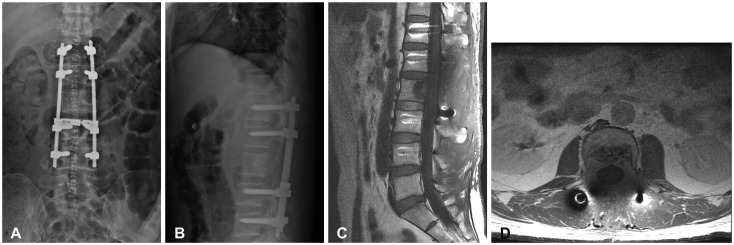
A 45-year-old man presented with a one-month history of progressive back pain and hypesthesia in both L3 dermatomes. There were no motor weakness. He had undergone a right temporo-occipital craniotomy and partial removal of a huge HPC followed by whole brain radiotherapy at other university hospital in 2003 (Fig. 1A). He underwent two more craniotomies with recurrent tumors removal at our institute in 2012 and 2013 (Fig. 1B). Thereafter, he has been treated by gamma knife radiosurgery three times successfully for local recurrent and residual HPC. Since then, no local recurrence has been found. In March 2016, however, the first extracranial metastasis was found in the L2 vertebral body. Diagnostic workup including thoracolumbar magnetic resonance imaging (MRI), bone scintigraphy and thoracolumbar computed tomography (CT) revealed a solitary L2 body lesion. CT scans showed well demarcated osteolytic mass in the posterior aspect of L2 vertebral body with retropulsion to anterior epidural space (Fig. 2A, B). T1- and T2-weighted MRIs showed an isointense tumor with strong contrast enhancement compressing the spinal cord (Fig. 2C, D). Bone scintigraphy also showed hot uptake in L2 body. In consideration of the patient's relatively young age and a solitary lesion at the L2 body, we planned subtotal en bloc corpectomy and radical removal of the tumor via a transpedicular approach. Angiography performed prior to surgery revealed hypervascular tumor supplied by multiple arteries from the bilateral L2 and right L1 segmental arteries. Preoperative embolization of the feeders was done successfully through a super-selection of bilateral L2 segmental arteries using gelform particles one day prior to surgery. The patient underwent a L2 total laminectomy and radical total removal of the tumor with subtotal corpectomy. At surgery, a gray and reddish tumor filled the L2 vertebral body, which was curetted and drilled out easily under surgical microscope, and the tumor cavity filled with minced autologous bone. There was no invasion of spinal dura mater. Trans-pedicular screw fixation was performed from T12 to L4 to prevent postoperative instability. Postoperative MRI and X-ray showed the tumor was completely removed (Fig. 3).
Fig. 1. Postcontrast axial T1-weighted magnetic resonance findings of intracranial HPC at preoperation and postoperation. A: Preoperative axial T1-weighted magnetic resonance imaging with gadolinium administration showing an intracranial HPC at the right temporo-occipital region. B: Postoperative axial T1-weighted magnetic resonance imaging with gadolinium administration showing gross total removal of the residual-recurrent intracranial HPC. HPC, hemangiopericytoma.
Fig. 2. Computed tomogram of the spine at the level of L2 reveals invasion of the tumor to vertebral body and anterior epidural space (A and B). T1-weighted image enhanced with gadolinium revealing an isointense lesion in posterior aspect of L2 vertebral body with retropulsion to anterior epidural space (C and D).
Fig. 3. Postoperative lateral & anteroposterior X-ray (A and B) and saggital & axial MRI (C and D). Grossly total removal of tumor at L2 vertebral body and replacement of bone graft. Transpedicular screw fixation from T12 to L4.
