Abstract
Background
Opioids are recently recommended for those who do not gain adequate pain relief from the use of acetaminophen or nonsteroidal anti-inflammatory drugs. Medical opioids are administered in various routes, and transdermal opioid products that can make up for the weaknesses of the oral or intravenous products have been developed. This study is to evaluate the clinical usefulness of fentanyl matrix in terms of the long-term improvement in pain and physical and mental functions.
Methods
This was a multicenter, open, prospective, observational study that was conducted in 54 institutions in Korea. Patients with non-cancerous chronic pain completed questionnaires, and investigators also completed questionnaires. A total of 1,355 subjects participated in this study, and 639 subjects completed the study. Subjects received transdermal fentanyl matrix (12 µg/hr, 25 µg/hr, or 50 µg/hr depending on the patient's response and demand). Subjects visited at 29 ± 7 days, 85 ± 14 days, and 169 ± 14 days after administration, respectively, to receive drug titration and fill out the questionnaires. The results were analyzed using the intention-to-treat (ITT) analysis, full analysis set (FAS), and per-protocol (PP) analysis. The FAS analysis included only 451 participants; the PP analysis, 160 participants; and the ITT analysis, 1,355 participants.
Results
The intensity of pain measured by the Numeric Rating Scale decreased from 7.07 ± 1.78 to 4.93 ± 2.42. The physical assessment score and mental assessment score of the Short-Form Health Survey 12 improved from 28.94 ± 7.23 to 35.90 ± 10.25 and from 35.80 ± 11.76 to 42.52 ± 10.58, respectively. These differences were significant, and all the other indicators also showed improvement. Adverse events with an incidence of ≥ 1% were nausea, dizziness, vomiting, and pruritus.
Conclusions
The long-term administration of fentanyl matrix in patients with non-cancerous pain can reduce the intensity of pain and significantly improves activities of daily living and physical and mental capabilities.
Keywords: Chronic non-cancer pain, Fentanyl matrix, Physical functioning, Emotional functioning
Chronic pain is defined as pain that persists beyond normal tissue healing time that is assumed to be 3 months by the International Association for the Study of Pain.1) Various chronic pain problems include spinal pain, arthritis, ischemic pain syndromes, visceral pain syndromes, neuropathic pain syndromes, and headache.2) It is an important and common medical problem around the world and a major reason for hospital visits.
Pain-related medical expenses ranged from $560 billion to $635 billion in 2010, which was greater than those for coronary artery disease, cancer, and acquired immune deficiency syndrome (AIDS) in the United States. Therefore, any increase in the cost should be suppressed through effective treatment.3) A World Health Organization (WHO) study on primary care patients in countries of Europe, North America, Africa, and Asia reported that 22% of patients complained of chronic pain that lasted for more than six months.4) A survey on 46,394 subjects in 15 European countries and Israel reported that 1 out of 5 persons suffered pain for 6 months,5) and 9% of the total population in the United States experienced moderate or severe chronic non-cancerous pain.6) In an Australian study, the prevalence of chronic pain was 19.2%.7) Although much effort is being made to control chronic pain, causal treatment is not possible in most cases and pain is difficult to be completely resolved. Thus, it is important to establish an appropriate goal for pain control rather than to expect a pain-free condition. Unless treated appropriately, pain may worsen the underlying disease and health-related quality of life, mental distress including anxiety, depression, and sleep disorder, and social problems including reduction in productivity and labor force.4)
The use of opioids are recently recommended for those who do not gain adequate pain relief from the use of acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs).8) Medical opioids can be administered in various routes, and each route of administration has its own merits and demerits. Oral products are easy to administer, but difficult to use in patients with difficulty in oral intake. Demerits of intravenous (IV) injections include difficulty in vascular access, patient's pain associated with the use of a syringe, and the risk of stabbing. Transdermal products are designed to make up for such weaknesses of oral or IV products.
Among these opioids, fentanyl is a potent synthetic opioid analgesic with a short half-life that selectively acts on the µ-receptor, and its primary pharmacological actions are analgesic and sedative. Transdermal fentanyl matrix, with controlled release, extends the duration of action; it has been known that it takes 1.2 to 40 hours until minimal effective serum concentrations are produced and the steady state is maintained for 3 days.9) Plasma concentrations correlate with the delivery rate. According to Muijsers and Wagstaff,10) the mean maximum plasma concentration (Cmax) values of transdermal fentanyl patches at 34 to 38 hours after application were 0.6, 1.4, 1.7, and 2.5 ng/mL, respectively, at a delivery rate of 25, 50, 75, and 100 µg/hr, respectively.
