Abstract
Background and Objectives
Recently, the osteoporosis treatment has attracted attention, and several drugs have been developed. Among these, bisphosphonates (BPs), parathyroid hormone (PTH) and anti-receptor activator of nuclear factor kappa B ligand (RANKL) monoclonal (MAb) denosumab (DMAb) are the major osteoporosis agents. Several studies demonstrated that the effect of osteoporosis agents is evaluated by lumar or hip dual energy X-ray absorptiometry (DXA). However, private clinic commonly use the radial DXA. On the other hand, rheumatoid arthritis (RA) is sometimes associated with osteoporosis but there is no established treatment approach. In addition, glucocorticoids (GCs) are often used in the treatment of RA and sometimes induce osteoporosis. The present study assessed the effect of DMAb on osteoporosis in patients divided into RA and RA + GC patients by radial DXA.
Patients
The therapeutic effect of denosumab was assessed in female osteoporosis patients using radial dual-energy X-ray absorptiometry (radial DXA) in three groups: those with postmenopausal osteoporosis (PO group), PO with rheumatoid arthritis (RA group), and PO with RA receiving glucocorticoids (RA + GC group).
Methods
In total, 427 PO patients 60 years of age or older with a young adult mean value of <70 %, as determined by radial DXA, were treated with denosumab. The denosumab treatment group comprised a PO group (n = 205), RA group (n = 156), and RA + GC group (n = 66). The control group comprised a PO group (n = 44) and RA group (n = 33) who received oral bisphosphonate. Bone mineral density (BMD) was determined by using radial DXA. The bone turnover marker type I collagen cross-linked N-telopeptide (NTx) was also measured.
Results and Conclusions
Radial DXA revealed a significant increase in BMD in the denosumab treatment group but not in the bisphosphonate treatment group. The onset of an increase in BMD with denosumab was slower in the RA group than in those without RA. The effect of denosumab in preventing increased NTx levels was smaller in the RA and RA + GC groups than in the PO group. Adherence to denosumab treatment was statistically significantly greater than for bisphosphonate treatment.
Key Points
| Unlike bisphosphonates, the therapeutic effect of denosumab can be assessed using radial dual-energy X-ray absorptiometry (radial DXA) in clinics. |
| Radial DXA revealed the efficacy of denosumab in rheumatoid arthritis (RA) and RA + glucocorticoid groups that responded poorly to bisphosphonate treatment. |
| Adherence to denosumab treatment was significantly higher than that for bisphosphonate treatment. |
Introduction
In recent years, the treatment of osteoporosis has become a focus of attention, and many therapeutic agents have been developed [1]. Among these, bisphosphonates, parathyroid hormone (PTH) and the anti-receptor activator of nuclear factor-κB ligand (RANKL) monoclonal antibody (MAb) denosumab are major osteoporosis agents. Several studies have demonstrated that the effect of bisphosphonates and PTH is not detected by radial dual-energy X-ray absorptiometry (DXA) (except for one report), during the effect of denosumab is confirmed [2–5]. Radial DXA was selected for use as it determines the bone mineral density (BMD) of cortical bone rather than trabecular bone and age-related bone loss is more common in cortical bone [6]; in addition, radial DXA is more commonly used in clinics [7].
Rheumatoid arthritis (RA) is commonly associated with osteoporosis but there is no established treatment approach. Moreover, glucocorticoids are often used as a treatment for RA and sometimes induce osteoporosis.
