Abstract
In plant-associated Pseudomonas species, the production of several secondary metabolites and exoenzymes is regulated by the GacS/GacA two-component regulatory system (the Gac-system). Here, we investigated if a mutation in the GacS sensor kinase affects the production of volatile organic compounds (VOCs) in P. fluorescens SBW25 (Pf.SBW25) and how this impacts on VOCs-mediated growth promotion and induced systemic resistance of Arabidopsis and tobacco. A total of 205 VOCs were detected by Gas Chromatography Mass Spectrometry for Pf. SBW25 and the gacS-mutant grown on two different media for 3 and 6 days. Discriminant function analysis followed by hierarchical clustering revealed 24 VOCs that were significantly different in their abundance between Pf.SBW25 and the gacS-mutant, which included three acyclic alkenes (3-nonene, 4-undecyne, 1-undecene). These alkenes were significantly reduced by the gacS mutation independently of the growth media and of the incubation time. For Arabidopsis, both Pf.SBW25 and the gacS-mutant enhanced, via VOCs, root and shoot biomass, induced systemic resistance against leaf infections by P. syringae and rhizosphere acidification to the same extent. For tobacco, however, VOCs-mediated effects on shoot and root growth were significantly different between Pf.SBW25 and the gacS-mutant. While Pf.SBW25 inhibited tobacco root growth, the gacS-mutant enhanced root biomass and lateral root formation relative to the non-treated control plants. Collectively these results indicate that the sensor kinase GacS is involved in the regulation of VOCs production in Pf.SBW25, affecting plant growth in a plant species-dependent manner.
Keywords: two-component regulation, Pseudomonas, GC-MS, volatile organic compounds, plant growth promotion, ISR
Introduction
Microorganisms produce a variety of volatile organic compounds (VOCs), which are defined as low molecular weight compounds with high vapor pressure (Cordovez et al., 2015; Schmidt et al., 2015). Their physical and chemical properties allow dispersal over longer distances than other extracellular microbial metabolites. VOCs are structurally diverse and include many yet unknown compounds. For example, from 12 Streptomyces strains isolated from the plant rhizosphere, a total of 381 VOCs were detected with most of them structurally unknown (Cordovez et al., 2015). To date, microbial VOCs have been grouped into hydrocarbons, ketones/alcohols, acids, sulfur compounds, nitrogen-containing compounds and terpenes (Audrain et al., 2015; Schmidt et al., 2015). Bacteria can also release inorganic volatiles such as hydrogen cyanide (HCN), ammonia, and nitrous oxide (Audrain et al., 2015; Schmidt et al., 2015). Based on the structural diversity, several natural functions have been proposed for microbial VOCs. These include a role of VOCs as: (1) infochemicals in inter- and intra-organismal communications (Chernin et al., 2011; Schmidt et al., 2015), (2) antimicrobial agents (Kai et al., 2010; Cordovez et al., 2015), and (3) compounds that promote or inhibit plant growth (Blom et al., 2011; Cordovez et al., 2015). Indeed, several bacterial genera, including Bacillus, Pseudomonas, Streptomyces, Serratia, Arthrobacter, Collimonas, and Stenotrophomonas, can influence plant growth via VOCs (Ryu et al., 2003; Garbeva et al., 2014; Audrain et al., 2015; Cordovez et al., 2015; Kanchiswamy et al., 2015; Park et al., 2015). The bacterial VOCs acetoin and 2,3-butanediol from Bacillus are well-known for their role in plant growth promotion and induction of systemic resistance (ISR) against pathogen infection (Ryu et al., 2003, 2004). Over the past years, several other bacterial VOCs, including indole, 1-hexanol, pentadecane, 13-tetradecadien-1-ol, 2-butanone, and 2-methyl-n-1-tridecene, have been implicated in plant growth promotion (Blom et al., 2011; Park et al., 2015).
Bacterial VOCs are synthesized via diverse pathways including aerobic, heterotrophic carbon metabolism, fermentation, amino-acid catabolism, terpenoid biosynthesis, fatty acid degradation, or sulphur reduction (Penuelas et al., 2014). Because the production of certain VOCs appears to be dependent on cell density, quorum sensing (QS) has been suggested as a possible regulatory system of bacterial VOCs production (Audrain et al., 2015). For example, 2-amino-acetophenone (2-AA) produced by P. aeruginosa is controlled by the multiple virulence factor regulator (MvfR), a known QS system (Kesarwani et al., 2011; Que et al., 2013). In contrast, no significant effects on VOCs production were found for a QS-mutant of Burkholderia ambifaria LMG19182 (Groenhagen et al., 2013). In Pseudomonas, the production of various secondary metabolites and exoenzymes is under the regulation of the GacS/GacA two-component regulatory system (referred to here as the Gac-system). The Gac-system consists of the membrane-bound sensor kinase GacS and the cytoplasmic transcriptional response regulator GacA. Mutations (spontaneous or site-directed) in the gacS or gacA genes generally abolish secondary metabolite production (Zuber et al., 2003). In P. protegens Pf-5, P. chlororaphis 30-84, and P. fluorescens strains SBW25 (Pf.SBW25) and F113, mutations in the gacA or gacS genes have significant effects on iron homeostasis and expression of genes involved in virulence, biofilm formation, motility, stress responses, and survival (Chancey et al., 1999; Martínez-Granero et al., 2005, 2006; Blom et al., 2011; Cheng et al., 2013; Wang et al., 2013). Production of the volatile HCN is regulated by the Gac-system in P. chlororaphis 30-84, P. fluorescens F113, and P. protegens strains CHA0 and Pf-5 (Chancey et al., 1999; Aarons et al., 2000; Duffy and Defago, 2000; Hassan et al., 2010) and also the production of 2R, 3R-Butanediol by P. chlororaphis O6 was shown to be Gac-dependent (Han et al., 2006). To date, however, the role of the GacS sensor kinase in the overall regulation of VOCs produced by plant growth-promoting rhizobacteria is not known. In this study, we analysed the VOC profiles of wild-type Pf.SBW25 and its gacS mutant grown on different media and after different incubation periods. We subsequently determined if and how a mutation in the gacS gene affects VOCs-mediated growth promotion and rhizosphere acidification of Arabidopsis and tobacco, and ISR in Arabidopsis.
