Abstract
Objective
Currently available measles vaccines are administered by subcutaneous injections and require reconstitution with a diluent and a cold chain, which is resource intensive and challenging to maintain. To overcome these challenges and potentially increase vaccination coverage, microneedle patches are being developed to deliver the measles vaccine. This study compares the cost-effectiveness of using microneedle patches with traditional vaccine delivery by syringe-and-needle (subcutaneous vaccination) in children’s measles vaccination programs.
Methods
We built a simple spreadsheet model to compute the vaccination costs for using microneedle patch and syringe-and-needle technologies. We assumed that microneedle vaccines will be, compared with current vaccines, more heat stable and require less expensive cool chains when used in the field. We used historical data on the incidence of measles among communities with low measles vaccination rates.
Results
The cost of microneedle vaccination was estimated at US$0.95 (range US$0.71–US$1.18) for the first dose, compared with US$1.65 (range US$1.24–US$2.06) for the first dose delivered by subcutaneous vaccination. At 95 % vaccination coverage, microneedle patch vaccination was estimated to cost US$1.66 per measles case averted (range US$1.24–US$2.07) compared with an estimated cost of US$2.64 per case averted (range US$1.98–US$3.30) using subcutaneous vaccination.
Conclusions
Use of microneedle patches may reduce costs; however, the cost-effectiveness of patches would depend on the vaccine recipients’ acceptability and vaccine effectiveness of the patches relative to the existing conventional vaccine-delivery method. This study emphasizes the need to continue research and development of this vaccine-delivery method that could boost measles elimination efforts through improved access to vaccines and increased vaccination coverage.
Electronic supplementary material
The online version of this article (doi:10.1007/s40268-016-0144-x) contains supplementary material, which is available to authorized users.
Key Points
| Use of microneedle patch technology in measles vaccination programs potentially reduces costs and extends vaccine coverage in hard-to-reach communities. |
| Acceptability of new technology relative to the conventional vaccine-delivery method is one of the key elements of cost-effectiveness of the microneedle patch. |
Introduction
The measles vaccine has been available since 1963, and following its widespread use, global estimated measles deaths have decreased dramatically from 2.6 million in 1980 to 145,700 in 2013 [1, 2]. Following substantial decreases in measles incidence, an expert advisory panel was convened by the World Health Organization (WHO) and concluded that measles can and should be eradicated. In 2012, a group of experts identified the research priorities for global measles control and eradication including the need for innovative strategies for increasing vaccination coverage and improving vaccine delivery [3].
Currently available measles vaccines are commonly packaged in multi-dose vials of lyophilized dried powder that require a well-functioning cold chain (i.e., 2–8 °C) for transportation and storage [4, 5]. These vaccines then require reconstitution with a diluent, before being administered by subcutaneous injection using a conventional syringe and needle. Once the vial is opened and the vaccine is reconstituted, it has a shelf life of approximately 6 h, after which unused reconstituted vaccine must be discarded. Discarding such unused vaccine can lead to notable levels of wastage. Therefore, delivering the vaccine requires a complex logistical system and well-trained healthcare personnel. To address such shortcomings, new methods of vaccine delivery are being researched and developed, such as aerosolized vaccines, dry powder, and microneedle patches [6].
We focus this analysis on microneedle patches, which have shown particular promise to date [4, 6–8]. These patches consist of micron-scaled needles made of either metal or a polymer. The metal needles are pre-coated with the vaccine, while the polymer needles deliver the vaccine by dissolving into the skin (i.e., dermis and epidermis) [4, 6, 7]. Microneedle patches are likely to be fabricated with an inexpensive plastic applicator that provides an audible feedback (a snapping noise) when a user correctly uses the device on a patient. Thus, the microneedle patch may eliminate the need for in-the-field vaccine reconstitution prior to administration, reducing the need for sharps handling and waste disposal, and thus reducing vaccine wastage. The simplicity of using patches can also potentially reduce program costs because patients can be vaccinated by minimally trained health personnel or perhaps even self-administered by a patient or a patient’s parent or caregiver [4, 8]. In addition, preliminary findings have shown that the microneedle patches will be more thermostable than currently available vaccines [4], reducing necessary cold chain volumes and easing transportation. Prototype microneedle patches, containing the measles vaccine, successfully generated neutralizing antibody responses in a small animal model [4]. In light of these positive results, we assessed the cost-effectiveness of using the microneedle patch vs. a subcutaneous injection in measles vaccination programs, primarily for low- and middle-income countries. This technology may also be suitable for vaccinating against other diseases. This analysis will aid practicing public health officials, and decision makers in organizations who are contemplating funding investments in the research and development of the microneedle technology.
Methods
We built a simple spreadsheet-based, incidence-of-measles model (Electronic Supplementary Material Appendix 1) to calculate the potential vaccinations and related costs for a hypothetical population of approximately 11 million that consists of 1,000,000 children (9.2 % of the total population) under 5 years of age. Actual size of the population and the birth cohort do not impact the findings and conclusion of our analysis because the costs and efficiency of two different vaccine-delivery methods are the primary focus of this research. We used historical data recording the incidence of measles among communities where children had low, or even no, rates of measles vaccination (Electronic Supplementary Material Appendix 2; Table 1). We assumed that the risk of measles infection was equal throughout the population of children. We then used published data that provide estimates of a reduction in the incidence of measles for every 1 % increase in children effectively vaccinated against measles [5]. Note that Hall and Jolley reported higher levels of reduction in measles incidence once 80 % or more of children were effectively vaccinated [5]. This is due to the “herd immunity” effect, and the use of their estimates incorporates that effect into our model. We then used the model to estimate the comparative impact and cost-effectiveness of potentially different levels of measles vaccine effectiveness and vaccination coverage as a result of the two different types of vaccine administration technologies (conventional subcutaneous injection vs. microneedle patches).
Table 1.
