Abstract
We review the biofunctional effects of the flower buds of Camellia sinensis and C. sinensis var. assamica, such as antihyperlipidemic, antihyperglycemic, antiobesity, and gastroprotective effects in vivo, and antiallergic, pancreatic lipase inhibitory, and amyloid β (Aβ) aggregation inhibitory activities in vitro. Although the biofunctional effects of tea leaves have been extensively studied, less attention has been given to those of the flowers and seeds of the tea plant. Our studies focused on the saponin constituents of the extracts of the flower buds of C. sinensis cultivated in Japan and China, and C. sinensis var. assamica cultivated in India, and we review their beneficial biofunctions for health promotion.
Keywords: Camellia sinensis, Camellia sinensis var. assamica, Flower buds, Chakasaponin, Floratheasaponin, Floraassamsaponin, Biofunctional effect
Introduction
Tea made from the leaves of the plant Camellia sinensis has been used since ancient times for medicinal purposes, and is now consumed as a popular beverage. Cultivated in more than 30 countries, >4 million tons of tea are produced annually. Depending on the processing of the leaves, tea is classified into two major types—green tea and black tea. While black tea is the major type of tea produced and consumed worldwide, green tea is more popular than black tea in China and Japan. Tea has been studied for its beneficial health effects, such as reduction of body weight, alleviation of metabolic syndrome, prevention of cardiovascular diseases and cancer, and protection against neurodegeneration. With regard to the mechanisms responsible for metabolic syndrome, decreased absorption of lipids by tea polyphenols such as (−)-epigallocatechin 3-O-gallate at the intestine, and activation of adenosine monophosphate (AMP)-activated protein kinase by tea polyphenols that are bioavailable in the liver, skeletal muscle, and adipose tissues have been demonstrated. Activation of AMP-activated protein kinase decreases gluconeogenesis and fatty acid synthesis and increases catabolism, leading to body weight reduction and alleviation of metabolic syndrome [1].
Although the biofunctional effects of tea leaves have been studied extensively, less attention has been given to those of the flowers and seeds of the tea plant. We have previously reported the various tea saponins, asssamsaponins, and camelliasaponins (classified as acylated oleanane-type triterpene oligogycosides) from the seeds of C. sinensis and C. sinensis var. assamica [2–9]. Among them, theasaponins E1, E2, E5, and assamsaponin C (at a low dose of 5.0 mg/kg) showed protective effects on ethanol-induced gastric mucosal lesions in rats [3, 5, 6], and theasaponin E1 inhibited gastric emptying, and elicited an accelerating effect on gastrointestinal transit [4]. Foliatheasaponins from the leaves of Japanese C. sinensis (Tencha) have also been reported to display such effects [10]. With regard to the biofunctions of the tea flower (‘Chaka’ in Japanese), we have reported the beneficial biofunctions of the extract for health promotion—antihyperlipidemic [11, 12], antihyperglycemic [12, 13], gastroprotective [13], and antiobesity effects [14] in vivo, with antiallergic [15], pancreatic lipase inhibitory [16], and amyroid β (Aβ) aggregation inhibitory effects [17].
Here, we focused on the saponin constituents of the flower bud extracts of C. sinensis cultivated in Japan (Japanese Chaka), Anhui, Sichuan, and Fujian provinces of China (Anhui, Sichuan and Fujian Chaka), and C. sinensis var. assamica cultivated in India (Indian Assam Chaka) and on their biofunctional effects. We further divided the review into three parts—(A) Japanese and Anhui Chaka, (B) Fujian Chaka, and (C) Indian Assam Chaka, based on their saponin constituents and the variety of plant.
The biofunctional effects of Japanese and Anhui Chaka
With regard to the biofunctional effects of Japanese and Anhui Chaka, we documented the antihyperlipidemic, antihyperglycemic, and gastroprotective effects of the methanolic (MeOH) extract in mice and rats, and provided findings on the antiallergic activity and inhibitory action of pancreatic lipase, rat intestinal α-glucosidase, and rat lens aldose reductase (in vitro).
Antihyperlipidemic and antihyperglycemic effects
With regard to antihyperlipidemic properties, we observed the effect of the MeOH extract from Japanese and Anhui Chaka on increased plasma triglyceride (TG) levels in olive oil-loaded mice [11]. The MeOH extract (34.1 % from the material) (500–1,000 mg/kg, p.o.) and n-BuOH-soluble fraction (15.8 %) (500 mg/kg, p.o.) of Japanese Chaka significantly inhibited increased plasma TG levels in olive oil-loaded mice [11].
With regard to the antihyperglycemic properties, we also observed the effect of the MeOH extract of Japanese Chaka on increased serum glucose levels in sucrose-loaded rats, rat intestinal α-glucosidase and rat lens aldose reductase. Briefly, the MeOH extract (1,000 mg/kg, p.o) and n-BuOH-soluble (500 mg/kg, p.o.) fraction significantly inhibited plasma glucose elevation in rats. In addition, the extract significantly inhibited rat lens aldose reductase (IC50 72 µg/mL), although inhibition of rat intestinal α-glucosidase by the extract was weak [13].
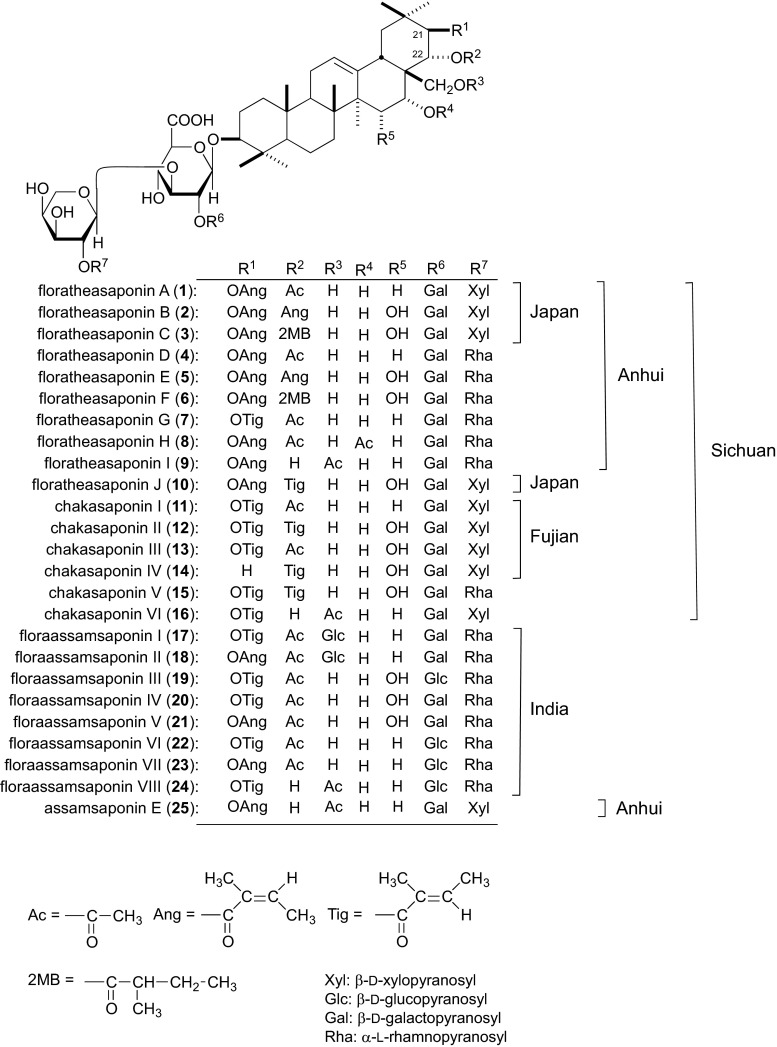
In the course of our studies on the saponin composition of tea plants, acylated oleanane-type triterpene oligoglycosides, floratheasaponins A (1)–J (10), were isolated from Japanese Chaka and Anhui Chaka, and their structures were elucidated on the basis of chemical and physicochemical evidence [11, 15, 18] (Fig. 1). However, although we were not able to isolate floratheasaponins from Fujian Chaka, instead we obtained chakasaponins I (11)–IV (14) [11, 16]. These findings were supported by qualitative and quantitative analyses of floratheasaponins and chakasaponins in Chaka from different regional origins. Briefly, the floratheasaponin content in the Fujian and Sichuan Chaka is lower than those in Japan, Taiwan, and India [13, 19]. Furthermore, the floratheasaponin content in Japanese Chaka varies markedly during the blooming periods, and is abundant at half-bloom [13]. In addition, the caffeine content in the flower buds is lower than the tea leaves [13].