Materials and Methods
Bacterial Strains, Media, and Culture Conditions
Pseudomonas fluorescens SBW25 (Pf.SBW25) and its gacS-mutant (referred to here as the Gac-mutant) (Cheng et al., 2013, 2015) were pre-cultured in liquid King’s B (KB) broth supplemented with rifampicin (50 μg/ml) and with rifampicin (50 μg/ml) and kanamycin (100 μg/ml), respectively, at 25°C for 24 h. The pathogen Pseudomonas syringae pv. tomato DC3000 (Pst) was cultured in KB broth supplemented with rifampicin (50 μg/ml) at 25°C for 24 h. Bacterial cells were collected by centrifugation, washed three times with 10 mM MgSO4 and resuspended in 10 mM MgSO4 to a final density of OD600 = 1.0 (∼109 CFU/ml).
Collection and Analysis of VOCs
For the collection of bacterial VOCs, 100 μL of a cell suspension of Pf.SBW25 and the Gac-mutant (OD600 = 0.1) were inoculated individually in 90-mm-diameter glass Petri dish containing 20 ml of KB agar or 1/5th strength Potato Dextrose Agar (1/5th PDA, Oxoid) media with three replicates each. Plates containing the agar media only served as controls. To collect the headspace VOCs, the lid of these Petri dishes were designed with an outlet connected to the traps filled with an adsorbent (Tenax). The Tenax traps were pre-conditioned at 260°C with a Helium flow rate for 45 min and cooled afterward to room temperature. Petri dishes connected to the Tenax traps were sealed with parafilm and incubated at 25°C. After 3 and 6 days of incubation, headspace VOCs was analyzed by GC-Q-TOF-MS (Agilent 7890B GC and the Agilent 7200A QTOF, Santa Clara, CA, USA). VOCs were thermally desorbed from the Tenax traps using an automated thermal desorption unit (model UnityTD-100, Markes International Ltd., Llantrisant, UK) at 210°C for 12 min (He flow 50 ml/min) and captured on a cold trap at -10°C. The compounds released were transferred onto the analytical column (30 mm × 0.25 mm ID RXI-5MS, film thickness 0.25 μm – Restek 13424-6850, Bellefonte, PA, USA) with a split ratio of 1:20 (v/v). The temperature program of the GC oven started at 39°C (2-min hold) and rose to 95°C at a rate of 3.5°C min-1, to 165°C at 6°C min-1, to 250°C at 15°C min-1 and finally to 300°C at 40°C min-1 (20 min-hold). VOCs were detected by the MS operating at 70 eV in EI mode. Mass scanning was done from 30 to 400 m/z with a scan time of 4 scans s-1.
Mass signals from GC-MS raw data that were generated using an untargeted metabolomics approach were extracted and aligned by MetAlign software (Lommen and Kools, 2012). MSClust was used to remove signal redundancy per metabolite and to reconstruct compound mass spectra as previously described (Tikunov et al., 2012). VOCs detected for both the media and the bacterial strains [Fold Change (FC) < 2] were removed from the analyses. VOCs were annotated by comparing their mass spectra with those of commercial NIST14 (National Institute of Standards and Technology, USA1) and Wiley database 9th edition. The linear retention indices (RI) and the accurate mass of selected VOCs were compared with those in the library. Processed VOCs data were log transformed and auto-scaled using the average as an offset and the standard deviation as scale [raw value-average (offset)/SD (scale)] with GeneMaths XT Version 2.11 (Applied Maths, Belgium)). Log transformed data were subjected to One-Way ANOVA and VOCs that showed a significant difference (P < 0.05) at least under one of the conditions analysed were used in the hierarchical cluster analysis (Pearson’s correlation coefficient with UPGMA algorithm) and discriminant analysis. To select VOCs affected by the Gac-mutation, Student’s t-Test (for independent samples) was performed between wild-type and mutant for both media and time points following the criteria: P < 0.05 (t-Test), peak intensity of at least 104 and fold change (FC) > 2.
VOCs-Mediated Plant Growth Promotion
To determine the role of the GacS in VOCs-mediated growth promotion of Arabidopsis and tobacco (Nicotiana benthamiana), seedlings were exposed to the VOCs emitted by the wild type Pf.SBW25 or the Gac-mutant. Arabidopsis seeds (Col-0) were surface sterilized and were sown on square plates (100 × 100 mm) containing 50 ml of half-strength Murashige and Skoog (½MS) agar medium (Murashige and Skoog, 1962) supplemented with 0.5% (w/v) sucrose as previously described (Van De Mortel et al., 2012). Plates were sealed with parafilm and incubated in a climate chamber (21°C/21°C day/night temperature; 250 μmol light m-2s-1 at plant level during 16 h/d; 70% relative humidity). Dual-dish plates were prepared with a large round Petri dish (145-mm-diameter) containing 100 ml ½ MS medium fixed with a small Petri dish (35-mm-diameter) positioned inside the large Petri plate. Arabidopsis or tobacco seedlings were grown in the large Petri plate, whereas in the small Petri plate the bacteria were cultured on KB agar medium. Six days after sowing of surface-sterilized Arabidopsis seeds, 10 μl of bacterial suspension (∼109 CFU/ml) was spot-inoculated in the small petri dishes and incubated at 25°C overnight. On the next day, the 7-day-old Arabidopsis seedlings were transferred from square Petri dish to half strength MS media in the large Petri plate. Using this experimental set-up, the plants and bacterial cultures were physically separated in the dual-dish plate and bacteria-plant interactions were only possible via VOCs. Plates were sealed with parafilm and then incubated in the climate chamber (21°C/21°C day/night temperature; 250 μmol light m-2s-1 at plant level during 16 h/d; 70% relative humidity). After 11 days of incubation, plant fresh, and dry weights were determined. Differences in shoot and root biomass were analysed statistically by one-way ANOVA, Tukey, P < 0.05). The experiment was performed three times, each time with at least five replicates per treatment. Experiments with tobacco seedlings were performed twice as described above for Arabidopsis.