Patients and Methods
A case-control study was conducted in which the study population comprised 427 female osteoporosis patients 60 years of age or older who received subcutaneous injection of denosumab 60 mg every 6 months between October 2013 and January 2016 and had a young adult mean (YAM) value of <70 %. To prevent hypocalcemia associated with denosumab treatment, all patients received an oral vitamin D3 preparation (cholecalciferol 0.01 mg/day or eldecalcitol 0.75 μg/day) as per the package insert of denosumab. Assessment was performed separately for the three groups: patients with postmenopausal osteoporosis (PO) (PO group), patients with PO with RA (RA group), and PO patients with RA receiving glucocorticoids (RA + GC group) (glucocorticoids were taken for at least 4 weeks until immediately before the start of the study). Patients with secondary osteoporosis probably due to other diseases such as cancer metastatic to bone and metabolic disorders were excluded from the study. All RA patients studied met the 1987 American College of Rheumatology criteria [8]. The control group consisted of 78 patients retrospectively sampled from those who had received an oral bisphosphonate every 4 weeks (minodronate 50 mg) or once a month (risedronate 75 mg) for the treatment of osteoporosis and provided written informed consent. Patient selection was matched to the denosumab treatment group (age ≥60 years, YAM value <70 %, observation period October 2013 to January 2016). No patient treated with glucocorticoids was in the control group. Similar to the denosumab treatment group, assessment was performed separately for the PO (44 patients) and RA (34 patients) bisphosphonate groups. In the control group, as with the denosumab treatment group, patients with secondary osteoporosis were excluded and patients were eligible for inclusion if they were 60 years of age or older and had a YAM value of <70 %; no patients in the bisphosphonate group had received glucocorticoid treatment.
A total of 427 female osteoporosis patients were treated with denosumab: 205 in the PO group, 156 in the RA group, and 66 in the RA + GC group. All patients in the RA + GC group received glucocorticoids at a dose of ≤5 mg/day (1–5 mg/day). Of the female osteoporosis patients who had received bisphosphonate treatment after providing informed consent for participation in previous clinical studies, 78 could be matched with those in the denosumab treatment group for age and YAM value; 44 and 34 were classified in the PO and RA groups, respectively. However, no patients in the bisphosphonate group had been taking glucocorticoids (Table 1).
Table 1.
Baseline characteristics
| DMAb (n = 427)a | BP (n = 78)b | p value | |
|---|---|---|---|
| Age | 69.2 ± 10.3 | 67.9 ± 8.4 | NS |
| Weight (kg) | 57.1 ± 10.8 | 56.3 ± 12.7 | NS |
| BMI (kg/m2) | 26.2 ± 8.6 | 28.7 ± 18.4 | NS |
| % YAM | 54.1 ± 13.0 | 56.3 ± 13.8 | NS |
| NTX (nmolBCE/mmolCr) | 64.7 ± 25.0 | 66.2 ± 31.1 | NS |
Data are presented as mean ± standard error
BMI body mass index, BP bisphosphonate-treated group, DMAb denosumab-treated group, nmolBCE/mmolCr nmol Bone Collagen Equivalents/nmol creatinine, NS not statistically significant, PO patients with postmenopausal osteoporosis, RA osteoporosis patients with rheumatoid arthritis, RA + GC osteoporosis patients with rheumatoid arthritis taking glucocorticoids, NTX type I collagen cross-linked N-telopeptide, YAM young adult mean
aPO (n = 205), RA (n = 156), RA + GC (n = 66)
bPO (n = 44), RA (n = 34)
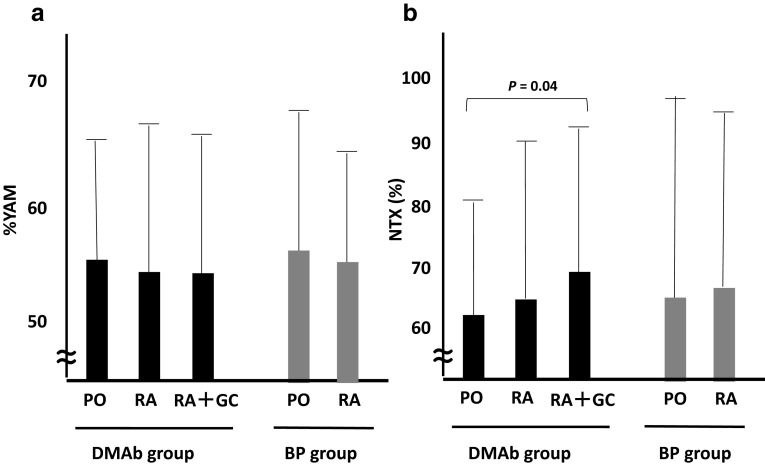
In accordance with pre-treatment YAM and urine type I collagen cross-linked N-telopeptide (NTx) values, the denosumab group was divided into three subgroups and the bisphosphonate group was divided into two subgroups. There is no statistical difference for YAM between any two groups, and no statistical difference was found for urine NTx among any two groups except for between the PO and RA + GC groups taking denosumab (Fig. 1). It is well-known that glucocorticoids strongly induce bone resorption and subsequently influence the NTx level; the difference in the RA + GC group may be due to this effect.