Fig. 1.
Chemical structures of acylated oleanane-type triterpene oligoglycosides from the flower buds of C. sinensis cultivated in Japan and China, and C. sinensis var. assamica cultivated in India
Floratheasaponins A (1)–C (3) from Japanese Chaka at 50 and 100 mg/kg p.o. significantly inhibited TG elevation in olive oil-loaded mice [11], and on serum glucose elevation in sucrose-loaded rats [13] (Table 1). Comparing the effects of 1–3 with those of theasaponins E1 and E2 from the seeds of C. sinensis suggests that the 21 and 22-acyl groups are essential, although the 23-aldehyde group is not relevant to the antihyperlipidemic activity [11].
Table 1.
Effects of floratheasaponins A (1)–C (3) on serum triglyceride (TG) elevation in olive oil-loaded mice and serum glucose elevation in sucrose-loaded rats.
Data were taken and reproduced from references [11] and [13]
| Treatment | Dose (mg/kg, p.o.) |
Serum TG (mg/100 mL) at 2.0 h |
Serum glucose (mg/100 mL) at 0.5 h |
|---|---|---|---|
| Normal | – | 140.8 ± 5.9** | 80.3 ± 2.7** |
| Control | – | 566.6 ± 22.2 | 147.6 ± 3.0 |
| Floratheasaponin A (1) | 25 | 411.0 ± 34.3 | – |
| 50 | 387.4 ± 74.7* | 122.5 ± 8.8 | |
| 100 | 158.3 ± 31.9** | 107.3 ± 3.6** | |
| Floratheasaponin B (2) | 25 | 411.7 ± 50.6 | – |
| 50 | 316.9 ± 63.0** | 120.3 ± 10.5 | |
| 100 | 161.9 ± 11.9** | 98.4 ± 7.2** | |
| Floratheasaponin C (3) | 25 | 348.5 ± 83.7** | – |
| 50 | 204.1 ± 40.3** | 119.8 ± 7.9** | |
| 100 | 143.1 ± 11.3** | 87.7 ± 5.5** | |
| Normal | – | 154.3 ± 9.3** | – |
| Control | – | 387.1 ± 39.2 | – |
| Orlistat | 6.25 | 266.4 ± 31.1* | – |
| 12.5 | 187.9 ± 25.5** | – | |
| 25 | 158.9 ± 28.7** | – | |
| Normal | – | – | 85.6 ± 3.6** |
| Control | – | – | 159.6 ± 7.0 |
| Metoformin hydrochloride | 50 | – | 134.8 ± 4.3* |
| 100 | – | 124.1 ± 5.5** |
Effects on serum TG levels in mice: each test sample was given orally to fasted ddY male mice, and olive oil (5 mL/kg, p.o.) was given 30 min later. Blood samples were collected at 2, 4, and 6 h after olive oil treatment. Serum TG levels were determined by an enzymatic method using a commercially available kit
Effects on serum glucose levels in rats: each test sample was given orally to fasted male Wistar rats, and a 20 % (w/v) sucrose solution (5 mL/kg, p.o.) was given 30 min later. Blood samples were collected at 0.5, 1, and 2 h after sucrose loading. Serum glucose levels were determined by an enzymatic method using a commercially available kit
Values represent the mean ± SEM (n = 5–15)
Significant difference where *p < 0.05 or **p < 0.01 was compared with controls
Gastromucosal protection
We further studied the protective effect of Japanese Chaka on ethanol and indomethacin-induced gastric lesions in rats. The MeOH extract (5–50 mg/kg) dose-dependently inhibited gastric lesions, and floratheasaponins A (1)–C (3) significantly inhibited ethanol-induced gastric lesions [13]. In fact, their effects were stronger than that of cetraxate hydrochloride (used as a positive control; Table 2).
Table 2.
Inhibitory effects of floratheasaponins A (1)–C (3) on gastric lesions induced by ethanol in rats.
Data were taken and reproduced from reference [13]
| Treatment | Dose (mg/kg, p.o.) |
Gastric lesions | |
|---|---|---|---|
| Length (mm) | Inhibition (%) | ||
| Control | – | 157.0 ± 15.5 | – |
| Floratheasaponin A (1) | 5 | 80.2 ± 14.7 | 48.9 |
| 10 | 72.9 ± 11.1** | 57.7 | |
| 20 | 24.2 ± 10.2** | 88.4 | |
| 50 | 0.0 ± 0.0** | 100.0 | |
| Floratheasaponin B (2) | 5 | 92.8 ± 24.2 | 40.9 |
| 10 | 55.4 ± 12.2** | 64.7 | |
| 20 | 24.9 ± 10.5** | 84.1 | |
| 50 | 11.5 ± 7.7** | 92.7 | |
| Control | – | 163.3 ± 10.3 | – |
| Floratheasaponin C (3) | 5 | 38.0 ± 7.1** | 76.7 |
| 10 | 22.3 ± 6.8** | 86.3 | |
| 20 | 18.5 ± 7.2** | 88.7 | |
| 50 | 0.0 ± 0.0 | 100.0 | |
| Control | – | 148.4 ± 9.8 | – |
| Cetraxate hydrochloride | 75 | 87.2 ± 7.4** | 41.2 |
| 150 | 51.0 ± 4.0** | 65.6 | |
| 300 | 30.5 ± 8.3** | 79.4 | |
Ethanol (99.5 %, 1.5 mL/rat, p.o.) was given to fasted male Sprague–Dawley rats 1 h before removal of the stomach, which was previously inflated by an injection of 10 ml of 1.5 % formalin to fix the inner and outer layers of the gastric walls. Subsequently, the stomach was incised along the greater curvature and the lengths of gastric lesions were measured, and the total length (mm) was expressed as a lesion index. The test sample was administered 1 h before the ethanol treatment
Values represent the mean ± SEM (n = 6–12)
Significant difference where **p < 0.01 was compared with controls
Antiallergic effects in vitro
Histamine, which is released from mast cells stimulated by an antigen or a degranulation inducer, is usually determined as a degranulation marker in in vitro experiments on immediate allergic reactions. β-Hexosaminidase is also stored in secretory granules of mast cells and basophils, and is also released concomitantly with histamine when mast cells are immunologically activated. Therefore, it is generally accepted that β-hexosaminidase is a degranulation marker of mast cells, and this assay has been used for the evaluation of antiallergic compounds as an alternative to passive cutaneous anaphylaxis reactions in laboratory animals [20].
Park et al. reported that the dammarane-type triterpene glycoside, ginsenoside Rh2, exhibited inhibitory activity on β-hexosaminidase release from rat basophilic leukemia (RBL-2H3) cells, an activity suggested to originate from cell membrane-stabilizing action [21]. Acylated oleanane-type triterpene oligoglycosides, floratheasaponins A (1)–F (6), were also expected to exhibit cell membrane-stabilizing action. Therefore, we examined the effects of these floratheasaponins (1–6) on the release of β-hexosaminidase induced by dinitrophenylated bovine serum albumin (DNP-BSA) from RBL-2H3 cells sensitized with anti-DNP immunoglobulin E.