VOCs-Mediated Induced Systemic Resistance
For the induced resistance (ISR) assay, leaves of 14-day-old Arabidopsis seedlings, exposed (or not) to VOCs from wild type Pf.SBW25 or the Gac-mutant, were inoculated in the centre of the leaf rosette with 2 μl cell suspension (∼109 CFU/ml) of the pathogen Pst. Five to 7 days after inoculation, disease incidence was assessed by determining the percentage of diseased leaves per plant. Leaves were scored as diseased when they exhibited necrotic or water soaked lesions surrounded by chlorotic or necrotic leaf tissue. Disease incidence was calculated for each plant with at least 20 plants per treatment. The experiment was performed at least twice. Statistically significant differences were determined by ANOVA (P < 0.05).
VOCs-Induced Rhizosphere Acidification
Next to the plant growth promotion and ISR assays, pH changes in the rhizosphere of Arabidopsis and tobacco seedlings were monitored by supplementing bromocresol green (0.02% w/v) into the ½ MS medium on which the seedlings were grown. Bromocresol green acts as a pH indicator with a yellow color at pH 3.8 and a blue color at pH 5.4. The pH changes were monitored and captured during 11 days of co-cultivation of Arabidopsis and tobacco seedlings with the bacterial strains.
Results
Gac-Regulation of VOCs Production by Pf.SBW25
Volatile organic compounds produced by wild type Pf.SBW25 and its Gac-mutant grown on KB or 1/5th PDA agar media were collected at different time points (3 or 6 days of incubation). A total of 205 putative VOCs were detected by GC-MS in the headspace of Pf.SBW25 and its Gac-mutant, of which 79 VOCs differed significantly in abundance (One-way ANOVA, P < 0.05) in at least one of the growth conditions (Figure 1). Discriminant function analysis of these 79 VOCs resulted in a model with 3 principal components, which explained 84% of the total variation. The first component explained 42% of the total variation and is related to the effect of different cultivation media on the VOCs produced by Pf.SBW25 and the Gac-mutant. VOCs detected for both Pf.SBW25 and the Gac-mutant were clearly separated by the type of cultivation medium (Figure 1). The second component explained 23% of the total variation and is related to the effect of the Gac-mutation on VOCs when grown on KB medium. The third component explained 19% of the total variation and is related to the effect of the Gac-mutation on VOCs when the strains are grown on 1/5th PDA (Figure 1). A total of 24 VOCs were significantly (P < 0.05, FC > 2) affected by the Gac-mutation at least under one of the growth conditions (Table 1). The VOC profiles of Pf.SBW25 and its Gac-mutant are shown in the Hierarchical Cluster Analysis (HCA; Figure 2A). For the majority of these metabolites, the effect of the Gac-mutation on VOCs emission was dependent on the cultivation medium (Figures 2A,B).
FIGURE 1.
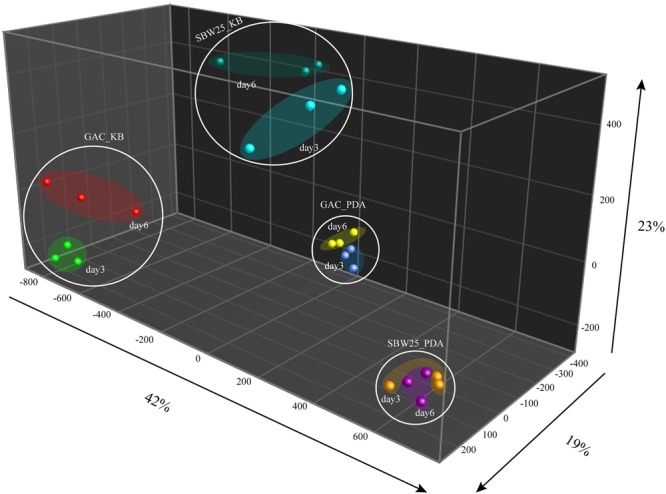
Discriminant function analysis based on 79 volatile organic compounds (VOCs) that were significantly different in their abundance at least under one of the conditions analyzed (ANOVA, P < 0.05). The first component explained 42% of the total variation that is primarily associated to variation in the abundance of VOCs when Pf.SBW25 and the Gac-mutant are grown in 1/5th PDA and KB media. The second component explained 23% of the total variation and particularly related to the effect of the mutation on VOCs when Pf.SBW25 and the Gac-mutant are grown on KB medium. The third component explained 19% of the total variation and is related to the effect of the Gac-mutation on the VOCs when Pf.SBW25 and the Gac-mutant are grown on 1/5th PDA.
Table 1.
List of 24 volatile organic compound (VOCs) significantly affected by the Gac-mutation of Pf.SBW25 under different growth conditions and incubation periods.