Fig. 1.
Patient baseline bone mineral density (a) and urine NTx (b). There is no statistical difference in % YAM among any two groups (a). There is no statistical difference in urine NTx among any two groups except for between the PO and RA + GC groups within the DMAb group (b). Bars indicate the standard deviation. BP bisphosphonate, DMAb denosumab, NTx type I collagen cross-linked N-telopeptide, PO patients with postmenopausal osteoporosis, RA osteoporosis patients with rheumatoid arthritis, RA + GC osteoporosis patients with rheumatoid arthritis taking glucocorticoids, YAM young adult mean
All RA patients enrolled in the study showed remission or a low 28-joint Disease Activity Score–erythrocyte sedimentation rate (DAS28-ESR) of <3.2 for 4 weeks before the start of denosumab treatment [9]. The dosage of both conventional systemic disease-modifying antirheumatic drugs (csDMARDs) and biological DMARDs (bDMARDs) in addition to glucocorticoids remained unchanged throughout the study period. Those patients who required changes in or discontinuation of antirheumatic therapy with glucocorticoids, csDMARDs, and bDMARDs because of changes in RA status or adverse reactions were excluded from the study. No patients in the bisphosphonate group required changes in their RA treatment. The objective of the present study was to assess the therapeutic effect of denosumab by radial DXA, and those patients who could not meet this objective, or who dropped out for reasons other than those related to the effect of denosumab or the response to denosumab, were excluded. The content of the study was explained to all patients participating in the study and written informed consent was provided in advance. This study was conducted according to the principles of the Declaration of Helsinki.
BMD was determined by radial DXA (DCS-600EXV; Hitachi Aloka Medical, Japan) every 24 weeks after the start of the study. The change in BMD between before treatment and after treatment (∆BMD) was calculated for each patient group to compare the effect of denosumab (Eq. 1):
| 1 |
For the denosumab treatment group, assessment of a marker of bone turnover was also performed. Levels of the marker of bone resorption, NTx, were measured in the second morning urine (enzyme-linked immunosorbent assay: nmol Bone Collagen Equivalents/nmol creatinine) every 24 weeks after the start of denosumab treatment. Percentage NTx (%NTx) values were calculated to compare the effect of denosumab in the patient groups as follows (Eq. 2):
| 2 |
Denosumab continuation was estimated using the Kaplan–Meier method and compared using a log-rank test (Mantel–Cox test). The last observation carried forward method was used in each analysis. Results are expressed as mean ± standard deviation or standard error (SE). This study deals with the treatment plan approved by the Institutional Review Board of Matsubara Mayflower Hospital (No. 177) (Kato city, Hyogo, Japan). Verbal informed consent was obtained from each patient to use their clinical data. All analysis proportions were analyzed for treatment differences with the Chi-square test and continuous variables were compared with Student’s t test. A statistically significant difference was defined as p < 0.05.