List of inputs and parameters used to estimate the cost-effectiveness using either microneedle patches or syringe and needle for measles vaccination
| Model input | Value | Comments | Source (References) |
|---|---|---|---|
| Children population aged under 5 years | 1,000,000 | Assumed intended target of vaccination programs using the two vaccination technologiesa | Assumed |
| Incidence of measles among children aged under 5 years | 10–100 % | Sensitivity analysis: incidence from studies among communities with low levels of measles vaccination | [10–12] |
| Impact of increase in vaccination coverage (1 % increase) upon incidence of measles | 0.4–11.4 % | 1 % increase in first dose of vaccine: 2 % fall in reported incidence. Above 80 % vaccine coverage, for 1 % increase in coverage incidence fall by 11.4 % For each percentage increase in coverage with the second dose, a 0.4 % fall in incidence |
[5] |
| Vaccine coverage | 0–100 % | Sensitivity analysis (range of coverage) | |
| Vaccination dropout rate | 7.7 % | Proportion of people who received first dose of MCV but did not receive the second dose | [13] |
| Vaccine efficacy | |||
| Single dose (MCV1 only) | 85 % | Sensitivity analyses assuming MCV1 vaccine effectiveness = 77 % and 94 % | [14] |
| Two dose (MCV1 + MCV2)b | 97.75 % | ||
| Relative vaccine compliance rate in microneedle technologyc | 90 % (80–100 %) | Microneedle patch as a new technology might have a lower compliance rate. Sensitivity analysis conducted at different rates | Assumed |
MCV1 first dose of measles-containing vaccine (MCV), MCV2 second dose of MCV
aThe two vaccine administration technologies are: syringe-and-needle (existing technology) and micro-needle patches (in development)
bWe assumed 85 % vaccine effectiveness for a single dose and 97 % effectiveness for two doses in the base model
cBecause vaccination by a microneedle patch is a new technology, we assumed vaccine acceptability or the compliance rate will potentially be lower than the traditional syringe-and-needle injection technology
In the base model, we assumed that 100 % of children in the target population were susceptible to measles. We set the vaccination coverage target at 95 %, vaccine effectiveness of 85 % for both vaccination methods, and vaccine compliance rates of 100 % for subcutaneous injection and 90 % for microneedle patches. We further assumed 7.7 % of those receiving the first dose of measles-containing vaccine (MCV) would drop out and not receive the second dose of vaccine. We assumed in this population of children that the WHO measles vaccination recommendations would be followed, and that children would receive two doses of MCV [9]. We further assumed that these two doses (the first dose: MCV1 and the second dose: MCV2) were administered in a 1-year period to susceptible children in the model population. We examined the impact of differing levels of coverage (from 0 to 100 %). We also varied the effectiveness of the vaccine by both dose and type of vaccine administration technology (Table 1).
We used previously published costs data for measles vaccination from Zambia [15], and adjusted those costs to 2010 $US, assuming a 3 % annual inflation rate (Tables 2, 3). We calculated different sets of costs for delivering and administering the first and second doses of measles vaccine using two different technologies (subcutaneous injections and microneedle patches). The first dose is considered a routine measles vaccine and the second dose is similar to a mass vaccination campaign, referred to as a supplementary immunization activity (SIA) [9]. We also allowed for technology-induced differences in vaccine storage (cold chain or cool chain), and differing levels of vaccine wastage factors (Tables 2, 3). Characterizations of costs for the first and second dose are summarized in Tables 2 and 3. We calculated the cost-effectiveness of vaccine technologies as the cost per measles case averted, estimated by dividing the total costs of the vaccination program by the total number of measles cases averted. We then compared the cost-effectiveness of conventional needle-based technology (subcutaneous injection, status quo) and the new technology (microneedle patch) in terms of costs of per case of measles averted. We took the perspective of the payer (budgetary impact to a government of a low- and middle-income country) of vaccination services. We did not account for medical care-associated savings as a result of measles cases averted, as we assumed that the type of vaccination technology will not alter the benefits from an averted case of measles (i.e., medical care costs saved are the same for both technologies). Because we considered a 1-year analytic time frame, we discounted model inputs and outputs, and the potential benefits of measles cases averted beyond a 1-year period.
Table 2.
Costs of delivering and administering a first dose of measles-containing vaccine using either subcutaneous needle-and-syringe injection or microneedle patches
| Variables | Cost per dose of vaccine administration ($US)a | |||||
|---|---|---|---|---|---|---|
| Subcutaneous injection | Microneedle patches | |||||
| Mean | Low | High | Mean | Low | High | |
| Vaccine (antigen) | 0.211 | 0.158 | 0.264 | 0.211 | 0.158 | 0.264 |
| Injection equipment | 0.179 | 0.134 | 0.224 | 0.000 | 0.000 | 0.000 |
| Microneedle and applicator | 0.00 | 0.00 | 0.00 | 0.060 | 0.045 | 0.075 |
| Cold chain (2–8 °C) | 0.041 | 0.031 | 0.051 | 0.000 | 0.000 | 0.000 |
| Cool chain (room temperature) | 0.000 | 0.000 | 0.000 | 0.005 | 0.004 | 0.006 |
| Transportation | 0.034 | 0.025 | 0.042 | 0.034 | 0.025 | 0.042 |
| Personnel | 0.110 | 0.082 | 0.137 | 0.055 | 0.041 | 0.069 |
| Supplies | 0.003 | 0.002 | 0.004 | 0.004 | 0.003 | 0.005 |
| Needle disposal | 0.001 | 0.001 | 0.002 | 0.000 | 0.000 | 0.000 |
| Sub-total: cost per dose | 0.579 | 0.434 | 0.723 | 0.369 | 0.277 | 0.461 |
| Wastage factorc | 3.740 | 3.740 | 3.740 | 1.870 | 1.870 | 1.870 |
| Costs of wastage factor | 1.069 | 0.802 | 1.336 | 0.579 | 0.434 | 0.724 |
| Total costs ($US) per dose | 1.648 | 1.236 | 2.060 | 0.948 | 0.711 | 1.185 |
aCosts associated with subcutaneous syringe-and-needle injection were based on Dayan et al. [15], adjusted to 2010 $US. Costs for microneedle patches were based either on subcutaneous syringe-and-needle injection costs (e.g., vaccine antigen, transportation, and supplies) or expert opinion. See text for further details
bThe wastage factor was defined as the number of vaccine doses wasted per vaccine dose administered, and the wastage factor values for the subcutaneous syringe and needle were based on the results of published studies [15, 18]. The wastage cost included the costs of vaccine, cold (or cool chain) storage, and transport; we assumed microneedle patches required cool chain storage, were single dose packaged, and had 50 % less wastage than syringe-and-needle vaccines
Table 3.