This study is to evaluate the clinical usefulness of fentanyl matrix with respect to the long-term improvement in pain and physical and mental functions.
METHODS
This study was conducted at 54 institutions in Korea from September 28, 2009 to December 15, 2010. A total of 1,355 subjects used transdermal fentanyl matrix (Durogesic Dtrans Patch; Janssen Pharmaceuticals Korea, Seoul, Korea), and 639 (47.16%) of them completed the study. The remaining 716 subjects (52.84%) were withdrawn early due to loss to follow-up, adverse events (AEs), patient's choice, pain relief or pain resolution, patient's uncooperativeness, and others (Table 1). The mean age (± standard deviation [SD]) of the subjects was 60.52 ± 13.18 years, ranging widely from 20 to 95 years). The mean height and weight were 161.30 ± 9.19 cm and 62.06 ± 10.82 kg, respectively. Patients who were suitable for inclusion criteria participated in the study. Inclusion criteria and exclusion criteria are described in Table 2. If a patient discontinued the treatment before the end of the study period, end-of-treatment assessment and follow-up assessment were performed.
Table 1. Causes of Dropout.
| Subjects and early dropout causes | No. (%) |
|---|---|
| Total no. of subjects | 1,335 (100) |
| Subjects who completed the study | 639 (47.16) |
| Subjects who dropped out of the study early | 716 (52.84) |
| Lost to follow-up | 289 (40.36) |
| Adverse event | 116 (16.20) |
| Patient's choice | 109 (15.22) |
| Pain relief or pain resolution | 90 (12.57) |
| Patient's uncooperativeness | 22 (3.07) |
| Consent withdrawal | 7 (0.98) |
| Protocol violation | 4 (0.56) |
| Judgment by investigator | 1 (0.14) |
| Others | 78 (10.89) |
Table 2. Inclusion and Exclusion Criteria.
| Inclusion criteria |
| Aged 20 years or older |
| Complaining of chronic pain |
| Inadequate pain control despite previous treatment (e.g., tramadol, codeine, oral morphine, oxycodone) |
| Ability to complete the questionnaire judged by the investigator |
| Provision of written informed consent form |
| Exclusion criteria |
| Experience of treatment with fentanyl matrix within the past 4 weeks |
| Current or past history of drug or alcohol abuse |
| Likely to complain of pain more than necessary for actual pain on account of, e.g., traffic accident insurance |
| Cannot use transdermal analgesics due to skin disease |
| Serious mental disease |
| History of hypersensitivity to opioid analgesics |
| Chronic lung disease or respiratory failure |
| Pregnant or possibly pregnant |
| Considered by the investigator to be ineligible to participate in this study based on warning, precaution, and contraindication in the package insert of fentanyl matrix |
| Considered by the investigator to be in a condition to threaten the patient's well-being |
Study subjects were patients who visited the participating institutions with a complaint of non-cancerous chronic pain that was inadequately controlled despite treatment with opioids (such as tramadol, codeine, oral morphine, and oxycodone) and who were prescribed the fentanyl matrix by an investigator. The fentanyl matrix was administered for 6 months. Fentanyl matrix was not provided free of charge. The initial dose of the drug was determined based on the patient's opioid dose used as well as the degree of narcotic tolerance, patient's general condition including body size, age, and degree of weakness, and medical condition. The initial dose for opioid-naive patients was 12 µg/hr. A fentanyl matrix dose conversion table created based on the equianalgesic potency conversion and daily oral morphine dosage were referred to when oral or non-oral opioids were switched to the fentanyl matrix in opioid-experienced patients (Tables 3 and 4).11,12) If necessary, the dose was reduced or increased to either 12 µg/hr, 25 µg/hr, or 50 µg/hr to determine the minimum optimal dose of the fentanyl matrix depending on the patient's response and adjuvant analgesic demand.