The MeOH extract (38.5 % from the material) from Anhui Chaka exhibited a significant inhibitory effect on the release of β-hexosaminidase from RBL-2H3 cells (inhibition 43.5 ± 2.4 % at 100 µg/mL, p < 0.01). The ethyl acetate (EtOAc)- and n-BuOH-soluble fractions inhibited antigen-induced degranulation in RBL-2H3 cells at 100–30 µg/mL (inhibition 18.1 ± 4.0 %, p < 0.05 and 60.1 ± 2.9 %, p < 0.01, respectively), although the H2O-soluble fraction did not show such an effect. Floratheasaponins (1–6) were found to show inhibitory effects on antigen-induced degranulation. In particular, floratheasaponins B (2) and E (5) exhibited significant inhibitory effects of 59.8 ± 3.6 % (p < 0.01) and 52.3 ± 3.2 % (p < 0.01) at 3 µM, respectively, and their activities were, in fact, more potent than those of two antiallergic compounds, tranilast and ketotifen fumarate [15].
Biofunctional effects of Fujian Chaka
We investigated the effects of the antihyperlipidemic, antihyperglycemic, and antiobesity properties of the MeOH extract (31.1 % from the material) from Fujian Chaka, along with their ability to inhibit gastric emptying, and accelerate gastrointestinal transit.
Antihyperlipidemic and antihyperglycemic effects
The MeOH extract (500 mg/kg, p.o.) of Fuijian Chaka also inhibited plasma TG elevation in olive oil-loaded mice and plasma glucose elevation in sucrose-loaded mice [12]. Chakasaponins I (11)–IV (14) isolated from Fujian Chaka, and their chemical structures were determined [12, 16]. In addition, chakasaponins V (15) and VI (16) together with floratheasaponins A (1)–I (9), chakasaponins I (11)–III (13), and assamsaponin E (25) were isolated from the dried flower buds of C. sinensis cultivated in Sichuan province of China (Sichuan Chaka) [22]. Chakasaponins I (11)–III (13) from the n-BuOH-soluble fraction of Fujian Chaka at 50 mg/kg significantly inhibited plasma TG and glucose elevation in mice [12] (Table 3), without affecting the enzyme activity of rat intestinal α-glucosidase. The MeOH extract and EtOAc- and n-BuOH-soluble fractions significantly inhibited pancreatic lipase, and the IC50 values of 11–13 were 0.17, 0.18 and 0.53 mM, respectively, without the desacyl derivative of 12 affecting it, suggesting that the 21 and 22-acyl groups are essential for the activity [16].
Table 3.
Effects of chakasaponins I (11)—III (13) and escin IIa on plasma triglyceride (TG) and glucose elevations in olive oil and sucrose-loaded mice.
Data were taken and reproduced from reference [12]
| Treatment | Dose (mg/kg, p.o.) |
Plasma TG (mg/100 mL) at 2.0 h |
Plasma glucose (mg/100 mL) at 0.5 h |
|---|---|---|---|
| Normal | – | 115.5 ± 12.4** | 128.5 ± 5.3** |
| Control | – | 440.5 ± 45.2 | 226.9 ± 8.2 |
| Chakasaponin I (11) | 25 | 435.7 ± 67.4 | – |
| 50 | 284.2 ± 9.6** | 199.6 ± 8.7** | |
| 100 | – | 196.8 ± 8.2** | |
| Normal | – | 152.4 ± 13.5** | 116.5 ± 7.9** |
| Control | – | 553.8 ± 49.8 | 221.5 ± 14.4 |
| Chakasaponin II (12) | 25 | 431.8 ± 49.8** | – |
| 50 | 249.5 ± 31.1** | 185.7 ± 9.9** | |
| 100 | – | 178.5 ± 8.3** | |
| Normal | – | 124.0 ± 8.4** | 126.5 ± 4.0** |
| Control | – | 407.2 ± 73.0 | 238.7 ± 5.9 |
| Chakasaponin III (13) | 25 | 394.1 ± 81.4 | – |
| 50 | 214.4 ± 62.7* | 215.9 ± 10.6** | |
| 100 | – | 189.0 ± 10.2** | |
| Normal | – | 91.9 ± 9.4** | – |
| Control | – | 440.3 ± 60.2 | – |
| Orlistat | 5 | 371.3 ± 41.5 | – |
| 10 | 203.8 ± 52.1** | – | |
| Normal | – | – | 124.8 ± 7.3** |
| Control | – | – | 218.7 ± 4.0 |
| Acarbose | 10 | – | 162.4 ± 11.7** |
| 20 | – | 153.8 ± 10.2** | |
| Normal | – | 114.9 ± 18.1** | 122.2 ± 6.3** |
| Control | – | 546.7 ± 59.4 | 263.3 ± 16.2 |
| Escin IIa | 50 | 464.2 ± 45.3 | 254.2 ± 14.0 |
| 100 | 308.9 ± 61.4* | 206.4 ± 12.9** |
Effects on plasma TG levels in mice: each test sample was given orally to fasted male ddY mice and olive oil (5 mL/kg, p.o.) was given 30 min later. Blood samples were collected at 2, 4, and 6 h after olive oil treatment. Plasma TG levels were determined by an enzymatic method using a commercially available kit
Effects on plasma glucose levels in mice: each test sample was given orally to fasted male ddY mice, and a 10 (w/v) % sucrose solution (10 mL/kg, p.o.) was given 30 min later. Blood samples were collected at 0.5, 1, and 2 h after sucrose loading. Plasma glucose levels were determined by an enzymatic method using a commercially available kit
Values represent the mean ± SEM (n = 5–9)
Significant difference where *p < 0.05 or **p < 0.01 was compared with controls
Antiobesity effects in high-fat diet-fed mice and Tsumura Suzuki Obese Diabetes (TSOD) mice
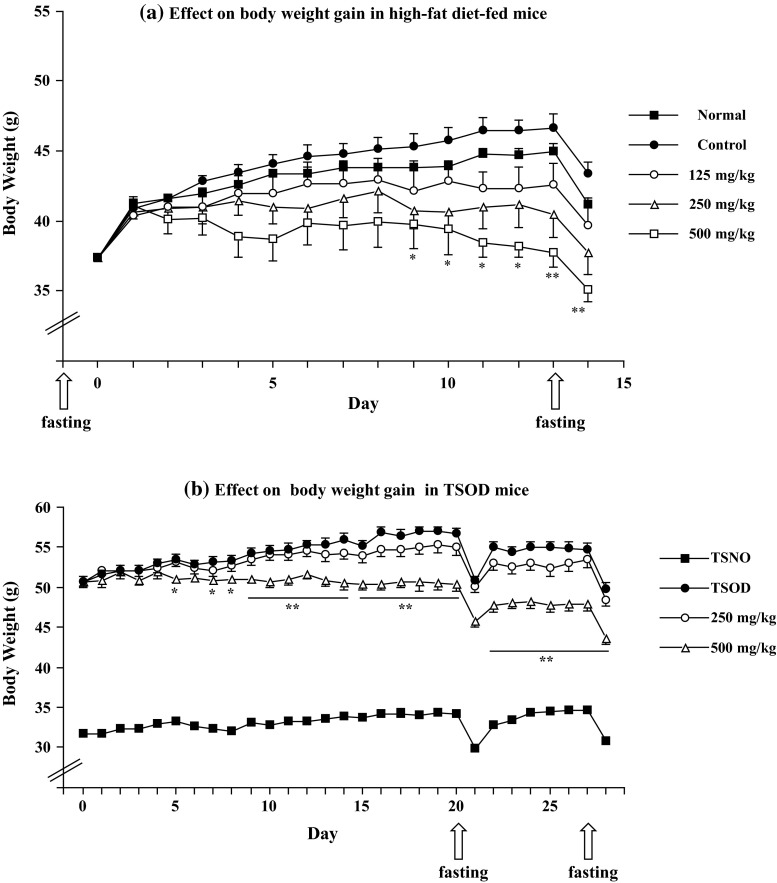
The effects of the MeOH extract on body weight gain in high-fat diet-fed mice and an experimental animal model of metabolic syndrome, TSOD mice, have been reported [14]. The MeOH extract (500 mg/kg/day, p.o.) markedly inhibited body weight gain 9–14 days after administration in high-fat diet-fed mice (Fig. 2a). The MeOH extract (250 and/or 500 mg/kg/day, p.o.) significantly suppressed liver weight, liver TG and the weight of visceral fat. A positive control, bezafibrate (50 and 100 mg/kg/day, p.o.), significantly reduced body weight gain (43.4 ± 0.9 g at 50 mg/kg, p < 0.05 and 43.0 ± 1.1 g at 100 mg/kg, p < 0.05 vs 46.2 ± 0.5 g, control group) on day 14 after fasting. The compound also reduced the weight of visceral fat and plasma TG at 100 mg/kg. Peroxisome proliferator-activated receptor (PPAR)α agonists including bezafibrate are reported to increase liver weight at higher doses in rats [23]. Consistent with this report, an increase in liver weight was observed in bezafibrate-treated mice. However, the MeOH extract did not show such an effect, and this result together with the lack of effect on plasma TG levels suggested that the extract might not act as a PPARα agonist.