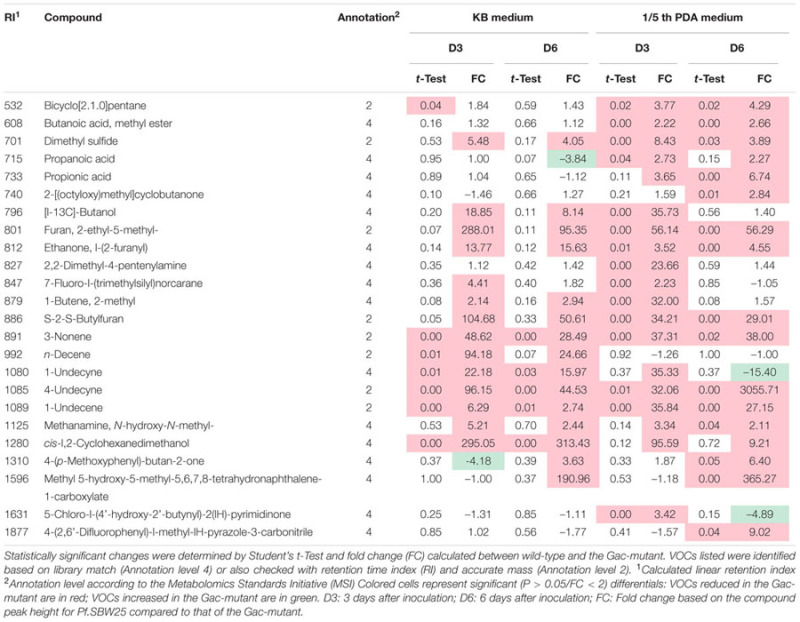 |
FIGURE 2.
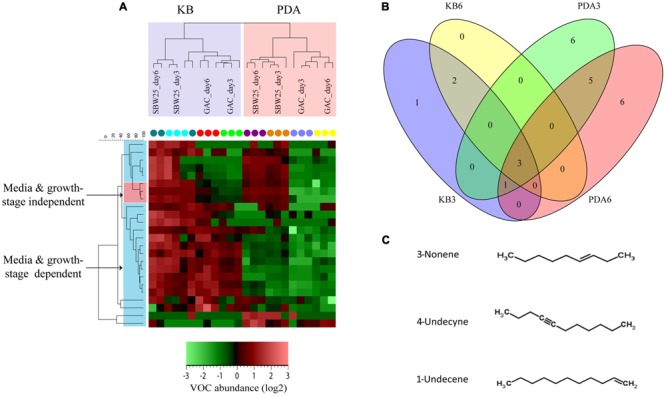
VOCs profiling of Pf.SBW25 and the gacS-mutant (Gac-mutant). (A) Hierarchical cluster analysis (HCA) based on 24 VOCs that were significantly different (P < 0.05 and fold change > 2) for the Pf.SBW25 and the Gac-mutant grown on 1/5th PDA and KB media after 3 and 6 days of inoculation. Columns represent the different isolates (in triplicates), whereas rows represent the VOCs (green: low abundance, red: high abundance). (B) Venn diagram showing the unique and shared VOCs detected for Pf.SBW25 and the Gac-mutant grown on 1/5th PDA and KB media after 3 (D3) and 6 (D6) days of inoculation. Yellow and blue cycles represent VOCs detected for strains grown on KB at D3 and D6, respectively; Green and red cycles represent VOCs detected for strains grown on 1/5th PDA at D3 and D6, respectively. (C) Group of alkenes significantly affected by the Gac-mutation of Pf.SBW25 grown on KB and 1/5th PDA media at D3 and D6 after inoculation.
On KB medium, the emission of 3 VOCs belonging to the class of acyclic alkenes was significantly reduced in the Gac-mutant (Table 1). These VOCs are 3-nonene (RI: 891), 1-undecene (RI: 1089), and 4-undecyne (RI: 1085). The VOC n-decene (RI: 992) was only detected at 3 days of incubation (Table 1; Figure 2C). On 1/5th PDA, the emission of 7 VOCs was significantly reduced in the Gac-mutant, independent of the incubation time (Table 1). These VOCs are bicyclo[2.1.0]pentane (RI: 532), dimethyl sulfide (RI: 701), S-2-S-butylfuran (RI: 886) and the three alkenes described above for KB medium.
VOCs-Mediated Effects on Plant Growth and ISR
Since the Gac-mutant established much lower cell densities than wild-type Pf.SBW25 on 1/5th PDA medium but not on KB medium, the plant growth-promotion and ISR assays were performed with the bacterial strains grown on KB only. When Arabidopsis seedlings were exposed to VOCs from Pf.SBW25 or from the Gac-mutant, plant growth, and root architecture were altered: the primary roots were longer and more lateral roots were formed by the VOCs-exposed plants than by non-exposed (medium only) control plants (Figure 3A). Both shoot and root biomass of Arabidopsis seedlings, exposed to VOCs from either Pf.SBW25, or the Gac-mutant, increased approximately fourfold as compared to control plants (Figure 3B). Leaves of Arabidopsis plants exposed to VOCs from Pf.SBW25 or the Gac-mutant were also visually greener than those of control plants (Figure 3A). To test for VOCs-mediated ISR, Arabidopsis leaves were inoculated with a suspension of the pathogen Pst. Results showed that Pst disease incidence was significantly reduced in plant seedlings exposed to VOCs from either Pf.SBW25 or from the Gac-mutant (Figure 3C). Collectively, these results show that a mutation in the GacS sensor kinase of Pf.SBW25 did not alter VOCs-mediated growth promotion of Arabidopsis seedlings nor ISR against Pst.
FIGURE 3.