Costs of delivering and administering a second dose of measles-containing vaccine using either subcutaneous needle-and-syringe injection or microneedle patches
| Variables | Cost per dose of vaccine administration ($US)a | |||||
|---|---|---|---|---|---|---|
| Subcutaneous injection | Microneedle patches | |||||
| Average | Low | High | Average | Low | High | |
| Vaccine (antigen) | 0.211 | 0.158 | 0.264 | 0.211 | 0.158 | 0.264 |
| Injection equipment | 0.179 | 0.134 | 0.224 | 0.000 | 0.000 | 0.000 |
| Microneedle and applicator | 0.00 | 0.00 | 0.00 | 0.060 | 0.045 | 0.075 |
| Cold chain (2–8 °C) | 0.041 | 0.031 | 0.051 | 0.000 | 0.000 | 0.000 |
| Cool chain (room temperature) | 0.000 | 0.000 | 0.000 | 0.005 | 0.004 | 0.006 |
| Transportation | 0.034 | 0.025 | 0.042 | 0.034 | 0.025 | 0.042 |
| Personnel | 0.110 | 0.082 | 0.137 | 0.055 | 0.041 | 0.069 |
| Supplies | 0.003 | 0.002 | 0.004 | 0.004 | 0.003 | 0.005 |
| Needle disposal | 0.001 | 0.001 | 0.002 | 0.000 | 0.000 | 0.000 |
| Social mobilization (SIAs) | 0.103 | 0.077 | 0.129 | 0.103 | 0.077 | 0.129 |
| Supervision (SIAs) | 0.007 | 0.005 | 0.009 | 0.004 | 0.003 | 0.004 |
| Planning and training (SIAs) | 0.034 | 0.025 | 0.042 | 0.017 | 0.013 | 0.021 |
| Administration (SIAs) | 0.007 | 0.005 | 0.009 | 0.007 | 0.005 | 0.009 |
| Additional personnel (SIAs) | 0.020 | 0.015 | 0.025 | 0.020 | 0.015 | 0.025 |
| Additional transportation (SIAs) | 0.006 | 0.004 | 0.007 | 0.006 | 0.004 | 0.007 |
| Sub-total: cost per dose | 0.755 | 0.566 | 0.944 | 0.524 | 0.393 | 0.656 |
| Wastage factorb | 1.100 | 1.100 | 1.100 | 0.550 | 0.550 | 0.550 |
| Costs of wastage factor | 0.314 | 0.236 | 0.393 | 0.138 | 0.103 | 0.172 |
| Total costs ($US) per dose | 1.069 | 0.802 | 1.337 | 0.695 | 0.521 | 0.868 |
SIAs supplemental immunization activities
aCosts associated with subcutaneous syringe-and-needle injection were based on Dayan et al. [15], adjusted to 2010 $US. Costs for microneedle patches were based either on subcutaneous injection costs (e.g., vaccine antigen, transportation, and supplies) or expert opinion. See text for further details
bThe wastage factor was defined as the number of vaccine doses wasted per vaccine dose administered, and the wastage factor values for the subcutaneous syringe and needle were based on the results of published studies [15, 18]. The wastage cost included the costs of vaccine, cold (or cool chain) storage, and transport; we assumed microneedle patches required cool chain storage, were single dose packaged, and had 50 % less wastage than syringe-and-needle vaccines
Vaccine Coverage and Effectiveness in Immunization
We initially used a value of 85 % effectiveness for the first dose of MCV (Table 1). In comparison, Demicheli et al. reviewed eight cohort and five case-control studies reporting measurements of the effectiveness of the measles, mumps, and rubella vaccine [16]. They concluded that a single dose of the measles, mumps, and rubella vaccine “is at least 95% effective in preventing clinical measles…”. Kidd et al. [14] estimated the following levels of measles vaccine effectiveness in three regions of Burkina Faso: in Bogodogo 94 % (95 % confidence interval [CI] 45–99); in Zorgho 87 % (95 % CI 37–97); and, in Sahel 84 % (95 % CI 41–96). More recently, Yang et al. estimated that in Guangzhou Province, China, for the period 2009–2012, in children aged 8 months to 14 years, a single dose of measles vaccine was 89.1 % (95 % CI 44.5–97.9) effective, and two or more doses were 97.8 % (95 % CI 88.3–99.6) effective [12]. On the basis of the results from these studies, we assumed that a second dose boosted vaccine effectiveness to 97.75% (among those receiving the second dose). We further assumed, based on WHO data, that 7.7 % of those receiving the first dose of MCV would drop out and not receive the second dose [17]. In the sensitivity analyses, we consider the impact of different levels of vaccine effectiveness applied to the two vaccine administration technologies (Table 1). We used the following general equation to estimate the impacts of measles vaccination programs:
where the percentage of children effectively vaccinated is the percent of the model population vaccinated multiplied by vaccine effectiveness. The values used in these equations are shown in Table 1. We estimated the impacts of vaccine coverage values ranging from 0 to 100 %. Users of our spreadsheet model can also explore the impact of changes in assumed vaccine effectiveness.
Vaccination Program Costs
We assumed that the first dose of MCV was administered as part of a routine vaccination program, while the second dose of MCV would be similar to the mass vaccination campaign, referred to as a SIA. In 2009, the WHO reported that 132 countries deliver the second dose of MCV as part of routine vaccination programs, while 49 countries conduct “regular, nationwide campaigns… (SIA)”. Routine vaccines are usually delivered in healthcare facilities (e.g., health posts and clinics), whereas vaccines in SIAs are delivered outside the healthcare facilities to reach children who do not have easy access to the health system [9].