Results
Changes in Bone Mineral Density as Determined by Radial Dual-Energy X-Ray Absorptiometry After Denosumab Treatment
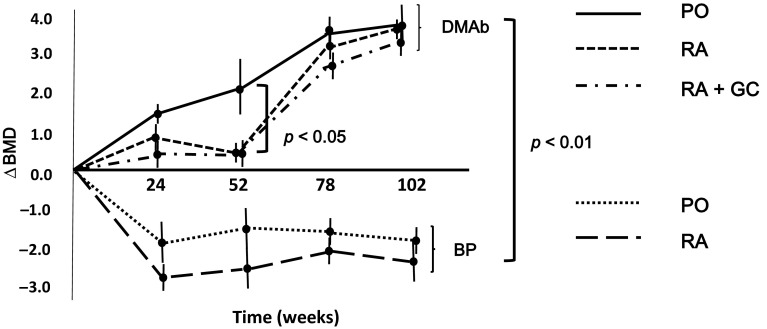
There were statistically significant differences in the increase in BMD as determined by radial DXA between denosumab and bisphosphonate treatments (p < 0.01). No increase in BMD was observed in the bisphosphonate treatment group, whereas BMD was found to increase over time in the denosumab treatment group (administration every 6 months). The therapeutic effect of denosumab was noted in each of the PO, RA, and RA + GC groups. However, the effect was slower in onset in the RA and RA + GC groups than in the PO group. At week 52, significant differences were found between the increase in BMD between the PO group and the other groups (Fig. 2).
Fig. 2.
Changes in BMD after the start of DMAb treatment as determined by radial DXA (∆BMD = [radial DXA value for each week] − [radial DXA value at the start of treatment]). In the PO group, the radial DXA value increased with the number of doses (over time). Treatment with DMAb was also associated with increases in the radial DXA value in the RA and RA + GC groups. However, the efficacy of DMAb in increasing radial DXA values was slower in onset than in the PO group. At week 52 of DMAb treatment, statistically significant differences were found in BMD between the PO and RA or RA + GC groups (p < 0.05). Treatment with bisphosphonate showed no increase in the radial DXA value, and the effect of bisphosphonate was statistically significantly inferior to that of DMAb (p < 0.01). Data are expressed as mean ± standard error. ∆BMD change in bone mineral density, BMD bone mineral density, DMAb denosumab, PO patients with postmenopausal osteoporosis, RA osteoporosis patients with rheumatoid arthritis, RA + GC osteoporosis patients with rheumatoid arthritis taking glucocorticoids, radial DXA radial dual-energy X-ray absorptiometry
All denosumab-treated patients received oral cholecalciferol or eldecalcitol as a vitamin D3 preparation in accordance with their hepatic or renal condition. There is no statistical difference between patients using cholecalciferol or eldecalcitol in ∆BMD at 102 weeks (3.0 ± 0.6 vs. 3.2 ± 0.8 [mean ± SE]).
Changes in Urinary Type I Collagen Cross-Linked N-Telopeptide Levels After the Start of Denosumab Treatment
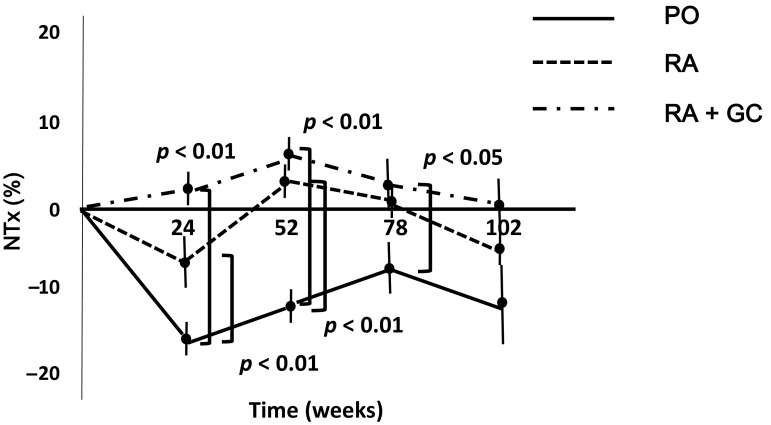
Analysis of the rate of change (%) in the levels of NTx showed that bone resorption was rapidly inhibited in the PO group after the start of denosumab treatment and that the inhibitory effect lasted until the end of the observation period at week 102. The inhibitory effect of denosumab on bone resorption was smaller in the RA and RA + GC groups than in the PO group. The effect of denosumab in preventing an increase in NTx levels was statistically significantly greater in the PO group than in the other groups up to week 78 (Fig. 3).