Table 3. Equivalence of Opioids.
| Active substance | Equianalgesic dose (mg) | |
|---|---|---|
| Intramuscular | Oral | |
| Morphine | 10 | 30 (repeat dose) |
| 60 (single dose or intermittent dose) | ||
| Hydromorphone | 1.5 | 7.5 |
| Methadone | 10 | 20 |
| Oxycodone | 15 | 30 |
| Levorphanol | 2 | 4 |
| Oxymorphone | 1 | 10 (rectal) |
| Heroin | 5 | 60 |
| Meperidine | 75 | - |
| Codeine | 130 | 200 |
Table 4. Table for Conversion of Oral Morphine to Fentanyl Matrix.
| Oral morphine (mg/day) | Fentanyl matrix dose (βg/hr) |
|---|---|
| < 135 | 25 |
| 135–224 | 50 |
| 225–314 | 75 |
| 315–404 | 100 |
| 405–494 | 125 |
| 495–584 | 150 |
| 585–674 | 175 |
| 675–764 | 200 |
| 765–854 | 225 |
| 855–944 | 250 |
| 945–1,034 | 275 |
| 1,035–1,124 | 300 |
The most common diagnosis was low back pain (125 subjects, 27.72%), followed by spinal stenosis (98 subjects, 21.73%), osteoarthritis (57 subjects, 12.64%), postoperative neuropathic pain (22 subjects, 4.88%), failed back surgery syndrome (FBSS; 17 subjects, 3.77%), and spinal cord injury (14 subjects, 3.10%). While 294 subjects (65.19%) had no history of pain treatment, 157 subjects (34.81%) had a history of treatment. Regarding the previous treatments, nerve block was most common (74 subjects, 47.13%), followed by an exercise therapy (60 subjects, 38.22%) and surgery (23 subjects, 14.65%) (Table 5).
Table 5. Characteristics of the Patients in the Full Analysis Set.
| Characteristic | No. (%) |
|---|---|
| Total | 451 |
| Age (yr) | |
| Mean ± standard deviation | 60.52 ± 13.18 |
| Median (range) | 61 (20–95) |
| Age group | |
| 20s | 11 (2.44) |
| 30s | 13 (2.88) |
| 40s | 64 (14.19) |
| 50s | 118 (26.16) |
| 60s | 120 (26.61) |
| 70s | 102 (22.62) |
| 80s | 21 (4.66) |
| ≥ 90s | 2 (0.44) |
| Diagnosis | |
| Low back pain | 125 (27.72) |
| Spinal stenosis | 98 (21.73) |
| Osteoarthritis | 57 (12.64) |
| Postsurgical neuropathic pain | 22 (4.88) |
| Posttraumatic neuropathic pain | 17 (3.77) |
| Failed back surgery syndrome | 17 (3.77) |
| Spinal cord injury | 14 (3.10) |
| Rotator cuff tear | 12 (2.66) |
| Complex regional pain syndrome | 10 (2.22) |
| Diabetic neuropathy | 5 (1.11) |
| Post-stroke pain | 2 (0.44) |
| Postherpetic neuralgia | 1 (0.22) |
| Others | 71 (15.74) |
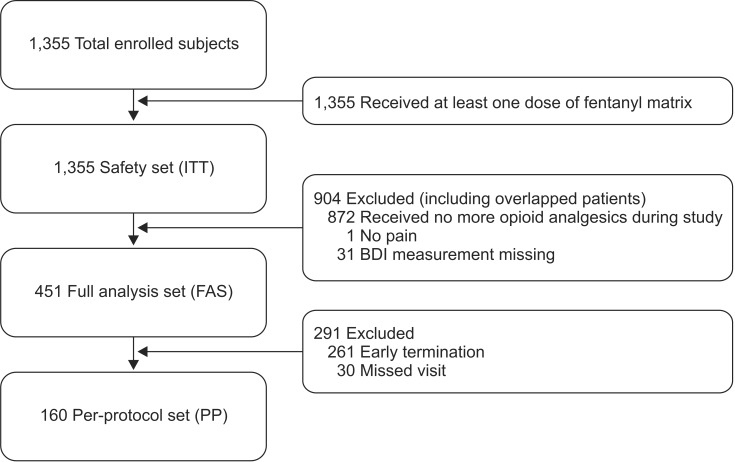
Subjects made 2 to 4 visits (at 29 ± 7 days, 85 ± 14 days, and 169 ± 14 days after administration, respectively) to have drug titration and submit clinical questionnaires. The intensity of pain and degree of interference with activities of daily life were assessed using Numeric Rating Scale (NRS). Mental function was assessed using the Korean version Beck Depression Inventory (K-BDI). Physical and mental health status were assessed by the Korean version Short-Form Health Survey 12 (SF-12). Patients and investigators respectively made global assessment of the effect of the drug at a visit compared with the previous visit on a 5-point scale themselves. In addition, the investigator's Clinical Global Impression of Improvement (CGI-I) measured symptom improvement from baseline on a 7-point scale. Data from patients were analyzed by intention-to-treat (ITT) analysis, full analysis set (FAS), and per-protocol (PP) analysis. Efficacy data were primarily analyzed by the FAS analysis in principle, and additional analysis was performed by the PP analysis. The FAS population for efficacy assessment included 451 subjects who met all of the inclusion criteria and none of the exclusion criteria, received at least one dose of the fentanyl matrix, and had primary efficacy endpoint measured at least once. The 451 subjects consisted of 188 males (41.69%) and 263 females (58.31%). Safety of the drug were analyzed by the ITT analysis. The results were compared among the analyses (Fig. 1).