Fig. 2.
Effects of the MeOH extract of Fujian Chaka on body weight gain in high-fat diet-fed mice and Tsumura Suzuki Obese Diabetes (TSOD) mice. a Male ddY mice were fed a high-fat diet (45 kcal % fat, D12451; Research Diet, Inc.) or normal diet (10 kcal % fat, D12450B; Research Diet, Inc.) for 14 days. The test sample was given orally once a day. b TSOD and Tsumura Suzuki Non-obesity (TSNO) mice were fed a standard laboratory chow MF (Oriental Yeast Co., Ltd.) for 28 days. The test sample was given orally once a day. Each value represents the mean with SEM (n = 6–10). Significant difference where *p < 0.05 or **p < 0.01 was compared with controls.
Data were taken from reference [14]
The extract (500 mg/kg/day, p.o.) also significantly suppressed body weight gain of TSOD mice after one week (Fig. 2b). Three weeks later, a glucose tolerance test was performed using an intraperitoneal injection of glucose. The MeOH extract (250–500 mg/kg/day, p.o.) significantly suppressed an increase in plasma glucose levels 2 h after glucose loading. After 4 weeks, liver weight, weight of visceral fat, and plasma total cholesterol levels were significantly suppressed by the extract (500 mg/kg/day, p.o.).
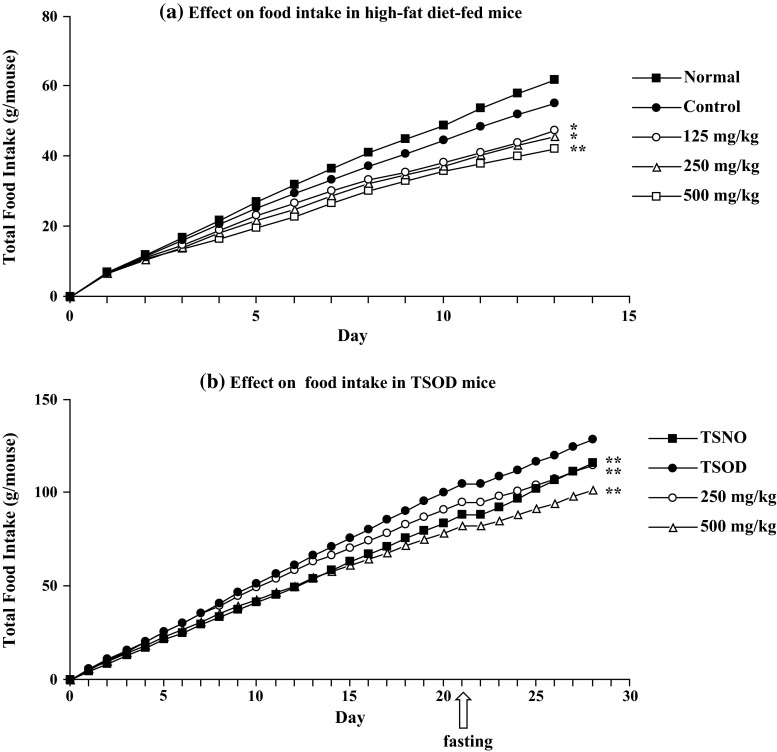
We speculated that the strong reduction of body weight within a week after extract treatment was mainly due to reduction of food intake. Therefore, the effect of the extract on food intake was examined in high-fat diet-fed mice and TSOD mice. As shown in Fig. 3a and b, the extract inhibited food intake in a dose-dependent manner. Furthermore, this effect was also observed in normal diet-fed mice; the total intake for 5 days in the MeOH extract-treated group (500 mg/kg/day, p.o.) was 19.3 g (p < 0.01) vs 21.0 g in the control group, although an obvious toxic effect was not observed (except for body weight gain) [14].
Fig. 3.
Effects of the MeOH extract of Fujian Chaka on food intake in high-fat diet-fed mice and TSOD mice. a Male ddY mice were fed a high-fat diet (45 kcal % fat) or normal diet (10 kcal % fat) for 14 days. The test sample was given orally once a day. b TSOD and TSNO mice were fed a standard laboratory chow MF (Oriental Yeast Co., Ltd.) for 28 days. The test sample was given orally once a day. Each value represents the mean for 6–10 mice. Significant difference where *p < 0.05 or **p < 0.01 was compared with controls. Refer to Fig. 2 for other abbreviations.
Data were taken from reference [14]
Next, we examined the n-BuOH-, ethyl acetate (EtOAc)- and H2O-soluble fractions from the MeOH extract. The n-BuOH-soluble fraction (16.4 % from the material) inhibited food intake at a dose of 250 mg/kg/day, p.o. (Fig. 4a), but the EtOAc- and H2O-soluble fractions (3.2 and 11.5 % from the material) had no such effect, when the fraction was given orally according to its yield.
Fig. 4.
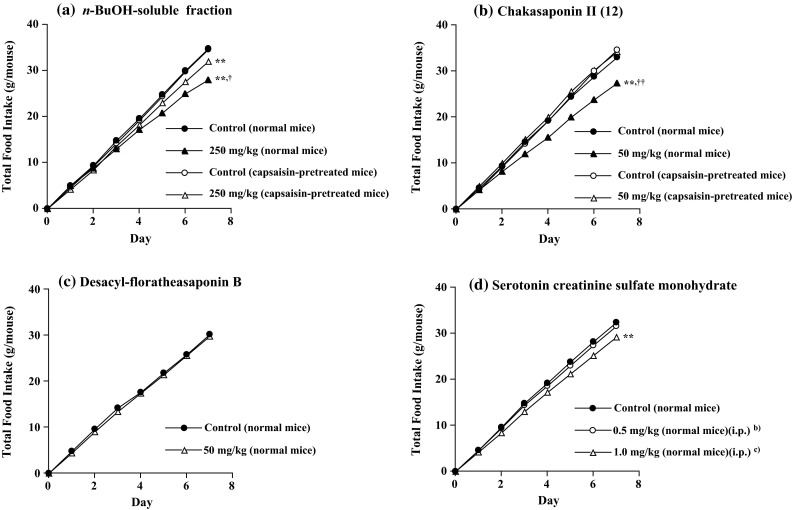
Effects of the BuOH-soluble fraction of Fujian Chaka, chakasaponin II (12), desacyl-floratheasaponin B, and 5-HT on food intake in normal mice and/or capsaicin-pretreated mice. Male ddY mice were fed a standard laboratory chow MF (Oriental Yeast Co., Ltd.) for 8 days. The test sample was given orally once a day. b, c These doses are equivalent to approximately 0.2–0.4 mg/kg serotonin. Each value represents the mean of 5 or 6 mice. Significant differences where **p < 0.01 and † p < 0.05 or †† p < 0.01 were compared with controls and capsaicin-treated group, respectively.