Fig. 3.
Changes in the urinary levels of NTx, a marker of bone turnover, associated with denosumab treatment were marked in the PO group. Up to week 78 of denosumab treatment, urinary NTx levels were statistically significantly lower in the PO group than in the RA and RA + GC groups. %NTx = . Data are expressed as mean ± standard error. NTx type I collagen cross-linked N-telopeptide, PO patients with postmenopausal osteoporosis, RA osteoporosis patients with rheumatoid arthritis, RA + GC osteoporosis patients with rheumatoid arthritis taking glucocorticoids
Adherence to Denosumab or Bisphosphonate Treatment
For both denosumab and bisphosphonate treatments, withdrawal from the study was found to occur most frequently at week 24 in the early stages of treatment. Adherence to denosumab treatment was statistically significantly higher than that for bisphosphonate treatment (Fig. 4).
Fig. 4.
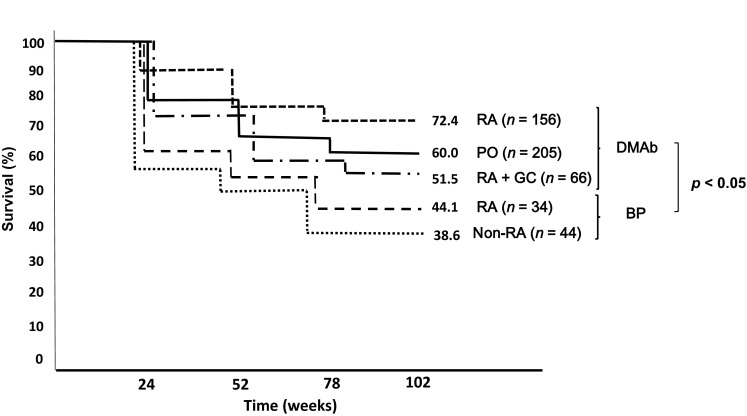
Adherence to DMAb treatment remained higher than that for BP treatment (Kaplan–Meier method). BP bisphosphonate, DMAb denosumab, PO patients with postmenopausal osteoporosis, RA osteoporosis patients with rheumatoid arthritis, RA + GC osteoporosis patients with rheumatoid arthritis taking glucocorticoids
Adverse Reactions
One 75-year-old patient in the RA group discontinued treatment with denosumab because of an adverse reaction (osteonecrosis of the jaw [ONJ]). The patient was found to have ONJ during tooth extraction at a dental clinic after 60 weeks of denosumab treatment (after the second administration). However, she had been treated with bisphosphonate for 8 years before the start of denosumab treatment, and therefore it is difficult to determine whether the ONJ was attributable to denosumab or bisphosphonate treatment.
Discussion
The number of patients with osteoporosis has increased with the aging of the population worldwide. Against this backdrop, various therapeutic agents for osteoporosis have recently been developed and applied clinically [1, 10]. Among many treatments for osteoporosis, bisphosphonate, denosumab, and PTH are especially highly evidence based and effective. These agents have been shown to increase the BMD of the lumbar spine and total hip [3–5, 11], although radial DXA has revealed little effect for agents other than denosumab. Given that radial DXA determines cortical BMD and that age-related bone loss is attributed to the porosity of cortical bone, radial DXA is of significance [6]. Most of the clinics at which osteoporosis is often treated use radial DXA to determine BMD. It is likely that medical devices such as central (spine/hip) DXA and computed tomography are not used because of cost and space issues [7]. In this context, it is significant that we assessed the effect of denosumab using radial DXA.