Fig. 1. Efficacy and safety validation group. ITT: intention-to-treat, BDI: Beck Depression Inventory.
Patients who violated major inclusion/exclusion criteria, received no dose of the fentanyl matrix, or had no post-baseline efficacy data were excluded from the FAS population. The PP analysis included data from patients who were included in the ITT analysis and had completed all assessments at 24 ± 1 weeks after administration (169 ± 14 days) per protocol. However, patients who completed all assessments until 12 ± 1 weeks (85 ± 14 days) and then discontinued the treatment before the last assessment (169 ± 14 days) and those who completed all assessments scheduled for the last assessment at the time of discontinuation were included in the PP analysis. Depending on the nature of efficacy endpoints, t-test, paired t-test, or non-parametric tests of Wilcoxon signed-rank test, Wilcoxon rank-sum test, and chi-square test were used to test the difference from baseline.
For safety assessment, frequency and severity of AEs were investigated, and the rate of early withdrawal due to an AE was determined. For this, the ITT analysis was performed using all data obtained from patients who received at least one dose of the fentanyl matrix. This analytical approach was applied to the AE data analysis as well. Frequency of abnormal behavior following the drug treatment was also investigated.
RESULTS
In the FAS population, the mean initial dose was 13.43 ± 4.10 µg/hr, and the mean dose at the end point was 15.87 ± 9.39 µg/hr, with an increase of 2.44 µg/hr. The mean duration of administration was 105.15 ± 64.80 days, and the mean dose was 15.15 ± 7.72 µg/hr (range, 6.00 to 131.54 µg/hr) (Table 6).
Table 6. Fentanyl Dose in Patients in the Full Analysis Set (n = 451).
| Classification | Mean ± SD | Median (range) |
| Initial dose (µg/hr) | 13.43 ± 4.10 | 12 (6–25) |
| Dose at the end point (µg/hr) | 15.87 ± 9.39 | 12 (6–150) |
| Total dose administered | 1,708.19 ± 1,702.84 | 1,500 (12–23,809) |
| Total duration of dosing (day) | 105.15 ± 64.80 | 115 (1–197) |
| Mean dose (µg/hr) | 15.15 ± 7.72 | 12 (6–131.54) |
SD: standard deviation.
The mean ± SD pain intensity measured by NRS was 7.07 ± 1.78 at baseline and 4.93 ± 2.42 at the last assessment, and the change in pain intensity from baseline to the last assessment was statistically significant (p < 0.001). Regarding the degree of improvement in pain intensity, improvement of 30% or more from baseline to the last assessment was observed in 204 of 451 subjects (45%).
The degree of interference with activities of daily life due to pain was assessed in 7 domains as follows. The NRS score in the general activity domain was 6.87 ± 2.22 at baseline and was reduced by 2.1 to 4.77 ± 2.59 at the last assessment, and the change was statistically significant (p < 0.001). The NRS score in the mood domain was 6.83 ± 2.28 at baseline and was reduced by 2.16 to 4.67 ± 2.73 at the last assessment, and the change was statistically significant (p < 0.001). The NRS score in the ability to walk domain was 6.24 ± 2.79 at baseline and 4.33 ± 2.74 at the last assessment, showing statistically significant change (p < 0.001). The NRS score in the ability to perform normal work domain was 6.89 ± 2.28 at baseline, which was the highest score among 7 NRS domains, and was reduced by 2.06 to 4.83 ± 2.60, and the change was statistically significant (p < 0.001). The NRS score in the relations with others domain was 5.91 ± 2.92 at baseline and was reduced by 1.78 to 4.13 ± 2.71 at the last assessment. The change was smaller than that in other domains, but statistically significant (p < 0.001). The NRS score in the sleep domain was 6.07 ± 2.86 at baseline and 4.00 ± 2.91 at the last assessment, showing statistically significant change (p < 0.001). The NRS score in enjoyment of life domain was 6.45 ± 2.72 at baseline and 4.56 ± 2.83 at the last assessment, showing statistically significant change (p < 0.001) (Table 7).
