Abstract
Background
Non-functioning pituitary adenomas (NFPA) invading into the cavernous sinus are surgically challenging. To decrease recurrence rate, surgeon makes a strong endeavor to resect tumor gross totally. However, gross total resection (GTR) is difficult to achieve with cavernous sinus invasion. Recently, a new classification system for cavernous invasion of pituitary adenomas was suggested. The aim of this study is to validate this new classification system and to identify limitations and considerations in designing treatment strategies for patients with NFPA involving the cavernous sinus.
Methods
Between January 2000 and January 2012, 275 patients who underwent operation for NFPA were enrolled in the study. Median age was 50 years (15–79 years). There were 145 males and 130 females. The median follow-up duration was 4 years (range 1–12.5 years).
Results
Related to extent of tumor removal, GTR was obtained in 184 patients (66.9%), near total resection (NTR) was obtained in 45 patients (16.3%), and sub-total resection (STR) was obtained in 46 patients (16.7%) of a total 275 patients. There were statistically significant differences between the extent of resection and the new Knosp classification (p<0.001). In the high-grade group of the new Knosp classification, there was no difference in recurrence between patients who underwent GTR or NTR only and those who underwent STR with adjuvant radiation therapy (p=0.515).
Conclusion
In case of high risk of surgical complications, STR with adjuvant radiation therapy can be considered as an alternative strategy for safe treatment of cavernous-invading adenomas.
Keywords: Pituitary adenoma, Cavernous sinus, Recurrence, Radiation therapy
INTRODUCTION
Transsphenoidal surgery is the first-choice treatment modality for most pituitary adenomas. Because of its low invasiveness and high success rate, transsphenoidal surgery can be appropriately applied to microadenomas and also to macroadenomas with parasellar extension. Lesions involving the cavernous sinus are surgically challenging due to the close proximity of critical neurovascular structures and the deep intracranial location. Visualization and dissection of the complex anatomy, including the carotid artery and its branches and numerous cranial nerves, are often hindered by robust blood flow in this space. Although technical advances have increased the ability to operate within the cavernous sinus [1], the region remains treacherous even in the hands of experienced surgeons [2].
Invasion into the cavernous sinus is the most common cause for incomplete resection, resulting in higher recurrence rate. To prevent high recurrence from residual tumors, radiotherapy or radiosurgery have been widely recommended in cases of subtotal or partial tumor removal [3,4,5,6,7]. Treatment strategies must be established early whether an intended subtotal resection (STR) is followed by adjuvant radiotherapy or a tumor is removed completely. This decision is critical due to the high propensity for recurrence in non-functioning pituitary adenomas (NFPA) involving cavernous-invading adenomas.
A grading system proposed by Knosp et al. [8] for evaluating parasellar extension of the tumor has been widely used to evaluate carvernous sinus invasion (CSI) on preoperative magnetic resonance (MR) imaging [9,10,11,12]. A new modified Knosp classification proposed by Micko et al. [13] suggests the addition of grade 3A and 3B to distinguish differential outcomes of adenomas invading between the superior and inferior cavernous sinus compartments. Previously, Ceylan et al. [14] also suggested a similar classification for evaluating tumor extension within lateral and medical corridors.
The aim of this study was to validate the new classification and to identify any limitations and considerations in designing treatment strategies for patients with NFPA involving the cavernous sinus.
MATERIALS AND METHODS
We performed a retrospective analysis of 542 operative cases of NFPA that were performed in our department between January 2000 and January 2012. Symptomatic patients with NFPA underwent surgery, although those with large mass incidentally found on MRI also underwent surgery. Patients with insufficient data, [loss of medical record, loss of MRI or poor image quality (n=42), follow-up less than a year (n=204), and, those initially treated at another institution (n=21)] were excluded. A total of 275 patients were enrolled in this study.