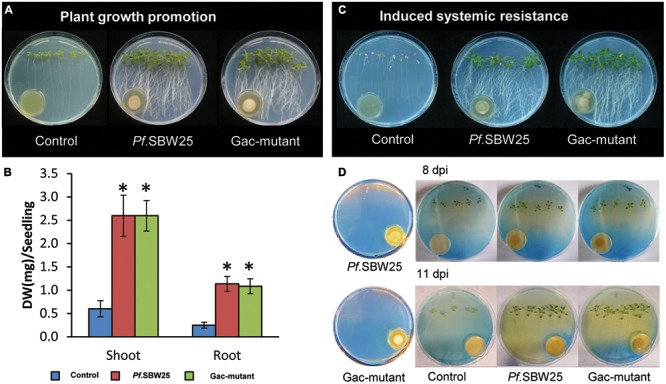
Growth promotion of Arabidopsis after 11 days of exposure to VOCs of Pf.SBW25 or the Gac-mutant (A). Quantitative analysis of the dry weight (DW) of Arabidopsis after 11 days of exposure to VOCs of Pf.SBW25 or the Gac-mutant (B). Bars represent standard deviation of the mean of 4 independent biological replicates (with 8–10 seedlings per replicate). Asterisks indicate a statistical difference as compared to controls (exposed to medium only) using Student’s t-Test (p < 0.05, n = 4). Induced systemic resistance of Arabidopsis against the leaf pathogen Pseudomonas syringae pv. tomato (Pst) (C). Rhizosphere acidification of Arabidopsis after 8 or 11 days of exposure to VOCs of Pf.SBW25 or the Gac-mutant (D). Orange colour of the medium reflects pH decline.
VOCs-Mediated Acidification of the Rhizosphere
When bromocresol green was added to the plant growth medium as a pH indicator, we observed more rhizosphere acidification (yellowish discoloration) in the VOCs-exposed Arabidopsis plants than the control plants (Figure 3D). This difference may, for a large part, be explained by the substantial increase in root biomass of the seedlings exposed to VOCs from Pf.SBW25 or the Gac-mutant. However, acidification in the Pf.SBW25-exposed seedlings was observed not only around the roots but across the entire plate, whereas for the Gac-mutant-exposed plants acidification was located mostly in the area surrounding the roots (Figure 3D). In the absence of the Arabidopsis seedlings, Pf.SBW25 and the Gac-mutant did not cause acidification of the medium. These results suggest that Pf.SBW25 and the Gac-mutant induce a quantitatively different VOCs-mediated rhizosphere acidification in Arabidopsis.
VOCs-Mediated Effects on Tobacco
To investigate if the observed VOCs-mediated phenotypic changes by Pf.SBW25 are typical for Arabidopsis or also appear in other plant species, we conducted similar assays with tobacco (N. benthamiana). When tobacco seedlings were exposed to VOCs of Pf.SBW25 or the Gac-mutant, the root architecture was differentially affected. When exposed to VOCs from Pf.SBW25, growth of the primary roots was inhibited (Figure 4A). This apparent visual difference, however, was not supported by quantitative measurements of the root biomass (Figure 4B). In contrast, VOCs from the Gac-mutant promoted root growth and induced more lateral roots relative to the Pf.SBW25 and control treatments (Figure 4A). Biomass quantifications of both shoot and root supported this latter observation (Figure 4B). For tobacco, no VOCs-mediated rhizosphere acidification was observed (Figure 4C). Collectively, these results indicate that VOCs-mediated plant growth promotion and rhizosphere acidification by Pf.SBW25 is plant species specific.
FIGURE 4.
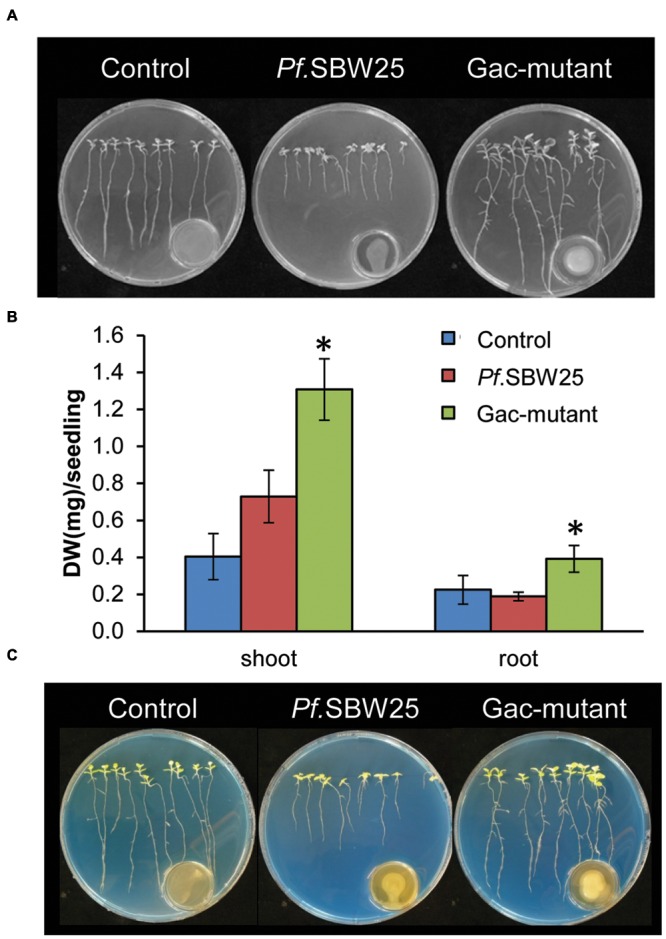
Growth promotion of tobacco (Nicotiana benthamiana) after 11 days of exposure to VOCs of Pf.SBW25 or the Gac-mutant (A). Quantitative analysis of the dry weight (DW) of tobacco after 11 days of exposure to VOCs of Pf.SBW25 or the Gac-mutant (B). Bars represent standard deviation of the mean of 4 independent biological replicates (with 8–10 seedlings per replicate). Asterisks indicate a statistical difference as compared to controls (exposed to medium only) using Student’s t-Test (p < 0.05, n = 4). Rhizosphere acidification of tobacco after 11 days of exposure to VOCs of Pf.SBW25 or the Gac-mutant (C). Blue colour of the medium reflects no pH decline.