Table 7. Degree of Interference with Daily Life Activities (n = 451).
| Variable | Visit 1 | Visit 2 | Visit 3 | Visit 4 | p-value* |
|---|---|---|---|---|---|
| General activity | < 0.001 | ||||
| Mean ± SD | 6.87 ± 2.22 | 5.65 ± 2.32 | 5.08 ± 2.47 | 4.77 ± 2.59 | |
| Median (range) | 7 (0–10) | 6 (0–10) | 5 (0–10) | 5 (0–10) | |
| Mood | < 0.001 | ||||
| Mean ± SD | 6.83 ± 2.28 | 5.55 ± 2.42 | 4.98 ± 2.58 | 4.67 ± 2.73 | |
| Median (range) | 7 (0–10) | 6 (0–10) | 5 (0–10) | 4 (0–10) | |
| Ability to walk | < 0.001 | ||||
| Mean ± SD | 6.24 ± 2.79 | 5.21 ± 2.62 | 4.68 ± 2.67 | 4.33 ± 2.74 | |
| Median (range) | 7 (0–10) | 5 (0–10) | 5 (0–10) | 4 (0–10) | |
| Ability to perform normal work | < 0.001 | ||||
| Mean ± SD | 6.89 ± 2.28 | 5.72 ± 2.33 | 5.16 ± 2.47 | 4.83 ± 2.60 | |
| Median (range) | 7 (0–10) | 6 (0–10) | 5 (0–10) | 5 (0–10) | |
| Relations with others | < 0.001 | ||||
| Mean ± SD | 5.91 ± 2.92 | 4.82 ± 2.64 | 4.42 ± 2.65 | 4.13 ± 2.71 | |
| Median (range) | 7 (0–10) | 5 (0–10) | 4 (0–10) | 4 (0–10) | |
| Sleep | < 0.001 | ||||
| Mean ± SD | 6.07 ± 2.86 | 4.85 ± 2.72 | 4.27 ± 2.79 | 4.00 ± 2.91 | |
| Median (range) | 7 (0–10) | 5 (0–10) | 4 (0–10) | 3 (0–10) | |
| Enjoyment of life | < 0.001 | ||||
| Mean ± SD | 6.45 ± 2.72 | 5.44 ± 2.62 | 4.81 ± 2.70 | 4.56 ± 2.83 | |
| Median (range) | 7 (0–10) | 5 (0–10) | 5 (0–10) | 4 (0–10) |
SD: standard deviation.
*Wilcoxon signed-rank test.
Changes in depression were measured using the K-BDI designed to assess the existence and severity of psychological and physiological symptoms of depression. Patients were asked to choose the statement that best represented their mood in 21 areas including sadness, pessimism, past failure, loss of pleasure, guilt feelings, punishment feelings, self-dislike, self-criticalness, suicidal thought, agitation, worthlessness, etc. The K-BDI score that was 0.96 ± 0.59 at baseline was changed by 0.21 ± 0.47 to 0.74 ± 0.54 at the last assessment. The change was statistically significant (p < 0.001).
The SF-12 score was 31.60 ± 11.37 at baseline and was improved to 40.15 ± 11.13 at the last assessment, and the change was statistically significant (p < 0.001). The Physical Component Summary score was 28.94 ± 7.23 at baseline and was improved to 35.90 ± 10.25 at the last assessment, and the change was statistically significant (p < 0.001). The Mental Component Summary score was 35.80 ± 11.76 at baseline and 42.52 ± 10.58 at the last assessment, also showing statistically significant improvement (p < 0.001).