For all patients, postoperative CT scans were taken the day after surgery in order to detect hemorrhagic complications, and postoperative MRI was obtained at 6 months. All pre- and post-operative images were retrospectively reviewed. Extent of resection was categorized as 1) gross total resection (GTR) (no residual enhancing mass on post-operative MR image), 2) near total resection (suspicious residual enhancing mass on post-operative MR image, or residual mass less than 10% of the initial size), and 3) sub-total resection (significant residual mass more than 10% of initial size). Regrowth of a remnant tumor was regarded as a recurrence because usually most recurrent pituitary tumors originate from remnant tumors. The study protocol was reviewed and approved by the Institutional Review Board of Samsung Medical Center (SMC 2016-04-163), and adhered to the recommendations of the Declaration of Helsinki for biomedical research involving human subjects (1975).
Assessment of invasion into the cavernous sinus
Cavernous sinus invasion was evaluated by the new Knosp classification [13]. The Knosp grading system includes five categories: 1) grade 0, no invasion with all of the lesion medial to the cavernous carotid artery; 2) grade 1, invasion extending to, but not past, the medial aspect of the cavernous carotid artery; 3) grade 2, invasion extending to, but not past, the lateral aspect of the cavernous carotid artery; 4) grade 3, invasion past the lateral aspect of the cavernous carotid artery, but not completely filling the cavernous sinus; and 5) grade 4, tumor completely filling the cavernous sinus both medial and lateral to the cavernous carotid artery [10]. The new Knosp classification subdivides grade 3 into grade 3A (superior cavernous sinus compartment) and grade 3B (inferior cavernous sinus compartment) [13]. All images were evaluated by consensus in a blinded fashion by three different board-certified radiologists and neurosurgeons (ST Kim, DS Kong & MH Lee). In this study, grade 0, 1, and 2 cavernous invading adenomas were regarded as low-grade cavernous invading adenomas. grade 3 and 4 adenomas were classified as high-grade cavernous invading adenomas.
Extent of resection
GTR refers to no residual mass (100% volumetric resection) determined radiographically on postoperative MR imaging. Near-total resection (NTR) indicates >90% volumetric resection while STR indicates <90% volumetric resection.
Technique of adjuvant radiotherapy
Adjuvant radiotherapy was considered within 6 months of surgery. Sixteen patients were treated with adjuvant radiotherapy. Ten patients received conventional radiotherapy with a daily fraction of 2 Gy. The median total dose was 54 Gy (range, 50–60 Gy). Six patients underwent stereotactic radiosurgery (SRS) with single section. The median marginal dose was 14 Gy/50% (range, 13–22 Gy).
Statistical analysis
Cohort summary data were presented using median (range) and number (%), where appropriate. Categorical characteristics were compared using the chi-square test or Fisher's exact test. Progression-free survival (PFS) was compared using a log-rank test. The inter-observer reliability analysis of the new Knosp classification was performed using weighted kappa. Inter-observer reliability for the new Knosp classification ranged from 0.820 to 0.905. The level of agreement was near perfect [15]. p-values were considered significant at the level of 0.05. Bonferroni's correction was used for p-values and confidence intervals of weighted κ. Statistical analyses were carried out using commercial software (SPSS, version 20; IBM, Armonk, NY, USA).
RESULTS
Patient and tumor characteristics
A retrospective analysis of 275 cases of NFPA that were performed in our department was conducted. The median age was 50 years (15–79 years). There were 145 males and 130 females. Median follow-up duration was 4 years (range, 1–12.5 years). The presenting signs and symptoms were visual symptoms in 144 patients (52.3%) and headache in 90 patients (32.7%). Thirty-seven patients (13.4%) were diagnosed incidentally. The median tumor size was 28 mm (range: 9.3–90.1 mm). Eighty-one tumors (29.4%) extended to the supra-sellar region and reached the 3rd ventricle, ten tumors (3.6%) extended to the infra-sellar region with invasion of the sellar turcica. According to the new Knosp classification, grade 0 was reported in 23 patients (8.4%), grade 1 in 103 patients (37.4%), grade 2 in 83 patients (30.0%), grade 3A in 40 patients (14.5%), grade 3B in 9 patients (3.2%), and grade 4 in 17 patients (6.1%) (Table 1). In this series, there was cerebrospinal fluid leakage in one patient. Four patients had intracranial hemorrhage. Of these, three patients recovered without any neurologic deficits after re-operation, but one patient progressed to a vegetative state. Postoperative intracranial infection was found in two patients, of whom one patient expired due to severe ventriculitis.