The microneedle technology is not yet fully developed nor licensed for large-scale production and use; thus, there are no available data on the costs associated with its use in the field. Therefore, we made the following assumptions to calculate costs (Tables 2, 3 provide relevant sources).
Vaccine (antigen), transportation, and social mobilization costs were the same for both technologies (i.e., subcutaneous injection and microneedle patch).
Because microneedles can be applied by “minimally trained personnel”, we assumed that the costs of personnel, supervision, planning, and training for microneedle patches will at least be 50 % less than that of subcutaneous vaccinations.
Currently available vaccines required a cold chain (e.g., 2–8 °C) [18], whereas microneedle vaccine patches need only a cool chain (e.g., room temperature of 15–20 °C; the exact requirements have yet to be determined) [4].
For the syringe-and-needle vaccination method, we used vaccine wastage factors of 3.42 and 1.10 for MCV1 and MCV2, respectively [15], but for microneedles, because of greater heat stability, we assumed vaccine wastage would be 50 % less than the syringe-and-needle method.
We assumed, owing to the simplicity of design, that the fabrication of microneedle patches will cost one third of the cost of manufacturing subcutaneous injection equipment.
To address the uncertainties around the costs, for each cost item, we used mean, low, and high itemized costs to estimate the costs in a range (Tables 2, 3).
Because vaccination by microneedles is a new technology, we assumed there might be vaccine acceptability/compliance issues that will directly or indirectly affect the vaccine effectiveness. We thus considered a wide range of vaccine coverages (Table 1).
We did not include any costs associated with treating vaccine-related adverse events because the safety profile for microneedle patches has not been assessed. Two small studies (fewer than 100 persons per study group), that assessed the potential acceptability and efficacy of microneedle patches, did not note any serious adverse events associated with using microneedle patches. Neither study, however, used the measles vaccine [6, 7]. We did explore, using ranges of costs as a proxy (Tables 2, 3), the potential for increased costs as a result of factors such as treating vaccine-related adverse events.
Sensitivity Analyses
In addition to varying the percentage of persons vaccinated from 0 to 100 % [13], and allowing for variability in costs, we also conducted a number of sensitivity analyses. We analyzed the impact on the cost-effectiveness vaccination by altering the vaccine effectiveness of MCV1 from the baseline value of 85 % to either 77 % (10 % lower from baseline) and 94 % (10 % higher from baseline). We considered the impact on the number of measles cases in the model population with both changes in vaccination coverage and three different levels of vaccine effectiveness. The latter analysis allowed us to consider the trade-off from using a technology that provides a relatively lower level of effectiveness in an individual, but allowed a greater degree of vaccination coverage and thus produced an overall greater degree of vaccine-induced protection in the vaccinated population of children.
Results
Estimated Measles Incidence
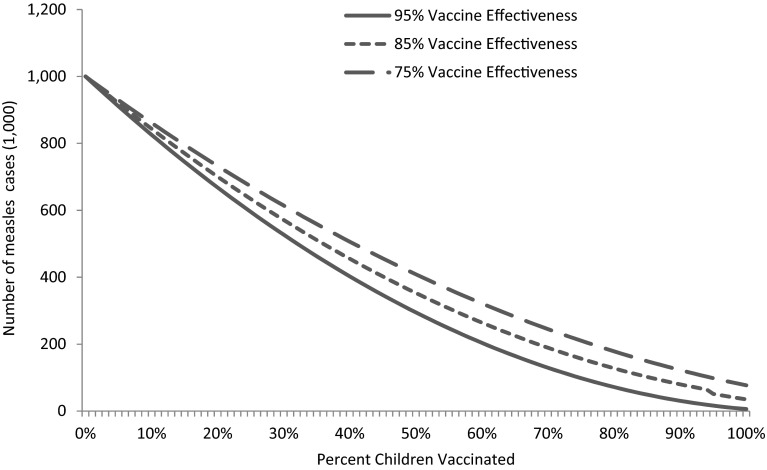
Without any vaccination, potentially almost the entire susceptible population of children would become infected by measles in the 1-year period (Fig. 1, at 0 % MCV coverage). As the vaccine coverage increases, the incidence of measles decreases (Fig. 1). Rate of decline by increasing vaccine coverage for microneedle patches is lower because of the underlying assumption of a lower compliance (e.g., vaccine acceptance) rate. The rate of decline in measles incidence with the second dose is non-linear owing to the fact that the second dose of vaccine is effective only to the persons who are not immunized by the first dose. The second dose acts as booster for immunization among children.
Fig. 1.
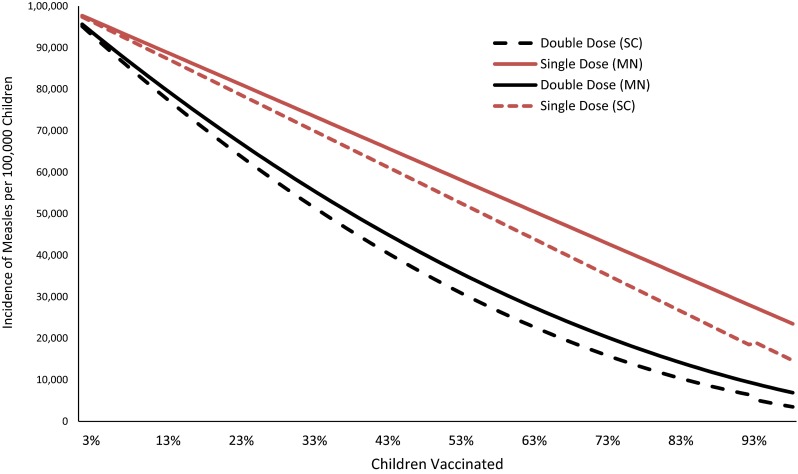
Impacts on measles incidence with changes in coverage with the first dose of measles-containing vaccine. Measles-containing vaccine effectiveness of 85 % and a vaccine dropout (those vaccinated with the first dose do not return for the second dose) rate of 7.7 %. Vaccine compliance rate (acceptability) was assumed to be 10 % lower for microneedle patches than the conventional technology. MN microneedle, SC subcutaneous
Estimated Total Costs per Administered Dose
We estimated total costs per administered dose of the first dose of MCV to be US$1.65 (range US$1.24–US$2.06) for subcutaneous vaccinations, and US$0.95 (range US$0.71–US$1.19) for vaccination using microneedle patches (Table 2). Similarly, we estimated the cost of the second dose of MCV by subcutaneous injection was US$1.07 (range US$0.80–US$1.34) compared with US$0.70 (range US$0.52–US$0.87) for the microneedle patch (Table 3). The largest cost component was wastage, responsible for 30–65 % of mean costs for MCV1 using the subcutaneous syringe-and-needle injection (Table 2).