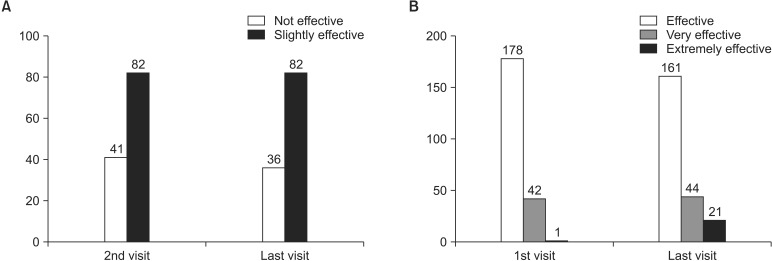
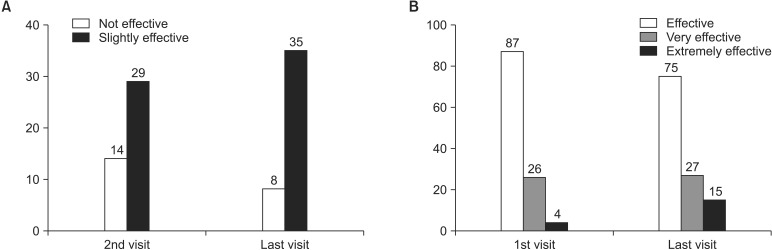
On the global assessment by patients, 41 subjects (11.92%) and 82 subjects (23.84%) at the second visit and 36 subjects (10.47%) and 82 subjects (23.84%) at the last assessment responded "not effective" and "slightly effective," respectively. Meanwhile, 178 subjects (51.74%), 42 subjects (12.21%), and 1 subject (0.29%) at the first assessment and 161 subjects (46.80%), 44 subjects (12.79%), and 21 subjects (6.10%) at the last assessment responded "effective," "very effective," and "extremely effective," respectively (Fig. 2). The increase in the number of subjects who responded the treatment was effective based on the global assessment was statistically significant (p = 0.001). On the global assessment by investigators, 14 investigators (8.75%) and 29 investigators (18.13%) at the second visit and 8 investigators (5.00%) and 35 investigators (21.88%) at the last assessment responded "not effective" and "slightly effective," respectively. Meanwhile, 87 investigators (54.38%), 26 investigators (16.25%), and 4 investigators (2.50%) at the first assessment and 75 investigators (46.88%), 27 investigators (16.88%), and 15 investigators (9.38%) at the last assessment responded "effective," "very effective," and "extremely effective," respectively. The increase in the number of investigators who responded the treatment was effective based on the global assessment was statistically significant (p = 0.015) (Fig. 3).
Fig. 2. Global assessment by patients. (A) Not effective vs. slightly effective. (B) Effective vs. very effective vs. extremely effective.
Fig. 3. Global assessment by investigators. (A) Not effective vs. slightly effective. (B) Effective vs. very effective vs. extremely effective.
On the 7-point scale CGI-I, "very much improved," "much improved," and "minimally improved" were considered improvement and the other answers were considered no improvement from baseline to the last assessment. Improvement was observed in 258 subjects (75.00%) at the first assessment and in 288 subjects (83.72%) at the last assessment. No improvement was observed in 86 subjects (25.00%) at the first assessment and in 56 subjects (16.28%) at the last assessment (p < 0.001). As in the FAS analysis, improvement at all efficacy endpoints was also significant in the PP analysis (Tables 8 and 9).
Table 8. Results of Per-Protocol Analysis (n = 160).
| Variable | Visit 1 | Visit 2 | Visit 3 | Visit 4 | p-value |
|---|---|---|---|---|---|
| Pain intensity | < 0.001* | ||||
| Mean ± SD | 7.46 ± 1.52 | 5.64 ± 1.80 | 4.66 ± 1.96 | 3.97 ± 2.09 | |
| Median (range) | 8 (3–10) | 6 (0–10) | 5 (1–10) | 3.5 (0–9) | |
| K-BDI | < 0.001† | ||||
| Mean ± SD | 1.11 ± 0.62 | - | - | 0.62 ± 0.53 | |
| Median (range) | 1.05 (0–3) | - | - | 0.48 (0–3) | |
| General activity | < 0.001* | ||||
| Mean ± SD | 7.34 ± 1.56 | 5.59 ± 1.86 | 4.64 ± 2.06 | 3.91 ± 2.14 | |
| Median (range) | 8 (3–10) | 6 (1–10) | 4 (0–10) | 4 (0–9) | |
| Mood | < 0.001* | ||||
| Mean ± SD | 7.15 ± 1.92 | 5.31 ± 2.01 | 4.28 ± 2.18 | 3.57 ± 2.28 | |
| Median (range) | 7 (0–10) | 5 (0–10) | 4 (0–10) | 3 (0–10) | |
| Ability to walk | < 0.001* | ||||
| Mean ± SD | 6.93 ± 2.23 | 5.42 ± 2.22 | 4.52 ± 2.28 | 3.71 ± 2.30 | |
| Median (range) | 7 (0–10) | 6 (0–10) | 4 (0–10) | 3 (0–9) | |
| Ability to perform normal work | < 0.001* | ||||
| Mean ± SD | 7.40 ± 1.79 | 5.73 ± 1.92 | 4.79 ± 2.11 | 4.06 ± 2.21 | |
| Median (range) | 8 (1–10) | 6 (1–10) | 5 (0–10) | 4 (0–10) | |
| Relations with others | < 0.001* | ||||
| Mean ± SD | 6.71 ± 2.50 | 4.90 ± 2.20 | 4.14 ± 2.28 | 3.46 ± 2.29 | |
| Median (range) | 7 (0–10) | 5 (0–10) | 4 (0–10) | 3 (0–10) | |
| Sleep | < 0.001* | ||||
| Mean ± SD | 6.65 ± 2.54 | 4.66 ± 2.24 | 3.58 ± 2.25 | 3.00 ± 2.36 | |
| Median (range) | 7 (0–10) | 5 (0–10) | 3 (0–10) | 2 (0–10) | |
| Enjoyment of life | < 0.001* | ||||
| Mean ± SD | 7.03 ± 2.36 | 5.33 ± 2.10 | 4.31 ± 2.25 | 3.73 ± 2.45 | |
| Median (range) | 8 (0–10) | 5 (0–10) | 4 (0–10) | 3 (0–10) |
Assessment on pain intensity, K-BDI, and degree of interference with activities of daily life due to pain.