Data were taken from reference [14]
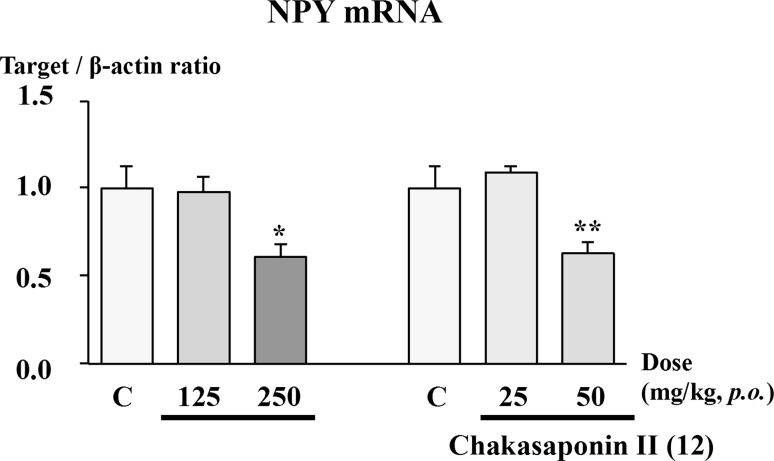
With regard to the effect of the n-BuOH-soluble fraction on appetite signals, the effects on neuropeptide Y (NPY) and agouti-related protein (AgRP) mRNA levels in the hypothalamus were examined. NPY is an important regulator of body weight through its effects on food intake and energy expenditure. The majority of neurons expressing NPY in the hypothalamus are found within the arcuate nucleus (ARC) and most co-express AgRP. The ablation of NPY/AgRP neurons in young mice reduces food intake and body weight, and an intracerebroventricular injection of NPY potently stimulates food intake in adult rats [24]. In our study, the n-BuOH-soluble fraction administrated at 250 mg/kg for 4 days significantly suppressed the expression of NPY mRNA (Fig. 5). These findings suggest that the n-BuOH-soluble fraction inhibited food intake by suppressing appetite signals.
Fig. 5.
Effects of n-BuOH-soluble fraction and chakasaponin II (12) on neuropeptide Y (NPY) mRNA levels in mice. The test sample was given orally to male ddY mice once a day. Four days later, the hypothalamus was dissected out, and the NPY mRNA levels were determined using real-time polymerase chain reaction. Each bar represents the mean ± SEM (n = 6). Significant difference where *p < 0.05 or **p < 0.01 was compared with controls
Data were taken and reproduced from reference [14].
Furthermore, a principal saponin, chakasaponin II (12), induced a similar suppression of food intake (50 mg/kg/day, p.o.) (Fig 4b). In addition, 12 (50 mg/kg/day, p.o.) significantly inhibited NPY mRNA levels in the hypothalamus similar to the n-BuOH-soluble fraction (Fig. 5). These results suggest that the saponins are active constituents of the extract. Furthermore, the desacyl derivative of 12, desacyl-floratheasaponin B, failed to produce the effect (Fig. 4c), suggesting that the 21 and 22-acyl groups are important for the activity.
Table 4.
Effects of chakasaponin II (12) on plasma GLP-1 and CCK levels in mice
| Treatments | Dose (mg/kg, p.o.) |
Food intake (g/30 min) |
Plasma GLP-1 (pg/mL) |
Plasma CCK (pg/mL) |
|---|---|---|---|---|
| Control | – | 1.31 ± 0.06 | 20.0 ± 3.4 | 419 ± 25 |
| Chakasaponin II (12) | 25 | 1.19 ± 0.08 | 25.3 ± 4.6 | 681 ± 70** |
| 50 | 1.02 ± 0.06** | 37.3 ± 9.9* | 702 ± 112** |
Male ddY mice (6 weeks old) were fed a high-fat diet (45 kcal % fat, D12451; Research Diets Inc.) for 7 days before the experiments. Test samples were given orally to the fasted mice 45 min before the mid-light satiety test. The high-fat diet was then given to the mice for 30 min, and the food intake within the 30-min period was measured for each group. A blood sample was collected from the portal vein under anesthesia, and plasma GLP-1 and CCK levels were measured by ELISA kits. Refer to Fig. 6 for abbreviations
Values represent the mean ± SEM (n = 7 or 8)
Significant difference where *p < 0.05 or **p < 0.01 was compared with controls
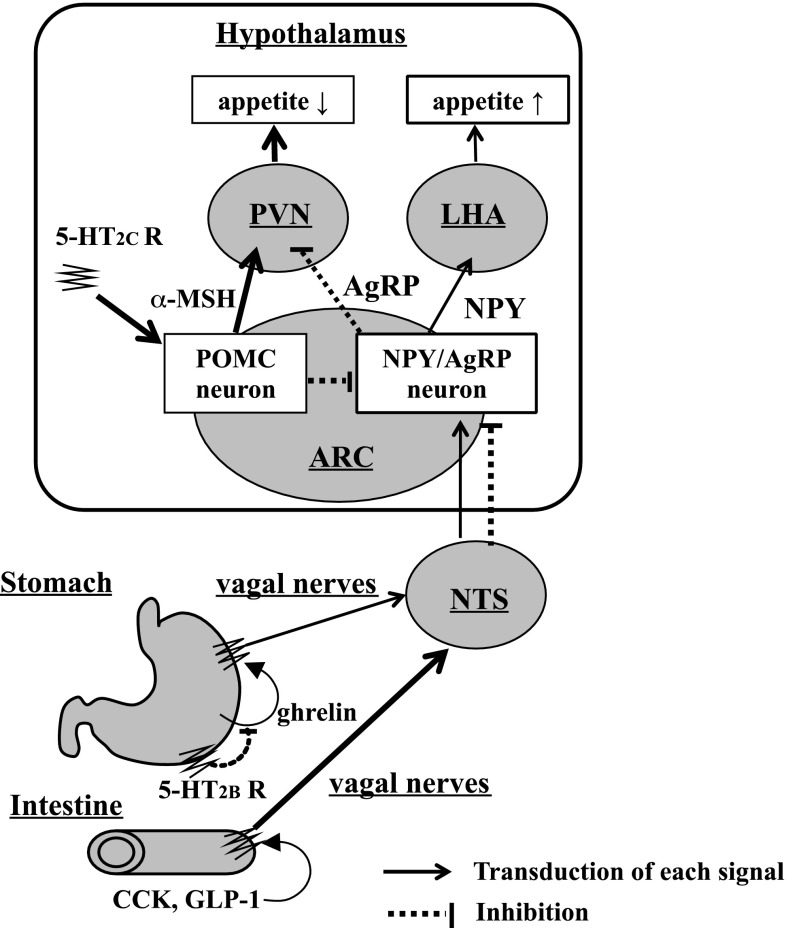
Recently, an anti-cancer drug, cisplatin, and selective serotonin reuptake inhibitors (SSRIs) have been reported to inhibit food intake, and the involvement of 5-hydroxytryptamine 2 (5-HT2) receptors in appetite control has been shown. Appetite is suppressed when the 5-HT2B receptor in gastric smooth muscle and the 5-HT2C receptor in the hypothalamus are activated. 5-HT produced during treatment with cisplatin or SSRIs binds to various receptor subtypes and is likely to stimulate 5-HT2B and 5-HT2C receptors. Stimulation of 5-HT2B receptor decreases plasma ghrelin levels, and suppresses appetite signals via afferent vagal nerves [25–27]. Consistent with previous reports, 5-HT (serotonin creatinine sulfate monohydrate, 1 mg/kg, i.p.) inhibited food intake in mice (Fig. 4d). In our study, we investigated the release of 5-HT from isolated ilea of mice and its retention in the tissues. Chakasaponin II (12) at 1.0 mM significantly enhanced the release of 5-HT into the medium and reduced its retention in the tissues [14]. This concentration is relatively high, but concentrations of saponin in the intestinal tract are thought to be generally high, since this type of compound is difficult to be absorbed [28]. Furthermore, the effects of n-BuOH-soluble fraction and chakasaponin II (12) on food intake were obviously reduced in the capsaicin-pretreated mice in which the capsaicin-sensitive sensory nerves were densensitized by pretreatment with a high dose of capsaicin (Fig. 4a, b). In our pre-examination, chakasaponin II (12) increased plasma cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1) levels in mice (Table 4). CCK and GLP-1 are known to influence satiety signals secreted from intestinal I-cells and L-cells [29]. These findings suggest that their inhibitory effects on food intake were initiated by excretion of CCK and GLP-1, and were mediated via capsaicin-sensitive sensory nerves, probably the afferent vagal nerves (Fig. 6).