Table 1. Clinical characteristics of tumors and patients with non-functioning pituitary adenomas.
| Variables | No. of patients |
|---|---|
| Number, sex (male:female) | 275 (145:130) |
| Age, yr (median, range) | 50 (15–79) |
| Clinical follow-up, yr (median, range) | 4.0 (1.0–12.5) |
| Initial symptoms, n (%) | |
| Visual symptom | 144 (52.3) |
| Headache | 90 (32.7) |
| Incidental | 37 (13.4) |
| Tumor size, mm (median, range) | 28 (9.3–90.1) |
| Radiologic findings, n (%) | |
| Supra-sellar extension | 81 (29.4) |
| Infra-sellar invasion | 10 (3.6) |
| Residual tumor, n (%) | |
| Gross total resection | 184 (66.9) |
| Near total resection | 45 (16.3) |
| Sub-total resection | 46 (16.7) |
| New Knosp classification, n (%) | |
| Grade 0 | 23 (8.4) |
| Grade 1 | 103 (37.4) |
| Grade 2 | 83 (30.0) |
| Grade 3A | 40 (14.5) |
| Grade 3B | 9 (3.2) |
| Grade 4 | 17 (6.1) |
Tumor resection according to the new Knosp classification system
Related to extent of tumor removal, GTR was obtained in 184 patients (66.9%), NTR was obtained in 45 patients (16.3%), and STR was obtained in 46 patients (16.7%) of a total 275 patients. Surgical outcomes are given in Table 2. There were statistically significant differences between the extent of resection and the new Knosp classification (Fisher's exact test with Monte Carlo method, p<0.001). According to the new Knosp classification, cohorts were categorized as low grade (grade 1 and 2) and high grade (grade 3A, 3B, and 4). There were differences in surgical outcomes between the low- and high-grade groups (chi-square test, p<0.001). Comparing extent of resection between tumors of grade 3A and grade 3B, the GTR rate was 50% in grade 3A and 44.4% in grade 3B, but there was no statistically significant difference (chi-square test, p=0.508).
Table 2. Tumor resection according to the new Knosp classification system.
| New Knosp classification | Extent of resection (n, % among Knosp classification) | p-value | ||
|---|---|---|---|---|
| Gross total resection | Near total resection | Subtotal resection | ||
| Grade 0 | 19 (82.6) | 1 (4.3) | 3 (13.0) | <0.001 |
| Grade 1 | 79 (76.7) | 18 (17.5) | 6 (5.8) | |
| Grade 2 | 61 (73.5) | 15 (18.1) | 7 (8.4) | |
| Grade 3A | 20 (50.0) | 10 (25.0) | 10 (25.0) | |
| Grade 3B | 4 (44.4) | 1 (11.1) | 4 (44.4) | |
| Grade 4 | 1 (5.9) | 0 (0) | 16 (94.1) | |
Tumor progression
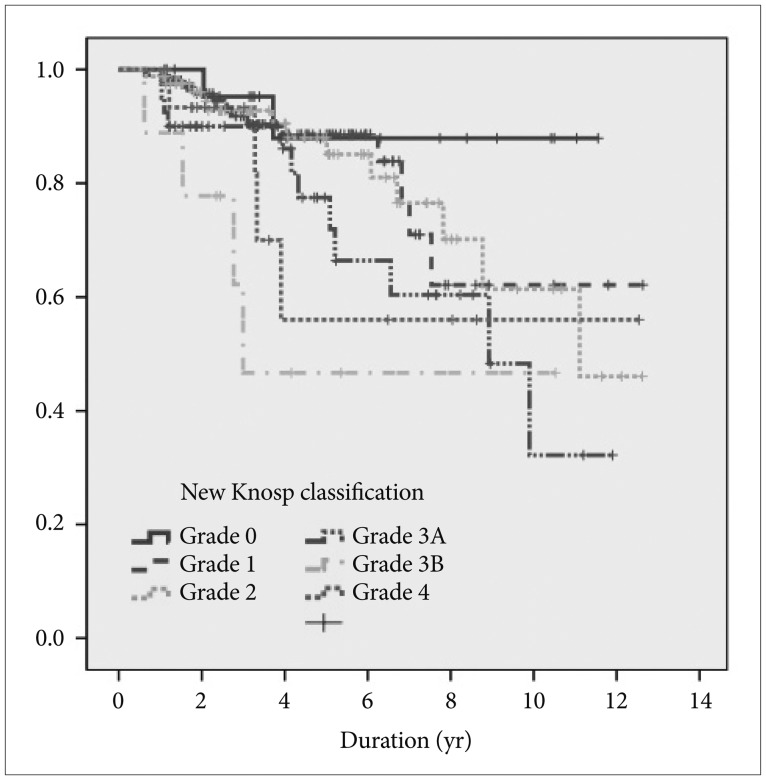