Cost per Case of Measles Averted
As a result of the non-linear nature of the measles cases-by-percent vaccinated (Fig. 1), the cost-per-case averted changed (i.e., increased) as coverage of vaccination increased (Fig. 2). For example, for subcutaneous vaccinations, at 50 % coverage, the mean cost per case averted was US$2.04 (range US$1.53–US$2.54) and at 95 % coverage, the mean was US$2.64 (range US$1.98–US$3.30). Similarly, for the cost of vaccinations using microneedle patches, at 50 % coverage, the mean cost per case averted was US$1.32 (range US$0.99–US$1.65) and the cost was US$1.66 per case averted (range US$1.24–US$2.07) at 95 % coverage (Table 4).
Fig. 2.
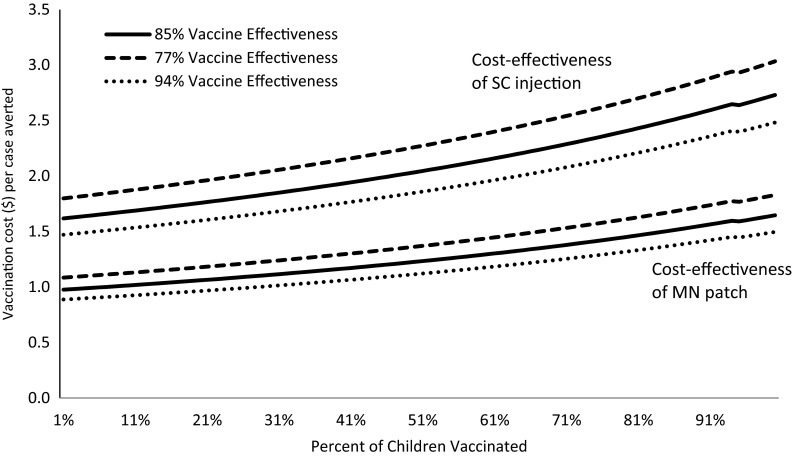
Costs per case of measles averted by percentage of the population vaccinated at three levels of vaccine effectiveness, using either a microneedle (MN) patch or subcutaneous (SC) injection. Vaccination coverage in the X-axis represents the first dose of measles but the costs per cases of measles averted also include the costs of the second dose. Costs of vaccines were calculated in 2010 US$. Cost estimates did not include potential medical treatment savings as a result of cases averted
Table 4.
Estimated total vaccination program costs and cost-effectiveness of using either a subcutaneous injection or microneedle patches by vaccination coveragea
| Vaccination coverage (%) | Subcutaneous injections | Microneedle patches | ||||||
|---|---|---|---|---|---|---|---|---|
| Total program costs (US$) | US$/case averted | Total program costs (US$) | US$/case averted | |||||
| Average | Low | High | Average | Low | High | |||
| 25 | 658,733 | 1.80 | 1.35 | 2.25 | 397,271 | 1.19 | 0.89 | 1.49 |
| 50 | 1,317,467 | 2.04 | 1.53 | 2.54 | 794,542 | 1.32 | 0.99 | 1.65 |
| 60 | 1,580,960 | 2.15 | 1.61 | 2.69 | 953,450 | 1.39 | 1.04 | 1.73 |
| 70 | 1,844,453 | 2.27 | 1.71 | 2.84 | 1,112,358 | 1.45 | 1.09 | 1.82 |
| 80 | 2,107,947 | 2.42 | 1.81 | 3.02 | 1,271,267 | 1.48 | 1.11 | 1.85 |
| 85 | 2,239,693 | 2.49 | 1.87 | 3.12 | 1,350,721 | 1.57 | 1.18 | 1.96 |
| 95 | 2,503,187 | 2.64 | 1.98 | 3.30 | 1,509,629 | 1.66 | 1.24 | 2.07 |
| 97 | 2,555,885 | 2.66 | 1.99 | 3.32 | 1,541,411 | 1.66 | 1.25 | 2.08 |
| 100 | 2,634,933 | 2.73 | 2.05 | 3.41 | 1,589,084 | 1.71 | 1.28 | 2.13 |
aCosts and cost-effectiveness estimates do not include potential medical treatment savings as a result of cases averted. We assumed a two-dose schedule of measles-containing vaccine with the second-dose coverage being 7.7 % less than the first-dose coverage, 85 % vaccine effectiveness for a single dose, and 97 % effectiveness for two doses. Costs of vaccination delivery and administration were as described in Tables 2 and 3. Costs are presented in 2010 US$. Epidemiological parameters and values are described in the text and Table 1
Costs of vaccination delivery and administration are described in Tables 2 and 3. Epidemiological parameters and values are described in the text and Table 1. For clarity, we omitted cost-effectiveness uncertainty bounds because of ranges in the costs of vaccination (Table 4). Because of the assumed reduced costs associated with using the microneedle patch (Tables 2, 3), the costs per case averted using the microneedle patch were always less than those associated with subcutaneous vaccinations. Regardless of the vaccination coverage levels compared, there were no overlaps in the ranges of cost-effectiveness—microneedle patches always cost less per case averted than syringe-and-needle delivery (Table 4; Fig. 2).