Fig. 6.
Appetite signals in the gastrointestinal−brain system. NPY neuropeptide Y, AgRP agouti-related protein, MSH melanocyte-stimulating hormone, POMC proopiomelanocortin, NTS nucleus tractus solitaries, ARC arcuate nucleus, PVN paraventricular nucleus, LHA lateral hypothalamic area, CCK cholescystokinin, GLP-1 glucagon-like peptide 1. CCK and GLP-1 secreted from the intestinal I-cells and L-cells stimulate each receptor and the signals are mediated through the afferent vagal nerves and NTS to reduce the expression of NPY and AgRP, and finally reduce the appetite. Stimulation of 5-HT2B receptor in the stomach by 5-HT from chromaffin cells in the intestine inhibits the release of ghrelin which stimulates the appetite through the afferent vagal nerves, and stimulation of the 5HT2C receptor in the hypothalamus stimulates POMC neurons to reduce the appetite
Effects on gastric emptying in mice
Previously, several saponins with similar structures, escins from the seeds of A. turbinata, were documented to show antihyperlipidemic effects in lipid emulsion-loaded mice, and inhibition of pancreatic lipase activity is suggested to be partly involved in this effect [30]. However, we considered that their antihyperlipidemic effects are mainly dependent on the inhibition of gastric emptying [31]. We, therefore, examined the effects of the MeOH extract and its n-BuOH-soluble fraction on gastric emptying in mice. The amount of phenol red in the stomach was determined 30 min after oral administration of carboxymethyl cellulose sodium salt (CMC-Na) containing phenol red.
The MeOH extract (125–500 mg/kg, p.o.) and n-BuOH-soluble fraction (125–250 mg/kg, p.o.) significantly inhibited gastric emptying in a dose-dependent manner. Furthermore, chakasaponins I (11) and II (12) (25–50 mg/kg) also inhibited gastric emptying in mice [12, 14] (Table 5).
Table 5.
Effects of chakasaponins I (11)–III (13) on gastric emptying and gastrointestinal transit in mice.
Data were taken and reproduced from references [12] and [16]
| Treatment | Dose (mg/kg, p.o.) |
Gastric emptying (%) |
Gastrointestinal transit (%) |
|---|---|---|---|
| Control | – | 75.0 ± 4.1 | 40.0 ± 2.5 |
| Chakasaponin I (11) | 25 | 68.5 ± 5.0 | 43.8 ± 1.3 |
| 50 | 50.2 ± 3.0** | 49.1 ± 4.3 | |
| 100 | 37.9 ± 1.4** | 81.6 ± 3.3** | |
| Control | – | 73.1 ± 2.1 | – |
| Chakasaponin II (12) | 25 | 65.2 ± 3.9 | 64.9 ± 10.8 |
| 50 | 56.9 ± 2.7* | 52.1 ± 5.8 | |
| 100 | 40.5 ± 6.7** | 75.6 ± 11.1** | |
| Control | – | 76.8 ± 2.4 | – |
| Chakasaponin III (13) | 25 | 70.7 ± 1.9 | 51.0 ± 3.4 |
| 50 | 52.0 ± 2.7** | 64.2 ± 6.3* | |
| 100 | 39.8 ± 1.7** | 68.5 ± 2.5** | |
| Control | – | 86.2 ± 1.3 | – |
| Escin IIa | 25 | 81.7 ± 6.5 | – |
| 50 | 69.6 ± 3.0* | – | |
| 100 | 53.8 ± 4.0** | – |
Effects on gastric emptying: a solution of 1.5 % carboxymethyl cellulose sodium salt (CMC-Na) containing 0.05 % phenol red as a marker was given (0.3 mL/mouse, p.o.) to fasted male ddY mice 30 min before being killed by cervical dislocation under anesthesia. The abdominal cavity was opened, the gastroesophageal junction and pylorus were clamped, and the stomach was removed, weighed, and placed in 10 mL of 0.1 M NaOH prior to homogenization, followed by the addition of 5 mL of the supernatant to 0.5 mL of 20 % trichloroacetic acid (w/v) for centrifugation. Next, 4 mL of supernatant was mixed with an equal volume of 0.5 M NaOH, and the amount of phenol red was determined by 560 nm absorbance. The test sample was given orally 30 min before the administration of the CMC-Na solution
Gastric emptying (%) = (1− amount of phenol red in test sample-treated group/amount of administered phenol red) × 100
Effects on gastrointestinal transit: a charcoal meal containing 1.5 % CMC-Na solution and 5 % charcoal as a marker was given (0.2 mL/mouse, p.o.) to fasted male ddY mice. Fifteen minutes later, the abdominal cavity was opened, and the gastrointestinal tract was removed. The traveled distance of the marker was measured and expressed as a percentage of the total length of the small intestine from the pylorus to the cecum. The test samples were given orally 1 h before administration of the charcoal meal
Values represent the means ± SEM (n = 5–10)
Significant difference where *p < 0.05 or **p < 0.01 is compared with controls
In a previous report, we observed that the inhibitory effects of escins on gastric emptying involved the release of dopamine and dopamine2 receptors via mechanisms involving capsaicin-sensitive sensory nerves, probably certain vagal afferent nerves [32]. Tominaga et al. reported that 5-HT inhibited gastric emptying in rats [33]. Consistent with the previous report, 5-HT (serotonin creatinine sulfate monohydrate, 10 mg/kg, i.p.) significantly inhibited gastric emptying under our experimental conditions, although the effective dose of 5-HT was higher than for food intake [14].
As described above, the inhibitory effects of escins on gastric emptying involved the capsaicin-sensitive sensory nerves, probably certain vagal afferent nerves [31]. Pretreatment with capsaicin partly reduced the inhibitory effects of the n-BuOH-soluble fraction and chakasaponins I (11) and II (12) on gastric emptying, suggesting that certain afferent vagal nerves are involved, at least in part, in the inhibition of gastric emptying as well as food intake. Bugajski et al. observed that long-term vagal electrical stimulation reduces food intake and body weight in rats [34]. Therefore, in addition to release of 5-HT, CCK, and GLP-1, other mechanisms of action including the direct stimulation of the vagal nerves by saponins warrant further study.
Acceleration of gastrointestinal transit
Gastrointestinal transit accelerating effects are thought to be beneficial for the prevention and treatment of the inhibition of gastrointestinal transit, such as the effects on the ileus in clinical situations. We have previously reported that escins and theasaponin E1 showed similar effects as those of the seeds of A. hippocastanum and C. sinensis var. assamica [4, 35] which also have similar structures. The MeOH extract (500 mg/kg, p.o.) and n-BuOH-soluble fraction (200 mg/kg, p.o.) from Fujian Chaka displayed an accelerating effect on gastrointestinal transit in mice. In a similar fashion, chakasaponins I (11)–III (13) (50 or 100 mg/kg, p.o.) also demonstrated such accelerating effects [16] (Table 5).
Saponin constituents and biofunctional effects of Indian Assam Chaka
Eight acylated oleanane-type triterpene oligoglycosides, floraassamsaponins I (17)–VIII (24), together with floratheasaponins D (4), G (7) and I (9), were isolated from Indian Assam Chaka, the flower buds of C. sinensis var. assamica, and their chemical structures were determined. Floraassamsaponins I (17) and II (18) have a sugar moiety at the 28-position, although other acylated oleanane-type triterpene oligoglycosides from Japanese and Chinese Chakas do not have a sugar moiety at this position.