Discussion
Microbial VOCs act as infochemicals in microbe-microbe interactions influencing inter- and intra-organismal communications (Schmidt et al., 2015). They can also have antagonistic activities by inhibiting bacterial and fungal growth (Chernin et al., 2011; Garbeva et al., 2014; Audrain et al., 2015; Cordovez et al., 2015; Schmidt et al., 2015), or by affecting gene expression, motility, biofilm formation, and antibiotic resistance of competing bacterial species (Kim et al., 2013; Audrain et al., 2015; Schmidt et al., 2015). Here, we show that VOCs production by plant growth-promoting Pf.SBW25 is regulated by the GacS sensor kinase. Our results show that a mutation in the gacS gene of Pf.SBW25 regulates, independently from growth medium or incubation time, the production of acyclic alkenes and in particular 3-nonene, 1-undecene, and 4-undecyne. These VOCs are produced by several Pseudomonas species and have been reported to have growth promoting-effects on Arabidopsis thaliana (Blom et al., 2011) and tobacco (Nicotiana tabacum cv. Xanthi-nc; (Park et al., 2015). For 1-undecene, Rui et al. (2014) recently identified the biosynthetic gene undA in P. aeruginosa PA14. Heterologous expression of undA homologs of Pseudomonas in E.coli conferred heterologous expression and production of 1-undecene (Rui et al., 2014). Our previous work showed that a mutation in the Gac-system of Pf.SBW25 caused major transcriptomic changes (Cheng et al., 2013). Revisiting these microarray data showed that the undA ortholog PFLU4307 in Pf.SBW25 was 9.7-fold down-regulated in the gacS-mutant. We did not find a so-called Gac-Box (Lapouge et al., 2008; Song et al., 2015) upstream of the undA gene in Pf.SBW25 (data not shown), suggesting that 1-undecene is not regulated by the Gac-system via small RNAs. The production of VOCs by Pf.SBW25 was also affected by the growth medium and growth stage (incubation time), confirming, and extending earlier observations made for VOCs produced by other bacterial and fungal genera (Blom et al., 2011; Schmidt et al., 2016).
Volatile organic compounds differentially produced by Pf.SBW25 and the Gac-mutant had no apparent differential effects on growth promotion and ISR in Arabidopsis. This observation suggests that the observed phenotypic effects in Arabidopsis are caused by VOCs not regulated by the sensor kinase GacS. Another possible explanation may be that other VOCs compensate for the growth-promoting or ISR-inducing VOCs regulated by GacS. Interestingly, tobacco plants did respond differentially to VOCs produced by Pf.SBW25 and the Gac-mutant. While VOCs produced by Pf.SBW25 repressed growth of the primary root of tobacco seedlings, VOCs from the Gac-mutant promoted tobacco growth relative to the untreated control. Visually, there was a clear difference between the biomass of the roots in the control and the root biomass of tobacco plants exposed to VOCs from Pf.SBW25. However, this visual difference was not supported by quantitative measurement, a discrepancy that may be due to differences in other root parameters such as root diameter. The phenotypic effects on root growth also suggests that some of the Gac-regulated VOCs may be toxic to the growth of tobacco seedlings or that the Gac-mutation enhances the production of other VOCs that trigger an increase in root growth and plant biomass. VOCs-mediated effects on plant growth are dose-dependent and can range from deleterious to beneficial effects (Blom et al., 2011; Schmidt et al., 2016). For example, indole has been shown to promote plant growth at low concentrations but to kill plants at high concentrations (Blom et al., 2011). The same was observed for sulfur-containing compounds such as dimethyl disulfide (Kai et al., 2010; Meldau et al., 2013). Therefore, the growth repression of tobacco seedlings by wild-type Pf.SBW25 can be due to Gac-regulated VOCs and/or due to specific VOCs that are emitted by Pf.SBW25 at higher concentrations than by the Gac-mutant. Pf.SBW25 does not produce HCN unlike several other Pseudomonas strains, which excludes effects of this VOC on plant growth observed in this study. The Gac-regulated VOCs that qualify for potential adverse effects on tobacco seedling growth include compounds putatively identified as 3-nonene (RI: 891), n-decene (RI: 992) 1-undecyne (RI: 1080), 4-undecyne (RI: 1085), 1-undecene (RI: 1089) and, cis-1,2-cyclohexanedimethanol (RI: 1280). The absence of these plant-growth-inhibitory VOCs in the Gac-mutant may explain in part the growth promotion observed for the Gac-mutant as compared to the Pf.SBW25-treated plants. Plant bioassays with these VOCs, applied alone and in combination in a dose-dependent manner, should be performed to pinpoint those VOCs with toxic or growth-promoting effects on tobacco seedlings. However, this chemical approach has, at this stage, several limitations that may lead to incomplete and inconclusive results. These limitations are: (i) there is a lack of pure references for several of the Gac-regulated VOCs, (ii) a wide range of concentrations and also a large number of combinations of multiple VOCs (at different concentrations) need to be tested, (iii) there is a lack of knowledge on the timing and duration of exposure of the plant seedlings to specific VOCs or combinations of VOCs, and (iv) there are technical limitations to determine if the concentrations used represent biologically relevant concentrations produced by the bacterium in situ.