PFS was 80% at 5 years and 60% at 10 years for the entire cohort of cases. A total of 169 of 184 patients (91.8%) who underwent GTR had no evidence of residual lesions on post-operative imaging. However, 15 patients (8.1%) experienced recurrence during the follow-up period. Of 45 patients who underwent NTR, 4 patients went on to be treated with radiation as an adjuvant therapy and none of the tumors recurred. However, 12 of 41 other patients (29.2%) who did not receive adjuvant treatment experienced recurrence. During the follow-up period, the other 29 patients (70.8%) experienced no recurrence without receiving adjuvant therapy. Of the 46 patients who underwent STR, 12 patients received radiotherapy as an adjuvant treatment, one of whom had recurrence (8.3%). A total of 18 of 34 other patients (52.9%) who did not receive adjuvant treatment experienced recurrence. There were no recurrences in the remaining 16 patients (47.1%) without adjuvant therapy during the follow-up period. The overall median survival time was not reached in grades 0, 1, and 4 of the new Knosp classification. Median survival time was 1.6 years in grade 2, 1.3 years in grade 3A, and 0.4 years in grade 3B. In terms of median survival, there was a statistically significant difference between the groups (log-rank test, p=0.042) (Fig. 1).
Fig. 1. Kaplan-Meier survival curve according to the new Knosp classification system.
Surgical resection in high-grade cavernous invading tumors
In a comparison of the grade 3A group vs. grade 3B and grade 4 groups, GTR or NTR rate was higher in grade 3A than in grades 3B and 4 (grade 3A: 30 cases, 75% vs. grade 3B and grade 4: 6 cases, 23%). There was a statistically significant difference between the groups (chi-square test, p<0.001). Although the GTR or NTR rates were higher in grade 3A, survival analysis showed no difference between the two groups (log-rank test, p=0.493). In a comparison of grade 3A and grade 3B only, the GTR or NTR rate was higher in grade 3A than in grade 3B (grade 3A: 30 cases, 75% vs. grade 3B, 5 cases, 55.5%). However, there was no significant difference (Fisher's exact test, p=0.220; log-rank test, p=0.285). In the 66 patients of the high-grade group, 25 patients (37.8%) received GTR and 11 patients (16.6%) received NTR. STR was performed in 30 patients (45.4%), and 10 patients underwent STR with radiation therapy (RT) or SRS.