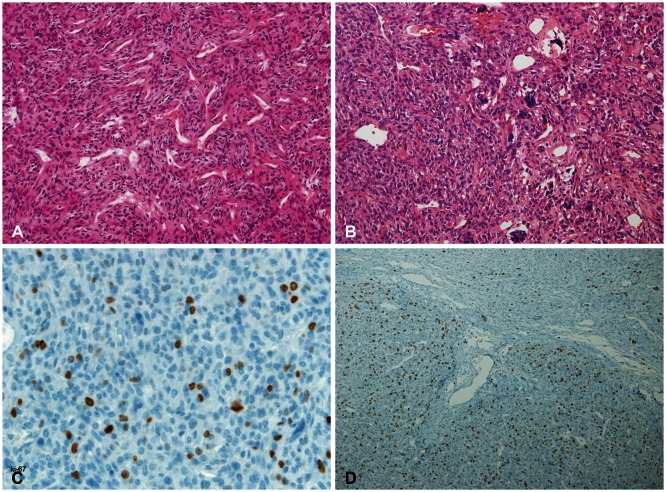
Histopathological finding revealed anaplastic HPC [World Health Organization (WHO) grade III] with numerous dilated, staghorn-type vessels with increased cellularity of the hyperchromatic tumor cells with oval nuclei which were surrounded by abundant blood vessels. Hematoxylin and eosin staining of metastatic spinal HPC (Fig. 4B) revealed more pronounced hypercellularity than intracranial HPC (Fig. 4A) and it showed increased cellular pleomorphism with mitotic count of ≥5 mitoses per 10 high-power fields in metastatic spinal HPC. Immunostaining of the tumor cells were negative for epithelial membrane antigen and CD34. The Ki-67 proliferation index of metastatic spinal HPC (Fig. 4D) was more increased by about 10% as compared with the cerebral lesion (Fig. 4C).
Fig. 4. Tumor histopathology. Right temporooccipital region of intracranial HPC (A and C) and metastatic spinal HPC (B and D) in 13 years later. Photomicrograph demonstrating a hemangiopericytoma with a highly cellular and vascular tumor consisting of compact neoplastic cells (A and B; H&E staining). Ki-67 proliferation index is more increased by about 10% in the metastatic spinal HPC as compared with intracranial HPC (C and D; Ki-67 immunohistochemistry). HPC, hemangiopericytoma.
Postoperatively, his back pain and hypesthesia in the both L3 dermatomes improved. Adjuvant radiotherapy was not undergone as the postoperative MR images confirmed complete tumor resection. He was discharged without neurological symptoms and has been recurrence-free for the past six months.
DISCUSSION
Extracranial metastatic tendency for the intracranial HPC has been well documented, with rates ranging from 14 to 50% [8]. However, reports of metastatic cases from the intracranial HPC to the vertebra have been extremely rare, so far. The most frequent site in the vertebrae is the vertebral body, because of its abundant vascularization and the presence of bone marrow inside [9].
Possible routes of spinal metastasis could be explained by three pathways: direct extension, via the lymphatics, and hematogenous pathway, which is the most frequent route. Pathogenetic mechanism of the vertebral body metastasis can be explained by the abundant tortuous vessels inside the vertebral body, which contribute to the easy deposits of the metastatic embolus [10]. The communication of the intracranial venous drain with the paravertebral venous plexus without any valve systems may contribute the intracranial HPC reach the spine via the venous routes. The characteristic anatomical connections between the spinal dura mater and the paravertebral venous plexus through the azygos and hemiazygous systems can be good routes for metastatic implantation in the spine. The well-known angiotropism of the HPC, the hemodynamic and the anatomical characteristics of the spinal circulation, and the persistence of the hematopoietic tissue inside the vertebral spongy bone may be the principal pathogenetic mechanisms for the spinal metastasis of the intracranial HPC [9,11,12,13].
Intracranial HPC has a strong tendency for local recurrence and not infrequent extracranial metastasis [3]. It is more commonly seen in adults, slightly higher number in males than in females, and the age of occurrence is younger than that of meningiomas [14]. So far, nine cases of metastatic HPC to the spine, including this case, have been reported. The mean duration between the onset of intracranial HPC and metastasis to the spine is 11.5 years, and the cervical, thoracic, and lumbar spine are equally involved (Table 1). In our case, it took 13 years until the first metastasis was detected. This data confirms that metastases can occur after many years in different locations with different intervals and may grow rapidly, even if the primary lesion has been well controlled. Therefore, spinal metastasis should be considered a possibility when a patient who had been treated previously for HPC presents with back or neck pain, and pain accompanied by weaknesses.