The observed plant-species-specific responses to bacterial VOCs may also be due to differences in VOCs-receptors or down-stream signal transduction pathways between the two plant species tested. To our knowledge, ethylene receptors are the only type of putative receptors reported so far in VOCs perception (Ryu et al., 2003; Bailly and Weisskopf, 2012). Loss of the positive regulator of the ethylene pathway EIN2 led to different VOCs-mediated growth responses in Arabidopsis by Bacillus strains IN937a and GB03 (Ryu et al., 2003). Bacillus VOCs failed to promote growth of ein2 Arabidopsis mutants suggesting that the ethylene pathway is involved in the response to bacterial VOCs (reviewed by Bailly and Weisskopf, 2012). Whether similar or other genes and pathways affect the differential responses of the two plant species to VOCs produced by Pf.SBW25 will be subject of future analyses.
Next to plant growth promotion, rhizosphere acidification was observed for Arabidopsis but not for tobacco seedlings. Farag et al. (2013) showed that Bacillus strain GB03 is able to induce rhizosphere acidification via VOCs. The possible mechanisms proposed include elevated proton exudation from roots and direct acidification of the environment by the bacterial VOCs themselves (Farag et al., 2013). VOCs-triggered acidification was also reported to be associated with the enhancement of iron assimilation and photosynthetic efficiency (Farag et al., 2013). Rhizosphere acidification is an efficient way to enhance iron uptake and could explain the enhanced greening of leaves of the VOC-exposed Arabidopsis seedlings observed in our study.
Conclusion
In this study, demonstrated the involvement of the GacS sensor kinase in the regulation of VOCs production in Pf.SBW25, which in turn affects plant growth in a species-dependent manner. The GacS significantly affects alkene production in Pf.SBW25 independent of the cultivation medium and growth stage. Future studies will focus on the role of these and other VOCs of Pf.SBW25 in the differential growth responses observed for Arabidopsis and tobacco.
Author Contributions
XC designed and performed the experiments and drafted the manuscript. VC, DE, and XC analyzed the GC-MS data. MV supervised the work and assisted the writing. JR supervised the work and assisted with the experimental design and writing. All authors revised the manuscript and approved submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was financially supported by the Netherlands Genomics Initiative (NGI-EcoLinc project). We would like to acknowledge Hans Zweers for GC-MS analysis.
Footnotes
References
- Aarons S., Abbas A., Adams C., Fenton A., O’Gara F. (2000). A regulatory RNA (PrrB RNA) modulates expression of secondary metabolite genes in Pseudomonas fluorescens F113. J. Bacteriol. 182 3913–3919. 10.1128/JB.182.14.3913-3919.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain B., Farag M. A., Ryu C. M., Ghigo J. M. (2015). Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 39 222–233. 10.1093/femsre/fuu013 [DOI] [PubMed] [Google Scholar]
- Bailly A., Weisskopf L. (2012). The modulating effect of bacterial volatiles on plant growth: current knowledge and future challenges. Plant Signal. Behav. 7 79–85. 10.4161/psb.7.1.18418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom D., Fabbri C., Connor E. C., Schiestl F. P., Klauser D. R., Boller T., et al. (2011). Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ. Microbiol. 13 3047–3058. 10.1111/j.1462-2920.2011.02582.x [DOI] [PubMed] [Google Scholar]
- Chancey S. T., Wood D. W., Pierson L. S., III (1999). Two-component transcriptional regulation of N-acyl-homoserine lactone production in Pseudomonas aureofaciens. Appl. Environ. Microbiol. 65 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., De Bruijn I., Van Der Voort M., Loper J. E., Raaijmakers J. M. (2013). The Gac regulon of Pseudomonas fluorescens SBW25. Environ. Microbiol. Rep. 5 608–619. 10.1111/1758-2229.12061 [DOI] [PubMed] [Google Scholar]
- Cheng X., Van Der Voort M., Raaijmakers J. M. (2015). Gac-mediated changes in pyrroloquinoline quinone biosynthesis enhance the antimicrobial activity of Pseudomonas fluorescens SBW25. Environ. Microbiol. Rep. 7 139–147. 10.1111/1758-2229.12231 [DOI] [PubMed] [Google Scholar]
- Chernin L., Toklikishvili N., Ovadis M., Kim S., Ben-Ari J., Khmel I., et al. (2011). Quorum-sensing quenching by rhizobacterial volatiles. Environ. Microbiol. Rep. 3 698–704. 10.1111/j.1758-2229.2011.00284.x [DOI] [PubMed] [Google Scholar]
- Cordovez V., Carrion V. J., Etalo D. W., Mumm R., Zhu H., Van Wezel G. P., et al. (2015). Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front. Microbiol. 6:1081 10.3389/fmicb.2015.01081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy B. K., Defago G. (2000). Controlling instability in gacS-gacA regulatory genes during inoculant production of Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 66 3142–3150. 10.1128/AEM.66.8.3142-3150.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M. A., Zhang H., Ryu C. M. (2013). Dynamic chemical communication between plants and bacteria through airborne signals: induced resistance by bacterial volatiles. J. Chem. Ecol. 39 1007–1018. 10.1007/s10886-013-0317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbeva P., Hordijk C., Gerards S., De Boer W. (2014). Volatile-mediated interactions between phylogenetically different soil bacteria. Front. Microbiol. 5:289 10.3389/fmicb.2014.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenhagen U., Baumgartner R., Bailly A., Gardiner A., Eberl L., Schulz S., et al. (2013). Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 39 892–906. 10.1007/s10886-013-0315-y [DOI] [PubMed] [Google Scholar]