With regard to new biofunctional effects of Indian Assam Chaka, the effect on 42-mer amyloid β-protein (Aβ42) aggregation in vitro was examined using the Thioflavin T method. Alzheimer’s disease is a neurodegenerative disorder characterized by Aβ42 aggregation. Previously, various phenolic constituents such as flavonoids and caffeoylquinic acids inhibited Aβ42 aggregation, although studies on this type of saponins have not been attempted [36–38]. The n-BuOH-soluble fraction and floraassamsaponins III (19), IV (20), and VII (23) significantly inhibited Aβ42 aggregation (inhibition 19: 73.4 ± 8.0 %, p < 0.01; 20: 68.8 ± 8.4 %, p < 0.01; and 23: 56.9 ± 13.2 %, p < 0.01 at 100 µM, respectively). Their effects were almost equivalent to significant suppression of a positive control, morin (inhibition 54.0 ± 11.2 %, p < 0.01 at 100 µM), which is known to be one of the most potent natural compounds with inhibitory effects [37]. Further studies (including in vivo investigations) are needed, since this type of compound shows less absorption as described above [28].
In conclusion, we have described the actions of new saponins (classified as acylated oleanane-type triterpene oligoglycosides), which were isolated from Japanese, Anhui, Sichun, and Fujian Chaka and Indian Assam Chaka. Biofunctions beneficial for health promotion, such as antihyperlipidemic, antihyperglycemic, antiobesity, and gastroprotective effects in mice and rats, as well as antiallergic, pancreatic lipase inhibitory, and Aβ42 aggregation inhibitory activities (in vitro) of the extract and newly identified saponin constituents (floratheasaponins, chakasaponin, and floraassamsaponins) were revealed. Furthermore, various bioactive flavonol glycosides and epicatechines, etc. were isolated from each Chaka [11, 12, 15, 16, 18, 22], apart from their quantitative analyses reported previously [39, 40]. On the basis of this experimental evidence, a variety of health/functional foods and beverages made of Chaka have been developed recently in Japan and Taiwan.
Acknowledgments
This work was supported in part by the Academic Frontier Project and a Grant-in-Aid for scientific research from Japan Society for the Promotion of Science [JSPS KAKENHI (Grant-in-Aid for Scientific Research (C) 18510194 and 25450144 to MH, 26460135 and 23790028 to NS, 16073221 to YM)] and the Hoh-ansha Foundation, Japan.
References
- 1.Yang CS, Zhang J, Zhang L, Huang J, Wang Y. Mechanisms of body weight reduction and metabolic syndrome allevation by tea. Mol Nutr Food Res. 2016;60:160–174. doi: 10.1002/mnfr.201500428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitagawa I, Hori K, Motozawa T, Murakami T, Yoshikawa M. Structures of new acylated oleanene-type triterpene oligoglycosides, theasaponins E1 and E2, from the seeds of tea plant, Camellia sinensis (L.) O. Kuntze. Chem Pharm Bull. 1998;46:1901–1906. doi: 10.1248/cpb.46.1901. [DOI] [PubMed] [Google Scholar]
- 3.Murakami T, Nakamura J, Matsuda H, Yoshikawa M. Bioactive saponins and glycosides. XV. Saponin constituents with gastroprotective effect from the seeds of tea plant, Camellia sinensis L. var. assamica Pierre, cultivated in Sri Lanka: structures of assamsaponins A, B, C, D, and E. Chem Pharm Bull. 1999;47:1759–1764. doi: 10.1248/cpb.47.1759. [DOI] [PubMed] [Google Scholar]
- 4.Murakami T, Nakamura J, Kageura T, Matsuda H, Yoshikawa M. Bioactive saponins and glycosides. XVII. Inhibitory effect on gastric emptying and accelerating effect on gastrointestinal transit of tea saponins: structures of assamsaponins F, G, H, I, and J from the seeds and leaves of the tea plant. Chem Pharm Bull. 2000;48:1720–1725. doi: 10.1248/cpb.48.1720. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa M, Morikawa T, Li N, Nagatomo A, Li X, Matsuda H. Bioactive saponins and glycosides. XXIII. Triterpene saponins with gastroprotective effect from the seeds of Camellia sinensis—theasaponins E3, E4, E5, E6, and E7. Chem Pharm Bull. 2005;53:1559–1564. doi: 10.1248/cpb.53.1559. [DOI] [PubMed] [Google Scholar]
- 6.Morikawa T, Li N, Nagatomo A, Matsuda H, Li X, Yoshikawa M. Triterpene saponins with gastroprotective effects from tea seed (the seeds of Camellia sinensis) J Nat Prod. 2006;69:185–190. doi: 10.1021/np058097w. [DOI] [PubMed] [Google Scholar]
- 7.Morikawa T, Matsuda H, Li N, Nakamura S, Li X, Yoshikawa M. Bioactive saponins and glycosides. XXVI. New triterpene saponins, theasaponins E10, E11, E12, E13, and G2, from the seeds of tea plant (Camellia sinensis) Heterocycles. 2006;68:1139–1148. doi: 10.3987/COM-06-10704. [DOI] [Google Scholar]
- 8.Yoshikawa M, Morikawa T, Nakamura S, Li N, Li X, Matsuda H. Bioactive saponins and glycosides. XXV. Acylated oleanane-type triterpene saponins from the seeds of tea plant (Camellia sinensis) Chem Pharm Bull. 2007;55:57–63. doi: 10.1248/cpb.55.57. [DOI] [PubMed] [Google Scholar]
- 9.Morikawa T, Matsuda H, Li N, Li X, Yoshikawa M. Bioactive saponins and glycosides part 29. Acylated oleanane-type triterpene saponins: theasaponins A6, A7, and B5 from the seeds of Camellia sinensis. Helv Chim Acta. 2007;90:2342–2348. doi: 10.1002/hlca.200790240. [DOI] [Google Scholar]
- 10.Morikawa T, Nakamura S, Kato Y, Muraoka O, Matsuda H, Yoshikawa M. Bioactive saponins and glycosides. XXVIII. New triterpene saponins, foliatheasaponins I, II, III, IV, and V, from Tencha (the leaves of Camellia sinensis) Chem Pharm Bull. 2007;55:293–298. doi: 10.1248/cpb.55.293. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa M, Morikawa T, Yamamoto K, Kato Y, Nagatomo A, Matsuda H. Floratheasaponins A–C, acylated oleananetype triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of tea plant (Camellia sinensis) J Nat Prod. 2005;68:1360–1365. doi: 10.1021/np0580614. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda H, Hamao M, Nakamura S, Kon’i H, Murata M, Yoshikawa M. Medicinal flowers. XXXIII. Anti-hyperlipidemic and anti-hyperglycemic effects of chakasaponins I–III and structure of chakasaponin IV from flower buds of Chinese tea plant (Camellia sinensis) Chem Pharm Bull. 2012;60:674–680. doi: 10.1248/cpb.60.674. [DOI] [PubMed] [Google Scholar]
- 13.Yoshikawa M, Wang T, Sugimoto S, Nakamura S, Nagatomo A, Matsuda H, Harima S. Functional saponins in tea flower (flower buds of Camellia sinensis): gastroprotective and hypoglycemic effects of floratheasaponins and qualitative and quantitative analysis using HPLC. Yakugaku Zasshi. 2008;128:141–151. doi: 10.1248/yakushi.128.141. [DOI] [PubMed] [Google Scholar]