Table 1. Summary of reported cases of metastatic intracranial HPC to spine.
| Study | Age | Sex | Duration (years) | Metastasis site | Extent of resection | RTx | F/U (months) | Outcome | Local recurrence | Other metastasis site |
|---|---|---|---|---|---|---|---|---|---|---|
| Kruse [19] | 22 | F | 8 | Lumbar | Surgery (detail NA) | No | 60 | Death | No | Femur |
| Scott et al. [20] | 38 | M | 16 | T12/L1 | Surgery (detail NA) | Yes | 36 | PD | Yes | Temporal bone |
| 19 | Upper cervical | Surgery (detail NA) | No | 36 | Death | |||||
| Nonaka et al. [15] | 40 | F | 9.5 | T8 | Partial | Yes | 24 | PD | Yes | Lung/femur |
| Woitzik et al. [6] | 40 | F | 8 | C6-T2 | Complete | Yes | 12 | PD | No | Liver/femur |
| 9 | L2 | - | Yes | NA | NA | |||||
| Lee et al. [8] | 48 | F | 6.5 | C6-7 | Partial | Yes | 7 | PD | No | No |
| Taniura et al. [21] | 30 | F | 4 | L4-S1 | Partial | Yes | 12 | PD | No | No |
| Cole and Schmidt [22] | 36 | F | 17 | C3 | Complete | Yes | 48 | PD | Yes | Liver |
| Fukuda et al. [23] | 36 | M | 17 | T10 | Complete | No | 36 | NED | No | No |
| Our case | 45 | M | 13 | L2 | Complete | No | 6 | NED | No | No |
NA, not available; PD, progression of disease; NED, no evidence of disease; F/U, follow up
There are several diagnostic tools to detect metastatic intracranial HPC to the spinal column. Isotope bone scan is an easy and useful method in detecting bone metastasis in HPCs. Since extracranial osseous metastases occur not infrequently, bone scan became essential for early detection of the lesions, making the treatment easier. Considering false negative results, a single negative scan is not sufficient to exclude bone metastasis, so the examination should be repeated [15]. Finally, gadolinium enhanced MRI is considered the most sensitive diagnostic method of detecting spinal metastasis [8]. In addition, CD99 and bcl-2 by immunohistochemistry are diagnostic biomarkers which are frequently positive in intracranial HPC [16]. Detection of STAT6 nuclear expression or NAB2-STAT6 fusion is also highly recommended to confirm intracranial HPC [17].
Histologically, the patient's intracranial HPC rarely showed mitosis and homogeneous cells were distributed in relatively uniform fashion without cellular pleomorphism. On the contrary, 50% of tumors in metastatic spinal HPC showed higher cellularity than before and although necrosis was not observed, cellular plemorphism increased. Above all, mitosis, which is an important tumor grade showed as many as 15 in 10 high-power fields and Ki-67 labeling index also showed greater than 10%, which is a high figure. According to the WHO criteria, mitosis is a criteria in differentiating HPC and anaplastic HPC and when mitosis is more than 5, it is classified as anaplastic HPC, WHO grade III. Therefore, the patient was diagnosed with anaplastic HPC (WHO grade III) which is considered a more severe grade compared to intracranial HPC [14,18].
At surgery, the surgeon should be prepared for a substantial amount of bleeding during metastatic spinal tumor excision because of the high vascularity of HPCs. The amount of bleeding can increase the rate of surgery-related morbidity and mortality, and can be the greatest barrier to perform a gross total resection [6]. As in our case, preoperative embolization can be used to reduce a significant amount of blood loss. Thus, en bloc resection with preoperative embolization would be a good method to reduce operative blood loss and it may also decrease the risk of recurrence. Current recommendation of treatment for metastatic spinal HPC is gross total resection of tumor, when possible. Although its role is not well established, radiotherapy can be used in the post-operative adjuvant in case of residual or high-grade tumors [5,7]. In our case, the patient did not undergo additional radiotherapy. In this case, the boundary between HPC and normal bone was clear hence the tumor invading the vertebral body was removed. After that, drilling was performed beyond the normal bone margin. Thus, it was concluded that radical total resection of tumor was done. After the surgery local radiotherapy was considered as adjuvant treatment. However, as the tumor was radically removed in this case, a short term regular follow up was decided upon patient's consent. The patient has been followed via outpatient clinic with no tumor recurrence for the last 6 months.