- Han S. H., Lee S. J., Moon J. H., Park K. H., Yang K. Y., Cho B. H., et al. (2006). GacS-dependent production of 2R, 3R-butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol. Plant Microbe Interact. 19 924–930. 10.1094/MPMI-19-0924 [DOI] [PubMed] [Google Scholar]
- Hassan K. A., Johnson A., Shaffer B. T., Ren Q. H., Kidarsa T. A., Elbourne L. D. H., et al. (2010). Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ. Microbiol. 12 899–915. 10.1111/j.1462-2920.2009.02134.x [DOI] [PubMed] [Google Scholar]
- Kai M., Crespo E., Cristescu S. M., Harren F. J., Francke W., Piechulla B. (2010). Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 88 965–976. 10.1007/s00253-010-2810-1 [DOI] [PubMed] [Google Scholar]
- Kanchiswamy C. N., Malnoy M., Maffei M. E. (2015). Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 6:151 10.3389/fpls.2015.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesarwani M., Hazan R., He J., Que Y. A., Apidianakis Y., Lesic B., et al. (2011). A quorum sensing regulated small volatile molecule reduces acute virulence and promotes chronic infection phenotypes. PLoS Pathog. 7:e1002192 10.1371/journal.ppat.1002192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Lee S., Ryu C. M. (2013). Interspecific bacterial sensing through airborne signals modulates locomotion and drug resistance. Nat. Commun. 4:1809 10.1038/ncomms2789 [DOI] [PubMed] [Google Scholar]
- Lapouge K., Schubert M., Allain F. H. T., Haas D. (2008). Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol. Microbiol. 67 241–253. 10.1111/j.1365-2958.2007.06042.x [DOI] [PubMed] [Google Scholar]
- Lommen A., Kools H. J. (2012). MetAlign 3.0: performance enhancement by efficient use of advances in computer hardware. Metabolomics 8 719–726. 10.1007/s11306-011-0369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Granero F., Capdevila S., ánchez-Contreras M. S., Martín M., Rivilla R. (2005). Two site-specific recombinases are implicated in phenotypic variation and competitive rhizosphere colonization in Pseudomonas fluorescens. Microbiology 151 975–983. 10.1099/mic.0.27583-0 [DOI] [PubMed] [Google Scholar]
- Martínez-Granero F., Rivilla R., Martín M. (2006). Rhizosphere selection of highly motile phenotypic variants of Pseudomonas fluorescens with enhanced competitive colonization ability. Appl. Environ. Microbiol. 72 3429–3434. 10.1128/AEM.72.5.3429-3434.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldau D. G., Meldau S., Hoang L. H., Underberg S., Wunsche H., Baldwin I. T. (2013). Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 25 2731–2747. 10.1105/tpc.113.114744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Park Y. S., Dutta S., Ann M., Raaijmakers J. M., Park K. (2015). Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem. Biophys. Res. Commun. 461 361–365. 10.1016/j.bbrc.2015.04.039 [DOI] [PubMed] [Google Scholar]
- Penuelas J., Asensio D., Tholl D., Wenke K., Rosenkranz M., Piechulla B., et al. (2014). Biogenic volatile emissions from the soil. Plant Cell Environ. 37 1866–1891. 10.1111/pce.12340 [DOI] [PubMed] [Google Scholar]
- Que Y. A., Hazan R., Strobel B., Maura D., He J., Kesarwani M., et al. (2013). A quorum sensing small volatile molecule promotes antibiotic tolerance in bacteria. PLoS ONE 8:e80140 10.1371/journal.pone.0080140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Z., Li X., Zhu X., Liu J., Domigan B., Barr I., et al. (2014). Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase. Proc. Natl. Acad. Sci. U.S.A. 111 18237–18242. 10.1073/pnas.1419701112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C. M., Farag M. A., Hu C. H., Reddy M. S., Kloepper J. W., Pare P. W. (2004). Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134 1017–1026. 10.1104/pp.103.026583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C. M., Farag M. A., Hu C. H., Reddy M. S., Wei H. X., Pare P. W., et al. (2003). Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100 4927–4932. 10.1073/pnas.0730845100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Cordovez V., De Boer W., Raaijmakers J., Garbeva P. (2015). Volatile affairs in microbial interactions. ISME J. 9 2329–2335. 10.1038/ismej.2015.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Etalo D. W., De Jager V., Gerards S., Zweers H., De Boer W., et al. (2016). Microbial small talk: volatiles in fungal-bacterial interactions. Front. Microbiol. 6:1495 10.3389/fmicb.2015.01495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Van Der Voort M., Van De Mortel J., Hassan K. A., Elbourne L. D., Paulsen I. T., et al. (2015). The Rsm regulon of plant growth-promoting Pseudomonas fluorescens SS101: role of small RNAs in regulation of lipopeptide biosynthesis. Microb. Biotechnol. 8 296–310. 10.1111/1751-7915.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikunov Y. M., Laptenok S., Hall R. D., Bovy A., De Vos R. C. (2012). MSClust: a tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics 8 714–718. 10.1007/s11306-011-0368-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Mortel J. E., De Vos R. C., Dekkers E., Pineda A., Guillod L., Bouwmeester K., et al. (2012). Metabolic and transcriptomic changes induced in Arabidopsis by the rhizobacterium Pseudomonas fluorescens SS101. Plant Physiol. 160 2173–2188. 10.1104/pp.112.207324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Lee S. H., Seeve C., Yu J. M., Pierson L. S., III, Pierson E. A. (2013). Roles of the Gac-Rsm pathway in the regulation of phenazine biosynthesis in Pseudomonas chlororaphis 30-84. Microbiologyopen 2 505–524. 10.1002/mbo3.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber S., Carruthers F., Keel C., Mattart A., Blumer C., Pessi G., et al. (2003). GacS sensor domains pertinent to the regulation of exoproduct formation and to the biocontrol potential of Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 16 634–644. 10.1094/MPMI.2003.16.7.634 [DOI] [PubMed] [Google Scholar]