SD: standard deviation, K-BDI: Korean-Beck Depression Inventory.
*Wilcoxon signed-rank test. †Paired t-test.
Table 9. Proportion of Subjects with Improvement of 30% or More in Pain Intensity.
| Variable | No. (%) | Confidence interval | |
|---|---|---|---|
| Lower limit | Upper limit | ||
| Improvement of ≥ 30% in pain intensity | 204/451 (45) | 0.38 | 0.52 |
In this study, 1,335 subjects who were administered at least one dose of fentanyl matrix were enrolled for the analysis of AEs and other safety data. These data were analyzed by the ITT analysis. A total of 279 cases of AEs occurred in 179 subjects (13.21%), and 228 cases of adverse drug reactions occurred in 156 subjects (11.51%). AEs with an incidence of ≥ 1% were nausea (65 cases in 65 subjects, 4.80%), dizziness (55 cases in 54 subjects, 3.99%), vomiting (43 cases in 41 subjects, 3.03%), and pruritus (22 cases in 21 subjects, 1.55%).
With regard to the relationship to the fentanyl matrix, 27 cases (9.68%) were considered "not related," 24 cases (8.60%) "doubtful," 107 cases (38.35%) "possible," 98 cases (35.13%) "probable," and 23 cases (8.24%) "very likely." With regard to the severity, 204 cases (73.12%) were "mild," 65 cases (23.30%) were "moderate," and 10 cases (3.58%) were "severe." With regard to action taken after the occurrence of AEs of fentanyl matrix, there was "no action" in 92 cases (32.97%), "dose modification" in 9 cases (3.23%), "temporary interruption" in 5 cases (1.79%), and "permanent discontinuation" in 173 cases (62.01%).
DISCUSSION
Use of opioids for the treatment of chronic pain is important in carefully selected patients. Representative opioid analgesics include methadone, oxycodone, fentanyl, hydromorphone, morphine, codeine, and meperidine. These opioids are effective for pain control, but adverse reactions such as nausea, vomiting, constipation, headache and dizziness have been frequently reported.4,13) Some studies have reported constipation and nausea as the most prevalent side effects.14) However, Yoo et al.15) argued that the greatest problem with the use of such opioids is vague anxiety about opioids, which limits continuous use. Fentanyl matrix, an opioid product, is a patch formulation developed to minimize gastrointestinal side effects of opioids.10) Allan et al.16) reported that transdermal fentanyl was preferred to sustained-release oral morphine by patients because it provided better pain relief, caused less constipation, and enhanced quality of life. Transdermal fentanyl has another advantage of allowing reduction in oral medication in elderly patients with chronic pain who take many medications.
Fentanyl matrix is a transdermal drug delivery system that steadily delivers fentanyl for 72 hours and has been reported to improve pain control and health-related quality of life in patients with non-cancerous chronic pain.10) Result of this study indicates that the long-term use of fentanyl matrix reduces pain intensity and significantly improves physical/mental functions including activities of daily life in patients with non-cancerous pain. With regard to the degree of improvement in pain intensity, improvement of 30% or more from baseline to the last assessment was observed in 204 of 451 subjects (45%).
In this study, 62.01% of the participants did neither come back to the clinics nor use the patches although AEs were only mild in them. It resulted in the decrease in the total number of subjects in the efficacy analysis. The results of our study were not significantly different from those in a previous study on fentanyl matrix.15) Insufficient use of opioid analgesics in patients with chronic non-cancerous pain has been mainly attributed to the fear of addiction, rather than AEs. Noble et al.17) showed in a meta-analysis that the rate of observed signs of opioid addiction was extremely low. Porter and Jick18) found 4 addiction cases among 12,000 patients prescribed with opioid analgesics, indicating that the long-term use of opioids are not associated with dependency. Nevertheless, Kissin19) claimed that there was no sufficient evidence to prove its safety against iatrogenic addiction among patients treated with opioids for chronic pain.