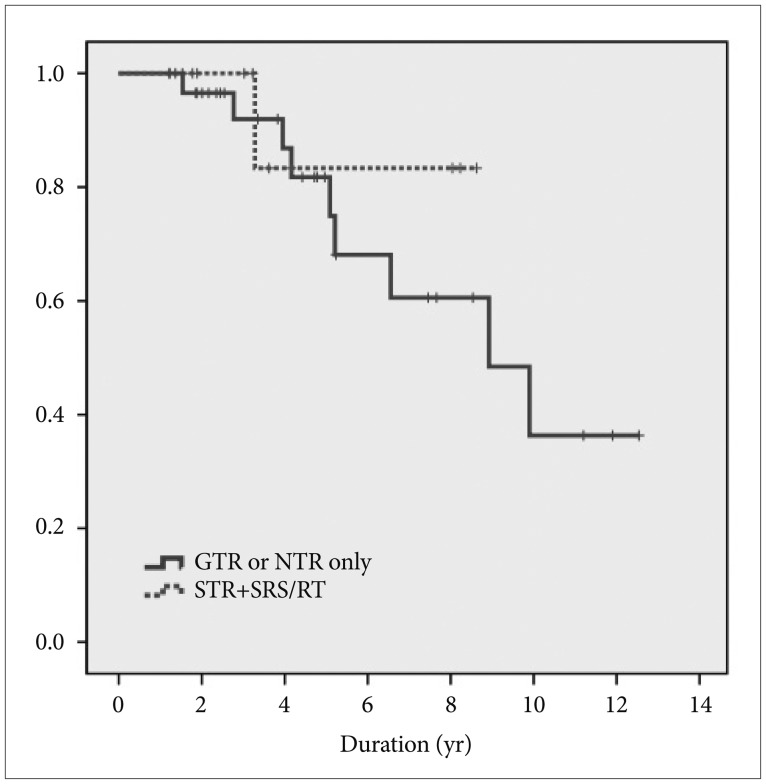
Recurrence rates in patients who underwent GTR or NTR were compared to the recurrence rate of patients who underwent STR with adjuvant RT. Of 36 patients who received GTR or NTR in the high-grade group, nine patients experienced recurrence (25%). Of 10 patients who underwent STR with adjuvant RT, only one patient experienced recurrence (10%). In the high-grade group, there was no difference in recurrence between patients who underwent GTR or NTR only and those who underwent STR with adjuvant RT (log-rank test, p-value=0.515) (Fig. 2). These data indicate that adjuvant RT is as effective as receiving only GTR or NTR for patients who undergo STR.
Fig. 2. Kaplan-Meier survival curve between GTR or NTR only and STR with SRS/RT in high-grade new Knosp classification (grade 3A, 3B, and 4) disease. GTR, gross total resection; STR, subtotal resection; NTR, near total resection; SRS, stereotactic radiosurgery; RT, radiotherapy.
DISCUSSION
Extent of resection and cavernous sinus invasion
This study is a retrospective analysis of a larger cohort of patients with long-term follow-up after surgery for NFPA. To decrease recurrence rate, surgeon makes a strong endeavor to resect tumor gross totally. However, GTR is difficult to achieve with cavernous sinus invasion, as safe complete resection lateral to the carotid artery remains nearly impossible. Radical dissection of low-grade invading tumors can be safely achieved with low morbidity, whereas high-grade tumors are high risk when pursued aggressively [10]. In a previous study, the extent of tumor resection varies significantly based on Knosp classification. Ceylan et al. [16] and Zhao et al. [11] reported only 65% and 62% of GTR rate from high CSI cases, respectively (Knosp grade 3, 4).
Considering the new classification system, the GTR rate might be higher in grade 3A than grade 3B. However, there was no significant difference between grades 3A and 3B in the present study. Using conventional microscopic transsphenoidal surgery, a surgeon would not aggressively remove the mass over the cavernous sinus. Microscopic cavernous sinus invasion was difficult to identify due to limited visualization. Tumors extending beyond the sella and invading the cavernous sinus were not considered cured by microscopic transsphenoidal surgery alone [17]. Thus, preoperative prediction for the feasibility of GTR through MR imaging is essential for cavernous-invading adenomas. The recent introduction of endoscopes into trans-sphenoidal surgery, combined with new extended and expanded approaches, has prompted skull base surgeons to reconsider the classic limitations of trans-sphenoidal approaches [18]. Angled endoscopes allow a more panoramic and tailored view of the surgical field. Woodworth et al. [10] described that high-grade (Knosp classification 3 or 4) cases that extend in a medial to lateral trajectory can be removed by endoscopic techniques. Ceylan et al. [16] also reported that more challenging cavernous sinus invasive tumors, such as grade 3A, can be removed by GTR with an endoscopic approach.