In conclusion, intracranial HPC can metastasize to spine even after a complete resection and radiotherapy of the primary lesion. Therefore, it is important to monitor patients carefully on a long-term not only for the intracranial but also for extracranial metastases.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Fredriksson F, Nordborg C, Hallén T, Blomquist E. Haemangiopericytoma presenting with acute intracerebral haemorrhage--a case report and literature review. Acta Oncol. 2013;52:753–758. doi: 10.3109/0284186X.2012.716163. [DOI] [PubMed] [Google Scholar]
- 2.Dufour H, Métellus P, Fuentes S, et al. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery. 2001;48:756–762. doi: 10.1097/00006123-200104000-00011. discussion 762-3. [DOI] [PubMed] [Google Scholar]
- 3.Thomas HG, Dolman CL, Berry K. Malignant meningioma: clinical and pathological features. J Neurosurg. 1981;55:929–934. doi: 10.3171/jns.1981.55.6.0929. [DOI] [PubMed] [Google Scholar]
- 4.Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74. [PubMed] [Google Scholar]
- 5.Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. 2004;100:1491–1497. doi: 10.1002/cncr.20109. [DOI] [PubMed] [Google Scholar]
- 6.Woitzik J, Sommer C, Krauss JK. Delayed manifestation of spinal metastasis: a special feature of hemangiopericytoma. Clin Neurol Neurosurg. 2003;105:159–166. doi: 10.1016/s0303-8467(02)00140-3. [DOI] [PubMed] [Google Scholar]
- 7.Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25:514–522. [PubMed] [Google Scholar]
- 8.Lee JK, Kim SH, Joo SP, et al. Spinal metastasis from cranial meningeal hemangiopericytomas. Acta Neurochir (Wien) 2006;148:787–790. doi: 10.1007/s00701-006-0766-9. [DOI] [PubMed] [Google Scholar]
- 9.Batson OV. The function of the vertebral veins and their role in the spread of metastases. 1940. Clin Orthop Relat Res. 1995;(312):4–9. [PubMed] [Google Scholar]
- 10.Batson OV. The vertebral vein system. Caldwell lecture, 1956. Am J Roentgenol Radium Ther Nucl Med. 1957;78:195–212. [PubMed] [Google Scholar]
- 11.Cappuccio M, Gasbarrini A, Van Urk P, Bandiera S, Boriani S. Spinal metastasis: a retrospective study validating the treatment algorithm. Eur Rev Med Pharmacol Sci. 2008;12:155–160. [PubMed] [Google Scholar]
- 12.Arguello F, Baggs RB, Duerst RE, Johnstone L, McQueen K, Frantz CN. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65:98–106. doi: 10.1002/1097-0142(19900101)65:1<98::aid-cncr2820650121>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Lugassy C, Zadran S, Bentolila LA, et al. Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to intravascular cancer dissemination. Cancer Microenviron. 2014;7:139–152. doi: 10.1007/s12307-014-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonaka M, Kohmura E, Hirata M, Hayakawa T. Metastatic meningeal hemangiopericytoma of thoracic spine. Clin Neurol Neurosurg. 1998;100:228–230. doi: 10.1016/s0303-8467(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 16.Alawi F, Stratton D, Freedman PD. Solitary fibrous tumor of the oral soft tissues: a clinicopathologic and immunohistochemical study of 16 cases. Am J Surg Pathol. 2001;25:900–910. doi: 10.1097/00000478-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE. Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol. 1991;22:84–91. doi: 10.1016/0046-8177(91)90067-y. [DOI] [PubMed] [Google Scholar]
- 19.Kruse F., Jr Hemangiopericytoma of the meniges (angioblastic meningioma of Cushing and Eisenhardt). Clinico-pathologic aspects and follow-up studies in 8 cases. Neurology. 1961;11:771–777. doi: 10.1212/wnl.11.9.771. [DOI] [PubMed] [Google Scholar]
- 20.Scott M, Kellett G, Peale A. Angioblastic meningioma (hemangiopericytoma) of the cerebellar fossa with metastases to the temporal bone and the lumbar spine. Surg Neurol. 1974;2:35–38. [PubMed] [Google Scholar]
- 21.Taniura S, Taniguchi M, Mizutani T, Takahashi H. Metastatic hemangiopericytoma to the cauda equina: a case report. Spine J. 2007;7:371–373. doi: 10.1016/j.spinee.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Cole CD, Schmidt MH. Hemangiopericytomas of the spine: case report and review of the literature. Rare Tumors. 2009;1:e43. doi: 10.4081/rt.2009.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda Y, Watanabe K, Toyama Y, Mikami S, Matsumoto M. Metastasis of intracranial meningeal hemangiopericytoma to thoracic spine 17 years after surgical excision: a case report. J Orthop Sci. 2015;20:425–429. doi: 10.1007/s00776-013-0450-x. [DOI] [PubMed] [Google Scholar]