Cost-Effectiveness Analysis
Average costs presented in Table 4 to avert measles cases are based on the assumption that the microneedle patches are equally as effective as subcutaneous injections. However, this may not be the case in practice. In the context of two alternative vaccine-delivery methods not being equally effective, we need to compare the costs and outcomes in terms of the average cost-effectiveness ratio. When microneedle patches are equally or more effective than the subcutaneous injection, microneedle patches are cost saving. As the effectiveness of microneedle patches increases, the cost savings increases as more measles cases are averted by a more effective vaccine-delivery method (Fig. 3).
Fig. 3.
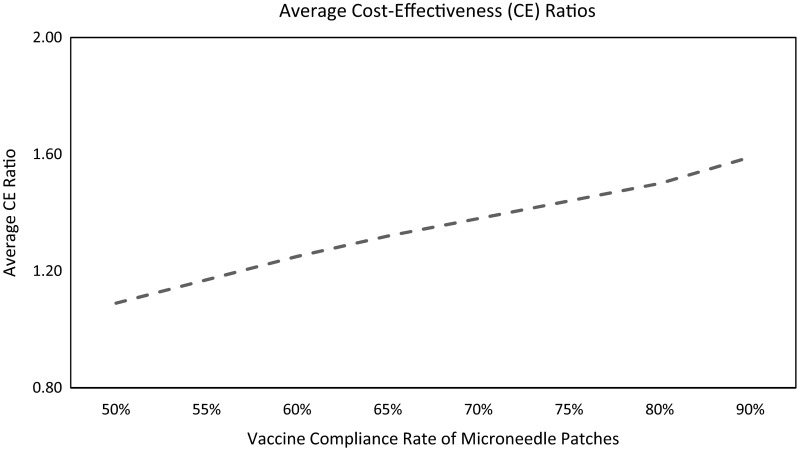
Average cost-effectiveness ratio of microneedle (MN) patches compared with subcutaneous (SC) injection at different levels of compliance rate of MN patches. Average cost-effectiveness ratio = (average cost-effectiveness of SC injection technology)/(average cost-effectiveness of MN patch technology). In cost-effectiveness ratios calculations, effectiveness of vaccines was set at 85 % and the vaccine coverage level was set at 95 % for both vaccination technologies
There can be, under certain conditions, a trade-off between vaccine effectiveness and coverage. For example, when the first dose (MCV1) was 95 % effective, and there was 55 % coverage, there were approximately 350,000 cases. The same impact could be achieved for a vaccination technology that was 75 % effective but achieved approximately 70 % coverage (because, say, it was easier to use effectively in the field) (top dotted line, Fig. 4).
Fig. 4.
Impact on the number of measles cases occurring by percentage of the population vaccinated with measles-containing vaccine (MCV) at three levels of vaccine effectiveness. Vaccination coverage in the X-axis represents the first dose (MCV1) of measles but the number of measles cases in the Y-axis includes both dosages (MCV1 + MCV2). MN microneedle, SC Inj. subcutaneous injection
This differential between effectiveness and coverage, however, disappeared when MCV1 was 95 % effective, and there was approximately 85 % vaccination coverage, causing approximately 200,000 cases. At 75 % effectiveness, even 100 % coverage did not achieve the same number of cases (lower dotted line, Fig. 4). In situations where ‘high’ levels of vaccination coverage (e.g., 85 % or more) have already been achieved and maintained, the potential advantages of the microneedle may not be sufficient to cause it to be widely used if microneedle-administered vaccines are notably less effective than syringe and needle.
Sensitivity Analyses
Because of the differences in vaccination costs (Tables 2, 3), even if microneedle patches are less effective than currently available syringe-and-needle vaccinations, they would still cost less per case averted (Fig. 2). For example, if MCV1 using a microneedle patch was only 77 % effective and subcutaneous vaccinations 94 % effective, it would still cost less per case averted to use a microneedle patch under any comparison of different levels of vaccination (Fig. 2). Cost-effectiveness of vaccination is also dependent on the existing incidence of measles (or existing immunity) in a given population. The average cost per case of measles averted is lower in a population with a high level of expected measles incidence (low level of existing immunity) and the costs per case averted by immunization increase in the population with a higher proportion of immunity against measles (Fig. 5).
Fig. 5.
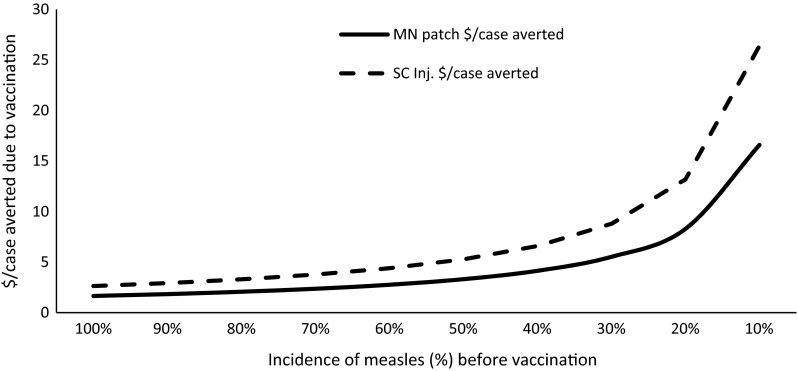
Existing incidence of measles and average cost per case averted for immunization. Note: costs are computed under the scenario of 95 % vaccine coverage and 85 % vaccine effectiveness. MN microneedle, SC Inj. subcutaneous injection
Discussion
We showed that in a variety of circumstances, the use of microneedle patches instead of subcutaneous injections for measles vaccination campaigns could substantially reduce costs. In addition to the cost advantages, microneedle patches potentially have several important logistical advantages (e.g., reduced cold chain requirements, fewer trained staff required) that could increase vaccine coverage, especially in hard-to-reach communities. Other vaccine-related technologies, such as aerosolized vaccines, could also offer advantages over the subcutaneous vaccination [19]. These technologies should also be carefully monitored and evaluated. However, many of those potential alternate technologies may still require the vaccine to be kept in cold chains, and thus do not offer the logistical advantages of the microneedle-based technologies.