Presentation of beneficial therapeutic effect increases patient enthusiasm for receiving treatment and enhances adherence to the continuation of treatment. However, in treatment with bisphosphonate or PTH, radial DXA is not helpful to assess the therapeutic effect, which hinders further treatment. Because denosumab inhibits bone resorption by penetrating cortical bone through the extracellular fluid [12], its effect can be adequately assessed by radial DXA, which determines cortical BMD [3–5]. Although bisphosphonates are most widely used in the treatment of osteoporosis, it was found that at most only 50 % of patients adhered to treatment for 1 year, partly because of limitations such as the need for bed rest and dietary restrictions other than fluid intake for at least 30 min after taking a bisphosphonate [13]. The result is similar to that obtained for patients receiving bisphosphonates in the present study (Fig. 4). The low adherence to bisphosphonate treatment in our study may be attributable to the inadequate presentation of the therapeutic effect (Figs. 2, 4) in addition to the complex instructions for taking a bisphosphonate.
In contrast to bisphosphonate treatment, at least 50 % of patients receiving denosumab adhered to the treatment even at week 102. This high adherence to denosumab treatment could be attributable to the simplicity of treatment (subcutaneous administration only every 6 months) and presentation of the preferable therapeutic effect (Figs. 2, 4). In addition, because denosumab is a human MAb prepared using transgenic mice, it is a stable pharmaceutical product with low immunogenicity [14], and therefore causes only slight pain at the time of injection and fewer adverse reactions. These factors may also contribute to the high adherence.
Denosumab also had an effect in the RA and RA + GC groups, although it was slower in onset in these groups than in the PO group (Fig. 2). A possible reason for this is that denosumab had an insufficient inhibitory effect on bone resorption (i.e., lowering of the NTx level) because of increased bone resorption due to the presence of RA or the impact of glucocorticoids in the RA and RA + GC groups (Fig. 3). This is the first reported attempt to assess the effect of denosumab in RA and RA + GC groups using radial DXA. However, a previous report showed that denosumab increases the BMD of both the lumbar spine and total hip; this result may be attributable to the effect of denosumab in reducing bone turnover by lowering the levels of both a marker of bone formation, serum procollagen 1 N-terminal peptide (P1NP), and one of bone resorption, serum C-telopeptide type 1 (sCTx-1) [11]. In the present study, denosumab insufficiently lowered NTx levels in the RA and RA + GC groups. This may be because the presence of RA at the start of treatment affected the levels of the marker of bone turnover; therefore, further investigations are needed to confirm this hypothesis.
It should also be noted that bisphosphonates have been shown to be effective in improving the BMD for the lumbar spine but not the total hip in osteoporosis patients with RA [15]. This is in marked contrast to the finding that bisphosphonate has an effect on both the lumbar spine and total hip in patients with PO alone [1], suggesting that patients with PO complicated by RA may be more refractory to treatment for osteoporosis. There is also a report showing that bisphosphonate has an effect on the lumbar spine and total hip in osteoporosis patients with RA [16]. However, in this report, bisphosphonate had no effect on the metacarpals, which are mainly composed of cortical bone. In contrast, denosumab had an effect in the RA and RA + GC groups in the present study. In addition, denosumab has recently been shown to be effective in the treatment of bone erosion in patients with RA [17]. The American College of Rheumatology recommends use of alendronate or risedronate in the treatment of glucocorticoid-induced osteoporosis [18]. Our results show that denosumab can also be recommended for osteoporosis in RA patients and for RA patients taking glucocorticoids.
Moreover, one patient experienced ONJ during denosumab treatment; however, it is difficult to determine whether the ONJ is attributable to denosumab or bisphosphonate treatment. Recently, it has been reported that RA is a risk factor for ONJ, and ONJ should be especially mentioned to long-term denosumab or bisphosphonate-treated RA patients (median 69 months, range 20–130 months) [19].
Compliance with Ethical Standards
Funding
This research received no specific grant from any funding agency in the public, commercial or non-profit sectors.