Role of adjuvant radiation therapy
Radiotherapy and radiosurgery have been helpful in preventing recurrence from residual NFPA [19,20]. In previous studies, patients who underwent surgery alone had a 48 to 94% 5-yr PFS and a 14 to 56% 10-yr PFS [3,6,20,21,22,23,24,25]. For patients who received adjuvant RT, 5-yr PFS was reported to range from 93 to 97% [3,4,5,7,24,26,27]. Previous studies recommended adjuvant SRS in patients with NFPA if postoperative follow-up MR images showed tumor recurrence or residual regrowth [11,26,27].
This study compared the clinical outcomes of patients who underwent GTR or NTR only and those who underwent STR with adjuvant RT for high-grade cavernous invading adenomas (Fig. 3). In the high-grade Knosp classification group (grade 3A, 3B, and 4), the two treatment modalities had no differences in outcome (GTR or NTR only vs. STR with adjuvant RT). In grade 3A patients who underwent GTR or NTR alone, 7 of the 28 patients (25.0%) eventually recurred (5-yr PFS, 88.2%; 10-yr PFS, 31.6%). This result suggests that GTR or NTR alone is not enough to control tumors in the high-grade group, even though surgeons attempt to achieve safe complete resection. Despite efforts to predict cavernous sinus invasion on pre-operative MR imaging, invasion cannot be ignored even with low-grade Knosp classification [8,9,12,28,29]. In addition, the authors suggest that adjuvant RT may be necessary in these groups although patients have already undergone GTR or NTR.
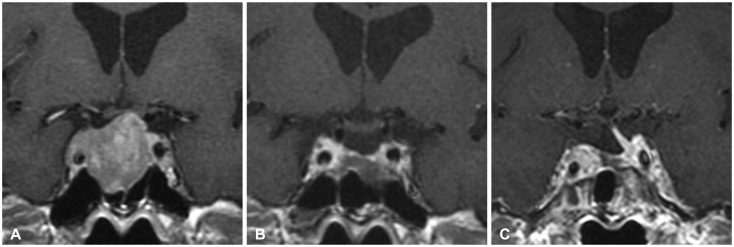
Fig. 3. A Knosp grade 3A macroadenoma in a 61-year-old man. A: Preoperative image revealing the tumor extending into cavernous sinus. B: Postoperative image showing gross total resection. C: Without any adjuvant treatment, the tumor was recurred 4 years after the operation.
This study has some limitations because treatment modalities were entirely dependent upon the microscopic technique. Microscopic views are limited in viewing structures within the cavernous sinus. We have converted to using an endoscopic transnasal approach rather than microscopic surgery and we believe that better outcomes will be achieved in high-grade cavernous invading adenomas in the future.
Illustrative cases
Case 1
A 61-yr-old male patient visited our department presenting with headache. Pre-operative MR image shows microadenoma with cavernous sinus invasion (Knosp grade 3A). The patient received operation via transsphenoidal approach, and 2 years after the operation, the MRI showed no residual tumor. 4 years after the operation, the patient visited our institute with headache and the MRI showed recurred tumor in superomedial portion of right cavernous sinus. The patient received gamma knife radiosurgery for adjuvant treatment (Fig. 3).
Case 2
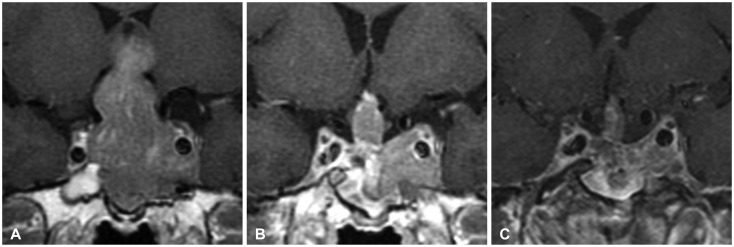
A 44-yr-old male patient visited our department presenting with diplopia. Pre-operative MR image shows macroadenoma with cavernous sinus invasion (Knosp grade 4). The patient received operation via transsphenoidal approach and received radiation therapy for remnant tumor. 3 months after the operation, the MRI showed residual tumor in the suprasellar area and left cavernous sinus. 9 years after the operation, the patient visited our institute and the MRI showed no growth in the treated region (Fig. 4).