In this article, we have computed costs of the measles vaccine based on a study by Dayan et al. [15]. Levin et al. [20] estimated the costs of routine (first-dose) and SIA (second-dose) measles vaccines (Table 5). We assumed constant average costs per vaccine (irrespective of coverage level), but Levin et al.’s estimates are dependent on the existing vaccination coverage. They assumed increasing marginal costs as coverage increases. The average costs for the routine vaccine are close to our estimates (subcutaneous injection), but the SIA vaccine (the second dose of MCV in our estimates) costs are somewhat different. Our estimates are country neutral and probably it is one of the benefits of being country neutral that we do not need to match with any other country’s estimate as long as we are within the range. Average costs of a measles case averted largely depend on the existing incidence of measles. In a population with a lower incidence of measles, relative costs are less in terms of costs per case averted (Fig. 5).
Table 5.
Comparison of measles vaccine costs per dose (2010 US$).
Source: Levin et al. [20]
| Levin et al.: estimates for different countries | Our estimatesa | |||||||
|---|---|---|---|---|---|---|---|---|
| Dose | Uganda | Ethiopia | Bangladesh | Tajikistan | Colombia | Brazil | SC inj. | Patches |
| Routine | $2.35 | $1.35 | $1.46 | $1.68 | $7.77 | $3.91 | $1.65 | 0.95 |
| SIA | $1.24 | $0.64 | $0.52 | $0.62 | $2.87 | $1.27 | $1.07 | 0.70 |
| Ratio | 1.9 | 2.1 | 2.8 | 2.7 | 2.7 | 3.1 | 1.54 | 1.36 |
In cost-effectiveness analyses, we assumed that microneedle patches were equally effective as subcutaneous injections in immunization against measles. Figure 6 illustrates the average costs of measles cases averted by microneedle patches. To be cost effective, patches should be at least 45 % effective relative to syringe-and-needle technology.
Fig. 6.
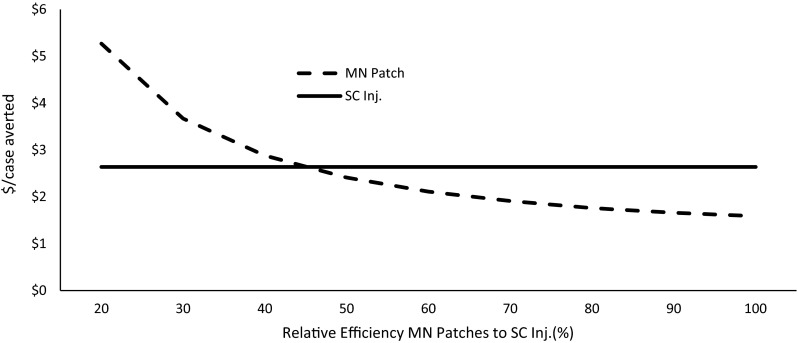
Relative effectiveness of microneedle (MN) patches to subcutaneous injections (SC Inj.) and average costs per case of measles averted. Note: Effectiveness of SC Inj. held constant for cost comparison of MN patches at different levels of efficiency relative to existing syringe-and-needle technology. Cost of illness is not included in the analysis
In most developing countries, immunization programs are limited and the effect of immunization of such programs is little felt [10]. To achieve the goal of global measles elimination [2, 3], innovative vaccination strategies that can reach all populations, particularly in areas that are difficult to reach, are critical for achieving the required high levels of measles vaccine coverage. Microneedle patches have potential to greatly aid in achieving this goal of measles elimination. A few researchers have also shown that, in the laboratory, microneedles can be used to vaccinate against other diseases such as influenza, polio, and hepatitis B [7, 21, 22].
Our analysis has several limitations. Our generic, non-country-specific approach to modeling may not realistically match the burden of measles and costs of vaccination in many countries where eliminating measles is still a significant challenge. As the microneedle technology moves closer to actual mass production and deployment, it may be worthwhile to build a complex disease transmission model, set for each specific country where the new technology could be used.
Another limitation is that we only included direct costs associated with vaccine delivery and excluded any costs associated with the medical care of measles cases, vaccine-related side effects, and indirect costs incurred by persons and communities (e.g., illness-related time lost from work). However, such costs likely would not vary by vaccination technology and excluding these indirect costs is not likely to bias the results toward either technology. Another potential limitation is that many of the vaccination costs used in this paper were measured in Zambia [15] and such costs may vary in other developing countries. However, what is most important and ‘drives’ the results presented here, is not the actual costs but rather the cost differential that may occur when a measles vaccination program switches from syringe-and-needle technology to the microneedle technology. The Excel tool (Electronic Supplementary Material Appendix 1) allows users to modify the itemized costs and see the impacts on the cost-effectiveness of vaccination methods.
In addition, because the microneedle patch is a new and untested vaccine-delivery method, we had to make several assumptions about the costs associated with vaccinations using microneedle patches, such as the assumption that a cool rather than a cold chain is required for microneedles. The microneedle technology is still in the early phases of research and development, and has not been licensed by the US Food and Drug Administration. Our cost-effectiveness comparison assumed that microneedle patches are already developed and in mass production, and so we did not account for costs associated with preclinical research including costs associated with obtaining intellectual property rights, implementing clinical trials, further product development, and manufacturing of microneedle patches. Similarly, the public acceptance rate of new vaccine technology would be determined by factors such as advertisements and other promotional activities. Costs of advertisement and promotions are not included in the analysis. Therefore, the assumed cost savings would not be fully realized until the new vaccine-delivery method was in routine use. Finally, the potential cost advantages of microneedle vaccinations would not be realized in situations where the microneedle patch confers notably less protection than vaccines delivered by subcutaneous injection, and vaccination coverage is already at ‘high levels’.