- 14.Hamao M, Matsuda H, Nakamura S, Nakashima S, Semura S, Maekubo S, Wakasugi S, Yoshikawa M. Anti-obesity effects of the methanolic extract and chakasaponins from the flower buds of Camellia sinensis in mice. Bioorg Med Chem. 2011;19:6033–6041. doi: 10.1016/j.bmc.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa M, Nakamura S, Kato Y, Matsuhira K, Matsuda H. Medicinal flowers. XIV. New acylated oleanane-type triterpene oligoglycosides with antiallergic activity from flower buds of Chinese tea plant (Camellia sinensis) Chem Pharm Bull. 2007;55:598–605. doi: 10.1248/cpb.55.598. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa M, Sugimoto S, Kato Y, Nakamura S, Wang T, Yamashita C, Matsuda H. Acylated oleanane-type triterpene saponins with acceleration of gastrointestinal transit and inhibitory effect on pancreatic lipase from flower buds of Chinese tea plant (Camellia sinensis) Chem Biodiv. 2009;6:903–915. doi: 10.1002/cbdv.200800153. [DOI] [PubMed] [Google Scholar]
- 17.Ohta T, Nakamura S, Nakashima S, Matsumoto T, Ogawa K, Fujimoto K, Fukaya M, Yoshikawa M, Matsuda H. Acylated oleanane-type triterpene oligoglycosides from the flower buds of Camellia sinensis var. assamica. Tetrahedron. 2015;71:846–851. doi: 10.1016/j.tet.2014.12.049. [DOI] [Google Scholar]
- 18.Sugimoto S, Yoshikawa M, Nakamura S, Matsuda H. Medicinal flowers. XXV. Structures of floratheasaponin J and chakanoside II from Japanese tea flower, flowerbuds of Camellia sinensis. Heterocycles. 2009;78:1023–1029. doi: 10.3987/COM-09-11799. [DOI] [Google Scholar]
- 19.Morikawa T, Miyake S, Miki Y, Ninomiya K, Yoshikawa M, Muraoka O. Quantitative analysis of acylated oleanane-type triterpene saponins, chakasaponins I–III and floratheasaponins A–F, in the flower buds of Camellia sinensis from different regional origins. J Nat Med. 2012;66:608–613. doi: 10.1007/s11418-012-0627-1. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda H, Nakamura S, Yoshikawa M. Degranulation inhibitors from medicinal plants in antigen-stimulated rat basophilic leukemia (RBL-2H3) cells. Chem Pharm Bull. 2016;6:96–103. doi: 10.1248/cpb.c15-00781. [DOI] [PubMed] [Google Scholar]
- 21.Park EK, Choo MK, Kim EJ, Han MJ, Kim DH. Antiallergic activity of ginsenoside Rh2. Chem Pharm Bull. 2003;26:1581–1584. doi: 10.1248/bpb.26.1581. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa M, Sugimoto S, Nakamura S, Matsuda H. Medicinal flowers. XXII. Structures of chakasaponins V and VI, chakanoside I, and chakaflavonoside A from flower buds of Chinese tea plant (Camellia sinensis) Chem Pharm Bull. 2008;56:1297–1303. doi: 10.1248/cpb.56.1297. [DOI] [PubMed] [Google Scholar]
- 23.Hays T, Rusyn I, Burns AM, Kennett MJ, Ward JM, Gonzalez FJ, Peters JM. Role of peroxisome proliferator-activated receptor-α (PPARα) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis. 2005;26:219–227. doi: 10.1093/carcin/bgh285. [DOI] [PubMed] [Google Scholar]
- 24.Shimpson KA, Martin NM, Bloom SR. Hypothalamic regulation of food intake and clinical therapeutic applications. Arq Bras Endocrinol Metab. 2009;53:120–128. doi: 10.1590/s0004-27302009000200002. [DOI] [PubMed] [Google Scholar]
- 25.Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004–2013. doi: 10.1053/j.gastro.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 26.Yakabi K, Kurosawa S, Tamai M, Yuzurihara M, Nahata M, Ohno S, Ro S, Kato S, Aoyama T, Sakurada T, Takabayashi H, Hattori T. Rikkunshito and 5-HT2C receptor antagonist improve cisplatin-induced anorexia via hypothalamic ghrelin interaction. Regul Pept. 2010;161:97–105. doi: 10.1016/j.regpep.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Hattori T (2010) Rikkunshito and ghrelin. Int J Pept 2010: Article ID 283549, 3 pages [DOI] [PMC free article] [PubMed]
- 28.Henschler D, Hempel K, Schultze B, Maurer W. The pharmakokinetics of escin. Arzneiimittelforschung. 1971;21:1682–1692. [PubMed] [Google Scholar]
- 29.Côté CD, Zadeh-Tahmasebi M, Rasmussen BA, Duca FA, Lam TK. Hormonal signaling in the gut. J Biol Chem. 2014;289:11642–11649. doi: 10.1074/jbc.O114.556068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu JN, Zhu XM, Han LK, Saito M, Sun YS, Yoshikawa M, Kimura Y, Zheng YN. Anti-obesity effects of escins extracted from the seeds of Aesculus turbinata BLUME (Hippocastanaceae) Chem Pharm Bull. 2008;56:12–16. doi: 10.1248/cpb.56.12. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda H, Li Y, Murakami T, Yamahara J, Yoshikawa M. Effects of escins Ia, Ib, IIa, and IIb from horse chestnuts on gastric emptying in mice. Eur J Pharmacol. 1999;368:237–243. doi: 10.1016/S0014-2999(99)00035-7. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda H, Li Y, Yoshikawa M. Possible involvement of dopamine and dopamine2 receptors in the inhibitions of gastric emptying by escin Ib in mice. Life Sci. 2000;67:2921–2927. doi: 10.1016/S0024-3205(00)00876-6. [DOI] [PubMed] [Google Scholar]
- 33.Tominaga K, Kido T, Ochi M, Sadakane C, Mase A, Okazaki H, Yamagami H, Tanigawa T, Watanabe K, Watanabe T, Fujiwara Y, Oshitani N, Arakawa T (2011) The traditional Japanese medicine rikkunshito promotes gastric emptying via the antagonistic action of the 5-HT3 receptor pathway in rats. Evid Based Complement Alternat Med 2011: Article ID 248481, 8 pages [DOI] [PMC free article] [PubMed]
- 34.Bugajski AJ, Gil K, Ziomber A, Zurowski D, Zaraska W, Thor PJ. Effect of long-term vagal stimulation on food intake and body weight during diet induced obesity in rats. J Physiol Pharmacol. 2007;58(Suppl. 1):5–12. [PubMed] [Google Scholar]
- 35.Matsuda H, Li Y, Yoshikawa M. Effects of escins Ia, Ib, IIa, and IIb from horse chestnuts on gastrointestinal transit and ileus in mice. Bioorg Med Chem. 1999;7:1737–1741. doi: 10.1016/S0968-0896(99)00100-5. [DOI] [PubMed] [Google Scholar]
- 36.Ono K, Hamaguchi T, Naiki H, Yamada M. Anti-amyloidogenic effects of antioxidants: implications for the prevention and therapeutics of Alzheimer’s disease. Biochim Biophys Acta. 2006;1762:575–586. doi: 10.1016/j.bbadis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 38.Miyamae Y, Kurisu M, Murakami K, Han J, Isoda H, Irie K, Shigemori H. Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid β-protein. Bioorg Med Chem. 2012;20:5844–5849. doi: 10.1016/j.bmc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Morikawa T, Ninomiya K, Miyake S, Miki Y, Okamoto M, Yoshikawa M, Muraoka O. Flavonol glycosides with lipid accumulation inhibitory activity and simultaneous quantitative analysis of 15 polyphenols and caffeine in the flower buds of Camellia sinensis from different regions by LCMS. Food Chem. 2013;140:353–360. doi: 10.1016/j.foodchem.2013.02.079. [DOI] [PubMed] [Google Scholar]
- 40.Morikawa T, Lee IJ, Okugawa S, Miyake S, Miki Y, Ninomiya K, Kitagawa N, Yoshikawa M, Muraoka O. Quantitative analysis of catechin, flavonoid, and saponin constituents in “tea flower”, the flower buds of Camellia sinensis, from different regions in Taiwan. Nat Prod Commun. 2013;8:1553–1557. [PubMed] [Google Scholar]