Recently, a new technique of fentanyl transdermal transport, fentanyl iontophoretic transdermal system (ITS), has been introduced. It is a patient-activated analgesic system indicated for the short-term management of acute postoperative pain in adult patients. Some study have shown that fentanyl ITS is an efficacious alternative to IV patient-controlled analgesia.20,21) Fentanyl ITS might be helpful as a next generation transdermal pain control. Still, transdermal fentanyl patch has been considered a useful pain controller in clinics. Some studies have reported that transdermal fentanyl patches improve postoperative pain relief and promote early functional recovery in total knee arthroplasty.22,23)
The limitation of this study lies in the interpretation of analysis results as a large proportion of patients was excluded from the analysis due to violation of inclusion criteria and dropping out of the study. It was an uncontrolled study, and no conclusion could be drawn on the efficacy. Active studies on the effective method of control of adverse reactions after the use of opioids and improvement of patients' recognition of opioid use are warranted in the future.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 2.Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians' (ASIPP) Guidelines. Pain Physician. 2008;11(2 Suppl):S5–S62. [PubMed] [Google Scholar]
- 3.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998;280(2):147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 5.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage. 2002;23(2):131–137. doi: 10.1016/s0885-3924(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 7.Henderson JV, Harrison CM, Britt HC, Bayram CF, Miller GC. Prevalence, causes, severity, impact, and management of chronic pain in Australian general practice patients. Pain Med. 2013;14(9):1346–1361. doi: 10.1111/pme.12195. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Grond S, Radbruch L, Lehmann KA. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin Pharmacokinet. 2000;38(1):59–89. doi: 10.2165/00003088-200038010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Muijsers RB, Wagstaff AJ. Transdermal fentanyl: an updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs. 2001;61(15):2289–2307. doi: 10.2165/00003495-200161150-00014. [DOI] [PubMed] [Google Scholar]
- 11.Foley KM. The treatment of cancer pain. N Engl J Med. 1985;313(2):84–95. doi: 10.1056/NEJM198507113130205. [DOI] [PubMed] [Google Scholar]
- 12.Ashburn MA, Lipman AG. Management of pain in the cancer patient. Anesth Analg. 1993;76(2):402–416. [PubMed] [Google Scholar]
- 13.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174(11):1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo BW, Kim JH, Park YS. Effect of long-term treatment and efficacy of transdermal opioid patch in the chronic low back pain. Int J Pain. 2010;1:15–20. [Google Scholar]
- 16.Allan L, Hays H, Jensen NH, et al. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ. 2001;322(7295):1154–1158. doi: 10.1136/bmj.322.7295.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605. doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123. doi: 10.1056/nejm198001103020221. [DOI] [PubMed] [Google Scholar]
- 19.Kissin I. Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety? J Pain Res. 2013;6:513–529. doi: 10.2147/JPR.S47182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindley EM, Milligan K, Farmer R, Burger EL, Patel VV. Patient-controlled transdermal fentanyl versus intravenous morphine pump after spine surgery. Orthopedics. 2015;38(9):e819–e824. doi: 10.3928/01477447-20150902-61. [DOI] [PubMed] [Google Scholar]
- 21.Viscusi ER, Grond S, Ding L, Danesi H, Jones JB, Sinatra RS. A comparison of opioid-related adverse events with fentanyl iontophoretic transdermal system versus morphine intravenous patient-controlled analgesia in acute postoperative pain. Pain Manag. 2016;6(1):19–24. doi: 10.2217/pmt.15.49. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto S, Matsumoto K, Iida H. Transdermal fentanyl patch improves post-operative pain relief and promotes early functional recovery in patients undergoing primary total knee arthroplasty: a prospective, randomised, controlled trial. Arch Orthop Trauma Surg. 2015;135(9):1291–1297. doi: 10.1007/s00402-015-2265-z. [DOI] [PubMed] [Google Scholar]
- 23.Hosseini H, Kargar S, Shiryazdi SM, Kargar S, Rezaie F, Neamatzadeh H. Fentanyl transdermal patch (Durogesic® DTRANS) for post abdominal laparotomy analgesia: a double blind randomized study. Minerva Chir. 2015;70(6):401–408. [PubMed] [Google Scholar]