Fig. 4. A Knosp grade 4 macroadenoma in a 44-year-old man. A: Preoperative image showing marked extension of tumor to supra sellar and cavernous sinus. B: Postoperative image showing residual tumor to receive a radiation therapy. C: No significant change 9 years after the operation.
In conclusion, in this study, there was a relatively high recurrence in patients within the high-grade Knosp classification group who underwent GTR or NTR alone. STR with adjuvant RT had a compatible outcome to GTR or NTR alone. In cases at high risk of surgical complications, STR with adjuvant RT can be considered as an alternative strategy for safe treatment of cavernous invading tumors. If GTR was archived on postoperative MR images, the possibility of residual tumor should be considered with close follow-up and imaging study. When recurrence or residual masses are identified, adjuvant RT can be considered for control of tumor growth.
According to the new Knosp classification, there was no statistically significant difference between grade 3A and 3B, as the present study is based on the conventional microscopic transsphenoidal surgery. Further studies with endoscopic surgery will be required to may bring about a result with a difference between grade 3A and 3B.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Krayenbühl N, Hafez A, Hernesniemi JA, Krisht AF. Taming the cavernous sinus: technique of hemostasis using fibrin glue. Neurosurgery. 2007;61(3 Suppl):E52. doi: 10.1227/01.neu.0000289712.72555.9c. discussion E52. [DOI] [PubMed] [Google Scholar]
- 2.DeMonte F, Smith HK, al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245–251. doi: 10.3171/jns.1994.81.2.0245. [DOI] [PubMed] [Google Scholar]
- 3.Chang EF, Zada G, Kim S, et al. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J Neurosurg. 2008;108:736–745. doi: 10.3171/JNS/2008/108/4/0736. [DOI] [PubMed] [Google Scholar]
- 4.Brada M, Rajan B, Traish D, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf) 1993;38:571–578. doi: 10.1111/j.1365-2265.1993.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 5.Gittoes NJ, Bates AS, Tse W, et al. Radiotherapy for non-function pituitary tumours. Clin Endocrinol (Oxf) 1998;48:331–337. doi: 10.1046/j.1365-2265.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 6.Lillehei KO, Kirschman DL, Kleinschmidt-DeMasters BK, Ridgway EC. Reassessment of the role of radiation therapy in the treatment of endocrine-inactive pituitary macroadenomas. Neurosurgery. 1998;43:432–438. doi: 10.1097/00006123-199809000-00020. discussion 438-9. [DOI] [PubMed] [Google Scholar]
- 7.McCollough WM, Marcus RB, Jr, Rhoton AL, Jr, Ballinger WE, Million RR. Long-term follow-up of radiotherapy for pituitary adenoma: the absence of late recurrence after greater than or equal to 4500 cGy. Int J Radiat Oncol Biol Phys. 1991;21:607–614. doi: 10.1016/0360-3016(91)90677-v. [DOI] [PubMed] [Google Scholar]
- 8.Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33:610–617. doi: 10.1227/00006123-199310000-00008. discussion 617-8. [DOI] [PubMed] [Google Scholar]
- 9.Vieira JO, Jr, Cukiert A, Liberman B. Evaluation of magnetic resonance imaging criteria for cavernous sinus invasion in patients with pituitary adenomas: logistic regression analysis and correlation with surgical findings. Surg Neurol. 2006;65:130–135. doi: 10.1016/j.surneu.2005.05.021. discussion 135. [DOI] [PubMed] [Google Scholar]
- 10.Woodworth GF, Patel KS, Shin B, et al. Surgical outcomes using a medial-to-lateral endonasal endoscopic approach to pituitary adenomas invading the cavernous sinus. J Neurosurg. 2014;120:1086–1094. doi: 10.3171/2014.1.JNS131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B, Wei YK, Li GL, et al. Extended transsphenoidal approach for pituitary adenomas invading the anterior cranial base, cavernous sinus, and clivus: a single-center experience with 126 consecutive cases. J Neurosurg. 2010;112:108–117. doi: 10.3171/2009.3.JNS0929. [DOI] [PubMed] [Google Scholar]