Conflict of interest
The author declares no conflict of interest associated with this manuscript.
References
- 1.Mandema JW, Zheng J, Libanati C, Perez Ruixo JJ. Time course of bone mineral density changes with denosumab compared with other drugs in postmenopausal osteoporosis: a dose-response-based meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3746–3755. doi: 10.1210/jc.2013-3795. [DOI] [PubMed] [Google Scholar]
- 2.Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT) J Clin Endocrinol Metab. 2014;99(7):2599–2607. doi: 10.1210/jc.2013-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid IR, Miller PD, Brown JP, Kendler DL, Fahrleitner-Pammer A, Valter I, et al. Denosumab Phase 3 Bone Histology Study Group. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25(10):2256–2265. doi: 10.1002/jbmr.149. [DOI] [PubMed] [Google Scholar]
- 5.Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie SA, Zhu Y, Foley K, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–1700. doi: 10.1210/jc.2013-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375(9727):1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi H, Fukunaga M, Nishikawa A, Orimo H. Changes in distribution of bone densitometry equipment from 1996 to 2006 in Japan. J Bone Miner Metab. 2010;28(1):60–67. doi: 10.1007/s00774-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 8.Arnett FC, Edworthy SM, Bloch DA, McShane DJ. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 9.Matsuno H, Okada M, Sakai Y, Abe C, Katayama K, Sagawa A, et al. The usefulness of a new triple combination treatment utilizing methotrexate, salazosulfapyridine, and bucillamine in rheumatoid arthritis. Mod Rheumatol. 2016;26(1):51–56. doi: 10.3109/14397595.2015.1059984. [DOI] [PubMed] [Google Scholar]
- 10.Albaum JM, Youn S, Levesque LE, Gershon AS, Cadarette SM. Osteoporosis management among chronic glucocorticoid users: a systematic review. J Popul Ther Clin Pharmacol. 2014;21(3):e486–e504. [PubMed] [Google Scholar]
- 11.Dore RK, Cohen SB, Lane NE, Palmer W, Shergy W, Zhou L, Denosumab RA Study Group et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis. 2010;69(5):872–875. doi: 10.1136/ard.2009.112920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zebaze RM, Libanati C, Austin M, Ghasem-Zadeh A, Hanley DA, Zanchetta JR, et al. Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone. 2014;59:173–179. doi: 10.1016/j.bone.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Cotté FE, Fardellone P, Mercier F, Gaudin AF, Roux C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21(1):145–155. doi: 10.1007/s00198-009-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9(10):767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 15.Lems WF, Lodder MC, Lips P, Bijlsma JW, Geusens P, Schrameijer N, et al. Positive effect of alendronate on bone mineral density and markers of bone turnover in patients with rheumatoid arthritis on chronic treatment with low-dose prednisone: a randomized, double-blind, placebo-controlled trial. Osteoporos Int. 2006;17(5):716–723. doi: 10.1007/s00198-005-0037-2. [DOI] [PubMed] [Google Scholar]
- 16.Jensen TW, Hansen MS, Hørslev-Petersen K, Hyldstrup L, Abrahamsen B, Langdahl B, Cimestra Study Group et al. Periarticular and generalised bone loss in patients with early rheumatoid arthritis: influence of alendronate and intra-articular glucocorticoid treatment. Post hoc analyses from the CIMESTRA trial. Ann Rheum Dis. 2014;73(6):1123–1129. doi: 10.1136/annrheumdis-2012-203171. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, et al. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis. 2016;75(6):983–990. doi: 10.1136/annrheumdis-2015-208052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2010;2(11):1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 19.Di Fede O, Bedogni A, Giancola F, Saia G, Bettini G, Toia F, et al. BRONJ in patients with rheumatoid arthritis: a multicenter case series. Oral Dis. 2016;22(6):543–548. doi: 10.1111/odi.12490. [DOI] [PubMed] [Google Scholar]