Conclusion
Use of microneedle patches in childhood measles vaccination programs may reduce the costs of immunization and potentially increase vaccination coverage, particularly in the hard-to-reach population in developing countries. Cost-effectiveness of patches depends on various factors such as vaccine acceptance rates and vaccine effectiveness of the microneedle patches relative to the subcutaneous vaccine-delivery method compared in this study. Potential benefits examined regarding the use of microneedle patches for use in measles vaccination programs may well be extended to other vaccines. This reinforces the need to complete research and development and conduct clinical trials of microneedle patches to determine their suitability for use in large-scale vaccination programs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Cathy Young who edited this manuscript. We also appreciate Charisma Atkins for sharing her knowledge and skills in developing the spreadsheet tool.
Compliance with Ethical Standards
Funding
The study was funded by the Centers for Disease Control and Prevention.
Conflict of interest
Drs. Adhikari, Goodson, Chu, Rota, and Meltzer declare no conflicts of interest.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Perry R, Gacic-Dobo M, Dabbagh A, et al. Global control and regional elimination of measles, 2000–2012. MMWR. 2014;63(5):103–107. [PMC free article] [PubMed] [Google Scholar]
- 2.Strebel P, Cochi S, Grabowsky M, et al. The unfinished measles immunization agenda. J Infect Dis. 2003;187(Suppl 1):S1–S7. doi: 10.1086/368226. [DOI] [PubMed] [Google Scholar]
- 3.Goodson JL, Chu SY, Rota PA, et al. Research priorities for global measles and rubella control and eradication. Vaccine. 2012;30(32):4709–4716. doi: 10.1016/j.vaccine.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edens C, Collins ML, Ayers J, et al. Measles vaccination using a microneedle patch. Vaccine. 2013;31(34):3403–3409. doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall R, Jolley D. International measles incidence and immunization coverage. J Infect Dis. 2011;204(Suppl 1):S158–S163. doi: 10.1093/infdis/jir124. [DOI] [PubMed] [Google Scholar]
- 6.Garrison L, Bauch C, Bresnahan B, et al. Using cost-effectiveness analysis to support research and development portfolio prioritization for product innovations in measles vaccination. J Infect Dis. 2011;204(Suppl 1):S124–S132. doi: 10.1093/infdis/jir114. [DOI] [PubMed] [Google Scholar]
- 7.Andersson N, Cockcroft A, Ansari NM, et al. Evidence-based discussion increases childhood vaccination uptake: a randomised cluster controlled trial of knowledge translation in Pakistan. BMC Int Health Hum Rights. 2009;9(Suppl 1):S8. doi: 10.1186/1472-698X-9-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norman JJ, Arya JM, McClain MA, et al. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–1862. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Measles vaccines: WHO position paper. Releve epidemiologique hebdomadaire Section d’hygiene du Secretariat de la Societe des Nations Wkly Epidemiol Rec Health Sect Secr Leag Nations. 2009;84(35):349–60. [PubMed]
- 10.Assaad F. Measles: summary of worldwide impact. Rev Infect Dis. 1983;5(3):452–459. doi: 10.1093/clinids/5.3.452. [DOI] [PubMed] [Google Scholar]
- 11.Dave KH. Measles in India. Rev Infect Dis. 1983;5(3):406–410. doi: 10.1093/clinids/5.3.406. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Xu J, Wang M, et al. Measles epidemic from 1951 to 2012 and vaccine effectiveness in Guangzhou, southern China. Hum Vaccin Immunother. 2014;10(4):1091–1096. doi: 10.4161/hv.27895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO/UNICEF. Immunization summary: 2014 edition. 2014 [cited 2014 06/06/2014]. http://www.who.int/immunization/monitoring_surveillance/en/. Accessed 22 Sep 2016.
- 14.Kidd S, Ouedraogo B, Kambire C, et al. Measles outbreak in Burkina Faso, 2009: a case–control study to determine risk factors and estimate vaccine effectiveness. Vaccine. 2012;30(33):5000–5008. doi: 10.1016/j.vaccine.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayan G, Cairns L, Sangrujee N, et al. Cost-effectiveness of three different vaccination strategies against measles in Zambian children. Vaccine. 2004;22(3–4):475–484. doi: 10.1016/j.vaccine.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Demicheli V, Rivetti A, Debalini MG, Di Pietrantonj C. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev. 2012;2:CD004407. doi: 10.1002/14651858.CD004407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO-UNICEF. Coverage estimates for 1980-2012, as of July 2013 2013 [12/27/2013]. http://www.who.int/entity/immunization_monitoring/data/coverage_estimates_series.xls. Accessed 22 Sep 2016.
- 18.Techathawat S, Varinsathien P, Rasdjarmrearnsook A, Tharmaphornpilas P. Exposure to heat and freezing in the vaccine cold chain in Thailand. Vaccine. 2007;25(7):1328–1333. doi: 10.1016/j.vaccine.2006.09.092. [DOI] [PubMed] [Google Scholar]
- 19.Low N, Bavdekar A, Jeyaseelan L, et al. A randomized, controlled trial of an aerosolized vaccine against measles. N Engl J Med. 2015;372(16):1519–1529. doi: 10.1056/NEJMoa1407417. [DOI] [PubMed] [Google Scholar]
- 20.Levin A, Burgess C, Garrison LP, Jr, et al. Global eradication of measles: an epidemiologic and economic evaluation. J Infect Dis. 2011;204(Suppl 1):S98–S106. doi: 10.1093/infdis/jir096. [DOI] [PubMed] [Google Scholar]
- 21.Edens C, Dybdahl-Sissoko NC, Weldon WC, et al. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo L, Qiu Y, Chen J, et al. Effective transcutaneous immunization against hepatitis B virus by a combined approach of hydrogel patch formulation and microneedle arrays. Biomed Microdevices. 2013;15:1077–1085. doi: 10.1007/s10544-013-9799-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.