- 12.Nishioka H, Fukuhara N, Horiguchi K, Yamada S. Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: predictive factors, strategies, and outcomes. J Neurosurg. 2014;121:505–510. doi: 10.3171/2014.3.JNS132214. [DOI] [PubMed] [Google Scholar]
- 13.Micko AS, Wöhrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. 2015;122:803–811. doi: 10.3171/2014.12.JNS141083. [DOI] [PubMed] [Google Scholar]
- 14.Ceylan S, Anik I, Koc K. A new endoscopic surgical classification and invasion criteria for pituitary adenomas involving the cavernous sinus. Turk Neurosurg. 2011;21:330–339. doi: 10.5137/1019-5149.JTN.4149-11.0. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 16.Ceylan S, Koc K, Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010;112:99–107. doi: 10.3171/2009.4.JNS09182. [DOI] [PubMed] [Google Scholar]
- 17.Taussky P, Kalra R, Coppens J, Mohebali J, Jensen R, Couldwell WT. Endocrinological outcome after pituitary transposition (hypophysopexy) and adjuvant radiotherapy for tumors involving the cavernous sinus. J Neurosurg. 2011;115:55–62. doi: 10.3171/2011.2.JNS10566. [DOI] [PubMed] [Google Scholar]
- 18.Juraschka K, Khan OH, Godoy BL, et al. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J Neurosurg. 2014;121:75–83. doi: 10.3171/2014.3.JNS131679. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan JP, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg. 2002;97(5 Suppl):408–414. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 20.Lee MH, Lee JH, Seol HJ, et al. Clinical concerns about recurrence of non-functioning pituitary adenoma. Brain Tumor Res Treat. 2016;4:1–7. doi: 10.14791/btrt.2016.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenman Y, Ouaknine G, Veshchev I, Reider-Groswasser II, Segev Y, Stern N. Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clin Endocrinol (Oxf) 2003;58:763–769. doi: 10.1046/j.1365-2265.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 22.Bradley KM, Adams CB, Potter CP, Wheeler DW, Anslow PJ, Burke CW. An audit of selected patients with non-functioning pituitary adenoma treated by transsphenoidal surgery without irradiation. Clin Endocrinol (Oxf) 1994;41:655–659. doi: 10.1111/j.1365-2265.1994.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 23.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236. doi: 10.1097/00006123-199702000-00001. discussion 236-7. [DOI] [PubMed] [Google Scholar]
- 24.Park P, Chandler WF, Barkan AL, et al. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery. 2004;55:100–106. doi: 10.1227/01.neu.0000126885.71242.d7. discussion 106-7. [DOI] [PubMed] [Google Scholar]
- 25.Turner HE, Stratton IM, Byrne JV, Adams CB, Wass JA. Audit of selected patients with nonfunctioning pituitary adenomas treated without irradiation - a follow-up study. Clin Endocrinol (Oxf) 1999;51:281–284. doi: 10.1046/j.1365-2265.1999.00865.x. [DOI] [PubMed] [Google Scholar]
- 26.Flickinger JC, Nelson PB, Martinez AJ, Deutsch M, Taylor F. Radiotherapy of nonfunctional adenomas of the pituitary gland. Results with long-term follow-up. Cancer. 1989;63:2409–2414. doi: 10.1002/1097-0142(19890615)63:12<2409::aid-cncr2820631206>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Gittoes NJ. Radiotherapy for non-functioning pituitary tumors--when and under what circumstances? Pituitary. 2003;6:103–108. doi: 10.1023/b:pitu.0000004801.95086.e2. [DOI] [PubMed] [Google Scholar]
- 28.Cottier JP, Destrieux C, Brunereau L, et al. Cavernous sinus invasion by pituitary adenoma: MR imaging. Radiology. 2000;215:463–469. doi: 10.1148/radiology.215.2.r00ap18463. [DOI] [PubMed] [Google Scholar]
- 29.Dickerman RD, Oldfield EH. Basis of persistent and recurrent Cushing disease: an analysis of findings at repeated pituitary surgery. J Neurosurg. 2002;97:1343–1349. doi: 10.3171/jns.2002.97.6.1343. [DOI] [PubMed] [Google Scholar]